Abstract
Objective. To determine how well skin symptoms considered specific to SSc are captured by patient reported outcomes currently used for assessing patients with SSc, the SHAQ, or skin disease, the Skindex-29; and how well these symptoms correlate with the extent of skin disease on physical exam and skin pathology.
Methods. SSc patients completed the scleroderma modification of the Health Assessment Questionnaire (SHAQ), Skindex-29 and a Skin Symptom Assessment questionnaire developed for this study. Correlations were assessed between the Skin Symptom Assessment and SHAQ, Skindex-29, modified Rodnan skin score, and skin pathological features including myofibroblast staining completed on the same date.
Results. Tight, hard and rigid/stiff skin symptoms correlated moderately highly with the modified Rodnan skin score (r = 0.445, P = 0.0008; r = 0.486, P = 0.0002; and r = 0.488, P = 0.0002, respectively). Tight skin symptoms correlated moderately with myofibroblast infiltration (r = 0.544, P = 0.0023) and hyalinized collagen (r = 0.442, P = 0.0164), while both hard and rigid/stiff skin correlated moderately with inflammation (r = 0.401, P = 0.0310 and r = 0.513, P = 0.0045), myofibroblast infiltration(r = 0.480, P = 0.0084 and r = 0.527, P = 0.0033) and hyalinized collagen (r = 0.453, P = 0.0137 and r = 0.478, P = 0.0087), while the SHAQ was not found to correlate with any of these pathological changes. In contrast, painful skin symptoms correlated moderately with the SHAQ (r = 0.413, P = 0.0073), and with the three domains of Skindex-29: Symptoms, Emotions and Functioning. Skindex-29 indicates that dcSSc patient skin symptoms are nearly as severe as those of patients with psoriasis or atopic dermatitis.
Conclusion. Patient reported skin symptoms correlate with clinical and pathological measures in the skin. A validated patient reported skin symptom instrument might considerably improve evaluation of SSc skin disease.
Keywords: systemic sclerosis, scleroderma, patient reported outcome, skin symptoms, skin pathology, quality of life, skin
Rheumatology key messages
Specific SSc patient reported skin symptoms correlate with modified Rodnan skin score and pathological changes on skin biopsy.
Results suggest scleroderma HAQ does not capture patient reported skin symptoms of tight, hard and rigid/stiff.
Skindex-29 may be useful in understanding the impact of skin symptoms on SSc patients.
Introduction
Skin sclerosis is the cardinal feature of SSc of either principal phenotype: dcSSc and lcSSc. The modified Rodnan skin score (MRSS) has become the primary outcome measure utilized in clinical trials of patients with dcSSc and possesses high intra- and moderate inter-observer reliability [1].
Although the MRSS provides a reliable estimate of the extent of skin involvement and degree of histopathology, measurement of the impact of skin disease on patient symptoms, quality of life and disability have relied upon mostly patient reported outcome (PRO) measures developed for other diseases [2]. The most commonly used SSc PRO, the scleroderma modification of the Health Assessment Questionnaire (SHAQ) captures mainly functional and quality of life measures, especially functional aspects of both upper and lower extremity mobility as it does in the HAQ. It represents a modification of the original HAQ’s eight domains with the addition of six scales assessing pain, patient global assessment, vascular symptoms (Raynaud’s), digital ulcers, lung involvement (breathlessness) and gastrointestinal involvement, but it does not include direct measures of skin symptoms [3]. Changes in the MRSS over time are variably correlated with PROs. For example, a <30% worsening in MRSS shows a significant change in the SHAQ disability index (SHAQ-DI) but not the SF-36, whereas improvement in MRSS was not found to correlate significantly with either measure [4]. In another study, the MRSS correlated with the HAQ-DI (r = 0.55), with the HAQ pain VAS (r = 0.47) and with the Psychosocial Adjustment to Illness Scale (r =0.3) [5].
Although important for understanding the effect of disease on patients’ function and quality of life, such instruments might be supplemented by one more closely capturing skin symptoms of SSc patients. Developing and validating PRO measures that are sensitive to change are particularly important in the context of drug approval [6]. In several settings of rheumatic disease, patients’ symptoms provide the most sensitive outcome measures [7–9], perhaps because these are closest to the biological and physiological variable effects of the disease [10].
