Abstract
The prevalence of vitamin D deficiency is increased among patients with CTDs. The active form of vitamin D (calcitriol) is a potent regulator of the immune system and may suppress inflammatory responses. This has led to claims that vitamin D may be a safe treatment, or a treatment adjunct, to reduce systemic inflammation in this patient population. It is important to note, however, that there is insufficient evidence from robust clinical trials to support these novel uses for vitamin D. In this review we examine the potential role of vitamin D as a treatment adjunct for CTDs. We will discuss how vitamin D may modulate the immune response and review the current evidence for using vitamin D to treat CTDs and their associated co-morbidities. We conclude that while there is much excitement about vitamin D in this context, further well-designed trials are needed to demonstrate its efficacy in the treatment of patients with CTDs.
Keywords: vitamin D, systemic lupus erythematosus, connective tissue disease, inflammation, cardiovascular disease
Rheumatology key messages
Patients with connective tissue diseases have a high prevalence of vitamin D deficiency.
CTD patients with vitamin D deficiency should be treated to optimise bone health.
Roles for vitamin D in CTD activity, prognosis and co-morbidities remain to be demonstrated.
Introduction
Vitamin D is a steroid hormone important for calcium homeostasis and the maintenance of bone health [1]. In humans, ∼80% of vitamin D is obtained by the photoconversion of 7-dehydroxycolesterol into pre-vitamin D3 by UV light on the skin [2, 3]. 7-dehydroxycolesterol subsequently undergoes non-enzymatic transformation into vitamin D3 (cholecalciferol). A second form, vitamin D2 (ergocalciferol), is obtained principally from the diet [4]. Both ergocalciferol and cholecalciferol are relatively biologically inert. Activation occurs in a two-stage process: initially, 25-α hydroxylation within hepatic microsomes, resulting in the formation of 25(OH)D [5]. While 25(OH)D has some activity at the vitamin D receptor (VDR), the 1-α hydroxylated form [1,25(OH)2D or calcitriol] has approximately 10 times greater potency. Although 1-α hydroxylase (CYP27B1) was first identified within the mitochondria of the kidney, other tissues contain this crucial enzyme [6].
Recently there has been interest in other roles of vitamin D, particularly in relation to inflammatory diseases [4]. The VDR has been identified in a number of tissues, including the skin and vasculature [7]. In the general population, large prospective observational studies have identified associations between vitamin D deficiency and a number of chronic illnesses [8].
The definitions of vitamin D deficiency and sufficiency remain controversial even within the general population. While a threshold of 20 ng/ml (50 nmol/l) has been proposed, based on the observation that serum PTH begins to rise at levels <20 ng/ml [9], demineralized osteoid is identified at post mortem with concentrations <30 ng/ml (75 mmol/l) [10]. A consensus opinion in 2005 suggests a target of 28–32 ng/ml (70–80 nmol/l) [11]. A number of high-risk groups have been reported, including pregnant women, older adults and various ethnic groups [12]. It is not known whether these different groups require different target vitamin D concentrations.
Low vitamin D has been reported in patients with CTDs, including SLE [13], primary SS [14] and idiopathic inflammatory myopathy (IIM) [15]. While the prevalence of vitamin D deficiency is well-recognized, its role in the development, progression and clinical manifestations of CTDs is not clear. Many rheumatologists remain uncertain about which patients should be tested, how deficiency should be treated and what benefits to expect from such interventions.
This review will assess the evidence for the use of vitamin D to treat CTD manifestations beyond its effects on bone health. We will focus primarily on data from observational and interventional studies in SLE, although other CTDs will also be considered.
Prevalence of vitamin D deficiency in SLE
Compared with healthy controls, patients with SLE have an increased risk of vitamin D deficiency. On reviewing studies that included a healthy control comparator group, lower vitamin D levels were reported in SLE patients in 12/14 (86%) such studies (see supplementary Table S1, available at Rheumatology Online). Conversely, Mandal et al. [16] found no difference in 25(OH)D levels between SLE patients and healthy controls, although there was marked vitamin D deficiency among the control group. Furthermore, 39% of the SLE patients were taking steroids and calcium/vitamin D supplements, which may have masked any differences [16]. Similarly, Stockton et al. [17] also found no difference in 25(OH)D between SLE patients and controls, which may be explained by 13/24 (54%) patients taking vitamin D supplements compared with 2/21 (10%) controls. Both of these studies may therefore have underestimated the prevalence of vitamin D deficiency in the patient populations.
Causes of vitamin D deficiency in SLE
The true relationship between vitamin D and inflammation remains to be determined. CTDs are chronic, often debilitating diseases with high levels of morbidity. In healthy subjects, reduced physical activity and reduced sun exposure (but not dietary intake) are important determinants of vitamin D status [18]. Vitamin D deficiency in the context of chronic illness may simply reflect reduced outdoor activity, and thus UV exposure, in these patients (reverse causation). This is relevant in SLE due to the photosensitive nature of the disease and the recommendations of sunlight avoidance and high sun protection factor (SPF) sunblock use [19].
A further explanation for the association between vitamin D deficiency and autoimmune disease is that 25(OH)D may act as a negative acute phase reactant. In a meta-analysis, low serum 25(OH)D was reported following an acute event (including orthopaedic surgery and acute pancreatitis) in 6/8 studies, often in association with a decrease in serum albumin and an increase in CRP [20]. It is proposed that this decrease occurs due to reduced levels of the vitamin D binding protein [21].
