Abstract
Objective
To examine associations between antidepressant use and healthcare utilization in young adults beginning maintenance HD therapy. Young adults on hemodialysis (HD) have high healthcare utilization, despite having less medical comorbidity than older patients. Depression and other psychosocial factors may contribute. Yet, this association in young adult HD patients has not been well described.
Patients and Methods
Antidepressant use, hospitalizations, and emergency department (ED visits were examined in young adults (aged 18 to 44 years) initiating HD (January 1, 2001-December 31, 2013; n=130) at a Midwestern USA institution. Primary outcomes included: hospitalization and ED visits during the first year.
Results
A depression diagnosis was common (36.2%) at HD start, yet only 21.5% of the cohort were receiving antidepressant therapy. The antidepressant group was more likely to have diabetes mellitus (64.3% vs. 32.4%), coronary artery disease (28.6% vs. 11.8%), and heart failure (32.1% vs. 14.7%); all P<.05 compared to the untreated group. Overall, 52.3% had 1 or more hospitalizations and 25.4% had 1 or more ED visit in the first year. Risk of hospitalization during the first year was higher in the antidepressant group (HR 2.35; 95% CI 1.39, 3.96; P=.001) which persisted after adjustment for diabetes, coronary artery disease, and heart failure, (HR 1.94; CI 1.22, 3.1; P=.006). ED visit rates were similar between groups.
Conclusion
Depression and antidepressant use for a mood indication are common among young adult incident HD patients and associate with higher hospitalization rates during the first year. Further research should determine if antidepressants are a marker for other comorbidities or if treated depression impacts the heightened healthcare use in these individuals.
Keywords: dialysis, incident, mood disorder, suicide, kidney transplant, diabetes mellitus, substance abuse, youth
INTRODUCTION
In 2014, approximately 120,000 individuals developed end-stage renal disease in the United States1. Among this cohort was a small minority (11%) of young adults aged 22 to 44 years, who faced especially unique challenges from this new reality. The first several months after dialysis start represents a difficult transition time marked by hospitalizations, emergency department (ED) visits, hospital readmissions and premature death2–4. Young adult patients may be particularly susceptible during this time given the recent transition from pediatric to adult medical care5, abrupt alteration in health and/or social status, potential onset of major mental disorders in late teen/early adult years6,7, and recent completion of neuromaturation during their early to mid-20s8–10. Hence, despite having fewer medical comorbidities than older dialysis patients, young adults experience high rates of hospitalizations2,3, and an exceptionally high 30-day readmission rate (over 40%)1–3.
The reason for high healthcare use in young adults on maintenance hemodialysis (HD) is unclear. It is known that 1) psychosocial factors impact resource use as patients transition from pediatric to adult care teams11,12 and 2) depression, common among dialysis patients13–15, may associate with increased morbidity15–24. Hence, given that dialysis-dependency is a risk factor for depression and/or antidepressant use, we hypothesized that antidepressant use for depression may be a marker for the increased psychosocial distress that contributes to high healthcare utilization in this otherwise “healthy” cohort of younger HD patients.
In this study, we examined the association between antidepressant use, hospitalizations, and ED visits within the first year of HD start among young, incident HD patients. Identifying markers of psychosocial burden, such as depression, anxiety, and antidepressant pharmacotherapy in incident patients may allow for development of interventions to reduce healthcare use and costs while improving quality of life.
MATERIALS AND METHODS
Cohort selection
The Mayo Clinic Health System (MCHS) provides healthcare to nearly 400,000 residents in Southeast Minnesota, Northern Iowa, and Southwest Wisconsin. The Mayo Clinic Dialysis Services (MCDS) provides all inpatient and outpatient HD in the MCHS. The study included all young adult patients (age 18 to 44 years at initiation; n=161) initiating HD between January 1, 2001, and December 31, 2013, with Minnesota Research Authorization. Patients were excluded for lack of in-center maintenance dialysis therapy at MCDS beyond 30 days (n=31); leaving a final study cohort of 130 patients. The Mayo Clinic Institutional Review Board approved this study.
Data Collection
Patient demographics, comorbidities, and cause of kidney failure were obtained from electronic medical record (EMR) review25–27. Psychiatric history, substance use history, and patient-reported suicide attempts were obtained through review of clinical notes, patient-completed medical history forms, and outside medical records from institutions beyond MCHS. Records were also reviewed for physician diagnoses of depressive disorders using Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) terms of “depression” and “depressive disorder” with any modifiers, and “dysthymic disorder.” The same method was implemented for diagnoses of other psychiatric conditions. Psychiatric diagnoses contained in DSM-IV, except substance abuse disorders, were categorized into anxiety, bipolar, psychotic, adjustment, personality, and other disorders. Substance use history included tobacco use, alcohol abuse/dependence, and illicit substance abuse/dependence within six months prior to dialysis start. Illicit substances were classified as cannabis, heroin/cocaine, methamphetamines, non-opioid prescription pills, opioid (inappropriate use or not taken as prescribed), and other substances (including “street drugs” or psilocybin mushrooms). Those with alcohol abuse included individuals meeting DSM-IV criteria as well as patients with clinical documentation of “excessive” alcohol use, “abuse,” or prior involvement in an alcohol rehabilitation program.
