Abstract
Objectives. Musculoskeletal symptoms are common in SLE and are associated with significant morbidity. However, assessing their nature can be challenging, with implications for treatment decisions and measuring response. US has been shown to be valid and reliable for the assessment of other inflammatory arthritides, but data in SLE are more limited. The objectives of this systematic literature review were to determine the characteristics of musculoskeletal US abnormalities in SLE and to evaluate the metric properties of US in the detection and quantification of musculoskeletal symptoms.
Methods. We systematically searched the literature using the PubMed, Embase and Cochrane Library databases for studies using musculoskeletal US for assessing SLE. Studies were assessed for quality using the Quality Assessment of Diagnostic Accuracy Studies tool and for their metric qualities, including reliability and validity.
Results. Nine studies were identified. Most studies investigated construct validity. Rates of abnormality were highly variable: synovitis and tenosynovitis were reported in 25–94% and 28–65% of patients, respectively; power Doppler and erosions were reported in 10–82% and 2–41% of patients, respectively. There was poor to moderate association between US abnormalities and disease activity indices and immunological findings. There was moderate to high risk of bias and there were concerns about applicability in most studies.
Conclusion. US has potential value in the assessment of musculoskeletal symptoms in SLE. However, there is methodological variation between studies that may account for lack of consensus on US abnormalities. Studies that address these problems are required before US can used as an outcome measure in SLE.
Keywords: systematic literature review, systematic lupus erythematosus, ultrasound, arthritis, tenosynovitis, Power Doppler, BILAG
Rheumatology key messages
Ultrasound has a promising role in the detection and assessment of musculoskeletal pathology in SLE.
The literature data on ultrasound abnormality rates are highly variable.
Further criterion and discrimination validation is required before using ultrasound as an outcome-measure in SLE.
Introduction
SLE is a complex, chronic multisystem autoimmune disease with a variable spectrum of manifestations, ranging from mild musculoskeletal or cutaneous disease to potentially life-threatening renal, cardiac or CNS disease. Musculoskeletal manifestations are very common and can be the first presenting symptom in up to 50% of cases, and they affect up to 95% of patients during the clinical course [1]. Musculoskeletal presentations in SLE patients can include arthritis, tenosynovitis, transient or migratory arthralgia and non-specific musculoskeletal symptoms without definitive objective clinical signs [2]. Despite the absence of clinical synovitis in many patients, musculoskeletal manifestations cause significant disability, loss of function and socio-economic impact [3, 4].
The low frequency of clinical synovitis makes measurement of musculoskeletal disease activity more difficult. This has consequences for the selection of patients for immunosuppressive therapy and the measurement of response to such therapies. Clinical disease activity instruments have been validated, including BILAG 2004 [5] and SLEDAI [6]. However, these are based on the clinical detection of joint swelling, so may fail to capture disease activity in many patients. Further, the non-specific clinical features of many forms of musculoskeletal disease in SLE are difficult to distinguish from other non-inflammatory causes using clinical features alone. Therefore, there is an unmet need for a more sensitive and specific tool for assessing joints and tendons in SLE. High-resolution musculoskeletal US has demonstrated potential utility in the assessment of other forms of inflammatory arthritis, particularly in low disease activity states such as early arthritis or remission [7, 8].
As well as the assessment of SLE musculoskeletal disease activity, musculoskeletal imaging may also be used to understand the pathogenesis of arthritis. For example, SLE arthropathy has traditionally been classified clinically into Rhupus (RA/SLE overlap), Jaccoud’s arthropathy (little or no evidence of synovitis or erosion on conventional radiographs, but with extensive deformities in hands and feet) and non-erosive non-deforming arthropathy [9]. However, reports of erosive changes in patients previously considered to have non-erosive, benign disease (based on the use of newer more sensitive modalities such as MRI and US) challenge this concept [10–12].
Although there are growing numbers of reports on the use of US in SLE, the interpretation of these findings remains unclear, as well as the validity and reliability of this modality. Furthermore, US is an operator-dependent tool that can be affected significantly by acquisition and interpretation methods. It will be important to understand the potential methodological issues that can affect US use in SLE, given most of the current available validated definitions and scoring methods have been devised for use in inflammatory arthropathies. The objectives of this systematic review of literature were: to determine the characteristics of US abnormalities (corresponding to arthritis, tenosynovitis and erosions) of SLE patients, and to evaluate the metric properties of US in the detection and quantification of arthritis and tenosynovitis.
