Abstract
Objective. To quantify peptidylarginine deiminase 2 (PAD2) in SF of RA patients and OA patients, and to determine the association between PAD2 levels, disease activity and inflammatory markers in RA.
Methods. Blood and SF samples were obtained from 39 RA patients and 40 patients with OA. PAD2 content and PAD activity were measured by means of in-house assays. TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-12 were measured using flow cytometry.
Results. PAD2 levels and PAD activity were higher in SF from RA than OA patients (P < 0.0001 and P = 0.03, respectively), as were all cytokine levels (P < 0.0001–0.05). SF PAD2 levels were higher among anti-CCP-positive patients than among anti-CCP-negative patients (P = 0.005). PAD2 levels in SF from RA patients correlated with disease activity, as assessed by DAS28 (P < 0.005). Moreover, SF PAD2 levels correlated with circulating CRP and anti-CCP levels (P < 0.0006), as well as with leucocyte count, IL-6, IL-8 and IL-10 levels in SF (P < 0.0001–0.02). PAD activity in SF was higher in RA patients than in OA patients, and correlated with PAD2 concentration.
Conclusion. Extracellular PAD2 levels in SF correlate with disease activity in RA patients. Anti-CCP-positive RA patients have higher PAD2 levels in SF than anti-CCP-negative RA patients and OA patients. Since we could demonstrate enzymatically active PADs in SF, we propose that free, extracellular PAD is of pathogenic relevance.
Keywords: peptidylarginine deiminase, citrullination, rheumatoid arthritis, cytokines
Rheumatology key messages
Peptidylarginine deiminases 2 is elevated in synovial fluid of anti-CCP-positive RA patients.
Extracellular peptidylarginine deiminases 2 levels correlate with disease activity in RA patients.
Introduction
RA is a chronic, systemic autoimmune disease affecting 0.5–1% of the population worldwide [1]. The small joints of the hands and feet are most commonly involved, but large joints can also be affected. Synovial inflammation causes activation of phagocytes, osteoclasts [2] and synovial fibroblasts [3] leading to erosion of bone and cartilage, which becomes irreversible in later stages of the disease. RA represents at least two disease entities with different pathogeneses and prognoses: a subgroup consisting of 70–80% of RA patients have ACPAs [4], as measured using tests for antibodies against anti-CCP, and HLA-DR molecules containing the shared epitope capable of binding citrullinated peptides [5, 6]. Citrullination refers to post-translational conversion (deimination) of protein arginine residues into citrulline residues. ACPAs are associated with joint damage and radiographic progression [7–9], and anti-CCP-positive RA patients have more active disease with a worse outcome than patients without these autoantibodies [10, 11].
Citrullination is catalysed by a family of enzymes known as peptidylarginine deiminases (PADs), of which five isoforms exist in mammals: PAD1, PAD2, PAD3, PAD4 and PAD6. They are highly homologous, especially in their catalytic site [12]. However, the isoforms are expressed differently in different cells and tissues, have different physiological roles [12], and have different substrate specificities and efficacy [13–16]. In anti-CCP-positive RA, at least PAD2 and PAD4—primarily derived from macrophages and neutrophils—play important roles in generation of the citrulline-containing (citrullinated) self-antigens that become targets for ACPAs and autoreactive T cells in these patients [17–21]. Evidence suggests that PADs, and thereby protein citrullination, are associated with inflammation in general [18, 21–24] and are upregulated in a number of autoimmune diseases and inflammatory conditions, including multiple sclerosis, Alzheimer’s disease, psoriasis, SS and chronic obstructive pulmonary disease (reviewed in [25]). Moreover, citrullination seems to play a pathogenic role in certain cancers [26, 27]. The PADI4 gene, encoding the PAD4 enzyme, has been identified as a risk factor for development of RA [28–30]; however, a number of studies, especially in European populations, did not support this association [31–33]. Only one study has reported an association between the PADI2 gene and RA [34]. The different roles of PAD2 and PAD4 in the pathogenesis of RA are still unclear, as are those played by intracellular and extracellular citrullination. Nor is it clear whether both events are required to initiate and maintain disease. During inflammation, including that associated with RA, pro-inflammatory stimuli and increased cell death allow protein citrullination to occur. It is not known to what extent citrullination occurs intracellularly, as a result of calcium influx after membrane disruption [35, 36], or extracellularly, where the calcium content is sufficiently high for PADs to have catalytic activity [13, 16]. Intracellular PAD2 and citrullinated proteins in the synovium have been associated with anti-CCP levels [37]. As an example of an extracellular protein, citrullinated fibrinogen has been demonstrated in SF from RA patients, but not in SF from OA patients [38].
We recently showed that cell-free SF contained PAD2 and enzymatic activity capable of efficiently citrullinating fibrinogen in vitro [16]. Accordingly, Kinloch and colleagues [21] reported that PAD2, PAD4 and citrullinated proteins were present in SF from patients with RA or spondyloarthritis, but not in SF from OA patients. Taken together, these studies indicate that protein citrullination also takes place extracellularly in the SF of RA patients, catalysed by extracellular PADs. Supporting this notion, citrullinated fibrinogen is present at much lower levels in the circulation than in SF [38], making translocation of citrullinated proteins from the bloodstream unlikely.
