Abstract
Advances in pharmacological treatment options in RA have led to a dramatic potential for improvement in patients’ physical and psychological status. Despite advances, poor outcomes, including fatigue, pain, reduced physical activity and quality of life, are still observed. Reasons include non-adherence to medication, insufficient knowledge about the disease and lack of support in coping and effectively self-managing their condition. Motivational interviewing (MI) is a person-centred approach that relies on collaboration and empathy aiming to elicit a person’s own motivation for behaviour change. It has been implemented in a variety of long-term conditions, addressing issues such as lifestyle changes with beneficial effects, but it is yet to be widely recognized and adopted in the field of rheumatology. This review will explain the techniques underpinning MI and the rationale for adopting this approach in rheumatology with the aim to increase medication adherence and physical activity and improve patients’ coping strategies for pain and fatigue.
Keywords: motivational interviewing, rheumatoid arthritis, intensive management, remission rates, quality of life
Rheumatology key messages
Motivational interviewing might be able to contribute to improving quality of life in patients with RA.
More well-conducted trials assessing the effectiveness of motivational interview-based interventions in RA are needed.
Rheumatology health professionals need to adopt a motivational interview approach in their practices.
Introduction
RA is a long-term systemic condition characterized by inflammation of the synovium, resulting in chronic pain, joint damage and disability [1]. In recent decades there has been a dramatic shift in the management of RA, with new pharmacological strategies available such as biologics and anti-TNFs [2]. Despite new options, there is no cure for RA and only a minority of patients achieve full remission [3–6]. In addition, patients continue to endure pain and disabling fatigue despite controlling objective inflammation [7]. Despite improvements in outcomes and work productivity, patients with RA continue to have unmet needs due to symptoms that remain unaddressed [8]. Collaborative care and a good therapeutic alliance can contribute to better treatment and health outcomes in patients with musculoskeletal and other conditions through addressing a number of issues patients might find challenging, such as medication intake, lack of emotional support and involvement in their care [9–11]. Thus, if rheumatologists are going to achieve the goal of disease remission, additional strategies are needed that address RA in a more holistic manner rather than simply increasing immunosuppressive therapy. Holistic care requires involvement of a wider multidisciplinary team [12, 13], e.g. access to psychological interventions. The most common examples of psychological interventions in RA include stress management training, self-management skills and cognitive behavioural therapy (CBT) [14–18]. These approaches are employed to help patients develop coping strategies, improve their ability to self-manage and improve adherence to treatment. Psychological interventions, delivered by experts, have a robust evidence base [19, 20], however, access to such services is sporadic. It is for this reason that there is growing interest in psychological interventions that can be delivered by practitioners in routine care by non-psychological specialists, as reported in a systematic review by Alam et al. [21].
Motivational interviewing (MI) is a technique that has been developed and specifically fits this niche: a psychological intervention that can address key components of long-term disease management (coping strategies, self-management, medication adherence) and can be delivered as part of routine care by a patient’s health care professional [22, 23]. MI stresses behavioural change by having a patient come to their own conclusions about what is wrong now with their current behaviour or therapy and what it would be like if the future were changed by adopting a new behaviour or treatment. Thus MI is concentrating on the side effects and risks of today and the benefits of later when the change has been made, which is the part of MI that enables behaviour change (Table 1). Such a tool, if effectively implemented, would align well with existing guidelines for RA care from the British Society of Rheumatology and British Health Professionals [12].
Table 1.
Exploration of positive and negative experiences to enable behaviour change
| Advantages of current behaviour: smoking helps me release stress | Disadvantages of changed future behaviour: not smoking would make me feel more stressed |
| Disadvantages of current behaviour: smoking makes me feel out of breath and fatigued | Advantages of changed future behaviour: not smoking would save me a lot of money and make me feel healthier |
MI has been adopted as a central component of interventions in numerous studies—including an ongoing large multicentre trial in RA [24]—in a variety of long-term conditions such as FM [25] and diabetes [26] to address lifestyle changes (e.g. weight loss and smoking cessation) [27, 28]. The purpose of this review is to outline the concepts that underpin MI and summarize the existing evidence base for its effectiveness.