Several PRO instruments have been designed and validated for skin disease, in particular psoriasis [11, 12]. These typically assess quality of life, as well as symptoms and psychosocial aspects of skin disease in these patients [12]. The Skindex-29, developed as a PRO for skin diseases, has been used widely and is well validated for use in psoriasis [13]. It has also been applied to patients with other skin diseases, including cutaneous lupus erythematosus (CLE) [14]. However, the items in this questionnaire, while addressing pain, itching and stinging, do not address unique features of skin disease in SSc associated with dermal fibrosis, such as tightness and hardness of the skin. Our study’s purpose was to explore the relationship between measures of skin symptoms felt to be specific to SSc to traditional PRO instruments and to concurrently obtain measures of skin histopathology and physical exam (the mRSS).
Methods
Patients and data collection
The patients were recruited at the Boston University Scleroderma Center during routine clinic visits. All skin samples, clinical data and patient questionnaires were collected under a protocol approved by the institutional review boards as part of a larger study. Our analysis of the data did not require separate ethical approval.
Sequential systemic sclerosis patients were recruited with dcSSc according to classification at the start of the study [15, 16] of <3 years’ duration, measured from the first non-Raynaud’s symptom (n = 37), other evidence of progressive disease as assessed by the investigator (n = 7), or >3 years’ duration (n = 7) and two subjects with unknown disease duration, and with lcSSc of any disease duration.
Data collection occurred between August 2012 and March 2015 and included the SHAQ, Skindex-29, MRSS and a Skin Symptom Assessment questionnaire developed for this study. All patient responses on all forms were captured on electronic forms collected through a custom-built web application developed and programmed by the BU School of Public Health, Data Coordinating Center. The data were stored in an SQL database and SAS 9.3 was used to export the data and for data analysis. Patients entered responses directly into the clinical data repository during clinic visits using online questionnaires formatted for iPad.
The Skin Symptom Assessment (SSA) was developed informally by consensus of two BU Scleroderma Center rheumatologists with over 30 years’ combined experience in caring for patients with scleroderma (R.L. and R.S.) to assess skin symptoms not clearly captured using other available PRO instruments. Thus, the face validity of the SSA was assessed by two study investigators. However, the content validity of the SSA was not assessed formally with patients or patient groups. Items in the patients’ self-evaluation comprised six skin symptoms (tight, painful, red, hard, rigid/stiff and itchy) and were each scored on a five-level Likert Scale (not at all, a little bit, somewhat, moderately, severely). Each item was posed as a question: during the past week my skin has been X? The Skindex-29 was used as previously described [17].
Skin biopsy
An unselected subgroup of patients had a skin biopsy performed at the same visit as the MRSS, SSA and SHAQ assessments (n = 29). Skin biopsies were obtained as a routine component of our ongoing biomarker and registry studies at the time of the initial visit. Skin biopsies were all performed over the dorsal mid-forearm and placed in 10% formalin for 1–2 days before embedding, cutting and staining. Histological evaluation of inflammation and degree of fibrosis was performed on haematoxylin and eosin stained sections. Myofibroblasts were stained using immunohistochemistry to α-smooth muscle actin as described previously [18]. In all cases scoring was performed by a blinded observer (R.L.) using a 0–100, 10 cm visual analogue scale and converted into a score by measuring from the 0 anchor as described previously [18].
Statistical analyses
Correlations using Spearman’s rho were assessed between SSA, SHAQ, MRSS and Skindex-29, only when the compared measures were completed on the same date. The variance of MRSS explained by each skin symptom was calculated from partial r2 values using a regression model adjusted for age, sex and disease duration. All calculations were performed in SAS 9.3.
Results
Patient characteristics
Three groups of dcSSc patients were studied (Fig. 1, supplementary Table S1, available at Rheumatology Online). MRSS and SSA were available for 53 dcSSc patients, a subset of whom completed the SHAQ and Skindex-29 along with MRSS and SSA (n = 41). Further, skin biopsies during the study were performed on a subset of dcSSc patients (n = 29). lcSSc patients were only analysed for SSA and Skindex-29 as a comparison group for dcSSc.
Fig. 1.