In the present study, we quantify PAD2 in SF from 40 OA and 39 RA patients, and determine the PAD activity in the SF samples. We assess the association between these parameters and systemic measures of disease activity on the one hand, and the synovial content of leucocytes and pro-inflammatory cytokines on the other.
Methods
Collection of SF from RA and OA patients
SF samples were obtained during joint aspiration for therapeutic reasons from 39 patients with RA and 40 with OA. The patients fulfilled the ACR criteria for the diagnosis of RA [39]. Thirty-seven of the RA patients were receiving conventional synthetic DMARDs (csDMARDs), 27 were receiving glucocorticoids and 10 (nine anti-CCP-positive and one anti-CCP-negative) were receiving biologics (etanercept [3], adalimumab [2] and golimumab [1]), anti-CD20 (rituximab [2]) and anti-IL6R (tocilizumab [2]). Out of these, one patient was on adalimumab monotherapy and one patient was being treated with glucocorticoids only.
All samples were centrifuged at 1900 g for 10 min to remove cells, and stored at –80°C until use. The study was approved by the local ethics committee of the Institute of Rheumatology in Prague, Czech Republic and written informed consent was obtained from all patients prior to initiation of the study.
Measurement of anti-CCP, RF, CRP and leucocyte count
The levels of serum anti-CCP antibodies and IgM-RF were determined by standard ELISA kits (TestLine Clinical Diagnostics, Brunn, Czech Republic). Serum CRP was measured using an immuno-turbidimetric technique with an Olympus biochemical analyser, model AU 400 (Olympus, Tokyo, Japan) and SF leucocytes were counted using the Iris IQ200 (Beckman Coulter, CA, USA) analyser.
Disease activity assessment
Disease activity in RA patients was assessed according to DAS28 using the number of swollen and tender joints, ESR or CRP, and patient responses using the global visual analogue scale.
Quantification of PAD2 concentration in SF
The PAD2 concentration in SF samples was determined using a previously described assay [40]. In brief, ELISA Maxisorp plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with anti-PAD2 mAb DN2 (1 µg/ml) and incubated overnight at 4°C. Samples were pre-diluted 1:10 in dilution buffer [PBS, 0.5% Tween-20, 2% adult bovine serum (Sigma-Aldrich), 20 μg/ml mouse IgG isotype control (Novus Biologicals, Cambridge, UK), pH 7.4] and incubated for 2 h at room temperature. Biotinylated anti-PAD2 mAb DN6 (1 µg/ml) was added, followed by incubation with streptavidin-conjugated horseradish peroxidase (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) and development with o-phenylene-diamine substrate (Kem-En-Tec, Taastrup, Denmark). All standards and samples were measured in duplicate. Samples were blinded to the investigator. Absolute PAD2 concentrations were calculated by regression analysis for the standard curve with four-parameter logistic curve-fitting using MARS software (BMG Labtech, Ortenberg, Germany).
ELISA activity assay
PAD activity was determined using a previously described assay [16]. Maxisorp plates were coated with 100 µl fibrinogen (1.0 µg/ml) and incubated overnight at 4°C. Wells were washed thrice and blocked in washing buffer A (Tris-buffered saline (TBS), 0.05% Tween-20, pH 7.4) for 20 min at room temperature. SF samples were applied diluted 1:3 in 100 mM Tris–HCl, 1 mM DTT with 10 mM CaCl2. Following three washes in washing buffer B (PBS, 0.05% Tween-20, pH 7.4) the wells were incubated for 90 min at room temperature with 100 µl murine anti-cFib antibody (clone 20B2, ModiQuest, Oss, Netherlands) in washing buffer B. After three further washes, the wells were incubated with 100 µl HRP-conjugated polyclonal rabbit anti-mouse immunoglobulin antibodies (Dako, Glostrup, Denmark) diluted 1:1000 in washing buffer B. Finally, the plates were washed thrice in washing buffer B and developed with o-phenylene-diamine substrate. Optical density was measured at 490–650 nm using the SPECTROstar nano Microplate Reader (BMG Labtech). Data were processed using MARS software (BMG Labtech).
Measurements of cytokines in SFs
The BD Cytometric Bead Array Human Inflammatory Cytokine Kit II (Cat. no. 551811, BD Biosciences, Franklin Lakes, NJ, USA) was used to measure IL-8, IL-1β, IL-6, IL-10, TNF-α and IL-12p70 in 5 µl SF diluted in 20 µl PBS according to the manufacturer’s instructions [41]. A FACSCalibur flow cytometer (BD Biosciences) was used for data acquisition, and the data were subsequently analysed using the FCAPArray Software (SoftFlow, Burnsville, MN, USA).
Statistics
The Kruskal–Wallis test was used to test for differences in three group comparisons using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). The Mann–Whitney test was used to compare differences in groups. The PAD2 measurements are given as the mean of duplicate measurements. Spearman’s correlation (rs) coefficients and levels of significance were determined using GraphPad Prism. SAS enterprise 9.4 (SAS Institute, Cary, NC, USA) was used to test for interactions between two variables. The study was exploratory, and raw P-values are presented. P < 0.05 were considered significant.