Methods
Search strategy and selection
The search of articles was performed using the PubMed, Embase and Cochrane Library databases from 1 January 1950 to 1 August 2014 because musculoskeletal US was first reported in 1958 by Dussik et al. [13]. The MeSH terms used were (ultrasonography OR ultrasound OR ultrasonics) AND [lupus OR (systemic AND lupus AND erythematosus)]. The search was restricted to studies on humans and in the English language. Manual searches were also conducted to screen for grey literature and articles published in 2014 that were not yet available in the databases. The following journals were searched: Annals of the Rheumatic Diseases, Arthritis and Rheumatism, Arthritis Research and Therapy, Arthritis Care and Research, Nature Reviews (Rheumatology), Rheumatology, Seminars in Arthritis and Rheumatism, and Lupus. This systematic review was registered on PROSPERO (prospective registration of systematic reviews); http://www.crd.york.ac.uk/PROSPERO/ (registration number: CRD42014013312).
Titles and abstracts were screened by two reviewers (A.Z. and M.Y.). If an abstract was selected by a reviewer, the full-text article was retrieved and subsequently screened for eligibility criteria prior to selection for review. The inclusion criteria were: original research on the use of US for assessment of musculoskeletal symptoms in non-rhupus SLE patients; patients fulfilled the revised ACR classification criteria for SLE [14]. The following exclusion criteria were used: editorials, reviews, case reports, letters to the editor and conference abstracts; studies reporting rhupus patients only; and studies of paediatric lupus patients. Any disagreement in the selection process was resolved by consensus.
The same reviewers (A.Z. and M.Y.) extracted the data from the selected articles using a standardized template designed for this review. The following data were extracted: type of study, type of validity, number of patients, number of controls, blinding, type of joints and tendons scanned, scoring methodology used [i.e. OMERACT/EULAR [15] or binary (yes/no)], US definition of synovitis, tenosynovitis and other tendon abnormalities, US settings and the mode used [i.e. grey scale (GS) or power Doppler (PDo)].
Quality assessment
The methodological quality of each study was assessed independently by two reviewers (A.Z. and M.Y.) using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) instrument [16]. QUADAS-2 is a revised version from the previous QUADAS. This instrument is recommended for use in systematic reviews of diagnostic accuracy by the National Institute for Health and Care Excellence, the Agency for Healthcare Research and Quality and the Cochrane Collaboration [17]. It consists of four key domains, including patient selection, index test, reference standard, and flow and timing. The generic QUADAS-2 signalling questions usually used to judge the risk of bias were adapted for this systematic (supplementary Table S1, available at Rheumatology Online). Any discrepancy was resolved by discussion.
Studies were also assessed for their metric properties: criterion, construct and discriminant validity. Criterion validity is determined by comparison with an optimal reference standard, such as histology. Construct validity was determined by comparison with other techniques measuring similar properties, and we therefore included clinical examination, SLE-related autoimmune profile, disease activity indices, conventional radiograph and or MRI. Analysis of discriminant validity of US in assessing SLE musculoskeletal symptoms was included if the interobserver and/or intraobserver reliability were reported by the authors.
Statistical analysis
Descriptive statistics were used to report data. Frequencies and percentages were shown for categorical variables.
Results
Selection of studies
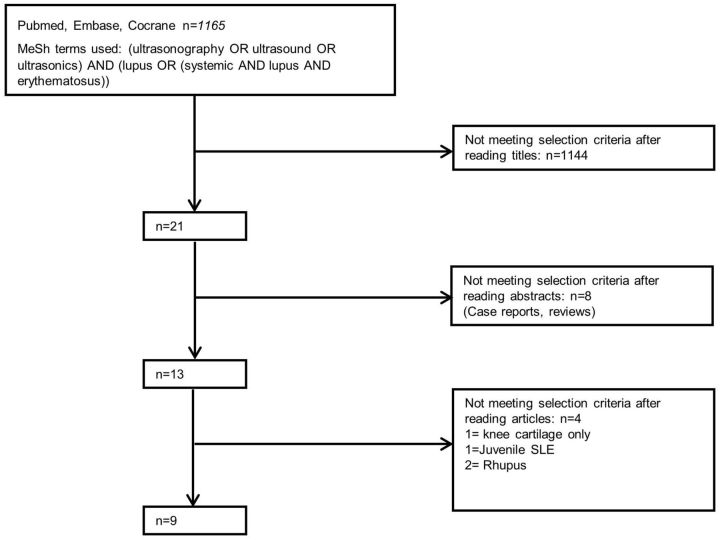
The search yielded 1165 citations, of which 1144 were rejected after reviewing the title and the abstracts. Subsequently, 21 full-text articles were retrieved and reviewed to determine eligibility for this review. Nine studies were included in the final analysis [12, 18–25]. Fig. 1 shows the flowchart of the systematic review process.
Fig. 1.
Flowchart of the systematic review process with number of articles included and excluded
Rhupus: RA/SLE overlap.
