The monosaccharide octulose was first isolated and identified about seventy years ago. Although this sugar has frequently been found in plants, bacteria, yeast and animals, its metabolic function has hardly been explored. It often occurs in small amounts, but occasionally it is highly abundant, notably in some resurrection plants. Recent results show that its synthesis may involve an alternative pentose phosphate pathway and that when present in large amounts it might confer important benefits such as ROS scavenging. It could also have value for nutrition and healthcare. The time is ripe for developing our understanding further.
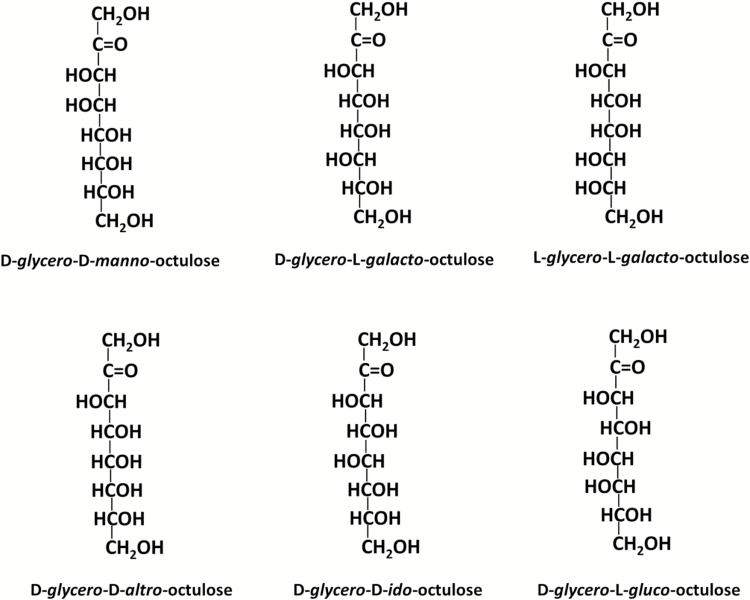
The eight-carbon monosaccharide octulose was found in plants, animals and humans many years ago (Bartlett and Bucolo, 1960; Charlson and Richtmyer, 1960; Bartlett, 1970). As an eight-carbon ketose, it can exist in isomeric forms; except for the 3-octulose D-gluco-L-glycero-3-octulose found in young leaves of Laurus nobilis (Sakata et al., 1989), the other reported octulose isomers in plants are all 2-octuloses and include D-glycero-D-manno-octulose, D-glycero-L-galacto-octulose, L-glycero-L-galacto-octulose, D-glycero-D-altro-octulose and D-glycero-D-ido-octulose (Table 1; see structures in Fig. 1). The amount present varies among plant species – for instance, only about 1 g of D-glycero-D-manno-octulose was obtained from 27 kg of avocado fruit (Charlson and Richtmyer, 1960), while D-glycero-D-ido-octulose makes up about 90% of total sugars in hydrated leaves of the resurrection plant Craterostigma plantagineum (430 mg g–1 lyophilized leaf material) (Bianchi et al., 1991).
Table 1.
Octulose-containing plant species
| Species | Octulose isomers | Source for octulose extraction | References |
|---|---|---|---|
|
Craterostigma plantagineum, C. agnewi and C. pumilum |
D-glycero-D-ido-octulose | Hydrated and dried leaves | Bianchi et al., 1991; Egert et al., 2015 |
| Fabiana imbricata | D-glycero-D-manno-octulose | Dried herbage | Richtmyer, 1970 |
| Laurus nobilis | D-gluco-L-glycero-3-octulose | Flush (young leaves) and leaves | Sakata et al., 1989 |
| Lindernia brevidens, L. numilarifolia, L. philcoxii and L. subracemosa | D-glycero-D-ido-octulose | Hydrated and dried leaves | Kutzer, 2004; Phillips et al., 2008 |
| Lindernia acicularis and L. exilis | D-glycero-D-ido-octulose | Dried leaves | Kutzer, 2004 |
| Medicago sativa | D-glycero-D-manno-octulose | Leaf petiole fraction of young shoots | Rendig et al., 1964 |
| Papaver somniferum | D-glycero-D-manno-octulose | Capsules | Haustveit and Wold, 1970 |
| Persea americana (avocado) | D-glycero-D-manno-octulose, D-glycero-L-galacto-octulose | Fruits | Charlson and Richtmyer, 1960; Sephton and Richtmyer, 1963a |
| Phalaris tuberosa | D-glycero-D-manno-octulose | Dry leaves | McComb and Rendig, 1970 |
| Primula officinalis | D-glycero-D-manno-octulose, D-glycero-L-galacto-octulose | Dried roots | Begbie and Richtmyer, 1966 |
| Sedum spectabile | D-glycero-D-manno-octulose, D-glycero-L-galacto-octulose | Whole plants | Charlson and Richtmyer, 1960; Sephton and Richtmyer, 1963b |
| Spinacia oleracea | D-glycero-D-altro-octulose, D-glycero-D-ido-octulose | Leaves and isolated chloroplasts | Arora et al., 1985; Flanigan et al., 2006; Williams and MacLeod, 2006 |
| Trifolium pratense | D-glycero-L-galacto-octulose | Leaves supplied with D-gulose and D-xylose | Haustveit et al., 1975a |
| Trifolium pratense | L-glycero-L-galacto-octulose | Leaves supplied with L-mannose and L-arabinose | Haustveit et al., 1975a |
| Trifolium pratense | D-glycero-D-altro-octulose | Leaves supplied with D-ribose and D-allose | Haustveit et al., 1975b |
Fig. 1.