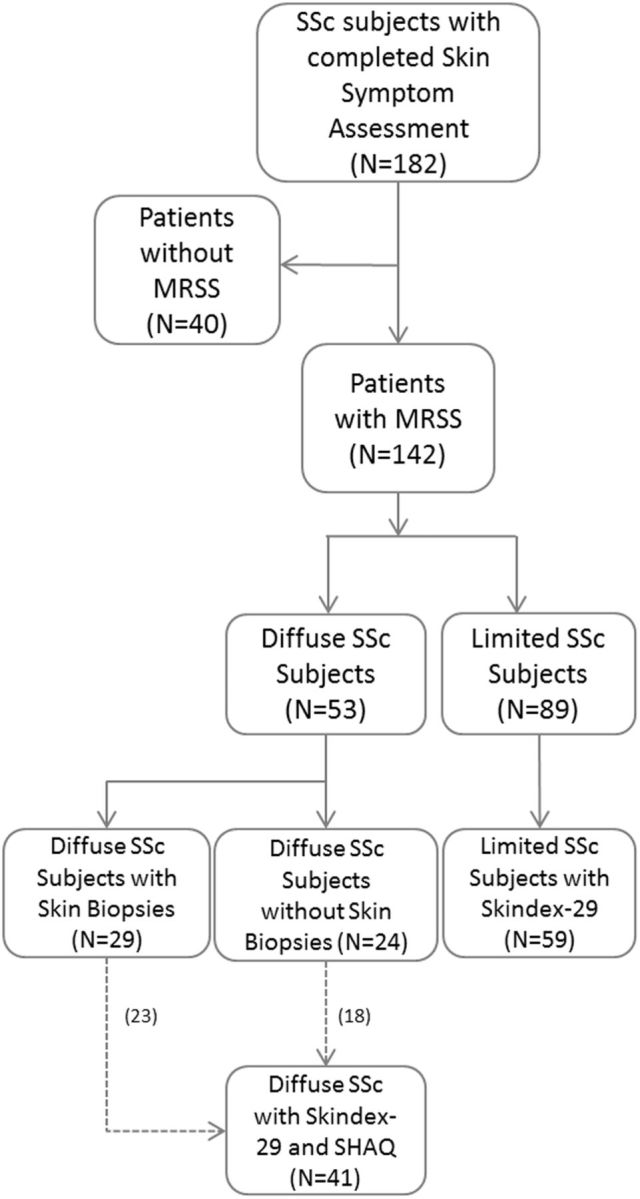
Identification of eligible records including sample size
MRSS: modified Rodnan skin score; SHAQ: scleroderma modification of the Health Assessment Questionnaire; n: number.
Patient reported skin symptoms
Examining the severity of skin symptoms in patients with dcSSc revealed significant levels of symptoms in all of the items queried. For all questions valued at a minimum of 1 (not at all) to a maximal score of 5 (severe), dcSSc patients showed elevated mean (s.d.) scores of tight [3.34 (1.23)], painful [2.54 (1.46)], red [2.52 (1.21)], hard [3.00 (1.39)], rigid/stiff [3.04 (1.26)] and itchy [2.66 (1.25)] skin. lcSSc patients also showed elevated mean scores of tight [2.41 (1.23)], painful [2.27 (1.29)], red [2.11 (1.16)], hard [2.00 (1.07)], rigid/stiff [2.32 (1.25)] and itchy [2.11 (1.21)] skin (Table 1). The main difference between these groups appears in expected items of tight skin, hard skin and rigid/stiff skin, all of which are much higher in dcSSc patients.
Table 1.
Descriptive statistics of skin symptom assessment in dSSc and lcSSc patients
| Type of cutaneous disease | Symptoms | n | Mean (s.d.) | Min | Max |
|---|---|---|---|---|---|
| Diffuse | Tight | 53 | 3.34 (1.23) | 1 | 5 |
| Painful | 53 | 2.54 (1.46) | 1 | 5 | |
| Red | 53 | 2.52 (1.21) | 1 | 5 | |
| Hard | 53 | 3.00 (1.39) | 1 | 5 | |
| Rigid/stiff | 53 | 3.04 (1.26) | 1 | 5 | |
| Itchy | 53 | 2.66 (1.25) | 1 | 5 | |
| Limited | Tight | 59 | 2.41 (1.23) | 1 | 5 |
| Painful | 59 | 2.27 (1.29) | 1 | 5 | |
| Red | 59 | 2.11 (1.16) | 1 | 5 | |
| Hard | 59 | 2.00 (1.07) | 1 | 5 | |
| Rigid/stiff | 59 | 2.32 (1.25) | 1 | 5 | |
| Itchy | 59 | 2.11 (1.21) | 1 | 5 |
Several of the skin symptom items appeared to be closely related. Not surprisingly, tight skin, hard skin and rigid/stiff skin correlated highly with each other (Table 2). Tight skin also correlated moderately highly with painful skin (r = 0.604) as did both hard (0.709) and rigid/stiff (r = 0.774). Notably, red and itchy skin both correlated significantly with all other skin symptoms, though not correlating as highly with each other, suggesting that they are capturing important different patient symptoms related to SSc skin disease.