Study characteristics
All nine studies included in this review were cross-sectional. Eight studies examined wrists and hand joints [12, 18, 19, 21–25], one study examined knees only [20] and one study included foot joints [25]. Six studies included tendons in their results [12, 18, 19, 21–23]. The study protocol was consistent in all studies, with clinical assessment, blood tests, conventional radiograph and US all performed on the same day.
A total of 459 SLE patients were included in the studies overall. Sample sizes ranged from 17 to 108 patients. Sample size calculations to determine the power of studies were not reported. There was heterogeneity in the populations studied, because only two studies clearly separated the rhupus group and the non-rhupus SLE group [22, 24]. Five studies included patients who did not complain of musculoskeletal symptoms at the time of the assessment [19, 21, 22, 24, 25]. Age and sex distributions were fairly consistent for most studies (mean or median age of patients between 37 and 47 years old and predominantly female patients). The disease duration was also similar across all studies, with mean or median between 10 and 15 years, apart from one study with shorter duration; median 6.2 years [21]. In five studies, normal volunteers were used as the control group [18–20, 22, 26], while three studies recruited RA patients as the control group [20, 23, 24]. The characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the included studies
| References | Year | Number of patients | Mean age, years | Mean disease duration, years | Joints assessed | Tendons assessed | US modes used | Synovitis definition used | GS and PDo scoring reported | Tenosynovitis reported | Tenosynovitis definition used | Tenosynovitis scoring reported | Erosions | No. of controls | US machine used | Frequency, MHz |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iagnocco et al. [18] | 2004 | 26 | 40.3 | 15.1 | Wrist, RCJ | Wrist flexors and extensors | GS, PDo | NV | B | Y | NV | B | Y | 15 | Agilent HP Image point Hx | 14 |
| Wright et al. [12] | 2006 | 17 | 37.6a | 13a | Wrist, second and third MCP and PIP | Second, third and fourth hand flexors | GS, PDo | OM | B | Y | OM | B | Y | 0 | Siemens Sonoline Antares | 5–13 |
| Delle Sedie et al. [19] | 2009 | 50 | 39.6 | 10.4 | Wrist, second and third MCP and PIP | ECU, second and third hand flexors | GS, PDo | OM | B | Y | OM | B | Y | 50 | Logiq 9 (GE) | 14 |
| Ossandon et al. [20] | 2009 | 26 | 40 | 15 | Knee | NA | GS, PDo | NV | B | N | NA | NA | Y | 15 | Agilent HP Image point Hx | 10 |
| Torrente-Segarra et al. [21] | 2013 | 58 | 43.1 | 6.2 | Wrist, all MCP and PIP | Hand and wrist flexors and extensors | GS, PDo | OM | B | Y | OM | B | N | 0 | Logic 5 Expert (GE) | 5–12 |
| Gabba et al. [22] | 2012 | 108 | 43.7 | 10.8 | Wrist, second and third MCP | Hand and wrist flexors and extensors | GS, PDo | OM | B | Y | OM | B | Y | 60 | Loqiq 9 (GE) | 8–15 |
| Iagnocco et al. [25] | 2014 | 62 | 42.8 | 11.2 | Wrist, all MCP, PIP and MTP | NA | GS, PDo | OM | B | N | NA | NA | N | 0 | MyLab70 Xvision Gold (Esaote) | 6–18 |
| Ball et al. [23] | 2014 | 50 | 46.7 | 12 | Wrist, second, third, fifth MCP and PIP | Hand and wrist flexors and t extensors | GS, PDo | OM | NA | Y | OM | NA | Y | 15 | Siemens Sonoline Antras | 13–15 |
| Buosi et al. [24] | 2014 | 62 | 39.9 | 11.7 | Wrist, all MCP, PIP and DRU | NA | GS, PDo | OM | OM | N | NA | NA | Y | 0 | MyLab60 Xvision (Esaote) | 6–18 |
aMedian. ECU: Extensor Carpi Ulnaris; GS: Grey scale; PDo: power Doppler; Y: Yes; N: No; NA: not applicable; NV; not validated; B: Binary; OM: OMERACT; GE: general electric.