Structures of octulose isomers described in this article (Jones and Sephton, 1960; Flanigan et al., 2006).
For the reported octulose-containing plant species, their relatives in the same genus or family may also contain the sugar. For example, except Lindernia rotundifolia, six investigated Lindernia species have been shown to synthesize D-glycero-D-ido-octulose (Table1: Kutzer, 2004; Phillips et al., 2008). Similarly, in the genus Craterostigma, three species synthesize substantial amounts of octulose in the hydrated state (Table 1: Egert et al., 2015). It is certainly reasonable to assume that it occurs in more higher plants than known to date.
Metabolic pathways involving octulose
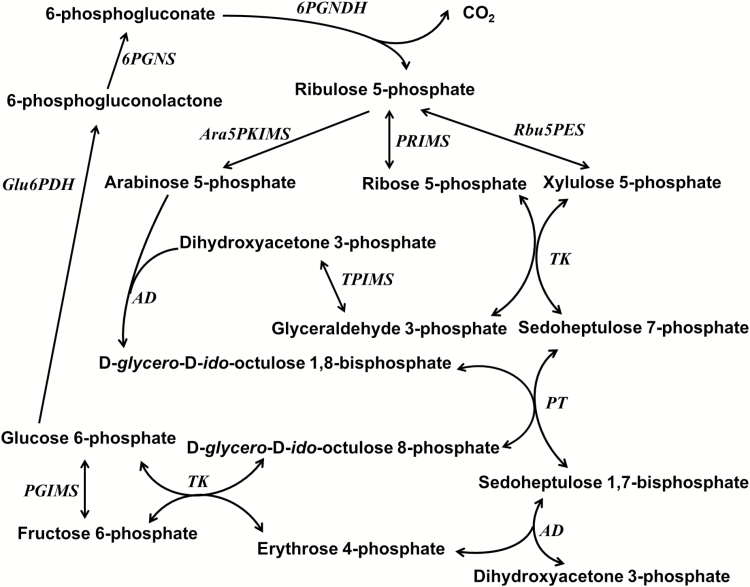
When octulose was first found to be present in various plant species, successful attempts were made to synthesize it in vitro (see Box 1). The possible physiological role of the sugar has mainly been explored by isotope labeling and GC/MS. Williams et al. (1978) proposed an alternative to the pentose phosphate pathway: this L-type pentose phosphate pathway in rat liver includes arabinose 5-phosphate, sedoheptulose 1,7-bisphosphate and the mono- and bisphosphates of D-glycero-D-ido-octulose as intermediates (Fig. 2). Williams and MacLeod (2006) proposed that D-glycero-D-ido-octulose 8-phosphate, D-glycero-D-altro-octulose 8-phosphate and D-glycero-D-altro-octulose 1,8-bisphosphate may also be reactants in a modified Calvin–Benson–Bassham pathway in spinach in which D-glycero-D-ido-octulose 8-phosphate is synthesized by the exchange reaction catalysed by transketolase using fructose 6-phosphate and glucose 6-phosphate as substrates, and D-glycero-D-altro-octulose 1,8-bisphosphate is synthesized via aldolase with ribose 5-phosphate and dihydroxyacetone phosphate as substrates (Flanigan et al., 2006). This reaction was confirmed using the recombinant C. plantagineum transketolase 7 and 10, which catalyses the exchange reaction and produces D-glycero-D-ido-octulose 8-phosphate using glucose 6-phosphate and fructose 6-phosphate as substrates (Zhang et al., 2016). Octulose phosphate was also identified in the protozoan parasite Trypanosoma brucei, and it was shown that octulose 8-phosphate is synthesized by transaldolase when ribose 5-phosphate and fructose 6-phosphate were used as acceptor and donor substrates, respectively (Creek et al., 2012; Creek et al., 2015). Using genetic approaches, the study with yeast by Clasquin et al. (2011) suggested that D-glycero-D-ido-octulose 1,8-bisphosphate might be synthesized by the aldol addition of dihydroxyacetone phosphate (DHAP) and ribose 5-phosphate, which is catalysed by the ubiquitous glycolytic enzyme fructose bisphosphate aldolase (Clasquin et al., 2011). This is consistent with the modified Calvin–Benson–Bassham pathways proposed by Flanigan et al. (2006) and Williams and MacLeod (2006).
