Abstract
Objective. To identify novel biomarker(s) for predicting advanced knee OA.
Methods. Study participants were derived from the Newfoundland Osteoarthritis Study and the Tasmania Older Adult Cohort Study. All knee OA cases were patients who underwent total knee replacement (TKR) due to primary OA. Metabolic profiling was performed on fasting plasma. Four thousand and eighteen plasma metabolite ratios that were highly correlated with that in SF in our previous study were generated as surrogates for joint metabolism.
Results. The discovery cohort included 64 TKR cases and 45 controls and the replication cohorts included a cross-sectional cohort of 72 TKR cases and 76 controls and a longitudinal cohort of 158 subjects, of whom 36 underwent TKR during the 10-year follow-up period. We confirmed the previously reported association of the branched chain amino acids to histidine ratio with advanced knee OA (P = 9.3 × 10 −7 ) and identified a novel metabolic marker—the lysophosphatidylcholines (lysoPCs) to phosphatidylcholines (PCs) ratio—that was associated with advanced knee OA (P = 1.5 × 10 −7 ) after adjustment for age, sex and BMI. When the subjects of the longitudinal cohort were categorized into two groups based on the optimal cut-off of the ratio of 0.09, we found the subjects with the ratio ⩾0.09 were 2.3 times more likely to undergo TKR than those with the ratio <0.09 during the 10-year follow-up (95% CI: 1.2, 4.3, P = 0.02).
Conclusion. We identified the ratio of lysoPCs to PCs as a novel metabolic marker for predicting advanced knee OA. Further studies are required to examine whether this ratio can predict early OA change.
Keywords: total joint replacement, knee osteoarthritis, metabolomics, metabolite ratios
Rheumatology key messages
Increased plasma ratios of branched chain amino acids to histidine are associated with knee osteoarthritis.
Increased ratio of lysophosphatidylcholines to phosphatidylcholines is associated with knee osteoarthritis.
Introduction
OA is the most common form of arthritis, affecting about 10% of the world’s population aged 60 years or older [ 1 ]. It is the major source of joint pain and disability [ 2 ], and imposes a substantial socioeconomic burden on society with a cost estimate between 1.0 and 2.5% of gross domestic product [ 3 ]. The pathological process of OA involves all structures within a synovial joint, including articular cartilage, synovium and underlying bone [ 4 ]. There is no known cure and total joint replacement is still the most effective therapy for advanced OA. In the past decade, the demand for joint replacement has been continually rising and the total knee replacement (TKR) rate has doubled [ 5 ]. Knee OA is a heterogeneous disease with variable clinical outcomes. Some patients do not have any symptoms or significant functional loss for many years after the onset of the disease whereas others can progress rapidly to an advanced stage [ 6 ], making it highly clinically relevant to identify those who are at high risk of progressing rapidly so that targeted interventions at an early stage of the disease can be implemented [ 5 , 7–9 ].
Few population-based cross-sectional or longitudinal studies have investigated predictive factors on TKR. Hawker et al. [ 10 ] surveyed a total of 48 218 individuals from two areas of Ontario, Canada, one area with a high rate of undergoing arthroplasty and one area with a low rate. They found that demonstrable need and willingness were greater in the high-rate area, suggesting these factors explain partially the observed geographical rate variation for this surgical procedure. In their follow-up study of 2128 subjects [ 10 ], they found that willingness to consider total joint anthroplasty was the strongest predictor of the time to first total joint anthroplasty. The rate of cartilage loss and radiographic severity of OA was studied for predicting knee replacement [ 11–13 ]. Cicuttini et al. [ 13 ] reported that for every 1% increase in rate of tibial cartilage loss there was a 20% increase in the risk of undergoing a knee replacement at 4 years. However, Toivanen and Dieppe et al. [ 14 , 15 ] found that radiographic measures of OA severity do not strongly correlate with pain and disability, and using functional measures as determinants of future TKR may be more appropriate. Zeni et al. [ 16 ] reported that age, knee extension range of motion and the knee outcome survey (Activities of Daily Living Subscale) together significantly predicted that a person would undergo TKR. However, the predictive capacities of these factors are limited. Research showed that biochemical changes occur in the early stage of OA progression and these metabolic changes can be reflected in SF [ 17 ]. These metabolic changes, once identified, could serve as predictive markers with great power.
However, obtaining SF samples is invasive and not practical in a clinical setting. We recently examined the correlation in metabolite profiles between SF and plasma [ 18 ]. We found that some metabolite ratios were highly correlated between SF and plasma and the plasma levels of these metabolite ratios could reflect joint metabolism and serve as surrogates for SF. Using these metabolite ratios, we undertook the current study to confirm the previously reported association of the branched chain amino acids to histidine ratio with knee OA and to identify novel metabolic markers for predicting advanced knee OA that requires TKR.