Study quality assessment
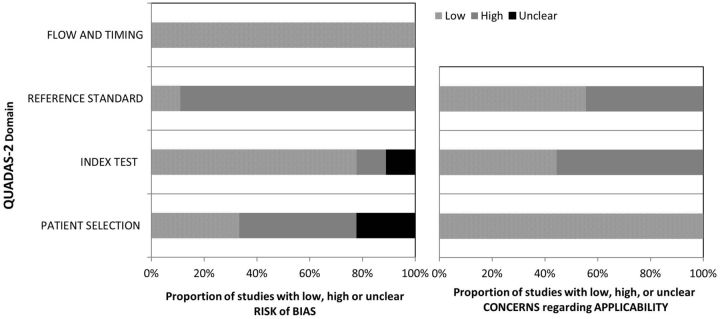
The quality of each study is summarized in Table 2. The overall quality of the studies included showed moderate to high risk of bias and applicability concerns as assessed using the QUADAS-2 instrument (Fig. 2). Only one study had low risk in both domains [22]. In terms of bias, two studies showed lower risk of bias than others [22, 24]. Most studies either did not clarify whether rhupus patients were separated in the analysis or did not report RF, which could have affected US findings [12, 18–24]. With regards to concerns about the applicability of each individual study to the proposed research question, only three studies showed low risk [12, 21, 26]. Most studies did not specify the period permitted for use of concomitant NSAIDS and oral prednisolone prior to US examination in the index test [12, 18, 19, 21–25], both potentially important confounding factors [27, 28].
Table 2.
QUADAS-2 Risk of bias and applicability concerns assessment
| Study | Risk of bias |
Applicability concerns |
|||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Wright et al. [12] | Low | Unclear | High | Low | Low | Low | Low |
| Iagnocco et al. [18] | High | Low | High | Low | Low | High | High |
| Delle Sedie et al. [19] | High | Low | High | Low | Low | High | High |
| Ossandon et al. [20] | Unclear | Low | High | Low | Low | High | Low |
| Torrente-Segarra et al. [21] | Unclear | Low | High | Low | Low | Low | Low |
| Gabba et al. [22] | Low | Low | Low | Low | Low | High | Low |
| Iagnocco et al. [25] | High | Low | High | Low | Low | High | High |
| Ball et al. [23] | High | High | High | Low | Low | Low | Low |
| Buosi et al. [24] | Low | Low | High | Low | Low | Low | High |
QUADAS-2: quality assessment of diagnostic accuracy studies.
Fig. 2.
Methodological quality of the studies included in the review
QUADAS-2: quality assessment of diagnostic accuracy studies.
Definition of US abnormality
All studies except two [18, 20] used the OMERACT definition of synovitis [15], and among those that looked at tenosynovitis, all except one [18] used the OMERACT definition of tenosynovitis [15]. Four studies described using semi-quantitative scoring for GS PDo in the methods [21, 23–25]. However, only one reported their findings in details [24]. The remaining reported binary findings (synovitis/no synovitis) or might have given a mean US score for synovitis without specifying GS or PDo and/or rates of different scores. The remaining five studies reported using a binary scoring system.
Characteristics of US abnormality in SLE
Synovitis
All nine studies used grade 1 GS with or without PDo to define synovitis. Overall, synovitis (based on a pre-specified definition) was detected in 25–94% of SLE patients, as shown in Table 3. GS was detected in various joints, assessed as follows: wrist 22–94% [12, 18, 21, 24, 25]; MCP joint 11–84% [12, 21, 24, 25]; proximal PIP joint 7–58% [21, 24, 25]; knees 42% [20]; and MTP joint 50% [25]. PDo was reported in 10–82% of SLE patients overall. The range of joints in which PDo was detected were: wrist 11–82% [12, 18, 21, 24, 25]; MCP 10–35% [12, 24, 25]; PIP 3–16% [24, 25]; and MTP 4.8% [25]. The one study that reported the semi-quantitative scale in scoring showed grade 3 GS in wrists (12%), in MCP (53%) and in PIP (24%), as well as grade 3 PDo in wrists (6%), MCP (7%) and PIP (4%) [24].
Table 3.