Fig. 2.
The L-type pentose phosphate pathway based on the report by Williams and MacLeod (2006). 6PGNDH, 6-phosphogluconate dehydrogenase; 6PGNS, 6-phosphogluconolactonase; AD, aldolase; Ara5PKIMS, arabinose 5-phosphate ketolisomerase; Glu6PDH, glucose 6-phosphate dehydrogenase; PGIMS, phosphoglucose isomerase; PRIMS, phosphoribose isomerase; PT, phosphotransferase; Rbu5PES, ribulose 5-phosphate 3-epimerase; TPIMS, triose phosphate isomerase; TK, transketolase.
Box 1. In vitro synthesis of octulose
D-glycero-D-altro-octulose, L-glycero-L-galacto-octulose and D-glycero-L-gluco-octulose have been synthesized in a condensation reaction catalysed by rabbit muscle aldolase using dihydroxyacetone phosphate (DHAP) with D-ribose, L-arabinose and D-lyxose, respectively (Jones and Sephton, 1960). Similarly, D-glycero-D-manno-octulose has been synthesized in a reaction using D-ribose, D-fructose 1,6-bisphosphate and rabbit muscle aldolase (Haustveit, 1976). In addition, Paoletti et al. (1979) used rabbit muscle aldolase to synthesize D-glycero-D-altro-octulose 1,8-bisphosphate or D-glycero-D-ido-octulose 1,8-bisphosphate by condensation of DHAP with ribose 5-phosphate or arabinose 5-phosphate. While studying methylthiolincosamide biosynthesis, Sasaki et al. (2012) observed that D-glycero-D-altro-octulose is formed via a transaldol reaction catalysed by a putative transaldolase (LmbR protein from Streptomyces lincolnensis) using D-fructose 6-phosphate or D-sedoheptulose 7-phosphate as the C3 donor and D-ribose 5-phosphate as the C5 acceptor.
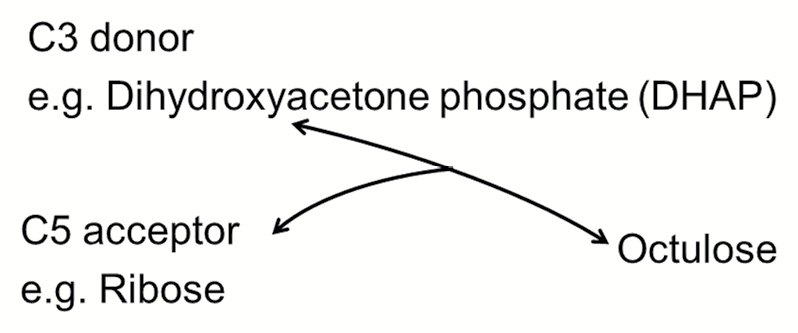
Various octulose isomers can be found in a single plant species (Table 1). Octulose synthesis can be catalysed by different enzymes, including aldolase, transaldolase or transketolase. However, it is also possible that different octulose isomers are synthesized in a similar way only with substrates of specific stereo configurations, as with the synthesis of D-glycero-D-manno-octulose and D-glycero-L-galacto-octulose in avocado and Sedum species (Charlson and Richtmyer, 1960; Sephton and Richtmyer, 1963a). Whether aldolase, transaldolase or transketolase catalyses the synthesis of octulose could be verified by transgenic approaches. Similar studies have been done in potato and tobacco (Haake et al. 1998; Henkes et al. 2001). The genetic transformation of octulose-containing plants, such as Spinacia oleracea or Medicago sativa, is possible and thus aldolase or transketolase mutants could be generated for analyzing octulose metabolism.