Results
Patient characteristics
The study included 39 patients with RA and 40 controls with OA (Table 1). As expected, the RA patients were younger than the OA patients (difference between mean ages of 12 years), and circulating levels of CRP, RF and anti-CCPs were significantly higher in RA than in OA patients (P < 0.0001, Table 1). Age, CRP, DAS28-ESR and DAS28-CRP did not differ significantly between anti-CCP-positive and anti-CCP-negative RA patients (Table 1).
Table 1.
Patient characteristics
| Patient characteristics | Anti-CCP-negative RA patients (n = 10, 50% females) | Anti-CCP-positive RA patients (n = 29, 75% females) | OA patients (n = 40, 60% females) | Kruskal–Wallis test |
|---|---|---|---|---|
| Mean (90% CI) | Mean (90% CI) | Mean (90% CI) | P-value | |
| Age, years | 53.8 (41.6, 66.0) | 54.6 (49.2, 60.0) | 66.3 (62.8, 69.8) | 0.002 |
| CRP, mg/l | 12.6 (6.26, 19.0) | 31.3 (18.55, 44.1) | 3.40 (1.97, 4.84) | <0.0001 |
| DAS28 ESR | 3.90a (3.19, 4.61) | 4.66a (4.05, 5.26) | NA | 0.06b |
| DAS28 CRP | 4.16 (3.34, 4.97) | 4.55 (4.10, 5.00) | NA | 0.21b |
| RF | 21.6 (7.5, 35.7) | 125.8 (64.7, 186.9) | 7.67 (6.3, 9.1) | <0.0001 |
| Anti-CCP, U/ml | 6.1 (2, 11) | 1067 (569, 1565) | 4.89 (3, 6) | <0.0001 |
aData not available for two patients.
bMann–Whitney test. NA: not applicable.
PAD2 levels in SF of RA and OA patients
We next assessed the levels of PAD2 in SF from RA and OA patients. PAD2 levels were 3-fold higher in SF from RA patients (median = 7.7 ng/ml) than in SF from OA patients (2.3 ng/ml) (Fig. 1, P < 0.0001). An influence of RF and heterophilic antibodies on these measurements was ruled out (supplementary Fig. S1, available at Rheumatology Online). We did not observe any differences between patients receiving biologics and those on conventional treatment in the subgroup of anti-CCP-positive RA patients.
Fig. 1.
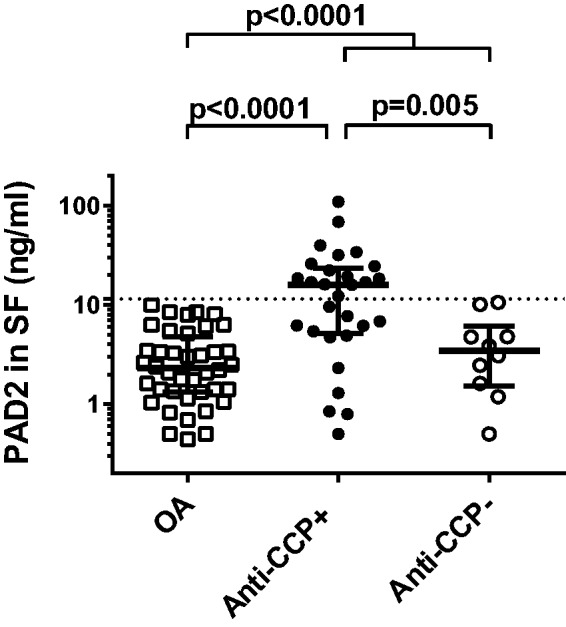
PAD2 concentration in SF
The concentration of PAD2 in SF from 40 patients with OA, 29 anti-CCP-positive RA patients and 10 anti-CCP-negative RA patients was measured by ELISA. Medians and interquartile ranges are shown. The dotted line represents a cut-off value of 11.45 ng/ml, determined by ROC analysis.
In the RA group, PAD2 levels were higher in SF from the anti-CCP-positive patients (15.9 ng/ml) than in SF from anti-CCP-negative patients (3.5 ng/ml) (P = 0.005), as shown in Fig. 1. Using a cut-off level of 11.45 ng/ml, as defined by receiver operating characteristic (ROC) analysis, the anti-CCP-positive patients could be separated from both OA and anti-CCP-negative patients, in both cases with a specificity of 100% and a sensitivity of 55%.