MI: an overview
MI is an evidence-based approach and focuses on constructive conversation with patients about behaviour change, initially described by Miller in 1983 [29] in alcohol counselling, where clients’ motivation for behaviour change was poor while denial and resistance were pronounced [30, 31]. MI concepts and approaches were later expanded upon by Rollnick and Miller [32]. Because of positive results in alcohol addiction, the use of MI extended to include substance abuse [33] and smoking [34]. For example, findings from the Project MATCH [35] indicated that MI is comparable in effectiveness to two other commonly used treatment approaches in treating alcoholism, CBT and a 12-step facilitation approach. MI interventions have been assessed in a variety of clinical populations, e.g. in diabetes and FM to address motivations for behaviour change as well as aspects of long-term illness such as medication adherence [36], pain, physical activity and diet [37]. Specific components of the interventions, for instance social support, targeting two domains simultaneously (such as diet and physical activity), increased contact frequency and the use of a specific cluster of self-regulatory behaviour change techniques (e.g. goal-setting and self-monitoring) were found to be associated with increased effectiveness [37–39].
In general, the aim of MI is for individuals to overcome the ambivalence that prevents them from making desired changes in their lives. The role of the clinician is collaborative and at its core lies empathic listening to facilitate an understanding of the patient’s perspective and decrease patient resistance. In addition to empathic listening, other principles that underpin MI are expressing empathy, rolling with resistance, supporting self-efficacy and developing discrepancy with the client (Table 2). A number of techniques are used to explore the individual’s values and beliefs and to elicit motivation for change, including open-ended questions, affirmations, reflections and summaries (Table 2). Typical examples of physician–patient consultations based on MI principles are provided in the supplementary data, available at Rheumatology Online. The techniques that are applied are adapted to the person’s state of readiness to change [40]. For a visual representation on the application and effect of MI in a health care consultation and the differences vs a non-MI session, see the following links to online video clips: http://www.youtube.com/watch?v=80XyNE89eCs and http://www.youtube.com/watch?v=URiKA7CKtfc [produced by the University of Florida, Department of Psychiatry; funded by Flight Attendant Medical Research Institute Grant #63504 (Co-Pls: Gold & Merlo)].
Table 2.
Core principles and techniques of MI
| Core principles of MI | MI techniques |
|---|---|
| Avoiding argument | Open-ended questions |
| Expressing empathy | Affirmations |
| Supporting self-efficacy | Reflections |
| Developing discrepancy | Summaries |
| Rolling with resistance |
MI: motivational interviewing.
Table 3.
Overview of reviews and meta-analyses evaluating the effectiveness of interventions based on MI in long-term conditions and health-related behaviours
| Authors | Aim/objective | Study design | Studies included | Outcome |
|---|---|---|---|---|
| Thompson et al. (2011) [39] | To review evidence on MI in relation to cardiovascular health | Systematic review | 13 studies: 5 primary source papers (RCTs, quasi, case–control) and 8 secondary studies (meta-analyses, systematic and literature reviews) | MI was useful to help nurses improve health behaviour in people with coronary risk factors |
| Hill and Kavookjian (2012) [41] | To examine the MI intervention literature regarding outcomes in improving HAART adherence in patients with HIV | Systematic review | Five RCTs | MI appeared to be a promising intervention based on results from three studies where medication adherence increased as a result of MI. Great variability in measuring adherence limited conclusions |
| Greaves et al. (2011) [37] | To review evidence on interventions promoting dietary and/or physical activity change in producing weight and behaviour changes in adults with a risk of developing type 2 diabetes | Systematic review of reviews | 30 systematic reviews (10 on physical activity interventions, 3 on dietary interventions and 17 on both) | Increased effectiveness of interventions was associated with the use of social support, established behaviour change techniques, contact frequency and self-regulatory techniques (e.g. goal-setting, self-monitoring) |
| Burke et al. (2003) [43] | To conduct a meta-analytic examination of the MI literature | Meta-analysis of controlled clinical trials investigating AMIs in treating problem behaviours (e.g. substance abuse, diet and exercise) | 30 controlled clinical trials: 15 examining AMIs for alcohol problems, 2 for smoking cessation, 5 for drug addiction, 2 for HIV-risk behaviours, 4 for diet and exercise, 1 for treatment adherence and 1 for eating disorders | AMIs were not effective in smoking cessation and HIV-risk behaviours. AMIs were moderately effective for diet and exercise and alcohol and drug problems. AMIs were equivalent to other active treatments but more time effective |
| Rubak et al. (2005) [42] | To evaluate the effectiveness of MI in different disease areas and to identify outcome factors | Systematic review and meta-analysis of RCTs using MI as the intervention | 72 RCTs | MI outperformed traditional provision of advice in the treatment of problem areas and behaviours in a range of diseases |
AMI: adaptation of MI; HAART: highly active antiretroviral therapy; MI: motivational interviewing; RCT: randomized controlled trial.
