From early RA to pre-RA
A desire to cure and even prevent RA has led to an increased interest in its earliest phases—those clinically apparent phases of disease before patients have developed the full set of characteristics that allow them to be classified as having RA and the phases of disease prior to the onset of symptoms. The series of review articles [1–4] that are featured in this and the next few issues of Rheumatology addresses key aspects of the transition to RA: the genetic factors that increase the risk of and protect against RA development [1]; the interactions between these genetic factors and environmental exposures in the initiation of RA and its very early pathogenesis [2]; strategies to predict RA development in at-risk populations, many of which integrate data relating to genetic and environmental risk factors [3]; and strategies to prevent RA in those at risk [4].
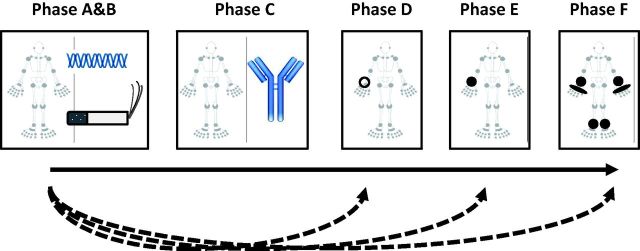
Although understanding of these early phases has increased considerably in the past few years, their existence was appreciated in the 1980s when it was first recognized that, in some individuals, the presence of RA-related autoantibodies preceded the onset of clinically apparent disease, often by many years. Recently a common lexicon has been proposed to allow a standardized approach to the description of individuals as they progress through at-risk phases towards the development of RA [5, 6]. Thus individuals with genetic risk factors (phase A) for RA would be exposed to relevant environmental risk factors (phase B). Some such individuals would proceed through phases associated with systemic autoimmunity (phase C), symptoms without clinical arthritis (phase D) and unclassified arthritis (phase E), before eventually developing RA (Fig. 1). Defining these phases in this manner has facilitated the study of mechanisms underlying the transitions between each of these phases, the biomarkers predictive of these transitions and how we think about preventive therapies. Primary prevention of seropositive RA would thus involve interventions to prevent the development of RA-related systemic autoimmunity (phase C) while secondary prevention of seropositive RA would involve approaches to prevent the development of RA in individuals with pre-existing systemic autoimmunity. In this scheme, it is important to understand that not all individuals progress inexorably through all the stages in sequence; individuals may skip stages (illustrated by the dashed lines with arrowheads in Fig. 1), may halt at an intermediate stage or may even revert to an earlier stage.
Fig. 1.
The five phases (A–E) an individual may pass through in the transition from health to the development of RA (phase F)
The open circle in phase D represents a painful but non-swollen joint. The filled shapes in phases E and F represent joints with clinically apparent soft tissue swelling. Reprinted from Raza K, Gerlag DM, Preclinical inflammatory rheumatic diseases: an overview and relevant nomenclature, Rheum Dis Clin N Am 2014;40:569–80, © 2014, with permission from Elsevier.
Division of the at-risk phases of RA into those illustrated in Fig. 1 helps focus attention on the specific factors that put individuals at risk for transition from one phase to the next. This is a critically important issue from the perspective of approaches to disease prevention because modulation of an environmental risk factor relevant for the transition from one particular phase to another may not have the same effect for other transitions. Recent evidence suggests that the HLA class II locus is associated less with the risk of developing ACPA and more with the risk of progression from a state of ACPA positivity (phase C) to having RA [7]; in contrast, environmental factors including smoking [7, 8] and pulmonary inflammation [9] may be more important in the transition to phase C. From the perspective of preventing RA, a smoking cessation campaign would therefore be a particularly important step to implement for first-degree relatives of patients with RA (phases A and B).
Very important questions remain to be addressed and this series of review articles highlights some of these. The triggers for and sites of initiation of the adaptive immune responses that characterize phase C in the transition to RA remain incompletely understood. While considerable data implicate cigarette smoke and other inhaled toxins, the microbiota at mucosal sites including the gut and lung may also play an important role [2]. The triggers for transition from phase C to phases D and E, the joint centric phases of disease, also remain poorly defined but are critical to understand in the context of developing approaches to secondary prevention.