Methods
Patients
We performed both cross-sectional and longitudinal studies. In the cross-sectional study, we used a two-stage case-control study design—discovery and replication. Knee OA patients for both phases were selected from the Newfoundland Osteoarthritis Study (NFOAS) that was initiated in 2011 and aimed at identifying novel genetic, epigenetic and biochemical markers for OA [ 19 ]. OA patients were recruited from those who underwent TKR due to primary OA between November 2011 and December 2013 in St Clare’s Mercy Hospital and Health Science Centre General Hospital in St John’s, the capital city of Newfoundland and Labrador, Canada. Healthy controls for both phases were selected from The Complex Diseases in the Newfoundland Population: Environment and Genetics study whose study participants were adult volunteers [ 20 ]. Both cases and controls were from the same source population of Newfoundland and Labrador. Knee OA diagnosis was made based on ACR clinical criteria for classification of idiopathic OA of the knee [ 21 ] and the judgement of the attending orthopaedic surgeons. Controls were those without family doctor diagnosed OA at any joints based on their medical information collected by a self-administered questionnaire.
For the longitudinal study, study participants were derived from the Tasmania Older Adult Cohort Study (TASOAC), which is a prospective, population-based study that was initiated in 2002 and was aimed at identifying the environmental, genetic and biochemical factors associated with the development and progression of OA at multiple sites (hand, knee, hip and spine) [ 22 ]. All the study subjects had X-ray data on their knee joint at baseline and subjects who underwent TKR due to primary OA during the 10-year follow-up period were identified.
This study was approved by the Health Research Ethics Authority of Newfoundland and Labrador as part of the NFOAS and by the Southern Tasmanian Health and Medical Human Research Ethics Committee as part of the TASOAC. Written consent was obtained from all the participants.
Demographics and anthropometrics
Demographic information was obtained by a self-administered questionnaire with the help of the research staff, if necessary. Anthropometric data including height and weight were retrieved from their hospital admission and medical records and BMI was calculated by dividing weight in kilograms by squared height in metres. Age was calculated at the time of the surgery for the NFOAS study participants and at the baseline for the TASOAC study participants.
Metabolic profiling
We used commercially available EDTA-treated tubes to collect blood. Blood samples were collected after at least 8 h fasting and plasma was separated from the whole blood using standard protocol. Briefly, the whole blood was centrifuged at 2000 g for 10 min and the resulting supernatant is designated plasma. Following centrifugation, the plasma was immediately transferred into a clean polypropylene tube using a pipette, and then stored at −80 °C until analysis. Metabolic profiling was performed on plasma by using the Waters XEVO TQ MS system (Waters, Mississauga, Ontario, Canada) coupled with Biocrates AbsoluteIDQ p180 kit (BIOCRATES Life Sciences AG, Innsbruck, Austria), which measures 186 metabolites including 90 glycerophospholipids, 40 acylcarnitines (including free L-carnitine), 21 amino acids, 19 biogenic amines, 15 sphingolipids and 1 hexose (>90% is glucose). The details of the 186 metabolites are listed in supplementary Table 1 , available at Rheumatology Online. The metabolic profiling method using this kit was described previously [ 19 ].
Statistical methods
Plasma metabolite ratios (4018), which were highly correlated between plasma and SF found in our previous study [ 18 ], were generated from the metabolite profiles and were compared between OA cases and controls using the Kolmogorov–Smirnov test. Linear regression was used to examine the association between each metabolite ratio and knee OA with adjustment for potential confounders including age, sex and BMI. The significance level was defined in the discovery stage at α = 0.05/4018 = 1.2 × 10 −5 with the Bonferroni method for correcting multiple testing. The identified metabolic ratios in the discovery stage were confirmed in the replication stage in both cross-sectional and longitudinal cohorts.
Receiver operating characteristic (ROC) analysis was used to examine predictive value(s) of the novel OA marker(s) with area under curve (AUC) calculated. Optimal cut-off values were determined using the Youden method [ 23 ] implemented in the OptimalCutpoints package in R and sensitivity and specificity for each novel marker for knee OA was calculated. Time to occurrence of TKR was estimated by the Kaplan–Meier method and statistical differences among groups were compared by the log-rank test. The follow-up time was censored if TKR happened or if the patient was lost to follow-up. The multivariable Cox proportional hazard model was utilized to calculate hazard ratios (HRs) with adjustment for age, sex and BMI. All analyses were performed using the PCIT, PROC and survival analysis packages implemented in R3.1.2 for Windows.
Results
In the cross-sectional study, 109 subjects (64 knee OA patients and 45 controls) were included in the discovery cohort and 148 subjects (72 knee OA patients and 76 age, BMI and sex matched controls) were included in the replication cohort. OA cases were older and had a greater BMI than that in controls in the discovery cohort (all P < 0.006) but no differences were found in the replication cohort ( Table 1 ).
T able 1 .