Efficacy of MI: evidence from reviews and meta-analyses
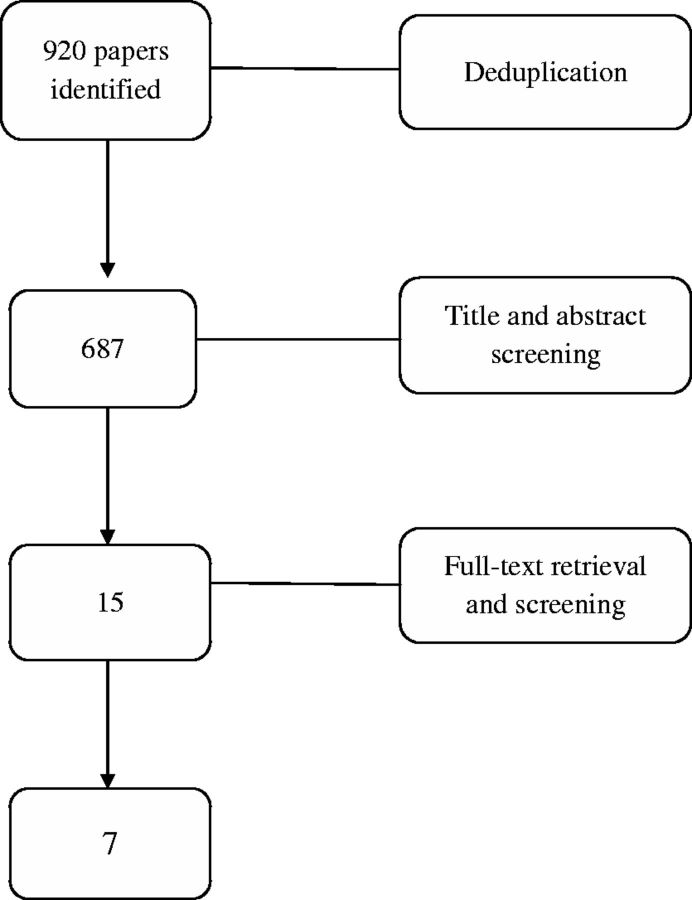
A systematic review of the effectiveness of MI-based interventions is beyond the scope of this article. However, a search for systematic reviews and meta-analyses examining the effects of MI interventions on patients with long-term conditions was undertaken. Seven databases were searched: MEDLINE, PsycARTICLES, PsycINFO, Embase, Web of Science, Ingenta Connect and Cumulative Index to Nursing and Allied Health Literature (CINAHL) from beginning to 4 July 2015. Key search terms included MI, chronic disease, long-term conditions, health behaviours, physical activity/exercise, treatment adherence, musculoskeletal conditions, diet and substance abuse. The terms were searched separately and combined with Boolean operators (AND/OR). A total of 920 papers were identified, which, after removal of duplicates and language filtering was reduced to 687. Title and abstract screening yielded 15 papers, of which, after full text retrieval, 5 were deemed relevant and were included: 3 systematic reviews [39, 41, 42], 1 systematic review of reviews [37] and 1 meta-analysis of controlled clinical trials on health behaviours related to long-term condition management such as substance abuse, diet and exercise [43]. The search strategy is presented in Fig. 1 and an overview of the studies identified is presented in Table 4. Overall, four of the five papers reported beneficial effects of MI on improving health behaviours in people with coronary risk factors [39] and on the risk of developing type 2 diabetes [37], on increasing medication adherence in patients with HIV [41] and on adherence to treatment of diseases as well as lifestyle changes such as body mass index, total blood cholesterol, systolic blood pressure, blood alcohol concentration and standard ethanol content [42]. The remaining paper by Burke et al. [43] reported that adaptations of MI were not effective in certain behaviours such as smoking cessation and HIV-risk behaviours, but that they were moderately effective for others such as diet and exercise and alcohol and drug problems. One reason that could be considered for the lack of effect of MI in certain domains such as smoking cessation and HIV-risk behaviours could be that the participants in those studies might have been ready for a change but might not have been in need of MI support. They could have developed their own motivation beforehand and might also have found their own ways of changing their behaviour. In contrast, other people may be in need of MI techniques as a means of enabling change.
Fig. 1.
Review process of studies using MI in interventions in musculoskeletal and rheumatic diseases
Table 4.