The missing loop: kinases and phosphatases
Many monosaccharides are recognized via their phosphate-ester derivatives, which are important intermediates in the catalysis and synthesis of carbohydrates in living organisms (Robyt, 1998). To participate in various aspects of metabolism, free monosaccharides are activated by an initial phosphorylation which is catalysed by sugar kinases. For example, hexokinase catalyses the phosphorylation of glucose to form glucose 6-phosphate and plays a central role in glucose sensing (Rolland et al., 2006). It is reasonable to hypothesize that there are also kinases catalysing the phosphorylation of octulose. In the resurrection plant C. plantagineum, the most abundant sugar in hydrated plants, D-glycero-D-ido-octulose, is converted to sucrose during dehydration and the phosphorylation of octulose should be essential in this process. However, the enzymatic activity of an octulose kinase has not yet been reported. As mentioned in discussion of its synthesis, octulose is involved in both photosynthesis and pentose phosphate pathways. Therefore, it is reasonable to hypothesize one or more phosphatases that dephosphorylate mono- and bisphosphates of octulose to produce free octulose, especially in plants which accumulate octulose. For some free monosaccharides, specific phosphatases exist to catalyse the dephosphorylation of their phosphate-ester derivatives (e.g. glucose, galactose and sedoheptulose: Van Schaftingen and Gerin, 2002; Conklin et al., 2006; Ceusters et al., 2013). To date, various sugar phosphatases have been identified, including L-galactose 1-phosphate phosphatase in Arabidopsis (Conklin et al., 2006). However, the phosphatases involved in octulose production have not been explored.
Besides traditional protein purification, which provides primary solutions for new protein characterization (Janson, 2012), knowledge from genomics, transcriptomics, proteomics and metabolomics, and approaches from genetic modification, have enhanced the identification and characterization of specific sugar kinases and phosphatases. For example, Groisillier et al. (2014) revealed that haloalkanoic dehalogenase-like enzymes in brown algae have specific mannitol-1-phosphatase activity, and Conklin et al. (2006) reported that the Arabidopsis VTC4 gene, which has been annotated as encoding a myo-inositol monophosphatase-like protein, encodes L-galactose 1-phosphate phosphatase. Similar approaches are possible for octulose-containing plant species, especially Spinacia oleracea and Medicago sativa, where there is full genome information and they can easily be genetically modified. Studies of the kinases and phosphatases involved in octulose metabolism might provide novel insights into carbohydrate metabolism in the same way that studies on sedoheptulose, a seven-carbon monosaccharide with a similar structure as octulose, have expanded basic understanding of carbohydrate metabolism (Clasquin et al., 2011; Nagy and Haschemi, 2013).
Interconnections between octulose and other monosaccharides
In the complex metabolic network of plants, the metabolism of octulose is probably interconnected with other metabolites, particularly seven- and nine-carbon sugars. When Begbie and Richtmyer (1966) and Sephton and Richtmyer (1963a) first isolated octulose in avocado and Primula officinalis, they also found heptoses, heptuloses and nonuloses. Numerous studies have shown how variations among family members of biosynthetic enzymes result in substrate preferences and further lead to the diversity of phytochemicals in a given plant species (Pichersky and Gang, 2000).