Table 2.
Correlation between skin symptom assessment questions (n = 53)
| Skin symptom assessment | Tight, Spearman’s r | Painful, Spearman’s r | Red, Spearman’s r | Hard, Spearman’s r | Rigid/stiff, Spearman’s r | Itchy, Spearman’s r |
|---|---|---|---|---|---|---|
| Tight | 1.00 | 0.60 | 0.48 | 0.71 | 0.77 | 0.47 |
| Painful | 0.60 | 1.00 | 0.51 | 0.69 | 0.73 | 0.47 |
| Red | 0.48 | 0.51 | 1.00 | 0.51 | 0.49 | 0.34 |
| Hard | 0.71 | 0.69 | 0.51 | 1.00 | 0.83 | 0.46 |
| Rigid/stiff | 0.77 | 0.73 | 0.49 | 0.83 | 1.00 | 0.53 |
| Itchy | 0.47 | 0.47 | 0.34 | 0.46 | 0.53 | 1.00 |
All are statistically significant with P < 0.001 except for the r value for the correlation of itchy and red, which has a P = 0.013.
Patient assessment of skin symptoms correlates with the MRSS
We next evaluated whether these patient reported skin symptom assessment items correlate with physical examination of the skin as assessed by the MRSS. Indeed, the MRSS correlated moderately with patient reported tight skin (r = 0.445, P = 0.0008), hard skin (r = 0.486, P = 0.002) and rigid/stiff skin (r = 0.488, P = 0.0002). The MRSS also correlated more weakly with painful skin (r = 0.35, P = 0.01; Table 3). The MRSS did not correlate significantly with red or itchy skin symptoms, suggesting that the MRSS does not capture some important patient symptoms. The proportion of the variance of MRSS explained by all factors (i.e. age, sex, disease duration and each skin symptom) ranges from 19.0 to 35.4%. Of the six skin symptoms compared, rigid/stiff explained 24.3% and had the highest full model while itchy explained the least at 4.9% (Table 4).
Table 3.
Skin symptom assessment correlation with SHAQ and modified Rodnan skin score
| Skin symptom assessment | MRSS, Spearman’s r (n = 53) | SHAQ, Spearman’s r (n = 41) |
|---|---|---|
| Tight | 0.44** | 0.015 |
| Painful | 0.35* | 0.41* |
| Red | 0.24 | 0.14 |
| Hard | 0.49** | 0.21 |
| Rigid/stiff | 0.49** | 0.25 |
| Itchy | 0.13 | 0.24 |
*P < 0.05,
**P < 0.001. MRSS: modified Rodnan skin score; SHAQ: scleroderma modification of the HAQ.
Table 4.
Proportion of modified Rodnan skin score variance explained by patient reported skin symptoms
| Variance explained by | Skin symptoms |
|||||
|---|---|---|---|---|---|---|
| Tight | Painful | Red | Hard | Rigid/ stiff | Itchya | |
| Specific skin symptom, % | 16.2 | 16.0 | 7.4 | 23.8 | 24.3 | 4.9 |
| Full modelb, % | 28.6 | 28.3 | 21.1 | 35.0 | 35.4 | 19.0 |
aTwo subjects missing.
bVariance explained by age, sex, disease duration and a specific skin symptoms.
Patient assessment of painful and tight skin, but not other measures of skin symptoms, correlate with the SHAQ
Comparing it to SSA items, the SHAQ score correlated most highly with painful skin (r = 0.413, P = 0.0073; Table 3). Thus, symptoms of red and itchy skin were not captured by either the MRSS or the SHAQ, while tight skin was partially captured by the MRSS but not by the SHAQ. Together these results suggested that certain dcSSc patient skin symptoms are not captured by existing measures and that others might be captured incompletely.