Overview of intervention studies evaluating the effectiveness of MI in rheumatic/musculoskeletal conditions
| Authors | Aim/objective | Study design | Intervention | Outcome |
|---|---|---|---|---|
| Chilton et al. (2012) [44] | To evaluate the effectiveness of MI to create change within musculoskeletal health care and identify the level of training received | Systematic review | Five studies within chronic pain, low back pain, FM and osteoporosis (cluster/non/and randomized trials, and quasi-experimental studies) | Inconclusive due to great variation in delivery modality, musculoskeletal conditions and type of MI intervention |
| Zwikker et al. (2014) [36] | To assess the effect of an intervention based on MI on changes in medication beliefs and adherence in RA | Single-centre researcher-blinded randomized clinical trial with two arms 1:1 | MI-guided group sessions led by a pharmacist vs brochures about prescribed DMARD (information only) | No superiority of intervention over control arm in changing beliefs about medication and increasing adherence-related outcomes such as walking and cholesterol levels |
| Karlsson et al. (2014) [45] | To develop and evaluate a method for smoking cessation support for patients with RA | Pilot study | Rheumatology nurse with MI and smoking cessation training provided individualized smoking cessation support every 4 weeks over 2 years | 43% of patients with RA within the smoking cessation programme stopped smoking |
| Ferguson et al. (2013) [46] | To adapt a psychological intervention based on CBT and MI for RA patients and assess its effectiveness in terms of improving adherence and quality of life | Pilot study | Up to six individual sessions of compliance therapy vs usual care | Significant improvement in mean post-intervention scores on both adherence measures, but not in the control group |
| Ang et al. (2013) [47] | To test the efficacy of MI in promoting exercise and improve symptoms in patients with FM | RCT | Six MI sessions vs an equal number of FM self-management lessons (education) | Despite a lack of benefit in the long-term, MI appeared to confer short-term benefits with regard to self-reported physical activity and clinical outcomes |
| Everett et al. (2012) [48] | To evaluate the 6 month effect of INC on patients with SLE participating in an ongoing CVD prevention counselling programme | Interventional study | INC incorporated patient-centred methods (tailored nutrition education, goal-setting and MI). Changes in select nutrients and diet habits, anthropometric measures and clinical outcomes were evaluated | A 6 month preliminary analysis suggested that INC using patient-centred methods was effective in promoting changes in nutrient intake, diet habits and possibly anthropometric measures (reduced sodium, fat, cholesterol and calorie intake and increased consumption of fruits, vegetables and fibre) |
| De Gucht et al. (2012) [49] | To examine the effects of a theory-based psychological intervention to increase physical activity among patients with RA | Interventional study | A 1 hour patient education session, one MI and two SR sessions vs patient education alone | The MI + SR intervention outperformed the control group in terms of sustained increases in physical activity at 32 weeks |
| Stockl et al. (2010) [50] | To evaluate adherence to injectable RA medications and assess health-related quality of life, work productivity and physical functioning | Observational cohort study | RA DTM programme vs specialty and community pharmacy services. DTM included patient-centred methods, MI elements, education and self-management skills training | Patients in the DTM programme had significantly higher injectable RA medication adherence compared with specialty and community patients. SF-12 physical components and HAQ-DI scores were significantly improved as well |
CBT: cognitive behavioural therapy; CVD: cardiovascular disease; DTM: disease therapy management; HAQ-DI: Health Assessment Questionnaire Disability Index; INC: individualized nutrition counselling; MI: motivational interviewing; RCT: randomized controlled trial; SF-12: 12-item Short Form Health Survey; SR, self-regulation.
In summary, MI is one of many interventions that have been developed to support patients with long-term conditions in improving their self-management. The impact of these interventions, however, can vary depending on the objectives and the particular characteristics of the chronic illness. Notably, Söderlund et al. [22] concluded that the effectiveness of MI delivery did not depend on the provider’s professional or academic background. A variety of health professionals such as nurses, doctors, midwives, dieticians and psychologists were equally successful in delivering MI appropriately [22].
Summary of the literature on the role of MI in the health care setting
The evidence from systematic reviews and meta-analyses indicates that MI-based interventions are effective—as compared with standard care or provision of information only—in the following outcomes: weight loss and increased physical activity [37], increased fruit and vegetable consumption [39], decreased alcohol intake [42], improved quality of life and self-care behaviours [39], increased medication adherence [41] and decreased total blood cholesterol and systolic blood pressure [42].