Octulose synthesis is catalysed by transketolase, transaldolase or fructose bisphosphate aldolase in vitro. All these enzymes can accept various substrates (Krüger and von Schaewen, 2003; Samland and Sprenger, 2009). Our study showed that the recombinant C. plantagineum transketolase 7 and 10 can catalyse the formation of both octulose phosphate and sedoheptulose phosphate (Zhang et al., 2016). Being important components of the Calvin cycle and the pentose phosphate pathway, reactions involving sedoheptulose are of primary endosymbiotic origin in plastids of eukaryotes (Richards et al., 2006; Price et al., 2012). Therefore it can be speculated that the metabolism of eight-carbon sugars might be derived from seven-carbon sugars. The study of Clasquin et al. (2011) provides a genetic proof for this hypothesis. They showed that in yeast the sedoheptulose-1,7-bisphosphatase deletion mutant accumulates sedoheptulose-1,7-bisphosphate and octulose-1,8-bisphosphate; the enzyme is both a selective sedoheptulose bisphosphatase and a selective octulose bisphosphatase. Another example of an interconnection between seven- and eight-carbon sugars is the presence of 3-deoxy-D-arabino-heptulosonic acid (DAH) and 3-deoxy-D-manno-octulosonic acid (KDO). DAH 7-phosphate is synthesized by an aldol condensation of phosphoenolpyruvate (PEP) and erythrose 4-phosphate catalysed by DAH 7-phosphate synthase, while KDO 8-phosphate is synthesized by an aldol condensation of PEP and arabinose 5-phosphate catalysed by KDO 8-phosphate synthase. Both reactions appear to occur by a common mechanism involving an attack from the ‘si’ face of PEP onto the ‘re’ face of the aldehyde group of the monosaccharide, followed by an attack by water onto the C2 position of PEP (Furdui et al., 2005). Based on sequence similarity and X-ray crystal structures, Birck and Woodard (2001) proposed that KDO 8-phosphate synthase and DAH 7-phosphate synthase are the result of a divergent evolutionary process from a common ancestor. These studies provide new evidence that the metabolism of seven-carbon sugars might be derived from eight-carbon sugars or vice versa. Thus studies on octulose, such as identification of specific phosphatases, could borrow ideas from those of sedoheptulose.
Physiological functions
Although studies on octulose have not received much attention, the sugar may be universal in the three main life-forms (microbes, plants and animals). As well as in plants, as already discussed, it has been found in bacteria (e.g. Streptomyces lincolnensis; Sasaki et al., 2012), fungi (e.g. Saccharomyces cerevisiae; Clasquin et al., 2011), a protozoan parasite (Trypanosoma brucei; Creek et al., 2012), animals (rat liver; Paoletti et al., 1979), human red blood cells (Bartlett and Bucolo, 1960) and human erythrocytes (Vanderheiden, 1965). Therefore, it is reasonable to assume that octulose is in fact much more widespread among living organisms.
As already noted, about forty years ago Williams et al. (1978) proposed an L-type pentose phosphate pathway involving octulose phosphates in liver cells. Although, only limited confirmation of this pathway has been obtained, the discoveries of sedoheptulokinase (Wamelink et al., 2008) and sedoheptulose 1,7-bisphosphatase (Clasquin et al., 2011) demonstrate that the full biochemical spectrum of the pentose phosphate pathway exceeds what we currently know (Stincone et al., 2015). Octulose could play an important role in the extended pentose phosphate pathway, and this needs further investigation.
Abundant octulose may also function as carbohydrate storage similar to starch (Norwood et al., 2000). Our studies showed that 75–80% of the sugars in phloem exudate of C. plantagineum is D-glycero-D-ido-octulose, indicating that this might be an important sugar transport form in this species (Zhang et al., 2016). It is intriguing that the desiccation-tolerant species in the genus Lindernia (e.g. L. brevidens) have significantly higher levels of D-glycero-D-ido-octulose than desiccation-sensitive species (e.g. L. subracemosa and L. rotundifolia): the presence of octulose correlates with desiccation tolerance. A reason for its high accumulation in some species could be that D-glycero-D-ido-octulose has a hydroxyl-scavenging ability superior to other common sugars (e.g. sucrose) (Zhang and Bartels, 2016). Such speculation needs to be proven directly. Although tools for direct genetic modifications are unsuited or lacking for resurrection plants, progress might be possible using specific inhibitors to limit octulose production and through this confirming its physiological role. For instance, oxythiamine can act as an inhibitor of transketolase, a key enzyme for octulose synthesis (Wang et al., 2013). Similar to the experiments done with sucrose by Matros et al. (2015), analogues of octulose can also be used in hydroxyl-scavenging reactions in plants in vivo to assess the function of octulose as an antioxidant.
Commercial value
Could octulose have beneficial effects in nutrition and healthcare for animals or people? One possibility is as an antioxidant: a derivative of octulose, 3,7-anhydro-1-deoxy-D-glycero-D-gulo-2-octulose, which was isolated from the roots of Brassica rapa, showed significant ROS reduction and protective effects on glutamate-induced cell death in neuronal HT-22 nerve cells (Wu et al., 2013). It may also be valuable in direct disease resistance: as proposed by Kovářová and Barrett (2016), some enzymes of the pentose phosphate pathway (possibly including enzymes linked to octulose biosynthesis) are essential to the parasites Trypanosoma brucei, Trypanosoma cruzi and various Leishmania species, and offer potential targets for new drugs (inhibitors) against one or other of the diseases caused by these parasites. It is also worth exploring the role of octulose in carbohydrate metabolism in higher animals and humans, as it is often ingested from different foods (e.g. alfalfa plants and avocado fruits). Last, more research on associated metabolic pathways and biological activities (e.g. antibacterial, antifungal, antiviral and anticancer) of octulose and its derivatives might be beneficial for developing novel drugs and cosmetics.