Finally, it is plausible that vitamin D deficiency may drive the development of autoimmune disease and potentiate the inflammatory response. An early study by Kamen et al. (2006) identified lower serum 25(OH)D observed in recently diagnosed SLE patients compared with healthy controls and suggested that vitamin D deficiency may be a risk factor for the development of SLE. In this study, however, the patients already had SLE [22], a condition that often has prolonged delays in diagnosis. Furthermore, the difference in vitamin D levels was only statistically significant for Caucasian patients (62% of the study cohort). In terms of leading to the development of autoimmunity, a small cross-sectional study of European Americans found significantly increased vitamin D deficiency in ANA-positive vs ANA-negative healthy controls [23]. In a linkage analysis study, admission to hospital for vitamin D deficiency (including osteomalacia or rickets) was associated with increased future risk of developing a number of immune-mediated diseases, including RA, SLE and SS [24]. Other large prospective studies of women have not identified any association between vitamin D intake (as assessed by dietary questionnaire) and the risk of developing SLE [25, 26]. However, these observations may reflect the inadequacy of using dietary questionnaires to estimate vitamin D status, particularly given the importance of cutaneous synthesis of vitamin D.
There is evidence that vitamin D deficiency may have a role once autoimmunity has developed. In a study by Zold et al. [27], patients who progressed from a UCTD to a clearly defined CTD had significantly lower vitamin D levels than those that did not progress.
How might vitamin D deficiency lead to the development of SLE?
Polymorphisms in the vitamin D pathway
The VDR genotype may provide a link between low serum 25(OH)D levels and SLE. The Fokl polymorphism (rs2228570) is notable since the FF genotype was associated with low 25(OH)D levels in a small genetic study [28] and an increased risk of SLE in a separate larger study [29]. A further VDR polymorphism, Bsml (rs1544410), has also been associated with an increased risk of SLE in Asian subjects, although it is not clear whether this is independent of serum 25(OH)D levels [29]. If vitamin D status is partly dependent on genetic variation, then a fixed definition of deficiency across populations may not be appropriate. The normal range for 25(OH)D may need to be redefined on the basis of the VDR genotype.
Vitamin D and immunomodulation
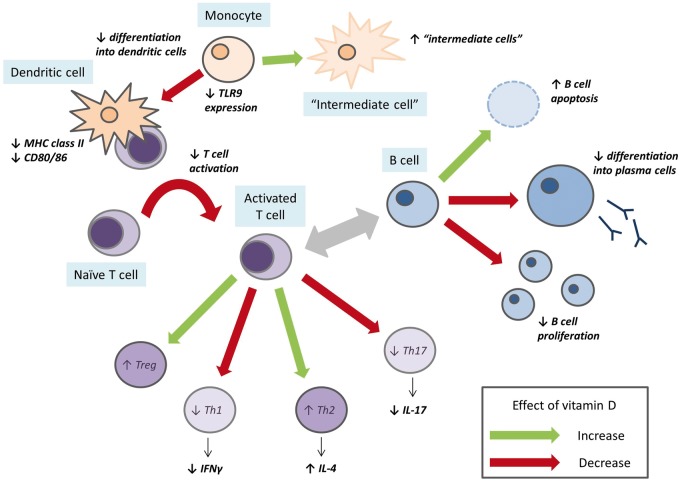
Vitamin D has broad immunomodulatory effects across both the innate and adaptive immune systems (Fig. 1). Of particular relevance to SLE, calcitriol reduces anti-dsDNA antibody production by inhibiting B cell proliferation, by reducing differentiation into plasma cells and by B cell apoptosis [30, 31]. A decrease in anti-dsDNA has also been demonstrated in vivo after 4 weeks of high-dose cholecalciferol [32]. This effect was not due to changes in memory B cells alone. Increases in naive and regulatory T cells and decreases in Th1 and Th17 cells were also observed. Furthermore, in terms of T cells, in vitro studies have also shown that the VDR is upregulated following T cell activation and that calcitriol can polarize cells towards the Th2 phenotype [33]. Similarly, in a clinical study, high-dose cholecalciferol resulted in a reduction in the IFN-γ:IL-4 ratio, representing a Th1 to Th2 shift [34]. In healthy subjects, high-dose vitamin D also increases the number of FoxP3+ regulatory T cells [35].
Fig. 1.
Summary of the effects of vitamin D on the innate and adaptive immune systems
A schematic representation, derived principally from in vitro studies, of the effects of 1,25(OH)2D on the immune system. The green arrows show an increase in response to 1,25(OH)2D and the red arrows show a decrease.
In the innate immune system, dendritic cells (DCs) and monocytes express both the VDR and CYP27B1. The expression of CYP27B1 in monocytes, and thus local calcitriol concentration, is under regulatory control by other immune cells. The effect of calcitriol on innate immune cells is predominantly immunosuppressive, with reduced monocyte differentiation and DC maturation, downregulation of MHC II and CD80/86, leading to reduced T cell activation [36–39]. This has relevance for SLE, as calcitriol attenuates monocyte expression of MHC II, CD40 and CD86 in response to lupus serum [40]. In a study of patients with type 1 diabetes mellitus, vitamin D supplementation resulted in reduced differentiation of monocytes into DCs and the expansion of an intermediate cell type phenotypically similar to tolerogenic DCs [41]. In addition, toll-like receptor 9 (TLR9) senses hypomethylated DNA present in immune complexes and other sources, promoting an inflammatory response [42]. Vitamin D downregulates TLR9 expression in healthy human monocytes, resulting in reduced IL-6 production in response to TLR9 stimulation [43].