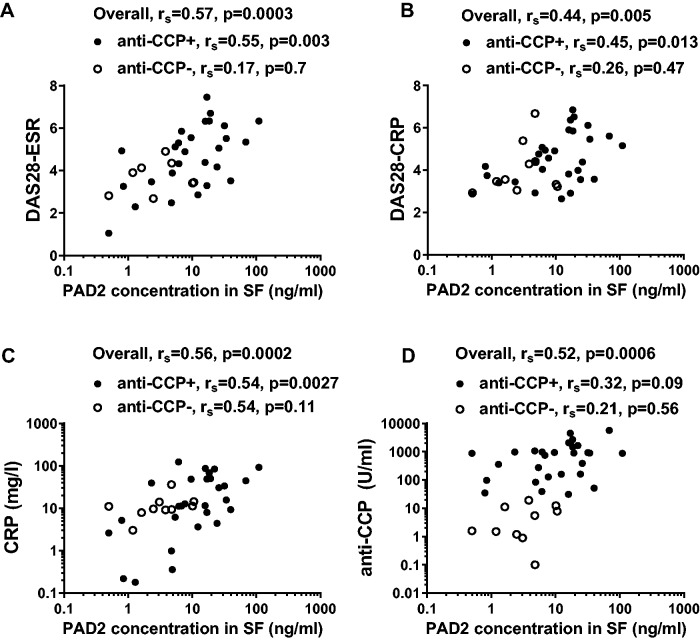
Association between PAD2 levels in SF and disease activity in RA patients
PAD2 levels in SF from RA patients correlated with disease activity assessed by DAS28-ESR and DAS28-CRP (Fig. 2A and B; P = 0.0003 and P = 0.005), as well as with serum CRP levels (Fig. 2C; P = 0.0002). These correlations were also significant in the subgroup of anti-CCP-positive patients alone, but not in the anti-CCP-negative patients (Fig. 2A–C). Moreover, PAD2 levels in SF correlated with serum anti-CCP levels in the RA patients as a whole (Fig. 2D, P = 0.006). Within RA patients, but not in the subgroups, anti-CCP levels correlated with DAS28-ESR (P = 0.013) and, with borderline-significance, with DAS28-CRP (P = 0.053) (data not shown).
Fig. 2.
SF PAD2 levels correlate with DAS28 and circulating CRP and anti-CCP levels
SF PAD2 levels in 29 anti-CCP-positive RA patients (closed circles) and 10 anti-CCP-negative RA patients (open circles) correlated with (A) DAS28-ESR, (B) DAS28-CRP, (C) circulating CRP levels and (D) anti-CCP levels. Spearman’s correlation coefficients (rs) and levels of significance are shown.
Leucocyte counts and cytokine levels in SF of RA and OA patients
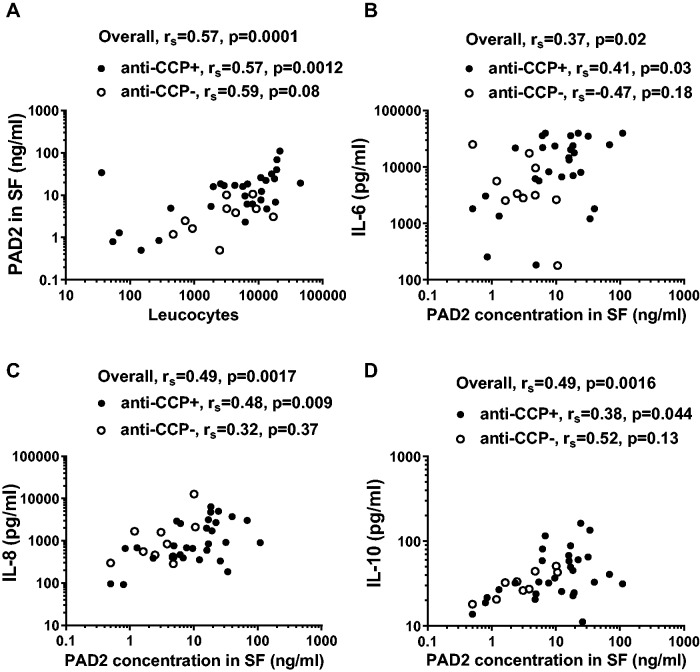
The median leucocyte levels were non-significantly higher in anti-CCP-positive RA patients (6661/μl) than in anti-CCP-negative RA patients (3191/μl), while both subgroups of RA patients had higher counts than OA patients (87/μl; P < 0.0001). The PAD2 levels correlated with the leucocyte count in all RA patients (Fig. 3A; P = 0.0001), which was significant for both anti-CCP-positive RA patients (Fig. 3A) and OA patients (rs = 0.34, P = 0.034; data not shown).
Fig. 3.
Correlation between SF levels of PAD2 with leucocyte counts and SF cytokines
(A) Correlation between PAD2 levels and leucocyte counts in SF from 29 anti-CCP-positive RA patients (closed circles) and 10 anti-CCP-negative RA patients (open circles). (B–D) The content of (B) IL-6, (C) IL-8 and (D) IL-10 in SF from 29 anti-CCP-positive and 10 anti-CCP-negative RA patients is shown as a function of PAD2 content. Spearman’s correlation coefficients (rs) and levels of significance are shown.