Characteristics and frequency of US abnormality in SLE patients
| Author | Clinical arthritis/ arthralgia (%) | US synovitis (OMERACT) (%) | GS (%) | PDo (%) | Tenosynovitis (%) | Erosion (%) | Reliability, κ |
|---|---|---|---|---|---|---|---|
| Wright et al. [12] | 100 | 94a | Wrist 94 MCP 71a | Wrist 82 MCP 35a | FT 65 | MCP 41b | Inter for synovitis 0.85 Inter for Erosion 0.78 |
| Iagnocco et al. [18] | 10 | 58a | Wrists 42c | Wrists 9.90d | FT 44.2a | Wrist 3.8 | inter 0.73–0.89 intra 0.82–1 |
| Delle Sedie et al. [19] | 60 | Wrist 80 Hand 50a | NS | NS | 28a | 2 | No |
| Ossandon et al. [20] | 40 | Knees 58a | 42a | 15a | NA | 0 | No |
| Torrente-Segarra et al. [21] | 48 | 25 | Wrists 25 MCP 11 PIP 7 | Wrist 13 | ET 39 FT 7 | NA | Inter ≥ 0.85 Intra ≥ 0.78 |
| Gabba et al. [22] | 36 | 25d | NS | 13b | 36b | 21b | Inter 0.68–0.96 |
| Iagnocco et al. [25] | 40 | NS | Wrist 22 MCP 24 PIP 9.7 MTP 50 | Wrist 11 MCP 10 PIP 3 MTP 5 | NA | NA | No |
| Ball et al. [23] | 100 | NS | NS | NS | 38b | No | |
| Buosi et al. [24] | 100 | Wrist 47 MCP 84 PIP 58 | Wrist 47 MCP 84 PIP 58 | Wrist 31 MCP 28 PIP 16 | NA | Wrist 18 MCP 13 PIP 4 | Inter 0.40–0.78 GS Inter 0.60–1 PDo, 0.53–0.77 erosion |
aPercentage of patients. bRecalculated from study tables after excluding Rhupus (not presented in original paper). cPercentage of joints. dData including Rhupus. NS: not specified; GS: grey scale; PDo: power Doppler; Rhupus: RA/SLE overlap; FT: flexor hand tendons; ET: extensor wrist tendons.
Erosion
Erosions were reported in 2–41% of SLE patients [12, 18, 19, 22–24]. Of these, only three studies clearly separated the non-rhupus and rhupus group [12, 22, 23]. Of the non-rhupus SLE group, only one study clearly defined the Jaccoud’s arthropathy subset, in which erosions were detected in 17% of patients [22]. Absence of erosion was reported in one study [20]. A higher prevalence of erosion (88%) was reported in the rhupus group [22].
Tenosynovitis and tendinopathy
Tenosynovitis was reported in 28–65% of the SLE patients, mainly affecting the extensor and flexor tendons of the wrists [12, 19, 21, 22, 25]. Tendon rupture was reported in one patient [12]. In the rhupus group, tenosynovitis was reported in 63% of the patients [22].
Metric properties of US
Validity
None of the included studies assessed criterion validity by comparison with a gold standard for histology or arthroscopy findings. Therefore, meta-analysis of sensitivity and specificity of the use of US compared with a gold standard test could not be performed. All nine studies (100%) assessed construct validity. The comparator most commonly used was disease activity indices (n = 9, 100%), followed by clinical examination (8, 89%), SLE-related autoantibodies (7, 78%) and conventional radiographs (2, 23%). Of the nine studies, only three reported positive associations between SLEDAI score and US findings [18, 22, 23], while one study reported a negative association (higher SLEDAI score was associated with an absence of tenosynovitis on US) [25]. All eight studies reported positive associations between clinical examination and US findings. Four studies reported US abnormalities in joints and tendons in asymptomatic SLE patients, ranging from 5 to 49% [20–22, 25]. A few studies reported positive associations between immunology findings and US (Table 4). However, these associations were not consistent across the literature. Comparing US with other imaging modality, US detected erosion in three out of eight patients (38%), for which conventional radiography failed to demonstrate this abnormality [12].
Table 4.
Correlation between clinical and immunological findings with US
| Author | Clinical assessment index | Was association of US with clinical findings specifically studied? | Was association of US with clinical indices found? | Blood investigations reported | Was association of US with laboratory investigations studied? | Was association of US with serology found? |
|---|---|---|---|---|---|---|
| Wright et al. [12] | SLAM, JAI, ACR/SLICC | Yes | No | ANA, ds DNA, Ro, RNP, RF, APL | No | NA |
| Iagnocco et al. [18] | SLEDAI | Yes | Yes | C3, ESR | Yes | No |
| Delle Sedie et al. [19] | ECLAM | Yes | No | ANA, ENA, dsDNA, RF, CCP, C3, C4, ESR, CRP | No | NA |
| Ossandon et al. [20] | SLEDAI, TJC/SJC | Yes | No | C3, ESR | Yes | No |
| Torrente-Segarra et al. [21] | SLEDAI, mHAQ | Yes | No | ESR, CRP, ds DNA, C3, C4, CH50 | Yes | Yes, with high ds DNA |
| Gabba et al. [22] | Musculoskeletal BILAG, ACR/SLICC, SLEDAI | Yes | Yes for SLEDAI and BILAG | ESR, CRP, ANA, ds DNA, anti CCP, C3 and C4 | Yes | Yes, with low C3 and C4 |
| Iagnocco et al. [25] | SLEDAI-2K, ECLAM | Yes | No | ANA, ENA, ds DNA, C3 and C4, CRP | Yes | No |
| Ball et al. [23] | JAI, SJC, TJC, BILAG, SELENA-SLEDAI, SLICC | Yes | Yes for SELENA-SLEDAI and BILAG MS | IL6 | Yes | Yes, with high IL6 |
| Buosi et al. [24] | SLEDAI-2K, HAQ, SJC, TJC, VAS | Yes | Yes for HAQ, DAS 28 SLEDAI (only second MCP PDo) | Homogeneous ANA | Yes | Yesa |
aReported sporadic associations between clinical and particular joints US positivity (e.g.: ANA is associated with synovial hypertrophy in second and fourth MCP and first PIP). NA: not applicable; JAI: Jaccoud’s arthropathy index; TJC: tender joint count; SJC: swollen joint count; Ro: Anti Ro/SSA antibody; mHAQ: modified Health assessment questionnaire; VAS: visual analogue scale; 2K: 2000; SELENA: Safety of Estrogens in Lupus Erythematosus National Assessment; C3: Complement component 3; C4: Complement component 4; CH50: 50% Haemolytic Complement.