Much less is known about the effects of vitamin D on neutrophil function. In animal models, calcitriol reduces neutrophil recruitment, possibly via an effect on IL-8 [44]. Vitamin D may also have direct effects on neutrophil function, as neutrophils express VDR mRNA and show differential gene expression in response to 1,25(OH)2D3 [45]. The relevance of these changes remains to be examined in the clinical setting.
Low vitamin D and lupus disease activity
The relationship between 25(OH)D and lupus disease activity remains unclear. While some studies have demonstrated an association [16, 28, 46–50], others have not [23, 51–54]. This discrepancy cannot be clearly explained, but there are many potential confounding factors. As an example, the largest of these studies was conducted by Amital et al. [50] and comprised 378 patients from several European and Israeli cohorts. A modest association was seen between the vitamin D level at a single time point and disease activity (r = −0.12, P = 0.018). In this study, disease activity was not defined using a standardized scoring system and no attempt was made to adjust for important cofounders such as the use of corticosteroids, immunosuppressant drugs, vitamin D supplements or for BMI. It is therefore difficult to conclude any causative association from this observation. Furthermore, even if confirmed, the strength of the correlation suggests that, at best, vitamin D accounts for only ∼1.4% of the variance in disease activity. Two other studies have also shown an association between lower vitamin D levels and lupus flares [55, 56]. These observational studies do not prove causation, as low levels may still be a consequence of systemic inflammation and the acute phase response, as described previously.
Interventional studies of vitamin D in SLE
Only a small number of interventional studies have investigated the effect of vitamin D on disease activity in SLE, the majority of which have been inconclusive. The largest observational study of 763 SLE patients explored the relationship between changes in vitamin D and disease activity. While it was not a true interventional trial, deficient patients were treated with 50 000 IU ergocalciferol (plus 200 IU cholecalciferol) as per local guidelines. Although this could be considered a ‘high-dose’ regimen, the mean increase in serum 25(OH)D was not reported. The authors identified small changes in disease activity [reduction in Safety of Estrogen in Lupus Erythematosus National Assessment–SLEDAI score of 0.22 (95% CI −0.41, −0.02) for a 20 ng/ml increase in vitamin D] and urinary protein:creatinine ratio [4% (95% CI 2, 5) decrease in PCR for a 20 ng/ml increase in vitamin D] in response to vitamin D therapy. The association between the change in serum 25(OH)D and disease activity was only apparent using a two-slope model and only when 25(OH)D was <40 ng/ml [57]. Such associations are also very modest and of uncertain clinical significance.
Two double-blind RCTs have also focused on the effect of vitamin D on laboratory markers of disease activity. Abou-Raya et al. [13] reported that 2000 IU/day for 12 months significantly reduced anti-dsDNA and anti-Sm titres and increased serum C4 compared with placebo. In this study, patients were allowed to continue baseline medication, but no details of any changes in medication over the 12 month trial were described. Furthermore, only a subset of the whole trial population was reported in the paper (in the intervention group, the change in SLEDAI is presented for 122/178 subjects). In contrast, a smaller study by Aranow et al. failed to find any change in expression of the IFN signature in response to vitamin D [58]. Although similar dosing regimens (2000 and 4000 IU/day) were used, the study was shorter (12 weeks), and again achieved incomplete vitamin D repletion (only 16/33 of treated patients at the end of the study). Recently another small high-dose crossover trial of 25 000 IU/month vs 300 000 IU loading and 50 000 IU/month also failed to show a change in disease activity. A limitation of this study was that only the high-dose regimen increased serum 25(OH)D levels, which may have been due to the high mean baseline 25(OH)D of 31.7 ng/ml [59].
A small trial randomized 40 adolescent SLE patients to 50 000 IU/week cholecalciferol or placebo and identified a significant difference in SLEDAI score and dsDNA positivity after 24 weeks [60]. The study does not clearly demonstrate that vitamin D therapy reduces disease activity; in the treated group, the SLEDAI score remained stable, but worsened in the placebo group.
These interventional studies highlight some of the difficulties in conducting clinical trials of vitamin D, including selecting the correct patient population, dose and treatment duration and determining the influence of potential threshold effects. Patients who are vitamin D deficient at baseline who receive adequate replacement may have the greatest benefit, pointing towards the need for a personalized approach. However, this hypothesis needs confirmation in well-conducted clinical trials.
SS
A cross-sectional study of 107 Turkish patients with SS found lower vitamin D levels in female but not male patients compared with controls [14]. This may reflect a true gender difference, although there were only 10 (9.3%) men in the study. In contrast, Agmon-Levin et al. [61] found no difference in serum 25(OH)D between SS patients and controls, although levels were lower in patients with peripheral neuropathy (18.6 vs 22.6 ng/ml) or lymphoma (13.2 vs 22.0 ng/ml). Furthermore, there is no evidence that genetic polymorphisms in the vitamin D pathway associate with the prevalence or severity of SS [62]. No studies have investigated the effect of vitamin D treatment in SS.