SF leucocyte count correlated with serum CRP levels (rs = 0.44, P = 0.005), but not with DAS28 (ESR/CRP) or circulating anti-CCP levels (data not shown). We further assessed SF from RA patients and OA patients for content of the pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-12, as well as of the anti-inflammatory cytokine IL-10 and the chemokine IL-8. All cytokine/chemokine levels in SF were significantly higher in RA than in OA patients (P < 0.0001–0.003), except for IL-1β, the levels of which were higher with borderline significance (P = 0.05) (Table 2). In the RA patients, none of the cytokine levels differed significantly between anti-CCP-positive and anti-CCP-negative patients (Table 2), although the median IL-6 level in anti-CCP-positive patients (14024 pg/ml) was considerably higher than that of anti-CCP-negative patients (3273 pg/ml). SF PAD2 levels correlated with the levels of IL-6, IL-8 and IL-10 in SF in all RA patients and also in the subgroup of anti-CCP-positive RA patients (Fig. 3). Moreover, SF PAD2 levels tended to correlate with IL-1β content among the RA patients (P = 0.07, data not shown). In the OA patients, PAD2 correlated with IL-1β, IL-8 and IL-10 (P < 0.01), but not with IL-6 (P = 0.95) (data not shown).
Table 2.
SF leucocyte count and cytokine/chemokine levels in RA and OA patients
| Cells and mediators of inflammation | Anti-CCP-negative patients (n = 10, 50% females) | Anti-CCP-positive patients (n = 28, 75% females) | Anti-CCP positive vs negative | OA patients (n = 40, 60% females) | Kruskal–Wallis test | |
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | P-value | Median (IQR) | P-value | ||
| Leucocytes/μl | 3193 (885–8434) | 6661 (1900–14773) | 0.32 | 87 (36–178) | <0.0001 | |
| TNF-α, pg/ml | 13.4 (6–27) | 14.4 (7–29) | 0.62 | 8.4 (0–13) | 0.01 | |
| IL-1β, pg/ml | 15.3 (10–24) | 20.7 (7–33) | 0.43 | 12.1 (0–23) | 0.12 | |
| IL-6, pg/ml | 3273 (2613–11 577) | 14 024 (3726–24 632) | 0.09 | 526.7 (218–1235) | <0.0001 | |
| IL-10, pg/ml | 30.0 (20–43) | 35.0 (25–64) | 0.15 | 12.0 (8–18) | <0.0001 | |
| IL-12, pg/ml | 12.1 (0–20) | 6.75 (0–16) | 0.85 | 0 (0–9) | 0.07 | |
| IL-8, pg/ml | 698.3 (385–1787) | 802.9 (429–2871) | 0.55 | 96.3 (61–341) | <0.0001 | |
IQR: interquartile range.
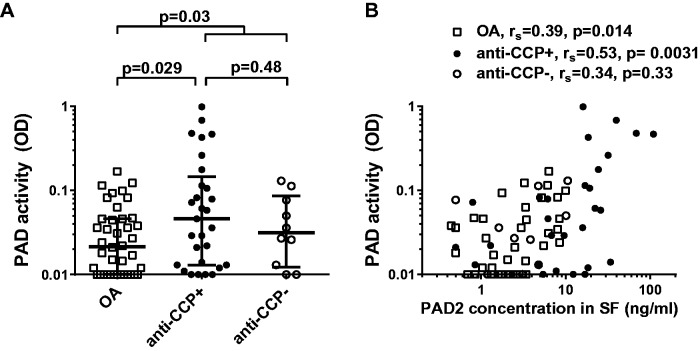
PAD activity in SF of RA patients and OA patients
Having established that PAD2 is present in SF of RA patients and OA patients, we measured whether it was catalytically active using an in-house assay for citrullination of fibrinogen at a site preferentially recognized by PAD2 [16]. In all RA patients and the subgroup of anti-CCP-positive RA patients, PAD activity in SF was significantly higher than in the OA patients (P = 0.030 and P = 0.029, respectively) (Fig. 4A). Despite differences in the level of PAD2 in SF, the anti-CCP-positive and anti-CCP-negative RA patients did not differ significantly with respect to SF PAD activity (Fig. 4A).
Fig. 4.
PAD activity in SF from patients with RA and OA
(A) PAD activity in SF samples from 40 OA patients, 29 anti-CCP-positive RA patients, and 10 anti-CCP-negative RA patients was measured by ELISA based on citrullination of matrix-bound fibrinogen. The data are presented as optical density at 490 nm of duplicate measurements. Medians and interquartile ranges are shown. (B) Association between PAD2 concentration and PAD activity in SF from OA patients (open squares), anti-CCP-positive RA patients (closed circles) and anti-CCP-negative RA patients (open circles). Spearman’s correlation coefficients (rs) and levels of significance are shown.
PAD activity in SF correlated with SF PAD2 levels in the subgroup of anti-CCP-positive RA patients and in the OA patients (Fig. 4B; P = 0.0031 and P = 0.014, respectively). Since the detecting antibody in the activity assay (anti-citrullinated fibrinogen) recognizes a citrullinated epitope, it is likely that ACPAs contained in the SF affect activity measurements by blocking this epitope. Indeed, anti-CCP levels and PAD activity, when tested statistically as dependent variables with respect to PAD2 among all 39 RA patients, were found to interact (P < 0.0001), adjusting the correlation coefficient from r = 0.53 to r = 0.75. PAD unadjusted activity in SF correlated only with levels of circulating CRP and leucocyte counts (P = 0.045 and P = 0.044, respectively).