Descriptive statistics of the study population
| Sample |
Discovery
|
Replication
|
Longitudinal
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| OA (n = 64) | OA-free (n = 45) | P-value | OA (n = 72) | OA-free (n = 76) | P- value | TKR (n = 36) | Non-TKR (n = 122) | P- value | |
| Age, mean ( s . d .), years | 65 (7) | 48 (6) | <0.0001 | 62 (5) | 61 (6) | 0.27 | 64 (7.5) | 63 (6.3) | 0.59 |
| Sex, %F | 59 | 77 | 0.08 | 48 | 50 | 0.86 | 56 | 58 | 0.99 |
| BMI, mean ( s . d .), kg/m 2 | 33.9 (7.3) | 30.09 (6.7) | 0.0059 | 30.3 (2.6) | 29.4 (3.6) | 0.08 | 29.7 (5.6) | 28.5 (4.8) | 0.25 |
| JSN score, mean ( s . d .) | — | — | — | — | — | — | 2.8 (2.4) | 2.5 (2.1) | 0.49 |
| Osteophyte score, mean ( s . d .) | — | — | — | — | — | — | 2.3 (3.2) | 1.0 (2.2) | 0.19 |
F: female; JSN: joint space narrowing.
In the 10-year follow-up study, 158 participants were included with baseline plasma samples available. Of these, 36 subjects underwent TKR during the follow-up period. There were no statistical differences in age, sex, BMI and baseline osteophyte and joint space narrowing scores between TKR patients and non-TKR patients. The detailed descriptive statistics of the study population are shown in Table 1 .
We first examined the association between knee OA and the branched chain amino acids to histidine ratio, which was reported in our previous study [ 18 ]. In the discovery cohort, the ratios of all three branched chain amino acids to histidine, namely valine to histidine, isoleucine to histidine and leucine to histidine, were significantly higher in knee OA patients than in controls (all P = 4.66 × 10 −9 ). We calculated the total concentration of the branched chain amino acids, and the ratio of the total branched chain amino acids (BCAAs) to histidine was also strongly associated with knee OA (P = 2.7 × 10 −23 ). The significance remained after adjustment for age, sex and BMI (P = 7.9 × 10 −8 ) ( Table 2 ). In the cross-sectional replication cohort, ratios of both valine and leucine to histidine were associated with knee OA after adjustment for age, sex and BMI (P < 0.035). The total BCAAs to histidine ratio was also significantly associated with knee OA (P = 0.01) ( Table 2 ).
T able 2 .
Multivariable regression results for the three BCAA to histidine ratios with knee OA a
| BCAA ratio |
Discovery
|
Replication
|
Combination
|
|||
|---|---|---|---|---|---|---|
| β ( s . e .) | P-value | β ( s . e .) | P-value | β ( s . e .) | P-value | |
| Valine to histidine | 1.2 (0.32) | 4.0 × 10 −4 | 0.23 (0.11) | 0.034 | 0.37 (0.12) | 1.6 × 10 −3 |
| Leucine to histidine | 1.7 (0.24) | 2.4 × 10 −10 | 0.23 (0.07) | 7.1 × 10 −4 | 0.57 (0.09) | 4.8 × 10 −10 |
| Isoleucine to histidine | 0.26 (0.11) | 0.023 | 0.06 (0.04) | 0.11 | 0.11 (0.04) | 4.3 × 10 −3 |
| Total BCAAs to histidine | 3.1 (0.54) | 7.9 × 10 −8 | −0.52 (0.20) | 0.01 | 1.1 (0.21) | 9.3 × 10 −7 |
a Age, sex and BMI were adjusted in the model. β: regression coefficient; BCAA: branched chain amino acid.
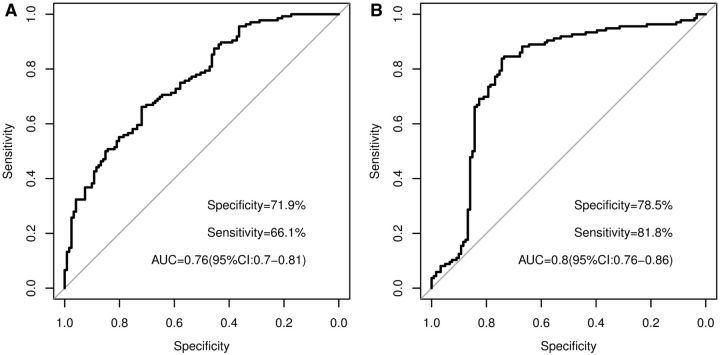
When the discovery and replication cohorts were combined, the total BCAAs to histidine ratio was strongly associated with knee OA (P = 9.3 × 10 −7 ) ( Table 2 ). ROC analysis showed that the total BCAAs to histidine ratio had an AUC of 0.76. With the optimal cut-off of 5.6, the ratio had a sensitivity of 66.1% and a specificity of 72.0% ( Fig. 1 A). However, the HR of 1.2 (95% CI: 0.59, 2.5) found in the longitudinal cohort was not statistically significant (P = 0.6).
F ig . 1 .