Seronegative RA presents its own challenges in the context of prediction and prevention. By definition, this form of RA is not associated with a presymptomatic autoantibody-positive phase, although as the number of autoantibodies recognized to be associated with RA increases, for example, those recognizing carbamylated proteins in addition to those recognizing citrullinated proteins, the proportion of patients who are truly seronegative decreases. Furthermore, the overall genetic component of risk for seronegative RA, and the genes that contribute to this risk, are not as well defined as for seropositive RA [1] and the environmental factors that interact with the genotype to trigger the development of seronegative RA have been very poorly described. Only when these, and the disease mechanisms operating at the earliest stages of seronegative RA, have been better characterized will it be apparent to what extent different approaches need to be taken for the primary prevention of seronegative and seropositive RA.
RA is certainly not the only rheumatic disease for which there is interest in risk, prediction and prevention. In the case of SSc, for example, the presence of presclerodermatous disease is well recognized and is typically characterized by the presence of autoantibodies (e.g. ANA) and RP, analogous to phases C and D in the context of RA. Similarly, natural history studies in SLE have demonstrated the prolonged presence and expansion of lupus-related autoantibodies in the preclinical phase of disease [10]. The use of equivalent terminologies to describe the at-risk phases of other chronic diseases will shed light on similarities and differences between diseases. This, in turn, may enhance our understanding of the approaches to screening populations and introducing more generally the concepts of autoimmune and rheumatic diseases prediction and prevention.
Acknowledgements
K.R. and L.K. receive funding for the study of the prediction of RA development from the FP7 HEALTH programme under the grant agreement FP7-HEALTH-F2-2012-305549. L.K. receives funding for studies of RA at-risk populations from the Innovative Medicines Initiative–funded BTCure project, within which also PhaDia/ThermoFischer contributes to the development of a multiplex antibody platform able to diagnose some RA at-risk patients. L.K. also receives funding for RA at-risk projects from the European Research Council and from several national Swedish foundations. V.M.H. receives funding for the study of RA at-risk populations from National Institutes of Health grants UM2 AR067678, UH2 AR067681, UM1 AI110503 and U01 AI101981.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: V.M.H. has received royalties through a Stanford University patent for the use of biomarkers to predict clinical phenotypes. K.R. has received honoraria from Pfizer, AbbVie and BMS and meeting expenses from Pfizer and Roche. The other author has declared no conflicts of interest.
References
- 1. Yarwood A, Huizinga TW, Worthington J. The genetics of rheumatoid arthritis: risk and protection in different stages of the evolution of RA. Rheumatology 2015; doi:10.1093/rheumatology/keu323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catrina AI, Deane KD, Scher JU. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology 2015; doi:10.1093/rheumatology/keu469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karlson EW, van Schaardenburg D, van der Helm-van Mil AH. Strategies to predict rheumatoid arthritis development in at-risk populations. Rheumatology 2016;54:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerlag DM, Norris JM, Tak PP. Towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology 2015; doi:10.1093/rheumatology/kev347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerlag DM, Raza K, van Baarsen LG, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis 2012;71:638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raza K, Saber TP, Kvien TK, Tak PP, Gerlag DM. Timing the therapeutic window of opportunity in early rheumatoid arthritis: proposal for definitions of disease duration in clinical trials. Ann Rheum Dis 2012;71:1921–3. [DOI] [PubMed] [Google Scholar]
- 7. Bos WH, Ursum J, de Vries N, et al. The role of the shared epitope in arthralgia with anti-cyclic citrullinated peptide antibodies (anti-CCP), and its effect on anti-CCP levels. Ann Rheum Dis 2008;67:1347–50. [DOI] [PubMed] [Google Scholar]
- 8. Hensvold AH, Magnusson PK, Joshua V, et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis 2015;74:375–80. [DOI] [PubMed] [Google Scholar]
- 9. Demoruelle MK, Weisman MH, Simonian PL, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum 2012;64:1756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]