References
- Arora KK, Cortis P, Bleakley PA, Williams JF. 1985. Identification and measurement of D-glycero D-ido octulose 1,8-bisphosphate: D-altro-heptulose 7-phosphotransferase enzyme in tissues with L-type pentose phosphate pathway activity. The International Journal of Biochemistry 17, 1329–1337. [DOI] [PubMed] [Google Scholar]
- Bartlett GR. 1970. Patterns of phosphate compounds in red blood cells of man and animals. In: Brewer GJ, ed. Red cell metabolism and function: Proceedings of the first international conference on red cell metabolism and function, held at the University of Michigan, Ann Arbor, October 1–3, 1969. Boston, MA: Springer US, 245–256. [Google Scholar]
- Bartlett GR, Bucolo G. 1960. Octulose phosphates from the human red blood cell. Biochemical and Biophysical Research Communications 3, 474–478. [DOI] [PubMed] [Google Scholar]
- Begbie R, Richtmyer NK. 1966. The isolation of some heptoses, heptuloses, octuloses, and nonuloses from Primula officinalis jacq. Carbohydrate Research 2, 272–288. [Google Scholar]
- Bianchi G, Gamba A, Murelli C, Salamini F, Bartels D. 1991. Novel carbohydrate metabolism in the resurrection plant Craterostigma plantagineum. The Plant Journal 1, 355–359. [DOI] [PubMed] [Google Scholar]
- Birck RM, Woodard WR. 2001. Aquifex aeolicus 3-Deoxy-D-manno-2-octulosonic acid 8-phosphate synthase: a new class of KDO 8-P synthase?Journal of Molecular Evolution 52, 205–214. [DOI] [PubMed] [Google Scholar]
- Ceusters J, Godts C, Peshev D, Vergauwen R, Dyubankova N, Lescrinier E, De Proft MP, Van den Ende W. 2013. Sedoheptulose accumulation under CO2 enrichment in leaves of Kalanchoë pinnata: a novel mechanism to enhance C and P homeostasis?Journal of Experimental Botany 64, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson AJ, Richtmyer NK. 1960. The isolation of an octulose and an octitol from natural sources: D-glycero-D-manno-octulose and D-erythro-D-galacto-octitol from the avocado and D-glycero-D-manno-octulose from Sedum species. Journal of the American Chemical Society 82, 3428–3434. [Google Scholar]
- Clasquin MF, Melamud E, Singer A et al. 2011. Riboneogenesis in yeast. Cell 145, 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N. 2006. Arabidopsis thaliana VTC4 encodes L-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. The Journal of Biological Chemistry 281, 15662–15670. [DOI] [PubMed] [Google Scholar]
- Creek DJ, Chokkathukalam A, Jankevics A, Burgess KE, Breitling R, Barrett MP. 2012. Stable isotope-assisted metabolomics for network-wide metabolic pathway elucidation. Analytical Chemistry 84, 8442–8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creek DJ, Mazet M, Achcar F et al. 2015. Probing the metabolic network in bloodstream-form Trypanosoma brucei using untargeted metabolomics with stable isotope labelled glucose. PLoS Pathogens 11, e1004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egert A, Eicher B, Keller F, Peters S. 2015. Evidence for water deficit-induced mass increases of raffinose family oligosaccharides (RFOs) in the leaves of three Craterostigma resurrection plant species. Frontiers in Physiology 6, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan IL, MacLeod JK, Williams JF. 2006. A re-investigation of the path of carbon in photosynthesis utilizing GC/MS methodology. Unequivocal verification of the participation of octulose phosphates in the pathway. Photosynthesis Research 90, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furdui CM, Sau AK, Yaniv O, Belakhov V, Woodard RW, Baasov T, Anderson KS. 2005. The use of (E)- and (Z)-phosphoenol-3-fluoropyruvate as mechanistic probes reveals significant differences between the active sites of KDO8P and DAHP synthases. Biochemistry 44, 7326–7335. [DOI] [PubMed] [Google Scholar]
- Groisillier A, Shao Z, Michel G et al. 2014. Mannitol metabolism in brown algae involves a new phosphatase family. Journal of Experimental Botany 65, 559–570. [DOI] [PubMed] [Google Scholar]