Despite the fact that the MI-based interventions described above were applied on outcomes related to conditions other than RA, such as cardiovascular disease, diabetes and HIV, these outcomes are relevant to RA as well. Some of the most common problem areas identified in RA include pain and fatigue [51], decreased physical activity [52], excess weight [53], medication non-adherence [54, 55] and impaired quality of life [56]. Therefore it is likely that interventions based on MI could also be beneficial for patients with RA.
According to the results of a systematic review of reviews [37], the components that increased intervention effectiveness were social support; approaches addressing both diet and physical activity; established behaviour change techniques such as identification of barriers, problem solving, action planning and increased contact frequency; and a combination of self-regulatory behaviour change techniques such as goal-setting, self-monitoring etc.
Moreover, no clear relationships were found between the effectiveness of the interventions and their setting, delivery mode, study population or delivery provider [37]. In most studies, MI was delivered in person; in one, the telephone was used for some sessions, while in another, behavioural audiotapes, a workbook and mailed material were included in the sessions. The interventions ranged between two and eight sessions and were administered over a period of 2–6 months.
What is the evidence for MI in RA?
Evidence for the use of MI is more limited in musculoskeletal diseases, and specifically in RA. The 687 papers identified in the previous section were screened for their relevance to musculoskeletal and rheumatic diseases based on title and abstract. Screening yielded 15 studies, of which 7 were included after full-text retrieval: 1 systematic review [44], 2 randomized controlled trials (RCTs) [36, 47], 2 interventional studies [48, 49] and 2 pilot studies [45, 46]. The systematic review of MI within musculoskeletal health, not including RA, could not provide direct comparative interpretations for the efficacy of interventions using MI [44]. Variations in modality, provider, duration, frequency and competency of MI delivery as well as variation in the fidelity of MI prevented conclusions as to its impact in musculoskeletal diseases. As a result, Chilton et al. [44] highlighted the need for well-designed RCTs with sufficient power to measure the effectiveness of MI in self-management and its application to promotion of lifestyle changes such as diet or physical activity.
While further systematic reviews on the use of MI and/or other psychological interventions in RA are not available, to the best of our knowledge, there are a number of studies that examined MI-based interventions in musculoskeletal and rheumatic diseases as identified in our search of the literature. The RCT by Zwikker et al. [36] assessed the effectiveness of a group intervention to change medication beliefs and improve medication adherence compared with a control group that received brochures at home about the DMARDs used at the time by patients with RA in The Netherlands. The intervention was based on MI principles and techniques and targeted patient beliefs about the necessity of and concerns about medication, such as side effects, as well as the resolution of perceived barriers in medication uptake [36]. In addition to addressing patient beliefs about the effect and necessity of the medication through provision of information and education on DMARDs, the intervention also focused on increasing patients’ self-efficacy. The sessions were delivered in a group format based on the social influence/modelling theory to increase communication regarding the medication. Despite the brevity of the intervention—two group sessions 1 week apart of 1.5 h duration with individual homework assignments between the two sessions and follow-up by the practitioners 8 weeks after the last group meeting—the intervention enhanced medication adherence rates in RA patients [36]. Patient education about RA and their treatment options as well as addressing barriers to medication adherence were the components of an MI-based telephone intervention with RA patients for three or six monthly sessions over a 7-month period [50]. However, the intervention was not more effective than the control arm that involved brochures on DMARDs provided to patients at home. The authors attributed the lack of effect to the possibility of regression to the mean [57] due to the likelihood of patients’ beliefs having changed before the intervention. A further possible explanation for the study findings include the Hawthorne effect [58], which refers to individuals adjusting their behaviour in response to being observed, as well as focusing only on patient-related factors or selection bias due to recruiting patients with long-standing RA (mean >14 years).
Further evidence for the efficacy of MI in rheumatology is provided from two pilot studies [45, 46]. Karlsson et al. [45] developed and evaluated a method of smoking cessation support for patients with RA, while Ferguson et al. [46] assessed the effectiveness of an intervention based on CBT and MI in terms of improving adherence and quality of life in patients with RA. The studies found that MI was associated with a significant increase in smoking cessation rates and adherence measures, respectively [45, 46]. MI was also reported to be beneficial in increasing physical activity in patients with RA [49] and FM [47], especially when combined with self-regulation components such as goal-setting or monitoring [49]. In addition, individualized counselling sessions that included MI elements as well as patient-centred methods such as tailored nutrition education and goal-setting were effective in promoting changes in dietary intake and patterns as well as anthropometric measures such as BMI in patients with SLE [48].