ROC curves for ( A ) total BCAAs to histidine and ( B ) lysoPCs to PCs ratio in the cross-sectional cohort
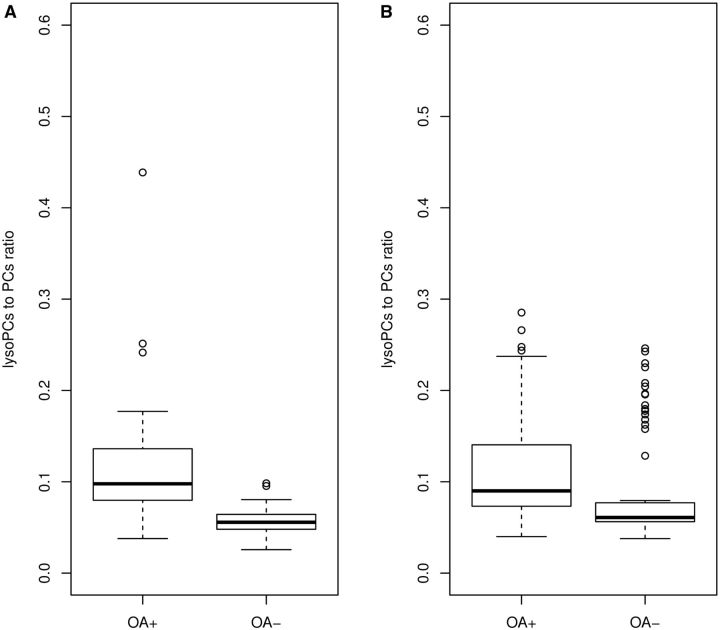
Of the 4018 plasma metabolite ratios that were highly correlated with those in SF in our previous study [ 18 ], we found that 35 metabolite ratios were significantly associated with knee OA with all P ⩽ 1.2 × 10 −5 in discovery cohort after adjustment of sex, age and BMI. In the cross-sectional replication cohort, 13 of them were replicated (P < 1.3 × 10 −3 ) ( supplementary Table 2 , available at Rheumatology Online). Eight of them were lysophosphatidylcholines (lysoPCs)/phosphatidylcholines (PCs), namely, lysoPC a C20:3/PC aa C36:0; lysoPC a C20:4/PC aa C36:3; lysoPC a C18:0/PC aa C36:5; lysoPC a C20:4/PC aa C38:6; lysoPC a C18:0/PC aa C40:6; lysoPC a C18:0/PC ae C34:3; lysoPC a C20:4/PC ae C36:1; and lysoPC a C20:4/PC ae C44:5. We therefore calculated the total lysoPCs to total PCs ratio from those eight significant ratios. The association between the ratio of the total lysoPCs to total PCs and knee OA was even stronger in the discovery cohort (P = 2.2 × 10 −16 ) and in the cross-sectional replication cohort (P = 7.6 × 10 −8 ). The ratio was on average 0.05 higher in knee OA patients than that of the controls ( Fig. 2 ).
F ig . 2 .
Boxplots for the lysoPCs to PCs ratio for OA and control groups in discovery and replication cohorts
( A ) Discovery cohort. ( B ) Replication cohort.
It appeared that the significant association between the lysoPCs to PCs ratio and knee OA was driven by a significantly higher concentration of lysoPCs and lower concentration of PCs in knee OA patients than in controls. In the discovery stage, the absolute concentration of lysoPCs in knee OA patients was on average 25.4 µM higher than in the controls (P = 1.2 × 10 −6 ), whereas that of the total PCs was on average 88 µM lower in knee OA patients than in controls (P = 5.6 × 10 −6 ). Similarly, in the cross-sectional replication cohort, the absolute concentration of lysoPCs in knee OA patients was on average 5.6 µM higher than in the controls (P = 2.3 × 10 −4 ), whereas that of the total PCs was on average 43 µM lower in knee OA patients than in controls (P = 0.006). ROC analysis showed that the total lysoPCs/total PCs ratio had an AUC of 0.80 based on the discovery and replication combined cohort. At the optimal cut-off of 0.09, it had a sensitivity of 81.8% and a specificity of 78.5% ( Fig. 1 B).
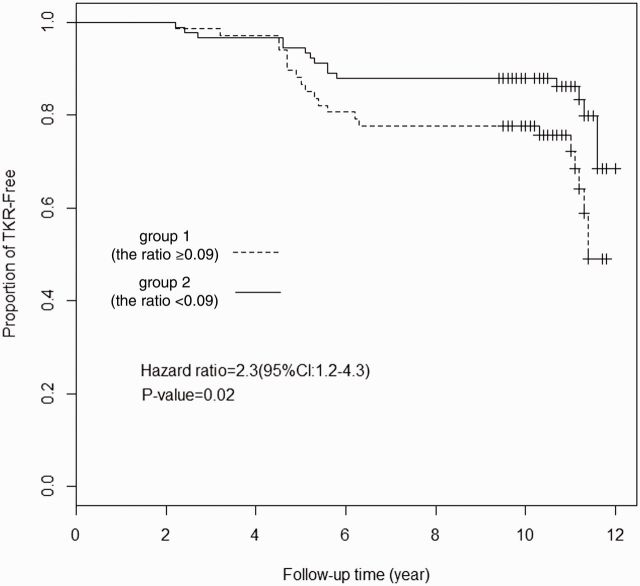
We categorized the subjects of the longitudinal cohort into two groups based on the optimal cut-off of 0.09 in the lysoPCs to PCs ratio at baseline, that is, group 1 (the ratio ⩾ 0.09) and group 2 (the ratio < 0.09). Kaplan–Meier analysis showed a significant Time-to-TKR in group 1 compared with group 2 (P = 0.02) ( Fig. 3 ). At end of the follow-up, 49% of subjects in group 1 remained TKR-free whereas 69% in group 2 remained TKR-free. Multivariable Cox regression analysis found an HR of 2.3 (95% CI: 1.2, 4.3; P = 0.02) for the occurrence of TKR in group 1 compared with group 2 after adjustment for age, sex and BMI.