Discussion
The role of PADs and citrullinated proteins in the pathogenesis of RA is not fully elucidated. It is not clear whether formation of the citrullinated self-antigens that are recognized by autoreactive T cells and ACPAs occurs locally in the synovium, and if so, whether it takes place intracellularly or extracellularly. Several investigations have shown [21–23] that citrullination is associated with inflammation, in general.
A prerequisite for extracellular citrullination is the presence of extracellularly located PAD. We show here that PAD2 is abundant in cell-free SF from anti-CCP-positive RA patients, and to a much lesser extent in SF from anti-CCP-negative patients or patients with OA. Indeed, PAD2 levels in SF exceeding 11.45 ng/ml were highly specific for anti-CCP-positive RA. Higher SF PAD2 and PAD4 levels in RA patients than in OA patients have also been shown by Kinloch et al. [21]. Using a less sensitive western blot assay, they did not detect PAD2 in cell-free SF from OA patients, however.
Notably, significant correlations were found between PAD2 levels in SF from RA patients on the one hand, and DAS28 levels, circulating CRP levels and anti-CCP levels on the other. The presence of PAD2 and citrullinated proteins in synovial cells has previously been associated with high levels of anti-CCP antibodies [37], and we extended this finding to free, soluble quantitative levels of PAD2 in SF. This association may reflect the more aggressive course of anti-CCP-positive RA [8, 10]. The association between ACPAs (measured as anti-CCP antibodies) and SF PAD2 levels is intriguing. It can be speculated that ACPAs form immune complexes with self-antigens citrullinated by PAD2 [42], and that binding of the complexes to synovial macrophages promotes PAD2 release [43]. Complement-activating immune complexes may also enhance presentation of self-antigens to T cells [44, 45], which induce differentiation of APCA-producing B cells into plasma cells [46].
It is a fair assumption that the levels of extracellular PAD2, which is produced by various leucocyte subsets within SF [18, 35], simply reflect the number of leucocytes in the inflamed joints, which is supported by the observed correlations between leucocyte count and PAD2 levels in SF in both OA and RA. Concordantly, the expression of PAD2 and PAD4 in synovial tissue from RA patients has been shown to correlate with general markers of inflammation, including cell infiltration [18]. Indeed, we found that SF from anti-CCP-positive RA patients contained higher leucocyte numbers than those of anti-CCP-negative RA patients, although the difference did not reach statistical significance. This was reflected in the PAD2 concentrations. It would seem that high PAD2 levels in SF are specific for anti-CCP-positive RA, but also that SF from some anti-CCP-negative RA patients and, to a lesser extent, OA patients contains PAD2. At present it is not known whether leucocytes leak PAD2 as a consequence of cell death or of active secretion. Supporting an active secretion, mast cells stimulated with ATP, a danger signal, release PAD2 and citrullinated proteins [47], while neutrophils stimulated with phorbol-12-myristate-13-acetate release PAD2, PAD4 and citrullinated proteins [48].
In accordance with many other studies, we observed increased levels of inflammatory cytokines and the chemokine IL-8 in the joints of RA patients compared with OA patients (for a meta-analysis see [49]). The levels of the measured cytokines, except IL-6, were roughly similar in anti-CCP-positive and anti-CCP-negative RA patients. Remarkably, however, the anti-CCP-positive RA patients had more than 4-fold higher IL-6 levels in SF than the anti-CCP-negative subgroup. This is in accordance with a report by Sanchez-Pernaute et al. [50], who found that fibrinogen citrullinated by PAD2 induced significantly higher production of IL-6 by synovial fibroblasts from RA patients than non-citrullinated fibrinogen. In our study, the IL-6 levels in SF correlated with PAD2 in the RA patients, and in anti-CCP-positive patients as a subgroup. Notably, a complete lack of correlation between PAD2 and IL-6 levels in SF was observed in the OA group. Elevated levels of IL-6 in SF from OA patients have been reported by others [51], but its source appears to be plasma cells [51], unlike RA where macrophages produce both IL-6 and PAD2. Our data on TNF-α should be interpreted with caution, since this cytokine is degraded during sample storage, and in many cases fell below the limit of detection.
We have previously quantified PAD2 in cell-free SF from four out of five RA patients, and shown that it was capable of citrullinating fibrinogen [16]. Here, we confirmed these findings in a larger cohort and showed that PAD activity correlated with PAD2 levels in both RA and OA. Within the RA group, PAD activity correlated only with SF leucocyte count and systemic CRP levels. It is likely that ACPAs in the samples from anti-CCP-positive RA patients may, to some extent, block the citrullinated epitope detected in the assay, and thereby obscure genuine correlations and differences between anti-CCP-positive and anti-CCP-negative RA patients. Higher extracellular PAD activity in SF from RA patients than in SF from OA patients has also been shown with other assays [48].