IIM
Little is known about the role of vitamin D in IIM. A single study of 149 IIM patients and 290 healthy subjects found a prevalence of vitamin D deficiency of 53–68% across the IIM subtypes compared with 21% in controls [15]. An inverse association between 25(OH)D and disease activity (assessed by physician global assessment) has been reported in JDM. However, the relationship was weak, with a 1 cm change in physician global assessment associated with only a 1.7 ng/ml change in 25(OH)D [63]. Similar to SS, there are also no associations between VDR polymorphisms and IIM. In a Hungarian study of 89 DM and PM patients, there were no differences in either VDR polymorphisms or haplotypes between the IIM patients and healthy subjects [64]. While there are also no intervention studies of vitamin D in IIM, a single in vitro study suggests that vitamin D may be able to module the inflammatory response. The cytokine CXCL10 is released from skeletal muscle in response to pro-inflammatory cytokines and is increased in the serum of IIM patients. VDR agonists can decrease CXCL10 secretion by human skeletal myocytes in response to stimuli [65].
SSc
The potential relationship between vitamin D and skin fibrosis is complex and beyond the scope of this review. Notably, the pro-fibrotic cytokine TGF-β1 is increased in the serum of vitamin D–deficient subjects, and the pro-fibrotic effect of TGF-β1 on epithelial fibroblasts is attenuated by exposure to 1,25(OH)2D3 [66–69]. Within fibroblasts, TGF-β1 signals via the phosphorylation of the Smad3 pathway. In animal models, vitamin D analogues reduce skin fibrosis via activation of the Th2 pathway [70].
A number of studies have identified that vitamin D deficiency is common in SSc [71–73]. Arnson et al. [74] measured 25(OH)D in 327 European SSc patients and 141 healthy controls. In this study, vitamin D levels were significantly lower in patients compared with controls [13.5 ng/ml (s.d. 9.0) vs 21.6 (9.7)] and were inversely associated with the severity of skin fibrosis. While reduced cutaneous synthesis due to skin thickening and reduced intestinal absorption have been postulated to contribute to vitamin D deficiency, analysis of vitamin D metabolites suggests that these processes actually remain intact [75]. Anti-vitamin D antibodies are prevalent in SSc, although there is no evidence that they contribute to disease development or progression [76].
A small study of oral calcitriol in scleroderma spectrum disorders failed to show any benefit over placebo, although only 2/27 patients had SSc while 20 had morphoea [77]. Cutaneous calcinosis is an important feature of lcSSc. Given that vitamin D regulates serum calcium and mobilizes calcium from bone, it is important to better understand any potential role of vitamin D in SSc to ensure that vitamin D therapy does not exacerbate calcinosis.
Vitamin D and CTD-related co-morbidity
Cardiovascular disease
A common observation among CTD patients is an increased risk of cardiovascular disease (CVD). In SLE, the relative risk for myocardial infarction is ∼2.5-fold across all age groups, but up to 52-fold in younger patients [78]. Vitamin D deficiency is a proposed risk factor for the development of CVD in the general population [79]. In SLE, an association between aortic stiffness and vitamin D deficiency has been demonstrated that may be mediated via increased disease activity [80]. Ravenell et al. [81] also found that lower vitamin D was associated with increased carotid plaque, although in this study it was also associated with reduced disease activity. The association between vitamin D and traditional CVD risk factors (e.g. hypertension, hyperlipidaemia, adiposity) is less clear. Some groups have demonstrated an association [82] while others have not [83]. There are currently no published interventional studies to demonstrate that vitamin D can improve cardiovascular outcomes in CTDs. However, in the general population, a meta-analysis of 22 trials demonstrated a significant reduction in all-cause mortality but only a trend towards reduced cardiovascular mortality [84]. Similarly, the Women’s Health Initiative interventional study of 36 282 post-menopausal women found that vitamin D supplementation did not affect CVD risk [85]. This study may have significantly underestimated the effect of vitamin D supplementation, as the control group was allowed personal supplementation (600 IU/day) that was greater than the intervention dose (400 IU/day). Several ongoing trials in the general population are under way and should help to resolve this question in due course.
Metabolic syndrome
The metabolic syndrome is present in ∼40% of SLE patients early after diagnosis [86]. Metabolic syndrome is an important risk factor for CVD and is associated with cumulative organ damage [87]. A small study of non-diabetic SLE patients found that low vitamin D was associated with increased insulin resistance and a trend towards increased metabolic syndrome, independent of BMI [88]. In the general population, increases in serum 25(OH)D were associated with significantly reduced risks of developing metabolic syndrome over 12 months [89].
Fatigue
Fatigue is common in SLE (∼80% of patients), often in association with poor sleep, anxiety and depression [90]. In an open-label study of 80 SLE patients there was a significant correlation between the change in serum 25(OH)D and the change in fatigue score over a 2 year period [91]. Further encouraging findings were seen in a randomized controlled trial of cholecalciferol in juvenile-onset SLE by Lima et al. In this study, there was also a significant reduction in fatigue at 24 weeks [60].
Which patients should be tested?
For the rheumatologist, there are few guidelines to advise which patients should be tested for vitamin D deficiency. The National Osteoporosis Society (NOS) advocates measurement in patients with bone disease (in whom vitamin D may be a treatment or in whom it should be corrected prior to other treatments) or patients with musculoskeletal symptoms that may be due to vitamin D deficiency. However, the NOS does not recommend screening asymptomatic individuals, even if they are at a high risk of vitamin D deficiency [92]. In contrast, the Endocrine Society (a US-based organization) recommends screening a number of patient groups, including those with chronic kidney disease, hepatic failure, obesity and African American and Hispanic populations [93]. Therefore an SLE/CTD population may include many patients in which screening for vitamin D deficiency is appropriate on the basis of assessment of bone health alone.