To sum up, MI has relevance to many aspects of RA care, from medication adherence to self-management. At present, the application of MI is infrequent and under-researched in RA, as evidenced from the limited literature in the field identified by the search that was undertaken, compared with other long-term conditions such as diabetes and cardiovascular disease. There is growing awareness that rheumatology practice could be enhanced through the use of psychological tools such as MI and, as such, there is a need for clinical trials to clarify the efficacy and acceptability of such interventions for patients and clinicians in the rheumatology field.
Implications
Most clinicians in rheumatology have little or no formal training in therapeutic techniques. As more evidence accumulates in other disease areas highlighting the importance of psychological/behavioural interventions, acknowledging that such tools are within the grasp of the average practitioner and not just relevant to psychologists, it is time the rheumatology community engaged more widely in behavioural interviewing techniques such as MI. Clinicians could be trained in a 2 day course in MI, which has been shown to be feasible and effective in an RCT that focused on training clinicians in MI [23]. Miller et al. [23] found that clinicians without previous MI training attending a 2 day workshop showed substantial gains in MI proficiency in the first 4 months after the training compared with individuals who used self-directed learning by book and videotapes, who showed no change. In addition, there was a significant change in patients’ response as evidenced by increased talk of change and lower resistance in the first 4 months of the sessions [23]. Therefore, it seems likely that clinicians will be able to effectively use MI in their routine practice, providing there is ongoing supervision and feedback available after the training has been completed.
Supplementary Material
Acknowledgements
This work is part of Treatment Intensities and Targets in Rheumatoid Arthritis ThErapy (TITRATE), which is funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant Reference Number RP-PG-0610-10066). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health. We wish to thank our colleagues on the TITRATE team. Prof David L. Scott, recipient of the grant, Prof Gabrielle Kingsley, Dr Naomi Martin, Fowzia Ibrahim, Richard Jenner, Isabel Neatrour, Dr Aneela Mian and all co-applicants for their invaluable contribution to the programme and the trial.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: H.L. has received funding over the last 24 months from the following funders as a principal investigator or co-applicant: National Institutes of Health Research; Arthritis Research UK; South London Membership Council; Guy’s and St Thomas’ Charity, London; European Union and the Health Foundation. L.P.’s salary is from an NIHR Programme Grant for Applied Research until April 2017. All other authors have declared no conflicts of interest.
References
- 1. Scott DL, Steer S. The course of established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:943–67. [DOI] [PubMed] [Google Scholar]
- 2. Scott D. Biologics‐based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther 2012;91:30–43. [DOI] [PubMed] [Google Scholar]
- 3. Quinn MA, Conaghan PG, O’Connor PJ. et al. Very early treatment with infliximab in addition to methotrexate in early, poor‐prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve‐month randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum 2005;52:27–35. [DOI] [PubMed] [Google Scholar]
- 4. Wakefield RJ, Freeston JE, Hensor E. et al. Delay in imaging versus clinical response: a rationale for prolonged treatment with anti–tumor necrosis factor medication in early rheumatoid arthritis. Arthritis Care Res 2007;57:1564–7. [DOI] [PubMed] [Google Scholar]
- 5. van der Heijde D, Klareskog L, Landewe R. et al. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 2007;56:3928–39. [DOI] [PubMed] [Google Scholar]
- 6. Flouri I, Markatseli TE, Voulgari PV. et al. Comparative effectiveness and survival of infliximab, adalimumab, and etanercept for rheumatoid arthritis patients in the Hellenic Registry of Biologics: low rates of remission and 5-year drug survival. Semin Arthritis Rheum 2014;43:447–57. [DOI] [PubMed] [Google Scholar]
- 7. Pollard L, Choy E, Gonzalez J, Khoshaba B, Scott D. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology 2006;45:885–9. [DOI] [PubMed] [Google Scholar]
- 8. Giacomelli R, Gorla R, Trotta F. et al. Quality of life and unmet needs in patients with inflammatory arthropathies: results from the multicentre, observational RAPSODIA study. Rheumatology 2015;54:792–7. [DOI] [PubMed] [Google Scholar]