- Haake V, Zrenner R, Sonnewald U, Stitt M. 1998. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. The Plant Journal 14, 147–157. [DOI] [PubMed] [Google Scholar]
- Haustveit G. 1976. Aldolase-catalysed formation of D-glycero-D-manno-octulose from D-ribose and D-fructose 1,6-diphosphate. Carbohydrate Research 47, 164–166. [DOI] [PubMed] [Google Scholar]
- Haustveit G, McComb EA, Rendig VV. 1975a. Biosynthesis of D- and L-glycero-L-galacto-octulose from pentoses and hexoses. Carbohydrate Research 39, 125–129. [DOI] [PubMed] [Google Scholar]
- Haustveit G, McComb EA, Rendig VV. 1975b. Biosynthesis of D-glycero-D-altro-octulose from D-ribose and D-allose. Carbohydrate Research 41, 363–365. [DOI] [PubMed] [Google Scholar]
- Haustveit G, Wold JK. 1970. Higher-carbon sugars of opium poppy (Papaver somniferum L.). Acta Chemica Scandinavica 24, 3059–3061. [DOI] [PubMed] [Google Scholar]
- Henkes S, Sonnewald U, Badur R, Flachmann R, Stitt M. 2001. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. The Plant Cell 13, 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson J-C. 2012. Protein purification: principles, high resolution methods, and applications. John Wiley & Sons. [Google Scholar]
- Jones JKN, Sephton HH. 1960. Synthesis of sugars from smaller fragments: part XII. Synthesis of D-glycero-D-altro-, L-glycero-L-galacto-, D-glycero-L-gluco-, and D-glycero-L-galacto-octulose. Canadian Journal of Chemistry 38, 753–760. [Google Scholar]
- Kovářová J, Barrett MP. 2016. The pentose phosphate pathway in parasitic trypanosomatids. Trends in Parasitology 32, 622–634. [DOI] [PubMed] [Google Scholar]
- Kruger NJ, von Schaewen A. 2003. The oxidative pentose phosphate pathway: structure and organisation. Current Opinion in Plant Biology 6, 236–246. [DOI] [PubMed] [Google Scholar]
- Kutzer M. 2004. Untersuchung zum Zuckerstoffwechsel der Wiederauferstehungspflanze Craterostigma plantagineum und einiger Lindernia-Arten, Ph. D. Thesis, Germany: University of Bonn. [Google Scholar]
- Matros A, Peshev D, Peukert M, Mock HP, Van den Ende W. 2015. Sugars as hydroxyl radical scavengers: proof-of-concept by studying the fate of sucralose in Arabidopsis. The Plant Journal 82, 822–839. [DOI] [PubMed] [Google Scholar]
- McComb EA, Rendig VV. 1970. Some nonfermentable free carbohydrates in the leaves of canary grass (Phalaris tuberosa). Journal of Agricultural and Food Chemistry 18, 1092–1094. [Google Scholar]
- Nagy C, Haschemi A. 2013. Sedoheptulose kinase regulates cellular carbohydrate metabolism by sedoheptulose 7-phosphate supply. Biochemical Society Transactions 41, 674–680. [DOI] [PubMed] [Google Scholar]
- Norwood M, Truesdale MR, Richter A, Scott P. 2000. Photosynthetic carbohydrate metabolism in the resurrection plant Craterostigma plantagineum. Journal of Experimental Botany 51, 159–165. [DOI] [PubMed] [Google Scholar]
- Paoletti F, Williams JF, Horecker BL. 1979. Synthesis and cleavage of octulose bisphosphates with liver and muscle aldolases. Archives of Biochemistry and Biophysics 198, 614–619. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Fischer E, Baron M, Van Den Dries N, Facchinelli F, Kutzer M, Rahmanzadeh R, Remus D, Bartels D. 2008. Lindernia brevidens: a novel desiccation-tolerant vascular plant, endemic to ancient tropical rainforests. The Plant Journal 54, 938–948. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gang DR. 2000. Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends in Plant Science 5, 439–445. [DOI] [PubMed] [Google Scholar]
- Price DC, Chan CX, Yoon HS et al. 2012. Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants. Science 335, 843–847. [DOI] [PubMed] [Google Scholar]
- Rendig V, McComb E, Hu C. 1964. Plant nutrition and feeding value, some nonfermentable free sugars in leaf-petiole fraction of alfalfa (Medicago sativa). Journal of Agricultural and Food Chemistry 12, 421–423. [Google Scholar]