Skin symptoms correlate with histological findings and myofibroblast infiltration on dcSSc skin biopsies
To further assess the value of patient reported skin symptom assessments, the histological features of skin biopsies from 29 dcSSc patients, who also completed the SSA and MRSS, were graded for inflammation, myofibroblast infiltration and the degree of fibrosis. Of the SSA items, patients’ grading of tight, hard and rigid/stiff skin correlated most highly with myofibroblast infiltration (supplementary Table S2, available at Rheumatology Online). Hard and rigid/stiff skin both correlated with the degree of fibrosis on skin biopsies, as well as with pathological evaluation of skin inflammation. In contrast to the observed correlations between tight, hard and rigid/stiff skin and all three pathological measures, patient reported skin tightness, while correlating with myofibroblast infiltration and fibrosis, did not correlate significantly with skin inflammation.
Histological findings and myofibroblast infiltration on dcSSc skin biopsies correlate with MRSS symptoms
We have previously shown that the MRSS correlates with skin pathology, particularly the myofibroblast score [18]. In this dataset, mostly consistent with previous results, the MRSS correlated with hyalinized collagen score and myofibroblast score (supplementary Table S2, available at Rheumatology Online). Significantly, tight, hard and rigid/stiff skin symptoms correlated similarly with myofibroblast score and MRSS, and almost as well as the MRSS with hyalinized collagen (supplementary Table S2, available at Rheumatology Online).
Comparing skin symptom assessments with Skindex-29 in SSc patients
The Skindex-29 is a skin PRO developed for use in patients with skin disease [19]. It includes items to assess symptoms, emotions and functioning domains that can be combined and scored separately. In dcSSc patients pain symptoms in the SSA correlated significantly with all three Skindex-29 domains (supplementary Table S3, available at Rheumatology Online). All skin symptoms correlated positively with the Skindex-29 symptoms score at a P < 0.05 confidence level. The Skindex-29 domains also correlated well with the SHAQ (0.49, 0.44 and 0.51, respectively, for symptoms, emotions and functioning domains; P < 0.05 for all correlations), but poorly with the MRSS (0.22, −0.09 and 0.04, respectively, for symptoms, emotions and functioning domains; P > 0.05 for all correlations).
dcSSc patients scored higher than lcSSc patients on all three domains (Table 5). Compared with patients with psoriasis [n = 44, symptom = 42 (21), emotions = 39 (27), functioning = 39 (27)] and eczematous dermatitis [n = 102, symptom = 48 (23), emotions = 41 (27), functioning = 26 (26)], dcSSc patients scored on average lower (less severe) in domains of symptoms and emotions, although about the same in the domain of functioning (Table 5) [13]. However, when patients with dcSSc were divided into tertiles based on SHAQ scores, severely affected patients scored more highly than the average scores of patients with psoriasis or eczematous dermatitis in all the Skindex domains. All these disease groups showed statistically higher scores in all domains than scores in patients with warts (n = 24, symptom = 23 (18), emotions = 22 (16), functioning = 6 (13)).
Table 5.
Mean Skindex-29 scores ranked by SHAQ severity in dSSc patients compared with lcSSc patient scores
| Skindex-29 | dcSSc—All (n = 41) | dcSSc—Milda (n = 12) | dcSSc—Moderate (n = 14) | dcSSc—Severe (n = 15) | lcSSc (n = 59) |
|---|---|---|---|---|---|
| Symptoms, mean (s.d.) | 37.50 (20.8) | 25.00 (13.0) | 40.56 (26.9) | 44.64 (15.3) | 26.13 (22.1) |
| Emotions, mean (s.d.) | 37.44 (25.3) | 20.14 (17.7) | 41.43 (25.7) | 47.56 (24.2) | 20.96 (20.1) |
| Functioning, mean (s.d.) | 24.95 (23.6) | 7.81 (7.33) | 27.84 (21.4) | 35.97 (27.0) | 14.50 (18.5) |
aSkindex-29 scores anchored by SHAQ, defined by tertiles of SHAQ scores. SHAQ: scleroderma modification of the HAQ.