How much vitamin D is enough?
There is currently no consensus regarding the optimum vitamin D treatment regime. The Institute of Medicine has focused only on dietary intake of vitamin D in the general population and recommends a conservative intake of ∼600 IU/day [94]. The Endocrine Society suggests a higher daily intake for at-risk individuals, aiming for 1500–2000 IU/day, with an upper limit of 10 000 IU [93]. In the UK, the NOS recommends treatment of deficiency [25(OH)D < 30 nmol/l] with a loading dose of up to 300 000 IU followed by a maintenance dose of ∼800–2000 IU/day after a period of 1 month [92]. There are no guidelines relating specifically to CTDs, either in terms of target vitamin D levels or recommended regimes. Our current practise is to focus on bone protection and follow the NOS recommendations, aiming for a target concentration of >30 ng/ml (75 nmol/l). Any additional effects of vitamin D beyond bone protection are currently theoretical and there is no convincing evidence to encourage a move away from or to enhance current guidelines, either in the scope of screening, the dose regimes or the ideal target vitamin D concentration.
Vitamin D toxicity
Vitamin D therapy is usually well-tolerated and vitamin D toxicity is rare. It has been proposed that chronic consumption of ∼40 000 IU/day and serum levels in excess of 80 ng/ml (200 nmol/l) are required before toxicity occurs [95]. Similarly, drug–vitamin D interactions are uncommon, but hypercalcaemia may occur when vitamin D is administered concurrently with calcium supplements and thiazide diuretics [96].
Summary and future areas of research
There remains considerable interest in the potential use of vitamin D as an adjunct in the treatment of CTDs. Although vitamin D deficiency is common across the CTDs, in observational studies there are numerous factors that confound the association between 25(OH)D and the presence of autoimmune disease. Furthermore, vitamin D levels vary considerably over time and many studies only measure 25(OH)D at a single time point. Mendelian randomization studies of vitamin D pathway polymorphisms may help to identify whether vitamin D deficiency predisposes individuals to developing autoimmune disease.
While experimental studies have demonstrated that 25(OH)D and 1,25(OH)2D3 are anti-inflammatory and immunoregulatory across a number of immune pathways, the results from clinical studies have been inconclusive. There is also a lack of well-designed interventional studies both in SLE and in other CTDs. Many of the studies have used relatively low doses of vitamin D and/or may not have achieved sufficient changes in 25(OH)D levels. It is likely that the optimum serum 25(OH)D level is different for individual patients, thus a personalized approach is likely to be needed. However, the absence of positive results may also point towards a more passive role for vitamin D in autoimmunity, with serum 25(OH)D levels acting as a negative acute phase reactant and thus reflecting the presence of systemic inflammation and general poor health rather than being causative. Well-designed trials are now needed in order to define the utility, if any, of vitamin D to influence or modify the primary disease or its co-morbidities beyond its role in bone health.
Supplementary Material
Acknowledgements
I.N.B. is a National Institute for Health Research (NIHR) Senior Investigator and is funded by Arthritis Research UK, the NIHR Manchester Biomedical Research Unit and the NIHR Manchester Wellcome Trust Clinical Research Facility. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Brincat M, Gambin J, Brincat M, Calleja-Agius J. The role of vitamin D in osteoporosis. Maturitas 2015;80:329–32. [DOI] [PubMed] [Google Scholar]
- 2. Saraff V, Shaw N. Sunshine and vitamin D. Arch Dis Child 2016;101:190–2. [DOI] [PubMed] [Google Scholar]
- 3. Holick MF. The cutaneous photosynthesis of pre-vitamin D3: a unique photo-endocrine system. J Invest Dermatol 1981;77:51–8. [DOI] [PubMed] [Google Scholar]
- 4. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 5. Ponchon G, Kennan AL, Deluca HF. Activation of vitamin D by liver. J Clin Invest 1969;48:2032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys 2012;523:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Zhu J, Deluca HF. Where is the vitamin D receptor? Arch Biochem Biophys 2012;523:123–33. [DOI] [PubMed] [Google Scholar]
- 8. Grober U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: update 2013: from rickets prophylaxis to general preventive healthcare. Dermatoendocrinol 2013;5:331–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998;351:805–6. [DOI] [PubMed] [Google Scholar]
- 10. Priemel M, von DC, Klatte TO. et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 2010;25:305–12. [DOI] [PubMed] [Google Scholar]
- 11. Dawson-Hughes B, Heaney RP, Holick MF. et al. Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713–6. [DOI] [PubMed] [Google Scholar]
- 12. van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab 2011;25:671–80. [DOI] [PubMed] [Google Scholar]
- 13. Abou-Raya A, Abou-Raya S, Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol 2013;40:265–72. [DOI] [PubMed] [Google Scholar]
- 14. Erten S, Sahin A, Altunoglu A, Gemcioglu E, Koca C. Comparison of plasma vitamin D levels in patients with Sjogren’s syndrome and healthy subjects. Int J Rheum Dis 2015;18:70–5. [DOI] [PubMed] [Google Scholar]