F ig . 3 .
Risk of remaining TKR-free as a function of lysoPCs to PCs ratio with the optimal cut-off of 0.09
The time of TKR with each group was plotted. TKR curves were estimated by Kaplan–Meier product limit distributions.
Discussion
We confirmed the previously reported association of the total BCAAs to histidine ratio with knee OA and identified a novel metabolic marker—the lysoPCs to PCs ratio—for predicting advanced knee OA. The strength of the current study includes both cross-sectional and longitudinal replication cohorts. We demonstrated that the lysoPCs to PCs ratio had a great sensitivity (81.8%) and specificity (78.5%) to distinguish advanced knee OA cases from healthy controls in our cross-sectional cohort. The subjects with the ratio of ≥0.09 were 2.3 times more likely to undergo TKR than those with the ratio of <0.09 in our longitudinal cohort.
We previously reported that the total BCAAs to histidine ratio was associated with knee OA. The study population was drawn from the UK and only females [ 24 ]. The confirmation of the association in the Newfoundland population in the current study extends the previous findings to the male population, and also demonstrates that the marker was not geography specific. While the potential mechanism for the association remains to be discovered, we found that the ratio was highly correlated between plasma and SF [ 18 ], suggesting this ratio reflects altered joint metabolism in knee OA patients. Compared with the cross-sectional results, the association in the longitudinal cohort did not reach statistical significance, most likely because the majority of subjects in the longitudinal cohort had radiographic OA at baseline.
Decreased lysoPC to PC ratio in cerebrospinal fluid has been reported to be associated with Alzheimer’s disease [ 25 ]. A study also suggested that an increased lysoPC to PC ratio in human spermatozoa and erythrocytes is a useful marker of reduced fertility [ 26 ]. PC to lysoPC ratio in human plasma has also been found as an indicator of the severity of RA [ 27 ]. In the current study, we found that knee OA patients had a significantly increased lysoPCs to PCs ratio in plasma. Put together, this may suggest that the lysoPC(s) to PC(s) ratio is not a disease-specific marker, but rather a marker for disease progression. Thus, it can be used to monitor disease progression in established OA patients. While we did not have severity grade data on our cross-sectional cohorts, the majority of the study participants in our longitudinal cohort had radiographic OA at baseline and the lysoPCs to PCs ratio can predict advanced OA indicated by TKR during the 10 year follow-up period, supporting this hypothesis.
Alternatively, it may indicate that these diseases shared a common pathological pathway with OA. Indeed, both RA and OA share some similar characteristics of joint structure damage although through different aetiologies. OA has also been found to be an independent risk factor for dementia [ 28 ] and accelerates and exacerbates Alzheimer’s disease pathology in mice [ 29 ].
The association could be driven by either an increased lysoPC concentration or a decreased PC concentration or both. The level of lysoPCs was found to be 18% higher in knee OA patients than in the controls in our cross-sectional cohorts. This is consistent with Kosinska et al. ’s report [ 30 ] in which the median concentration of lysoPCs in SF were 4.2-fold increased in late OA patients than that in controls, but the difference between early OA and controls was not significant. We also found that the level of PCs was 14% lower in knee OA patients that in the controls in our cross-sectional cohorts. Our result is consisted with Sivan’s study that the level of PCs in the SF is reduced in OA [ 31 ]. However, this is the opposite to what Kosinska et al. [ 30 ] reported in which PCs were found to be 2.7-fold increased in early OA and 5.4-fold increased in late OA. The reason for the contradiction is unclear, but LysoPCs are usually derived from PCs, and thus one would expect a low concentration of PCs when lysoPCs are increased.
LysoPCs can be made from PCs by phospholipase A2 (PLA2) [ 32 ]. Leistad et al. [ 33 ] showed that multiple PLA2 enzymes were highly expressed in OA chondrocytes in response to pro-inflammatory stimuli such as IL-1β, TNF and IL-6, which have been documented to be associated with OA. An increased expression of PLA2 would facilitate the conversion of PCs to lysoPCs, and thus result in an increased lysoPCs to PCs ratio in OA patients. We measured plasma levels of PLA2 in a subset (36 OA cases and 38 controls) of our cross-sectional cohort, but found no difference in plasma PLA2 levels between OA cases and controls (data not shown), suggesting the observed association is less likely to be caused by an overexpression of PLA2 enzyme. This is consistent with Smith et al. ’s report [ 34 ] in which plasma PLA2 level was significantly increased in active RA patients but there was no difference between OA patients and healthy controls. It is possible that PLA2 expression is increased in SF but could not be detected in plasma. An increased activity of PLA2 in SF has been reported in both human OA and animal OA models [ 35–39 ]. Due to the difficulty of obtaining SF samples from healthy subjects, we were unable to confirm this.