Reliability
In terms of discriminant validity, five studies (56%) reported inter and/or intrareader reliability [12, 18, 21, 22, 24]. The range of intrareader reliability was between 0.78 and 1.00 (good to excellent agreement), while the interreader reliability ranged between 0.68 and 0.96 (good to excellent agreement). One study had moderate interreader reliability [24].
Discussion
This systematic literature review (SLR) investigated evidence for the use of US in the evaluation of musculoskeletal manifestations in SLE. We have analysed heterogeneity in study design, populations studied, and confounding and reporting methodology. The current evidence supports further research into the use of US in assessing symptomatic SLE patients, among whom high prevalence of US abnormalities (including synovitis and tenosynovitis) can be found. However, many studies suffered from high risk of bias and lacked clarification of synovitis, tenosynovitis and erosion severity.
A first key question concerns the potentially superior ability of US to objectively measure joint and tendon inflammation compared with clinical examination. Rates of US-detected synovitis reported in these studies vary widely. For example, 10–82% of patients had PDo abnormality. Nevertheless, there are sufficient data in these studies to indicate the presence of US synovitis in symptomatic patients without clinical joint swelling. This may be important in the stratification of symptomatic patients for immunosuppressive therapy, and for the accurate measurement of response to such therapy. However, the inconsistency between studies in rates of abnormality needs to be resolved. This may relate to US reporting methodology or characteristics of the populations studied.
A number of US methodological issues may have influenced the studies in this review. The OMERACT definition of synovitis was used in most studies. However, most studies did not report the actual proportion of joints or patients with each GS and/or PDo score (grading GS and PDo for each joint using a semi-quantitative 0–3 scale). In most studies, grade 1 GS was considered sufficient to define synovitis. Grade 1 GS without PDo lacks specificity and can be found in OA [29] and anecdotally in joints with a tendency to hypermobility, presumed secondary to mechanical stress on joints. The exact significance of PDo as a marker of disease activity in SLE is not known, given the lack of other validity filters such as criterion and responsiveness in the current available evidence. The current evidence also lacks standardization regarding the use of steroids, NSAIDS and immunosuppressive therapy at the time of imaging, all which may have a significant confounding effect on GS and PDo synovitis scores [27].
A second key question concerns the ability of US to more sensitively detect bone erosion and also to detect a non-rhupus cause of symptoms. As for US-detected synovitis, there was great variability in the reported rates of erosion in SLE (from 2 to 41%). Most studies failed to clearly separate rhupus from pure SLE. Rhupus may be defined by clinical observation of RA-like synovitis with radiographic erosion. This distinction is more complex when incorporating US; some of the patients clinically defined as non-rhupus may be found to have more widespread synovitis and erosion on US. An immunopathogenic definition is probably more useful. Serology for RF and anti-CCP may be used to identify patients with immunopathogenesis more in keeping with RA than SLE. CCP+ Rhupus patients will also differ in concomitant and prior therapies, and differences in the time to initiation of MTX may be important in rate of erosion. Consistent evaluation and reporting of clinical phenotype will be important in achieving consistency in US evaluation.
In studies that did separate these phenotypes, erosions were found on US in clinical disease subtypes traditionally thought to be non-erosive. In the Gabba et al. study, US finding of erosion was detected in the non-deforming non-erosive arthropathy group and in Jaccoud’s arthropathy [albeit the small size sample (n = 6) in the latter]. This, along with MRI findings of erosion [10], challenge our previous clinical definitions of SLE-associated arthritis subtypes. Indeed, these observations may have influenced the recognition in the 2012 SLICC classification criteria that some SLE arthritis may in fact be erosive [30]. Our group has previously used US to show that bone erosion is not as specific for RA as once thought [31]. The nature and progression of US-detected erosions in SLE is poorly understood. From existing experience of disease progression, we might expect that these US erosions have different pathogenesis and clinical significance than they do in RA. This requires further study, and US and MRI provide tools for this.