- 15. Azali P, Barbasso HS, Kockum I. et al. Low serum levels of vitamin D in idiopathic inflammatory myopathies. Ann Rheum Dis 2013;72:512–6. [DOI] [PubMed] [Google Scholar]
- 16. Mandal M, Tripathy R, Panda AK. et al. Vitamin D levels in Indian systemic lupus erythematosus patients: association with disease activity index and interferon alpha. Arthritis Res Ther 2014;16:R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stockton KA, Kandiah DA, Paratz JD, Bennell KL. Fatigue, muscle strength and vitamin D status in women with systemic lupus erythematosus compared with healthy controls. Lupus 2012;21:271–8. [DOI] [PubMed] [Google Scholar]
- 18. Touvier M, Deschasaux M, Montourcy M. et al. Determinants of vitamin D status in Caucasian adults: influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors. J Invest Dermatol 2015;135:378–88. [DOI] [PubMed] [Google Scholar]
- 19. Cusack C, Danby C, Fallon JC. et al. Photoprotective behaviour and sunscreen use: impact on vitamin D levels in cutaneous lupus erythematosus. Photodermatol Photoimmunol Photomed 2008;24:260–7. [DOI] [PubMed] [Google Scholar]
- 20. Silva MC, Furlanetto TW. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr Res 2015;35:91–6. [DOI] [PubMed] [Google Scholar]
- 21. Waldron JL, Ashby HL, Cornes MP. et al. Vitamin D: a negative acute phase reactant. J Clin Pathol 2013;66:620–2. [DOI] [PubMed] [Google Scholar]
- 22. Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev 2006;5:114–7. [DOI] [PubMed] [Google Scholar]
- 23. Ritterhouse LL, Crowe SR, Niewold TB. et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis 2011;70:1569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramagopalan SV, Goldacre R, Disanto G, Giovannoni G, Goldacre MJ. Hospital admissions for vitamin D related conditions and subsequent immune-mediated disease: record-linkage studies. BMC Med 2013;11:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis 2008;67:530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hiraki LT, Munger KL, Costenbader KH, Karlson EW. Dietary intake of vitamin D during adolescence and risk of adult-onset systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res 2012;64:1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zold E, Szodoray P, Gaal J. et al. Vitamin D deficiency in undifferentiated connective tissue disease. Arthritis Res Ther 2008;10:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emerah AA, El-Shal AS. Role of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D level in Egyptian female patients with systemic lupus erythematosus. Mol Biol Rep 2013;40:6151–62. [DOI] [PubMed] [Google Scholar]
- 29. Mao S, Huang S. Association between vitamin D receptor gene BsmI, FokI, ApaI and TaqI polymorphisms and the risk of systemic lupus erythematosus: a meta-analysis. Rheumatol Int 2014;34:381–8. [DOI] [PubMed] [Google Scholar]
- 30. Linker-Israeli M, Elstner E, Klinenberg JR, Wallace DJ, Koeffler HP. Vitamin D3 and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin Immunol 2001;99:82–93. [DOI] [PubMed] [Google Scholar]
- 31. Chen S, Sims GP, Chen XX. et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007;179:1634–47. [DOI] [PubMed] [Google Scholar]
- 32. Terrier B, Derian N, Schoindre Y. et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Ther 2012;14:R221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boonstra A, Barrat FJ, Crain C. et al. 1α,25-dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol 2001;167:4974–80. [DOI] [PubMed] [Google Scholar]
- 34. Piantoni S, Andreoli L, Scarsi M. et al. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin D. Lupus 2015;24:490–8. [DOI] [PubMed] [Google Scholar]
- 35. Prietl B, Treiber G, Mader JK. et al. High-dose cholecalciferol supplementation significantly increases peripheral CD4+ Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur J Nutr 2014;53:751–9. [DOI] [PubMed] [Google Scholar]
- 36. Hewison M, Freeman L, Hughes SV. et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol 2003;170:5382–90. [DOI] [PubMed] [Google Scholar]
- 37. Sochorova K, Budinsky V, Rozkova D. et al. Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) and calcitriol (1,25-dihydroxyvitamin D3) exert potent immunomodulatory effects on dendritic cells and inhibit induction of antigen-specific T cells. Clin Immunol 2009;133:69–77. [DOI] [PubMed] [Google Scholar]
- 38. Penna G, Adorini L. 1α,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 2000;164:2405–11. [DOI] [PubMed] [Google Scholar]
- 39. Korf H, Wenes M, Stijlemans B. et al. 1,25-dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012;217:1292–300. [DOI] [PubMed] [Google Scholar]
- 40. Lerman M, Burnham J, Behrens E. 1,25-dihydroxyvitamin D3 limits monocyte maturation in lupus sera. Lupus 2011;20:749–53. [DOI] [PubMed] [Google Scholar]
- 41. Mauf S, Penna-Martinez M, Jentzsch T. et al. Immunomodulatory effects of 25-hydroxyvitamin D3 on monocytic cell differentiation and influence of vitamin D3 polymorphisms in type 1 diabetes. J Steroid Biochem Mol Biol 2015;147:17–23. [DOI] [PubMed] [Google Scholar]
- 42. Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med 2005;202:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dickie LJ, Church LD, Coulthard LR. et al. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology 2010;49:1466–71. [DOI] [PubMed] [Google Scholar]