Discussion
Skin involvement in patients with SSc leads to significant morbidity and, as measured by MRSS, is correlated with mortality [20]. The MRSS has frequently been the primary outcome measure in clinical trials, but an appropriate and validated PRO measure designed specifically for SSc-associated skin disease is not yet available. The most commonly used PRO instrument in SSc trials, the HAQ and the SHAQ, both address functionality and disability in SSc patients [21]. However, neither of these specifically addresses several questions relevant to symptoms of SSc skin disease.
We assessed patient responses on a simple questionnaire of SSc skin disease to explore the potential value of further developing a SSc skin PRO more systematically, according to established guidelines [6]. Our intent was to examine how well skin symptoms correlate with currently used outcomes and with dermal histopathology in advance of developing a validated PRO. We found that the SSA, like MRSS, is highly correlated with histopathology extent, although not every patient had a skin biopsy performed.
Several of our observations support the importance of developing a validated SSc PRO. First, several features of the SSA correlated more strongly with the MRSS than with the SHAQ, suggesting that while the SHAQ captures certain patient symptoms such as painful skin, it inadequately captures other patient symptoms such as rigid/stiff and tight skin. Second, neither the SHAQ nor the MRSS capture other symptoms of red and itchy skin seen significantly elevated in dcSSc patients. Finally, the SHAQ showed no correlation with changes in skin pathology, whereas the SSA did, again suggesting that important aspects of skin disease are not adequately captured with the SHAQ.
In clinical trials of agents showing clear efficacy for patients with SSc, more global PROs have shown statistically significant change. In the Scleroderma Lung Study, comparing oral CYC to placebo, the HAQ-DI, but not the SF-36, changed significantly [22]. In the ASTIS trial of immunoablation and autologous stem cell transplant compared with CYC, the area under the curve over time of the trial showed statistically significant changes in both the SF-36 and the HAQ-DI [23]. The MRSS was a secondary outcome in both these trials and in both changed significantly. However, patients in ASTIS also showed significant changes in other outcomes, notably in lung function, possible accounting for changes in HAQ-DI and SF-36. Both the SLS and ASTIS trials were comparatively large, with 145 and 156 patients, respectively, indicating that these PRO instruments can be effectively utilized in large studies of SSc patients. The use of instruments with greater specificity for skin symptoms might improve sensitivity to change of the instrument, a particularly important aspect in small early phase trials.
Hand involvement in SSc is a major aspect of patient morbidity and disability, and may be closely related to the degree of skin disease. Several measures are available to assess hand disease, such as the Côchin Hand Function Scales, which has been validated in SSc [21]. This measure evaluates mainly hand function and disability rather than symptoms. Indeed the SSA does not attempt to quantify functional limitations, another important aspect of skin disease, and this is a limitation of study presented here It would be important to consider this in developing a future skin PRO.
The Skindex-29 scores appeared to capture the severity of skin involvement in SSc, as patients in the most severely affected tertile showed a Skindex-29 score greater than psoriasis or eczematous dermatitis. This comparison may be particularly relevant in understanding the impact of skin symptoms in SSc patients.
In summary, we have shown that patient reported skin symptoms are highly correlated with both clinical and histological parameters, but may not be adequately captured by some of the most commonly used outcome measures in SSc clinical trials. The current study reinforces the need for the development of a valid and sensitive skin PRO for SSc, which could potentially enhance the ability to identify effective therapies.
Supplementary Material
Acknowledgements
R.L. received grants from the National Institute of Arthritis Musculoskeletal and Skin Disease (5P30AR061271, 1P50AR060780 and 2R01AR051089). M.H. received grants from the Scleroderma Research Foundation and K23 AR059763.
Funding: This work was supported by National Institutes of Health, Boston University Medical Center CTSI: UL1-TR000157.