Limitations of this study include the low number of anti-CCP-negative RA patients, which may have caused type II errors, and that the included RA patients were not treatment naïve. Their treatment may have suppressed inflammatory markers as well as PAD2 release. Our study should be regarded as hypothesis-generating and only raw P-values have been presented. However, all P-values remained significant after adjustment for multiple testing (except for the correlation between PAD2 and IL-6 levels in SF, P = 0.02), when PAD2 levels, leucocytes, cytokines and anti-CCP levels were regarded as independent variables. An effect of RF on PAD2 measurements was ruled out.
In conclusion, this study shows that soluble PAD2 is detectable in SF of both RA patients and OA patients, at especially high levels in anti-CCP-positive RA patients. Notably, PAD2 levels in SF correlate with disease activity and serum anti-CCP levels in RA. It remains to be determined whether PAD2 in SF is useful as a biomarker for subgroups of RA patients, that is, those with active disease and subsequent progression of structural joint damage, and whether it can be targeted therapeutically.
Supplementary Material
Acknowledgements
The authors thank Winnie Hansen for expert technical assistance with cytokine measurements. D.D. and C.H.N. designed the study. D.D. carried out all experiments. L.S. collected the SF samples and characterized the patients clinically. D.D. and C.H.N. wrote the paper, and L.S. revised it critically. All authors read and approved the final manuscript.
Funding: This study was supported by the Novo Nordisk Foundation and the Ministry of Health of the Czech Republic for conceptual development of research organization (No. 00023728, project SVV 262512), and the IMI project BTCure 115142-2.
Disclosure statement: D.D. and C.H.N. are inventors of two patents concerning therapeutic use of monoclonal antibodies against PADs. The other author has declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am 2001;27:269–81. [DOI] [PubMed] [Google Scholar]
- 2. Sato K, Takayanagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol 2006;18:419–26. [DOI] [PubMed] [Google Scholar]
- 3. Pap T, Meinecke I, Muller-Ladner U, Gay S. Are fibroblasts involved in joint destruction? Ann Rheum Dis 2005;64 (Suppl 4):iv52–4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 1998;101:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987;30:1205–13. [DOI] [PubMed] [Google Scholar]
- 6. Hill JA, Southwood S, Sette A. et al. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol 2003;171:538–41. [DOI] [PubMed] [Google Scholar]
- 7. Lindqvist E, Eberhardt K, Bendtzen K, Heinegard D, Saxne T. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 2005;64:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005;7:R949–R958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Syversen SW, Gaarder PI, Goll GL. et al. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis 2008;67:212–7. [DOI] [PubMed] [Google Scholar]
- 10. Ronnelid J, Wick MC, Lampa J. et al. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 2005;64:1744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miriovsky BJ, Michaud K, Thiele GM. et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis 2010;69:1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 2003;25:1106–18. [DOI] [PubMed] [Google Scholar]
- 13. Nakayama-Hamada M, Suzuki A, Kubota K. et al. Comparison of enzymatic properties between hPADI2 and hPADI4. Biochem Biophys Res Commun 2005;327:192–200. [DOI] [PubMed] [Google Scholar]
- 14. Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann Rheum Dis 2012;71:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assohou-Luty C, Raijmakers R, Benckhuijsen WE. et al. The human peptidylarginine deiminases type 2 and type 4 have distinct substrate specificities. Biochim Biophys Acta 2014;1844:829–36. [DOI] [PubMed] [Google Scholar]
- 16. Damgaard D, Senolt L, Nielsen M, Pruijn G, Nielsen CH. Demonstration of extracellular peptidylarginine deiminase (PAD) activity in synovial fluid of patients with rheumatoid arthritis using a novel assay for citrullination of fibrinogen. Arthritis Res Ther 2014;16:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lundberg K, Nijenhuis S, Vossenaar ER. et al. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res Ther 2005;7:R458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foulquier C, Sebbag M, Clavel C. et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum 2007;56:3541–53. [DOI] [PubMed] [Google Scholar]
- 19. Van SK, Tilleman K, De Ceuleneer M. et al. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res Ther 2010;12:R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feitsma AL, van der Voort EI, Franken KL. et al. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheum 2010;62:117–25. [DOI] [PubMed] [Google Scholar]
- 21. Kinloch A, Lundberg K, Wait R. et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum 2008;58:2287–95. [DOI] [PubMed] [Google Scholar]
- 22. Vossenaar ER, Smeets TJ, Kraan MC. et al. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum 2004;50:3485–94. [DOI] [PubMed] [Google Scholar]
- 23. Makrygiannakis D, Af KE, Lundberg IE. et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis 2006;65:1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapuy-Regaud S, Sebbag M, Baeten D. et al. Fibrin deimination in synovial tissue is not specific for rheumatoid arthritis but commonly occurs during synovitides. J Immunol 2005;174:5057–64. [DOI] [PubMed] [Google Scholar]