- 9. Pinto RZ, Ferreira ML, Oliveira VC. et al. Patient-centred communication is associated with positive therapeutic alliance: a systematic review. J Physiother 2012;58:77–87. [DOI] [PubMed] [Google Scholar]
- 10. Hall AM, Ferreira PH, Maher CG, Latimer J, Ferreira ML. The influence of the therapist-patient relationship on treatment outcome in physical rehabilitation: a systematic review. Phys Ther 2010;90:1099–110. [DOI] [PubMed] [Google Scholar]
- 11. Ferreira PH, Ferreira ML, Maher CG. et al. The therapeutic alliance between clinicians and patients predicts outcome in chronic low back pain. Phys Ther 2013;93:470–8. doi: 10.2522/ptj.20120137. [DOI] [PubMed] [Google Scholar]
- 12. Kennedy T, McCabe C, Struthers G. et al. BSR guidelines on standards of care for persons with rheumatoid arthritis. Rheumatology 2005;44:553–6. [DOI] [PubMed] [Google Scholar]
- 13. National Collaborating Centre for Chronic Conditions (UK). Rheumatoid arthritis: national clinical guideline for management and treatment in adults. NICE Clinical Guidelines no. 79. London, UK: Royal College of Physicians, 2009. [PubMed] [Google Scholar]
- 14. Hewlett S, Ambler N, Almeida C. et al. Self-management of fatigue in rheumatoid arthritis: a randomised controlled trial of group cognitive-behavioural therapy. Ann Rheum Dis 2011;70:1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cramp F, Hewlett S, Almeida C. et al. Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2013;8:CD008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain 2002;100:141–53. [DOI] [PubMed] [Google Scholar]
- 17. Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: a meta‐analysis of randomized controlled trials. Arthritis Care Res 2002;47:291–302. [DOI] [PubMed] [Google Scholar]
- 18. DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol 2004;23:207–18. [DOI] [PubMed] [Google Scholar]
- 19. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363:1589–97. [DOI] [PubMed] [Google Scholar]
- 20. Steed L, Cooke D, Newman S. A systematic review of psychosocial outcomes following education, self-management and psychological interventions in diabetes mellitus. Patient Educ Couns 2003;51:5–15. [DOI] [PubMed] [Google Scholar]
- 21. Alam R, Sturt J, Lall R, Winkley K. An updated meta-analysis to assess the effectiveness of psychological interventions delivered by psychological specialists and generalist clinicians on glycaemic control and on psychological status. Patient Educ Couns 2009;75:25–36. [DOI] [PubMed] [Google Scholar]
- 22. Söderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns 2011;84:16–26. [DOI] [PubMed] [Google Scholar]
- 23. Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol 2004;72:1050–62. [DOI] [PubMed] [Google Scholar]
- 24. Scott DL. Treatment Intensities and Targets in Rheumatoid Arthritis Therapy: Integrating Patients and Clinicians Views – the TITRATE Programme. London, UK: National Institute of Health Research, 2013. [Google Scholar]
- 25. Ang D, Kesavalu R, Lydon JR, Lane KA, Bigatti S. Exercise-based motivational interviewing for female patients with fibromyalgia: a case series. Clin Rheumatol 2007;26:1843–9. [DOI] [PubMed] [Google Scholar]
- 26. Pasma A, van’t Spijker A, Hazes JM, Busschbach JJ, Luime JJ. Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic review. Semin Arthritis Rheum 2013;43:18–28. [DOI] [PubMed] [Google Scholar]
- 27. Armstrong M, Mottershead T, Ronksley P. et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta‐analysis of randomized controlled trials. Obes Rev 2011;12:709–23. [DOI] [PubMed] [Google Scholar]
- 28. Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS. Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: a randomised controlled trial with a 12-month post-intervention follow-up. Int J Behav Nutr Phys Act 2013;10:40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller WR. Motivational interviewing with problem drinkers. Behav Psychother 1983;11:147–72. [Google Scholar]
- 30. Bien TH, Miller WR, Boroughs JM. Motivational interviewing with alcohol outpatients. Behav Cogn Psychother 1993;21:347–56. [Google Scholar]
- 31. Brown JM, Miller WR. Impact of motivational interviewing on participation and outcome in residential alcoholism treatment. Psychol Addict Behav 1993;7:211–8. [Google Scholar]
- 32. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother 1995;23:325–34. [DOI] [PubMed] [Google Scholar]
- 33. Saunders B, Wilkinson C, Phillips M. The impact of a brief motivational intervention with opiate users attending a methadone programme. Addiction 1995;90:415–24. [DOI] [PubMed] [Google Scholar]