One means of determining the truth of US abnormality is comparison with measures of disease activity in blood or other organs. Interestingly, there was poor to moderate association between US abnormalities and overall disease activity indices and immunological findings. It is unclear whether this is because of imprecision in US methodology and findings reported, or whether the increased sensitivity of US demonstrates musculoskeletal disease activity dissociated from other features of SLE. This emphasizes the need for validation of US against gold standard measures of synovitis (such as histology), or longitudinal studies that investigate the prognostic properties of US.
Since the submission of this article, Lins and Santiago [32] published a SLR looking at current evidence for US use in SLE. However, our search strategy yielded more results initially, followed by more stringent exclusion criteria, to specifically answer the research question. Unlike the paper by Lins and Santiago, this SLR was the first to assess the risk of bias in the included literature and their metric qualities. We also highlighted the issues regarding appropriate patient selection and lack of stratification of US findings. We investigated the variation in US findings in the literature and explored the discrepancies.
In conclusion, US has a promising role in the detection and assessment of joint and tendon pathology in SLE. However, further validation work is required before it can be used in clinical practice and as an outcome measure in clinical trials. Although variable prevalences of synovitis, tenosynovitis and erosions were detected by US in this review, there was little data available for the clinical consequences of these findings. With better longitudinal evidence, US may play an important role in better understanding the pathogenesis of musculoskeletal manifestations of SLE, and be crucial in facilitating targeted therapies and measuring response.
Supplementary Material
Acknowledgements
A.S.Z. is funded as a National Institute for Health Research (NIHR) Clinical lecturer and E.M.V. is funded as an NIHR Clinician scientist.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: E.M.V. is an NIHR Clinician Scientist and has received honoraria and research grant support from Roche and GSK. R.J.W. has received honoraria from Abbvie for US education. P.E. has received consultancy fees from BMS, Abbott, Pfizer, MSD, Novartis, Roche and UCB, as well as research grants paid to his employer from Abbott, BMS, Pfizer, MSD and Roche. All other authors have declared no conflicts of interest.
References
- 1. Zoma A. Musculoskeletal involvement in systemic lupus erythematosus. Lupus 2004;13:851–3. [DOI] [PubMed] [Google Scholar]
- 2. Ball EM, Bell AL. Lupus arthritis—do we have a clinically useful classification? Rheumatology 2012;51:771–9.. [DOI] [PubMed] [Google Scholar]
- 3. Drenkard C, Bao G, Dennis G, et al. The burden of systemic lupus erythematosus on employment and work productivity: data from a large cohort in the southeastern United States. Arthritis Care Res 2014;66:878–887. [DOI] [PubMed] [Google Scholar]
- 4. Eilertsen GO, Nikolaisen C, Becker-Merok A, Nossent JC. Interleukin-6 promotes arthritis and joint deformation in patients with systemic lupus erythematosus. Lupus 2011;20:607–13. [DOI] [PubMed] [Google Scholar]
- 5. Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology 2005;44:902–6. [DOI] [PubMed] [Google Scholar]
- 6. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 7. Wakefield RJ, D’Agostino MA, Naredo E, et al. After treat-to-target: can a targeted ultrasound initiative improve RA outcomes? Ann Rheum Dis 2012;71:799–803. [DOI] [PubMed] [Google Scholar]
- 8. Dale J, Purves D, McConnachie A, McInnes I, Porter D. Tightening up? Impact of musculoskeletal ultrasound disease activity assessment on early rheumatoid arthritis patients treated using a treat to target strategy. Arthritis Care Res 2014;66:19–26. [DOI] [PubMed] [Google Scholar]
- 9. van Vugt RM, Derksen RH, Kater L, Bijlsma JW. Deforming arthropathy or lupus and rhupus hands in systemic lupus erythematosus. Ann Rheum Dis 1998;57:540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sa Ribeiro D, Galvao V, Luiz Fernandes J, et al. Magnetic resonance imaging of Jaccoud’s arthropathy in systemic lupus erythematosus. Joint Bone Spine 2010;77:241–5. [DOI] [PubMed] [Google Scholar]