Alternatively, lysoPCs could be generated from PCs by the reactive oxygen species (ROS) in neutrophils [ 27 ]. Oxidative stress has been involved in the pathogenesis of OA [ 40–42 ]. Almost all the OA joint cells, including chondrocytes, synovial fibroblasts and adipocytes, can produce large amounts of ROS and nitric oxide in response to biomechanical or biochemical stimuli. Neutrophils are able to release a mixture of proteolytic enzymes combined with different ROS, which may trigger the destruction of the cartilage tissue [ 43 , 44 ].
Lysophospholipids are biologically active lipids regulating a variety of cellular functions [ 45 , 46 ]. Increased lysoPCs can induce both apoptotic and non-apoptotic cell death [ 47 ]. PCs are the main component (41–67%) of phospholipids, which are one of the major components of SF that independently mediate boundary lubrication [ 30 ]. The deficiency in PCs, especially unsaturated PC species with the long chain fatty acids, could increase friction and lead to articular cartilage damage [ 30 , 48–50 ].
Data on how an orthopaedic surgeon makes a judgment to replace a knee is still limited. Previous studies found that both structural changes such as cartilage loss measured by MRI and the patient’s willingness are predictors for TKR [ 10–13 ]. Our findings added more in the field and suggest that the lysoPCs to PCs ratio, which can be readily measured in blood, could assist clinicians in their decision-making process although further validation is necessary.
In conclusion, we confirmed that the total BCAAs to histidine ratio is associated with knee OA, which was previously reported, and identified the lysoPCs to PCs ratio as a novel potential marker for predicting advanced knee OA. Further studies are required to examine whether this ratio can predict early OA change.
Supplementary Material
Acknowledgements
We thank all the study participants who made this study possible, and all the staff in the NFOAS study who helped us in the collection of samples.
Funding : This study was funded by the Canadian Institutes of Health Research; Research & Development Corporation of Newfoundland and Labrador; and Memorial University of Newfoundland as part of the Newfoundland Osteoarthritis Study and by the National Health and Medical Research Council of Australia; Tasmanian Community Fund; the Arthritis Foundation of Australia; the University of Tasmania Grant-Institutional Research Scheme; Masonic Centenary Medical Research Foundation and Royal Hobart Hospital Research Foundation as part of the Tasmania Older Adult Cohort Study.
Disclosure statement : P.R. has been a consultant and speaker for Amgen, AbbVie, Celgene, Janssen, Pfizer, Novartis. All other authors have declared no conflicts of interest.
References
- 1. WHO Scientific Group on the Burden of Musculoskeletal Conditions at the Start of the New Millennium . The burden of musculoskeletal conditions at the start of the new millennium: report of a WHO scientific group . Geneva: : World Health Organization; , 2003. . [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M. et al. . Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 . Lancet 2012. ; 380 : 2163 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hiligsmann M, Cooper C, Arden N. et al. . Health economics in the field of osteoarthritis: an expert's consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) . Semin Arthritis Rheum 2013. ; 43 : 303 – 13 . [DOI] [PubMed] [Google Scholar]
- 4. Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis . Arthritis Res 2001. ; 3 : 107 – 13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carr AJ, Robertsson O, Graves S. et al. . Knee replacement . Lancet 2012. ; 379 : 1331 – 40 . [DOI] [PubMed] [Google Scholar]
- 6. Blanco FJ, Moller I, Romera M. et al. . Improved prediction of knee osteoarthritis progression by genetic polymorphisms: the Arthrotest Study . Rheumatology 2015. ; 54 : 1236 – 43 . [DOI] [PubMed] [Google Scholar]
- 7. Wise BL, Felson DT, Clancy M. et al. . Consistency of knee pain and risk of knee replacement: the Multicenter Osteoarthritis Study . J Rheumatol 2011. ; 38 : 1390 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dieppe P. Who should have a joint replacement? A plea for more 'phronesis' . Osteoarthritis Cartilage 2011. ; 19 : 145 – 6 . [DOI] [PubMed] [Google Scholar]
- 9. Dieppe P, Lim K, Lohmander S. Who should have knee joint replacement surgery for osteoarthritis? Int J Rheum Dis 2011. ; 14 : 175 – 80 . [DOI] [PubMed] [Google Scholar]