- 44. Takano Y, Mitsuhashi H, Ueno K. 1α,25-dihydroxyvitamin D3 inhibits neutrophil recruitment in hamster model of acute lung injury. Steroids 2011;76:1305–9. [DOI] [PubMed] [Google Scholar]
- 45. Takahashi K, Nakayama Y, Horiuchi H. et al. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1α,25-dihydroxyvitamin D3. Immunopharmacol Immunotoxicol 2002;24:335–47. [DOI] [PubMed] [Google Scholar]
- 46. Thudi A, Yin S, Wandstrat AE, Li QZ, Olsen NJ. Vitamin D levels and disease status in Texas patients with systemic lupus erythematosus. Am J Med Sci 2008;335:99–104. [DOI] [PubMed] [Google Scholar]
- 47. Mok CC, Birmingham DJ, Ho LY. et al. Vitamin D deficiency as marker for disease activity and damage in systemic lupus erythematosus: a comparison with anti-dsDNA and anti-C1q. Lupus 2012;21:36–42. [DOI] [PubMed] [Google Scholar]
- 48. Bonakdar ZS, Jahanshahifar L, Jahanshahifar F, Gholamrezaei A. Vitamin D deficiency and its association with disease activity in new cases of systemic lupus erythematosus. Lupus 2011;20:1155–60. [DOI] [PubMed] [Google Scholar]
- 49. Szodoray P, Tarr T, Bazso A. et al. The immunopathological role of vitamin D in patients with SLE: data from a single centre registry in Hungary. Scand J Rheumatol 2011;40:122–6. [DOI] [PubMed] [Google Scholar]
- 50. Amital H, Szekanecz Z, Szucs G. et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis 2010;69:1155–7. [DOI] [PubMed] [Google Scholar]
- 51. Kim HA, Sung JM, Jeon JY, Yoon JM, Suh CH. Vitamin D may not be a good marker of disease activity in Korean patients with systemic lupus erythematosus. Rheumatol Int 2010;31:1189–94. [DOI] [PubMed] [Google Scholar]
- 52. Orbach H, Zandman-Goddard G, Amital H. et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci 2007;1109:385–400. [DOI] [PubMed] [Google Scholar]
- 53. Lopez-Robles C, Rios-Fernandez R, Callejas-Rubio J, Ortego-Centeno N. Vitamin D deficiency in a cohort of patients with systemic lupus erythematous in the South of Spain. Lupus 2011;20:330–1. [DOI] [PubMed] [Google Scholar]
- 54. Souto M, Coelho A, Guo C. et al. Vitamin D insufficiency in Brazilian patients with SLE: prevalence, associated factors, and relationship with activity. Lupus 2011;20:1019–26. [DOI] [PubMed] [Google Scholar]
- 55. Birmingham DJ, Hebert LA, Song H. et al. Evidence that abnormally large seasonal declines in vitamin D status may trigger SLE flare in non-African Americans. Lupus 2012;21:855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dall’Ara F, Andreoli L, Piva N. et al. Winter lupus flares are associated with low vitamin D levels in a retrospective longitudinal study of Italian adult patients. Clin Exp Rheumatol 2015;33:153–8. [PubMed] [Google Scholar]
- 57. Petri M, Bello KJ, Fang H, Magder LS. Vitamin D in systemic lupus erythematosus: modest association with disease activity and the urine protein-to-creatinine ratio. Arthritis Rheum 2013;65:1865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aranow C, Kamen DL, Dall’Era M. et al. Randomized, double-blind, placebo-controlled trial of the effect of vitamin D3 on the interferon signature in patients with systemic lupus erythematosus. Arthritis Rheumatol 2015;67:1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andreoli L, Dall’Ara F, Piantoni S. et al. A 24-month prospective study on the efficacy and safety of two different monthly regimens of vitamin D supplementation in pre-menopausal women with systemic lupus erythematosus. Lupus 2015;24:499–506. [DOI] [PubMed] [Google Scholar]
- 60. Lima GL, Paupitz J, Aikawa NE. et al. Vitamin D supplementation in adolescents and young adults with juvenile systemic lupus erythematosus for improvement in disease activity and fatigue scores: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res 2016;68:91–8. [DOI] [PubMed] [Google Scholar]
- 61. Agmon-Levin N, Kivity S, Tzioufas AG. et al. Low levels of vitamin-D are associated with neuropathy and lymphoma among patients with Sjogren’s syndrome. J Autoimmun 2012;39:234–9. [DOI] [PubMed] [Google Scholar]
- 62. Zilahi E, Chen JQ, Papp G, Szanto A, Zeher M. Lack of association of vitamin D receptor gene polymorphisms/haplotypes in Sjogren’s syndrome. Clin Rheumatol 2015;34:247–53. [DOI] [PubMed] [Google Scholar]
- 63. Robinson AB, Thierry-Palmer M, Gibson KL, Rabinovich CE. Disease activity, proteinuria, and vitamin D status in children with systemic lupus erythematosus and juvenile dermatomyositis. J Pediatr 2012;160:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bodoki L, Chen JQ, Zeher M. et al. Vitamin D receptor gene polymorphisms and haplotypes in Hungarian patients with idiopathic inflammatory myopathy. Biomed Res Int 2015;2015:809895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Di LL, Sottili M, Antinozzi C. et al. The vitamin D receptor agonist BXL-01-0029 as a potential new pharmacological tool for the treatment of inflammatory myopathies. PLoS One 2013;8:e77745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Isik S, Ozuguz U, Tutuncu YA. et al. Serum transforming growth factor-beta levels in patients with vitamin D deficiency. Eur J Intern Med 2012;23:93–7. [DOI] [PubMed] [Google Scholar]
- 67. Ramirez AM, Wongtrakool C, Welch T. et al. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor β1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol 2010;118:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang GY, Cheng T, Luan Q. et al. Vitamin D: a novel therapeutic approach for keloid, an in vitro analysis. Br J Dermatol 2011;164:729–37. [DOI] [PubMed] [Google Scholar]