- 11. Ostendorf B, Scherer A, Specker C, Modder U, Schneider M. Jaccoud’s arthropathy in systemic lupus erythematosus: differentiation of deforming and erosive patterns by magnetic resonance imaging. Arthritis Rheum 2003;48:157–65. [DOI] [PubMed] [Google Scholar]
- 12. Wright S, Filippucci E, Grassi W, Grey A, Bell A. Hand arthritis in systemic lupus erythematosus: an ultrasound pictorial essay. Lupus 2006;15:501–6. [DOI] [PubMed] [Google Scholar]
- 13. Dussik KT, Fritch DJ, Kyriazidou M, Sear RS. Measurements of articular tissues with ultrasound. Am J Phys Med 1958;37:160–5. [PubMed] [Google Scholar]
- 14. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 15. Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 2005;32:2485–7. [PubMed] [Google Scholar]
- 16. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- 17. Reitsma JB, Rutjes AWS, Whiting P, et al. Chapter 9: Assessing methodological quality. In: Deeks JJ, Bossuyt PM, Gatsonis C, eds. Cochrane handbook for systematic reviews of diagnostic test accuracy. Version 1.0.0 The Cochrane Collaboration, 2009. http://srdta.cochrane.org/ (August 2014, date last accessed). [Google Scholar]
- 18. Iagnocco A, Ossandon A, Coari G, et al. Wrist joint involvement in systemic lupus erythematosus. An ultrasonographic study. Clin Exp Rheumatol 2004;22:621–4. [PubMed] [Google Scholar]
- 19. Delle Sedie A, Riente L, Scire CA, et al. Ultrasound imaging for the rheumatologist. XXIV. Sonographic evaluation of wrist and hand joint and tendon involvement in systemic lupus erythematosus. Clin Exp Rheumatol 2009;27:897–901. [PubMed] [Google Scholar]
- 20. Ossandon A, Iagnocco A, Alessandri C, et al. Ultrasonographic depiction of knee joint alterations in systemic lupus erythematosus. Clin Exp Rheumatol 2009;27:329–32. [PubMed] [Google Scholar]
- 21. Torrente-Segarra V, Lisbona MP, Rotes-Sala D, et al. Hand and wrist arthralgia in systemic lupus erythematosus is associated to ultrasonographic abnormalities. Joint Bone Spine 2013;80:402–6. [DOI] [PubMed] [Google Scholar]
- 22. Gabba A, Piga M, Vacca A, et al. Joint and tendon involvement in systemic lupus erythematosus: an ultrasound study of hands and wrists in 108 patients. Rheumatology 2012;51:2278–85. [DOI] [PubMed] [Google Scholar]
- 23. Ball E, Gibson D, Bell A, Rooney M. Plasma IL-6 levels correlate with clinical and ultrasound measures of arthritis in patients with systemic lupus erythematosus. Lupus 2014;23:46–56. [DOI] [PubMed] [Google Scholar]
- 24. Buosi AL, Natour J, Machado FS, Takahashi RD, Furtado RN. Hand ultrasound: comparative study between “no rhupus” lupus erythematosus and rheumatoid arthritis. Mod Rheumatol 2014;24:599–605. [DOI] [PubMed] [Google Scholar]
- 25. Iagnocco A, Ceccarelli F, Rizzo C, et al. Ultrasound evaluation of hand, wrist and foot joint synovitis in systemic lupus erythematosus. Rheumatology 2014;53:465–72. [DOI] [PubMed] [Google Scholar]
- 26. Ball EM, Gibson DS, Bell AL, Rooney MR. Plasma IL-6 levels correlate with clinical and ultrasound measures of arthritis in patients with systemic lupus erythematosus. Lupus 2014;23:46–56. [DOI] [PubMed] [Google Scholar]
- 27. Zayat AS, Conaghan PG, Sharif M, et al. Do non-steroidal anti-inflammatory drugs have a significant effect on detection and grading of ultrasound-detected synovitis in patients with rheumatoid arthritis? Results from a randomised study. Ann Rheum Dis 2011;70:1746–51. [DOI] [PubMed] [Google Scholar]
- 28. Larche MJ, Seymour M, Lim A, et al. Quantitative power Doppler ultrasonography is a sensitive measure of metacarpophalangeal joint synovial vascularity in rheumatoid arthritis and declines significantly following a 2-week course of oral low-dose corticosteroids. J Rheumatol 2010;37:2493–501. [DOI] [PubMed] [Google Scholar]
- 29. Keen HI, Wakefield RJ, Conaghan PG. A systematic review of ultrasonography in osteoarthritis. Ann Rheum Dis 2009;68:611–9. [DOI] [PubMed] [Google Scholar]
- 30. Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zayat AS, Ellegaard K, Conaghan PG, et al. The specificity of ultrasound-detected bone erosions for rheumatoid arthritis. Ann Rheum Dis 2015;74:897–903. [DOI] [PubMed] [Google Scholar]
- 32. Lins CF, Santiago MB. Ultrasound evaluation of joints in systemic lupus erythematosus: a systematic review. Eur Radiol 2015;25:2688–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.