- 25. Gudmann NS, Hansen NU, Jensen AC, Karsdal MA, Siebuhr AS. Biological relevance of citrullinations: diagnostic, prognostic and therapeutic options. Autoimmunity 2014;48:73–9. [DOI] [PubMed] [Google Scholar]
- 26. Cherrington BD, Zhang X, McElwee JL. et al. Potential role for PAD2 in gene regulation in breast cancer cells. PLoS One 2012;7:e41242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mohanan S, Cherrington BD, Horibata S. et al. Potential role of peptidylarginine deiminase enzymes and protein citrullination in cancer pathogenesis. Biochem Res Int 2012;2012:895343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suzuki A, Yamada R, Chang X. et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet 2003;34:395–402. [DOI] [PubMed] [Google Scholar]
- 29. Plenge RM, Padyukov L, Remmers EF. et al. Replication of putative candidate-gene associations with rheumatoid arthritis in > 4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am J Hum Genet 2005;77:1044–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoppe B, Haupl T, Gruber R. et al. Detailed analysis of the variability of peptidylarginine deiminase type 4 in German patients with rheumatoid arthritis: a case-control study. Arthritis Res Ther 2006;8:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barton A, Bowes J, Eyre S. et al. A functional haplotype of the PADI4 gene associated with rheumatoid arthritis in a Japanese population is not associated in a United Kingdom population. Arthritis Rheum 2004;50:1117–21. [DOI] [PubMed] [Google Scholar]
- 32. Martinez A, Valdivia A, Pascual-Salcedo D. et al. PADI4 polymorphisms are not associated with rheumatoid arthritis in the Spanish population. Rheumatology 2005;44:1263–6. [DOI] [PubMed] [Google Scholar]
- 33. Burr ML, Naseem H, Hinks A. et al. PADI4 genotype is not associated with rheumatoid arthritis in a large UK Caucasian population. Ann Rheum Dis 2010;69:666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang X, Xia Y, Pan J. et al. PADI2 is significantly associated with rheumatoid arthritis. PLoS One 2013;8:e81259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vossenaar ER, Radstake TR, van der Heijden A. et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis 2004;63:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asaga H, Yamada M, Senshu T. Selective deimination of vimentin in calcium ionophore-induced apoptosis of mouse peritoneal macrophages. Biochem Biophys Res Commun 1998;243:641–6. [DOI] [PubMed] [Google Scholar]
- 37. De Rycke L, Nicholas AP, Cantaert T. et al. Synovial intracellular citrullinated proteins colocalizing with peptidyl arginine deiminase as pathophysiologically relevant antigenic determinants of rheumatoid arthritis-specific humoral autoimmunity. Arthritis Rheum 2005;52:2323–30. [DOI] [PubMed] [Google Scholar]
- 38. Takizawa Y, Suzuki A, Sawada T. et al. Citrullinated fibrinogen detected as a soluble citrullinated autoantigen in rheumatoid arthritis synovial fluids. Ann Rheum Dis 2006;65:1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 40. Damgaard D, Palarasah Y, Skjodt K. et al. Generation of monoclonal antibodies against peptidylarginine deiminase 2 (PAD2) and development of a PAD2-specific enzyme-linked immunosorbent assay. J Immunol Methods 2014;405:15–22. [DOI] [PubMed] [Google Scholar]
- 41. Nielsen CH, Hegedus L, Rieneck K. et al. Production of interleukin (IL)-5 and IL-10 accompanies T helper cell type 1 (Th1) cytokine responses to a major thyroid self-antigen, thyroglobulin, in health and autoimmune thyroid disease. Clin Exp Immunol 2007;147:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao X, Okeke NL, Sharpe O. et al. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther 2008;10:R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Venrooij WJ, Pruijn GJ. An important step towards completing the rheumatoid arthritis cycle. Arthritis Res Ther 2008;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nielsen CH, Leslie RG, Jepsen BS. et al. Natural autoantibodies and complement promote the uptake of a self antigen, human thyroglobulin, by B cells and the proliferation of thyroglobulin-reactive CD4(+) T cells in healthy individuals. Eur J Immunol 2001;31:2660–8. [DOI] [PubMed] [Google Scholar]
- 45. Brimnes MK, Hansen BE, Nielsen LK, Dziegiel MH, Nielsen CH. Uptake and presentation of myelin basic protein by normal human B cells. PLoS One 2014;9:e113388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vossenaar ER, van Venrooij WJ. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis. Arthritis Res Ther 2004;6:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arandjelovic S, McKenney KR, Leming SS, Mowen KA. ATP induces protein arginine deiminase 2-dependent citrullination in mast cells through the P2X7 purinergic receptor. J Immunol 2012;189:4112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spengler J, Lugonja B, Ytterberg AJ. et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in RA synovial fluid. Arthritis Rheumatol 2015;67:3135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ. et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage 2012;20:1484–99. [DOI] [PubMed] [Google Scholar]
- 50. Sanchez-Pernaute O, Filkova M, Gabucio A. et al. Citrullination enhances the pro-inflammatory response to fibrin in rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis 2013;72:1400–6. [DOI] [PubMed] [Google Scholar]
- 51. Doss F, Menard J, Hauschild M. et al. Elevated IL-6 levels in the synovial fluid of osteoarthritis patients stem from plasma cells. Scand J Rheumatol 2007;36:136–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.