- 10. Hawker GA, Guan J, Croxford R. et al. . A prospective population-based study of the predictors of undergoing total joint arthroplasty . Arthritis Rheum 2006. ; 54 : 3212 – 20 . [DOI] [PubMed] [Google Scholar]
- 11. McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee . Ann Rheum Dis 1993. ; 52 : 258 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gossec L, Jordan JM, Lam MA. et al. . Comparative evaluation of three semi-quantitative radiographic grading techniques for hip osteoarthritis in terms of validity and reproducibility in 1404 radiographs: report of the OARSI-OMERACT Task Force . Osteoarthritis Cartilage 2009. ; 17 : 182 – 7 . [DOI] [PubMed] [Google Scholar]
- 13. Cicuttini FM, Jones G, Forbes A, Wluka AE. Rate of cartilage loss at two years predicts subsequent total knee arthroplasty: a prospective study . Ann Rheum Dis 2004. ; 63 : 1124 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toivanen AT, Arokoski JP, Manninen PS. et al. . Agreement between clinical and radiological methods of diagnosing knee osteoarthritis . Scand J Rheumatol 2007. ; 36 : 58 – 63 . [DOI] [PubMed] [Google Scholar]
- 15. Dieppe P, Judge A, Williams S. et al. . Variations in the pre-operative status of patients coming to primary hip replacement for osteoarthritis in European orthopaedic centres . BMC Musculoskelet Disord 2009. ; 10 : 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeni JA, Jr, Axe MJ, Snyder-Mackler L. Clinical predictors of elective total joint replacement in persons with end-stage knee osteoarthritis . BMC Musculoskelet Disord 2010. ; 11 : 86.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation . Arthritis Rheum 2012. ; 64 : 3963 – 71 . [DOI] [PubMed] [Google Scholar]
- 18. Zhang W, Likhodii S, Aref-Eshghi E. et al. . Relationship between blood plasma and synovial fluid metabolite concentrations in patients with osteoarthritis . J Rheumatol 2015. ; 42 : 859 – 65 . [DOI] [PubMed] [Google Scholar]
- 19. Zhang W, Likhodii S, Zhang Y. et al. . Classification of osteoarthritis phenotypes by metabolomics analysis . BMJ Open 2014. ; 4 : e006286.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fontaine-Bisson B, Thorburn J, Gregory A, Zhang H, Sun G. Melanin-concentrating hormone receptor 1 polymorphisms are associated with components of energy balance in the Complex Diseases in the Newfoundland Population: Environment and Genetics (CODING) study . Am J Clin Nutr 2014. ; 99 : 384 – 91 . [DOI] [PubMed] [Google Scholar]
- 21. Altman R, Alarcon G, Appelrouth D. et al. . The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip . Arthritis Rheum 1991. ; 34 : 505 – 14 . [DOI] [PubMed] [Google Scholar]
- 22. Khan HI, Aitken D, Zhai G. et al. . Association between hip and knee cartilage measured using radiographs and magnetic resonance imaging: the Tasmanian Older Adult Cohort Study . Rheumatology 2013. ; 52 : 2009 – 15 . [DOI] [PubMed] [Google Scholar]
- 23. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point . Biom J 2005. ; 47 : 458 – 72 . [DOI] [PubMed] [Google Scholar]
- 24. Zhai G, Wang-Sattler R, Hart DJ. et al. . Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis . Ann Rheum Dis 2010. ; 69 : 1227 – 31 . [DOI] [PubMed] [Google Scholar]
- 25. Mulder C, Wahlund LO, Teerlink T. et al. . Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer's disease . J Neural Transm 2003. ; 110 : 949 – 55 . [DOI] [PubMed] [Google Scholar]
- 26. Nimptsch A, Pyttel S, Paasch U. et al. . A MALDI MS investigation of the lysophosphatidylcholine/phosphatidylcholine ratio in human spermatozoa and erythrocytes as a useful fertility marker . Lipids 2014. ; 49 : 287 – 93 . [DOI] [PubMed] [Google Scholar]
- 27. Fuchs B, Schiller J, Wagner U, Hantzschel H, Arnold K. The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: investigations by 31P NMR and MALDI-TOF MS . Clin Biochem 2005. ; 38 : 925 – 33 . [DOI] [PubMed] [Google Scholar]
- 28. Huang SW, Wang WT, Chou LC. et al. . Osteoarthritis increases the risk of dementia: a nationwide cohort study in Taiwan . Sci Rep 2015. ; 5 : 10145.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kyrkanides S, Tallents RH, Miller JN. et al. . Osteoarthritis accelerates and exacerbates Alzheimer's disease pathology in mice . J Neuroinflammation 2011. ; 8 : 112. 2094-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kosinska MK, Liebisch G, Lochnit G. et al. . A lipidomic study of phospholipid classes and species in human synovial fluid . Arthritis Rheum 2013. ; 65 : 2323 – 33 . [DOI] [PubMed] [Google Scholar]