- 69. Slominski A, Janjetovic Z, Tuckey RC. et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab 2013;98:E298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Usategui A, Criado G, Del Rey MJ, Fare R, Pablos JL. Topical vitamin D analogue calcipotriol reduces skin fibrosis in experimental scleroderma. Arch Dermatol Res 2014;306:757–61. [DOI] [PubMed] [Google Scholar]
- 71. Caramaschi P, Dalla GA, Ruzzenente O. et al. Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol 2010;29:1419–25. [DOI] [PubMed] [Google Scholar]
- 72. Rios FR, Fernandez RC, Callejas Rubio JL, Ortego CN. Vitamin D deficiency in a cohort of patients with systemic scleroderma from the south of Spain. J Rheumatol 2010;37:1355. [DOI] [PubMed] [Google Scholar]
- 73. Rios-Fernandez R, Callejas-Rubio JL, Fernandez-Roldan C. et al. Bone mass and vitamin D in patients with systemic sclerosis from two Spanish regions. Clin Exp Rheumatol 2012;30:905–11. [PubMed] [Google Scholar]
- 74. Arnson Y, Amital H, Agmon-Levin N. et al. Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature. Autoimmun Rev 2011;10:490–4. [DOI] [PubMed] [Google Scholar]
- 75. Serup J, Hagdrup H. Vitamin D metabolites in generalized scleroderma. Evidence of a normal cutaneous and intestinal supply with vitamin D. Acta Derm Venereol 1985;65:343–5. [PubMed] [Google Scholar]
- 76. Carmel NN, Rotman-Pikielny P, Lavrov A, Levy Y. Vitamin D antibodies in systemic sclerosis patients: findings and clinical correlations. Isr Med Assoc J 2015;17:80–4. [PubMed] [Google Scholar]
- 77. Hulshof MM, Bouwes Bavinck JN, Bergman W. et al. Double-blind, placebo-controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. J Am Acad Dermatol 2000;43:1017–23. [DOI] [PubMed] [Google Scholar]
- 78. Manzi S, Meilahn EN, Rairie JE. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145:408–15. [DOI] [PubMed] [Google Scholar]
- 79. Wang TJ, Pencina MJ, Booth SL. et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reynolds JA, Haque S, Berry JL. et al. 25-hydroxyvitamin D deficiency is associated with increased aortic stiffness in patients with systemic lupus erythematosus. Rheumatology 2012;51:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ravenell RL, Kamen DL, Spence JD. et al. Premature atherosclerosis is associated with hypovitaminosis D and angiotensin-converting enzyme inhibitor non-use in lupus patients. Am J Med Sci 2012;344:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lertratanakul A, Wu P, Dyer A. et al. 25-hydroxyvitamin D and cardiovascular disease in patients with systemic lupus erythematosus: data from a large international inception cohort. Arthritis Care Res 2014;66:1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu PW, Rhew EY, Dyer AR. et al. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Care Res 2009;61:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chowdhury R, Kunutsor S, Vitezova A. et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. Br Med J 2014;348:g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cauley JA, Chlebowski RT, Wactawski-Wende J. et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health 2013;22:915–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Parker B, Urowitz MB, Gladman DD. et al. Impact of early disease factors on metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis 2015;74:1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Demir S, Artim-Esen B, Sahinkaya Y. et al. Metabolic syndrome is not only a risk factor for cardiovascular diseases in systemic lupus erythematosus but is also associated with cumulative organ damage: a cross-sectional analysis of 311 patients. Lupus 2016;25:117–84. [DOI] [PubMed] [Google Scholar]
- 88. Sabio JM, Vargas-Hitos JA, Martinez-Bordonado J. et al. Association between low 25-hydroxyvitamin D, insulin resistance and arterial stiffness in nondiabetic women with systemic lupus erythematosus. Lupus 2015;24:155–63. [DOI] [PubMed] [Google Scholar]
- 89. Pham TM, Ekwaru JP, Setayeshgar S, Veugelers PJ. The effect of changing serum 25-hydroxyvitamin D concentrations on metabolic syndrome: a longitudinal analysis of participants of a preventive health program. Nutrients 2015;7:7271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tench CM, McCurdie I, White PD, D’Cruz DP. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology 2000;39:1249–54. [DOI] [PubMed] [Google Scholar]
- 91. Ruiz-Irastorza G, Gordo S, Olivares N, Egurbide MV, Aguirre C. Changes in vitamin D levels in patients with systemic lupus erythematosus: effects on fatigue, disease activity, and damage. Arthritis Care Res 2010;62:1160–5. [DOI] [PubMed] [Google Scholar]
- 92. Francis RM, Aspray TJ, Bowring CE. et al. National Osteoporosis Society practical clinical guideline on vitamin D and bone health. Maturitas 2015;80:119–21. [DOI] [PubMed] [Google Scholar]
- 93. Holick MF, Binkley NC, Bischoff-Ferrari HA. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 94. Ross AC, Manson JE, Abrams SA. et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999;69:842–56. [DOI] [PubMed] [Google Scholar]
- 96. Robien K, Oppeneer SJ, Kelly JA, Hamilton-Reeves JM. Drug-vitamin D interactions: a systematic review of the literature. Nutr Clin Pract 2013;28:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.