Disclosure statement: M.H. received research grants from the Scleroderma Research Foundation and National Institutes of Health K23 AR059763. R.L. has received grants from Shire, Sanofi, Regeneron, Genentech, UCB, Human Genome Sciences, Precision Dermatology, Biogen, BMS, Inception, Stromedix, PRISM, Pfizer, Boston University, Bristol Myers Squibb and PRISM and consulted for Shire, Sanofi, Regeneron, Roche/Genentech, Biogen, Lycera, Novartis, Celgene, Bristol-Myers Squibb, Amira, Celdara, Celltex, Dart Therapeutics, Idera, Inception, Intermune, Medimmune, Precision Dermatology, Promedior, Zwitter, PRISM, UCB, Actelion, EMD Serono, Akros, Extera, Reneo, Scholar Rock and Merck. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Clements P, Lachenbruch P, Siebold J. et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995;22:1281–5. [PubMed] [Google Scholar]
- 2. Furst DE, Clements PJ, Steen VD. et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol 1998;25:84–8. [PubMed] [Google Scholar]
- 3. Steen VD, Medsger TA., Jr. The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 1997;40:1984–91. [DOI] [PubMed] [Google Scholar]
- 4. Khanna D, Furst DE, Clements PJ. et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol 2005;32:832–40. [PubMed] [Google Scholar]
- 5. Malcarne VL, Hansdottir I, McKinney A. et al. Medical signs and symptoms associated with disability, pain, and psychosocial adjustment in systemic sclerosis. J Rheumatol 2007;34:359–67. [PubMed] [Google Scholar]
- 6. Food and Drug Administration (FDA). Guidance for Industry: Patient-reported outcome measures: Use in medical product development to support labeling claims. Silver Spring, MD: Food and Drug Administration, 2009.
- 7. Katz JN, Gelberman RH, Wright EA. et al. Responsiveness of self-reported and objective measures of disease severity in carpal tunnel syndrome. Med Care 1994;32:1127–33. [DOI] [PubMed] [Google Scholar]
- 8. Irrgang JJ, Snyder-Mackler L, Wainner RS. et al. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am 1998;80:1132–45. [DOI] [PubMed] [Google Scholar]
- 9. Salaffi F, Stancati A, Carotti M. Responsiveness of health status measures and utility-based methods in patients with rheumatoid arthritis. Clin Rheumatol 2002;21:478–87. [DOI] [PubMed] [Google Scholar]
- 10. Ahmed S, Berzon RA, Revicki DA. et al. The use of patient-reported outcomes (PRO) within comparative effectiveness research: implications for clinical practice and health. Med Care 2012;50:1060–70. [DOI] [PubMed] [Google Scholar]
- 11. Fernandez-Penas P, Jones-Caballero M, Espallardo O. et al. Comparison of Skindex-29, Dermatology Life Quality Index, Psoriasis Disability Index and Medical Outcome Study Short Form 36 in patients with mild to severe psoriasis. Brit J Dermatol 2012;166:884–7. [DOI] [PubMed] [Google Scholar]
- 12. De Korte J, Mombers FM, Sprangers MA. et al. The suitability of quality-of-life questionnaires for psoriasis research: a systematic literature review. Arch Dermatol 2002;138:1221–7. discussion 27 [DOI] [PubMed] [Google Scholar]
- 13. Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clinics 2012;30:231–6, xiii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vasquez R, Wang D, Tran QP. et al. A multicentre, cross-sectional study on quality of life in patients with cutaneous lupus erythematosus. Brit J Dermatol 2013;168:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LeRoy EC, Black C, Fleischmajer R. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 16. Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 17. Chren MM, Lasek RJ, Flocke SA. et al. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol 1997;133:1433–40. [PubMed] [Google Scholar]
- 18. Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum 2006;54:3655–60. [DOI] [PubMed] [Google Scholar]
- 19. Chren MM, Lasek RJ, Quinn LM. et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Investig Dermatol 1996;107:707–13. [DOI] [PubMed] [Google Scholar]
- 20. Clements PJ, Hurwitz EL, Wong WK. et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum 2000;43:2445–54. [DOI] [PubMed] [Google Scholar]
- 21. Rannou F, Poiraudeau S, Berezne A. et al. Assessing disability and quality of life in systemic sclerosis: construct validities of the Cochin Hand Function Scale, Health Assessment Questionnaire (HAQ), Systemic Sclerosis HAQ, and Medical Outcomes Study 36-Item Short Form Health Survey. Arthritis Rheum 2007;57:94–102. [DOI] [PubMed] [Google Scholar]
- 22. Tashkin DP, Elashoff R, Clements PJ. et al. Cyclophosphamide versus placebo in scleroderma lung disease. New Engl J Med 2006;354:2655–66. [DOI] [PubMed] [Google Scholar]
- 23. van Laar JM, Farge D, Sont JK. et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 2014;311:2490–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