- 31. Sivan S, Schroeder A, Verberne G. et al. . Liposomes act as effective biolubricants for friction reduction in human synovial joints . Langmuir 2010. ; 26 : 1107 – 16 . [DOI] [PubMed] [Google Scholar]
- 32. Kita T, Kume N, Ishii K. et al. . Oxidized LDL and expression of monocyte adhesion molecules . Diabetes Res Clin Pract 1999. ; 45 : 123 – 6 . [DOI] [PubMed] [Google Scholar]
- 33. Leistad L, Feuerherm AJ, Faxvaag A, Johansen B. Multiple phospholipase A2 enzymes participate in the inflammatory process in osteoarthritic cartilage . Scand J Rheumatol 2011. ; 40 : 308 – 16 . [DOI] [PubMed] [Google Scholar]
- 34. Smith GM, Ward RL, McGuigan L, Rajkovic IA, Scott KF. Measurement of human phospholipase A2 in arthritis plasma using a newly developed sandwich ELISA . Br J Rheumatol 1992. ; 31 : 175 – 8 . [DOI] [PubMed] [Google Scholar]
- 35. Panula HE, Lohmander LS, Ronkko S. et al. . Elevated levels of synovial fluid PLA2, stromelysin (MMP-3) and TIMP in early osteoarthrosis after tibial valgus osteotomy in young beagle dogs . Acta Orthop Scand 1998. ; 69 : 152 – 8 . [DOI] [PubMed] [Google Scholar]
- 36. Kortekangas P, Aro HT, Nevalainen TJ. Group II phospholipase A2 in synovial fluid and serum in acute arthritis . Scand J Rheumatol 1994. ; 23 : 68 – 72 . [DOI] [PubMed] [Google Scholar]
- 37. Vignon E, Balblanc JC, Mathieu P, Louisot P, Richard M. Metalloprotease activity, phospholipase A2 activity and cytokine concentration in osteoarthritis synovial fluids . Osteoarthritis Cartilage 1993. ; 1 : 115 – 20 . [DOI] [PubMed] [Google Scholar]
- 38. Parks TP, Lukas S, Hoffman AF. Purification and characterization of a phospholipase A2 from human osteoarthritic synovial fluid . Adv Exp Med Biol 1990. ; 275 : 55 – 81 . [DOI] [PubMed] [Google Scholar]
- 39. Gilman SC, Chang J, Zeigler PR, Uhl J, Mochan E. Interleukin-1 activates phospholipase A2 in human synovial cells . Arthritis Rheum 1988. ; 31 : 126 – 30 . [DOI] [PubMed] [Google Scholar]
- 40. Ziskoven C, Jager M, Zilkens C. et al. . Oxidative stress in secondary osteoarthritis: from cartilage destruction to clinical presentation? Orthop Rev 2010. ; 2 : e23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boileau C, Martel-Pelletier J, Caron J. et al. . Protective effects of total fraction of avocado/soybean unsaponifiables on the structural changes in experimental dog osteoarthritis: inhibition of nitric oxide synthase and matrix metalloproteinase-13 . Arthritis Res Ther 2009. ; 11 : R41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis . Nat Rev Rheumatol 2012. ; 8 : 729 – 37 . [DOI] [PubMed] [Google Scholar]
- 43. Schiller J, Benard S, Reichl S, Arnhold J, Arnold K. Cartilage degradation by stimulated human neutrophils: reactive oxygen species decrease markedly the activity of proteolytic enzymes . Chem Biol 2000. ; 7 : 557 – 68 . [DOI] [PubMed] [Google Scholar]
- 44. Hilbert N, Schiller J, Arnhold J, Arnold K. Cartilage degradation by stimulated human neutrophils: elastase is mainly responsible for cartilage damage . Bioorg Chem 2002. ; 30 : 119 – 32 . [DOI] [PubMed] [Google Scholar]
- 45. Kabarowski JH, Xu Y, Witte ON. Lysophosphatidylcholine as a ligand for immunoregulation . Biochem Pharmacol 2002. ; 64 : 161 – 7 . [DOI] [PubMed] [Google Scholar]
- 46. Goetzl EJ. Pleiotypic mechanisms of cellular responses to biologically active lysophospholipids . Prostaglandins Other Lipid Mediat 2001. ; 64 : 11 – 20 . [DOI] [PubMed] [Google Scholar]
- 47. Hsieh CC, Yen MH, Liu HW, Lau YT. Lysophosphatidylcholine induces apoptotic and non-apoptotic death in vascular smooth muscle cells: in comparison with oxidized LDL . Atherosclerosis 2000. ; 151 : 481 – 91 . [DOI] [PubMed] [Google Scholar]
- 48. Mazzucco D, Scott R, Spector M. Composition of joint fluid in patients undergoing total knee replacement and revision arthroplasty: correlation with flow properties . Biomaterials 2004. ; 25 : 4433 – 45 . [DOI] [PubMed] [Google Scholar]
- 49. Chen Y, Crawford RW, Oloyede A. Unsaturated phosphatidylcholines lining on the surface of cartilage and its possible physiological roles . J Orthop Surg Res 2007. ; 2 : 14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Foy JR, Williams PF, 3rd, Powell GL. et al. . Effect of phospholipidic boundary lubrication in rigid and compliant hemiarthroplasty models . Proc Inst Mech Eng H 1999. ; 213 : 5 – 18 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.