- Richards TA, Dacks JB, Campbell SA, Blanchard JL, Foster PG, McLeod R, Roberts CW. 2006. Evolutionary origins of the eukaryotic shikimate pathway: gene fusions, horizontal gene transfer, and endosymbiotic replacements. Eukaryotic Cell 5, 1517–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtmyer NK. 1970. The isolation of D-manno-heptulose, perseitol, D-glycero-D-manno-octulose, and other compounds from pichi tops (Fabiana imbricata Ruiz & Pav.). Carbohydrate Research 12, 233–239. [Google Scholar]
- Robyt JF. 1998. Essentials of carbohydrate chemistry. Springer Science & Business Media. [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. 2006. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology 57, 675–709. [DOI] [PubMed] [Google Scholar]
- Sakata K, Hagiwara H, Yagi A, Ina K. 1989. The first naturally occurring 3-octulose, D-gluco-L-glycero-3-octulose, as the main constituent of Laurus nobilis flush. Agricultural and Biological Chemistry 53, 2539–2541. [Google Scholar]
- Samland AK, Sprenger GA. 2009. Transaldolase: from biochemistry to human disease. The International Journal of Biochemistry & Cell Biology 41, 1482–1494. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Lin CI, Lin KY, Liu HW. 2012. Construction of the octose 8-phosphate intermediate in lincomycin A biosynthesis: characterization of the reactions catalyzed by LmbR and LmbN. Journal of the American Chemical Society 134, 17432–17435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton HH, Richtmyer NK. 1963a. The isolation of a second octulose and of a heptose from the avocado: D-glycero-L-galacto-Octulose and D-glycero-D-galacto-heptose. The Journal of Organic Chemistry 28, 1691–1694. [Google Scholar]
- Sephton HH, Richtmyer NK. 1963b. Isolation of D-erythro-L-gluco-nonulose from the avocado. The Journal of Organic Chemistry 28, 2388–2390. [Google Scholar]
- Stincone A, Prigione A, Cramer T et al. 2015. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biological Reviews 90, 927–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaftingen E, Gerin I. 2002. The glucose-6-phosphatase system. The Biochemical Journal 362, 513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheiden BS. 1965. Phosphate esters of human erythrocytes. IV. Sedoheptulose-1,7-diphosphate, octulose-1,8-diphosphate, inosine triphosphate and uridine diphosphate. Biochemical and Biophysical Research Communications 21, 265–270. [DOI] [PubMed] [Google Scholar]
- Wamelink MM, Struys EA, Jansen EE, Levtchenko EN, Zijlstra FS, Engelke U, Blom HJ, Jakobs C, Wevers RA. 2008. Sedoheptulokinase deficiency due to a 57-kb deletion in cystinosis patients causes urinary accumulation of sedoheptulose: elucidation of the CARKL gene. Human Mutation 29, 532–536. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang X, Ma D et al. 2013. Inhibition of transketolase by oxythiamine altered dynamics of protein signals in pancreatic cancer cells. Experimental Hematology & Oncology 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JF, Blackmore PF, Clark MG. 1978. New reaction sequences for the non-oxidative pentose phosphate pathway. The Biochemical Journal 176, 257–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JF, MacLeod JK. 2006. The metabolic significance of octulose phosphates in the photosynthetic carbon reduction cycle in spinach. Photosynthesis Research 90, 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Cho JG, Lee DS et al. 2013. Carbohydrate derivatives from the roots of Brassica rapa ssp. campestris and their effects on ROS production and glutamate-induced cell death in HT-22 cells. Carbohydrate Research 372, 9–14. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bartels D. 2016. Physiological factors determine the accumulation of D-glycero-D-ido-octulose (D-g-D-i-oct) in the desiccation tolerant resurrection plant Craterostigma plantagineum. Functional Plant Biology 43, 684–694. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Linnemann TV, Schreiber L, Bartels D. 2016. The role of transketolase and octulose in the resurrection plant Craterostigma plantagineum. Journal of Experimental Botany 67, 3551–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]