- 34. Butler CC, Rollnick S, Cohen D. et al. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract 1999;49:611–6. [Google Scholar]
- 35. Allen J, Mattson M, Miller W. et al. Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. J Stud Alcohol 1997;58:7–29. [PubMed] [Google Scholar]
- 36. Zwikker HE, van den Ende CH, van Lankveld WG. et al. Effectiveness of a group-based intervention to change medication beliefs and improve medication adherence in patients with rheumatoid arthritis: a randomized controlled trial. Patient Educ Couns 2014;94:356–61. [DOI] [PubMed] [Google Scholar]
- 37. Greaves CJ, Sheppard KE, Abraham C. et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas ML, Elliott JE, Rao SM. et al. A randomized, clinical trial of education or motivational-interviewing-based coaching compared to usual care to improve cancer pain management. Oncol Nurs Forum 2012;39:39–49. [DOI] [PubMed] [Google Scholar]
- 39. Thompson DR, Chan SW, Astin F, Davidson PM, Ski CF. Motivational interviewing: a useful approach to improving cardiovascular health? J Clin Nurs 2011;20:1236–44. [DOI] [PubMed] [Google Scholar]
- 40. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390–5. [DOI] [PubMed] [Google Scholar]
- 41. Hill S, Kavookjian J. Motivational interviewing as a behavioral intervention to increase HAART adherence in patients who are HIV-positive: a systematic review of the literature. AIDS Care 2012;24:583–92. [DOI] [PubMed] [Google Scholar]
- 42. Rubak S, Sandbæk A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305–12. [PMC free article] [PubMed] [Google Scholar]
- 43. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003;71:843–61. [DOI] [PubMed] [Google Scholar]
- 44. Chilton R, Pires-Yfantouda R, Wylie M. A systematic review of motivational interviewing within musculoskeletal health. Psychol Health Med 2012;17:392–407. [DOI] [PubMed] [Google Scholar]
- 45. Karlsson M, Pettersson S, Lundberg I. Smoking cessation in patients with rheumatic disease. Scand J Rheumatol 2014;43:78.. [DOI] [PubMed] [Google Scholar]
- 46. Ferguson AM, Thomas VN, Lempp H. et al. Improving adherence in rheumatoid arthritis: a pilot study. Rheumatology 2013;52:107. [Google Scholar]
- 47. Ang DC, Kaleth AS, Bigatti S. et al. Research to encourage exercise for fibromyalgia (REEF): use of motivational interviewing, outcomes from a randomized controlled trial. Clin J Pain 2013;29:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Everett S, Haiduc V, Richey M, Erkan D. The short term effect of individualized nutrition counseling on nutrients and select cardiovascular risk factors in patients with systemic lupus erythematosus (SLE). Arthritis Rheum 2012;64:S1143. [Google Scholar]
- 49. De Gucht V. Motivational interviewing and self-regulation to increase physical activity in patients with rheumatoid arthritis. Int J Psychol 2012;47:455. [Google Scholar]
- 50. Stockl KM, Shin JS, Lew HC. et al. Outcomes of a rheumatoid arthritis disease therapy management program focusing on medication adherence. J Manag Care Pharm 2010;16:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stone AA, Broderick JE, Porter LS, Kaell AT. The experience of rheumatoid arthritis pain and fatigue: examining momentary reports and correlates over one week. Arthritis Rheum 1997;10:185–93. [DOI] [PubMed] [Google Scholar]
- 52. Eurenius E, Stenström CH. Physical activity, physical fitness, and general health perception among individuals with rheumatoid arthritis. Arthritis Care Res 2005;53:48–55. [DOI] [PubMed] [Google Scholar]
- 53. Okoro CA, Hootman JM, Strine TW, Balluz LS, Mokdad AH. Disability, arthritis, and body weight among adults 45 years and older. Obes Res 2004;12:854–61. [DOI] [PubMed] [Google Scholar]
- 54. Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum 2009;38:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van den Hoogen FH, Benraad B, Hekster YA, van Lankveld W. Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol 2009;36:2164–70. [DOI] [PubMed] [Google Scholar]
- 56. Strand V, Khanna D. The impact of rheumatoid arthritis and treatment on patients’ lives. Clin Exp Rheumatol 2009;28:S32–40. [PubMed] [Google Scholar]
- 57. Twisk JW, de Vente W. The analysis of randomised controlled trial data with more than one follow-up measurement. A comparison between different approaches. Eur J Epidemiol 2008;23:655–60. [DOI] [PubMed] [Google Scholar]
- 58. McNicholas N, Patel A, Chataway J. It is better to be in a clinical trial than not: lessons learnt from clinical neurology—the management of acute multiple sclerosis relapses. QJM 2012;105:775–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.