Antidepressant therapy
Antidepressant medication use was chosen as the marker for depression, as it reflects a depressive disorder of sufficient severity to justify pharmacotherapy. EMR review of medication lists and clinical notes were used to identify antidepressant pharmacotherapy at dialysis start. Antidepressant therapy use at HD start was defined as 1) active use of antidepressant medication and confirmed by 2) documented indication of treatment for a depressive disorder. Antidepressants taken for non-depressive disorder were recorded but patients were not classified in the antidepressant use group. Pharmacotherapy for depression was categorized as selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), tricyclic antidepressant (TCA), bupropion, or other (such as monoamine oxidase inhibitors or neuroleptics with a primary indication of mood disorder treatment). The untreated group at baseline was also followed for identification of antidepressant therapy initiation during the study period.
Outcomes
The two primary outcomes included hospitalization and ED visits (without subsequent hospitalization) within the first year of HD initiation. Patients were followed for one year starting at date of HD initiation and censored at: death, voluntary withdrawal of HD prior to death, kidney transplantation, renal function recovery allowing HD discontinuation, transfer to a non-MCDS facility for HD continuation, or transition to home hemodialysis or peritoneal dialysis therapy. Primary cause of hospitalizations and ED visits were categorized as cardiac, infection, gastrointestinal, dialysis vascular access, vascular (non-cardiac), neurologic, trauma, endocrine, pulmonary, malignancy-hematologic, pain, psychiatric, and other. Planned hospitalizations for procedures or events such as chemotherapy, obstetric care or delivery, and rehabilitation, were excluded from analyses.
Statistical analysis
Continuous variables were reported as mean ± standard deviation and median with interquartile range (IQR). Categorical variables were expressed as count and percentage. Categorical patient characteristics and rates were compared between antidepressant treated and non-treated groups using Chi-square test of independence and Fisher’s exact test for expected frequencies of <5. Age was compared using a two-sample t-test, and time on HD was compared using Wilcoxon rank-sum test because of its skewed distribution. Average length of hospital stay was assessed using generalized estimating equations with the log link function.
We assessed differences in time-to-first hospitalization along with hospitalization and ED visit rates between antidepressant and non-treated groups. Confidence intervals at the 95% level were calculated for rates using the Poisson exact method. Kaplan-Meier plots were used to compare time-to-first hospitalization and failure curves were compared using the log-rank test. Rates of hospitalizations and ED visits were compared using the Andersen-Gill model to allow for multiple hospitalizations or ED visits per patient. The model controlled for diabetes, coronary artery disease, and heart failure. The proportional hazards assumption was assessed by including an interaction between antidepressant use and logarithm of time as well as plotting the Schoenfeld residuals.
To determine the feasibility of using the diagnosis of a depressive disorder as the marker, in comparison to antidepressant pharmacotherapy, these analyses were repeated. We examined the relationship between depression, defined as a diagnosis of a depressive disorder at dialysis start (n=47 (36.2% of total cohort)), and healthcare utilization. P<.05 were considered statistically significant. Analyses were performed using SAS 9.4 (SAS Institute, Inc. Cary, NC).
RESULTS
Study Participants
From January 2001 to December 2013, 130 patients aged 18 to 44 years initiated maintenance HD therapy and remained on HD a minimum of 30 days in the MCDS. Baseline demographics, comorbid conditions, cause of kidney failure, and details surrounding dialysis initiation and follow-up are shown in Table 1. Overall, mean age was 33.8 ±8.3 years (median 35.9; IQR, 25.9-42.0) of which 56.2% were male, 76.9% were white, 39.2% had diabetes mellitus, 15.4% had coronary artery disease, and 18.5% had heart failure. The overall prevalence of depression was 36.2% (n=47). However, only one-fifth of the cohort (21.5%; n=28) was receiving 1 or more antidepressants for a mood indication at the time of HD initiation. The primary causes of kidney failure included diabetes (26.2%), failing kidney transplant (21.5%), and glomerulonephritis or tubulointerstitial disease (19.2%). Most patients (74.6%) had an outpatient nephrology evaluation completed within 12 months of dialysis initiation. Yet, 56% had their first dialysis session in a hospital setting. A catheter (tunneled n=81; temporary n=36) provided first dialysis access in 89.2%; an arteriovenous fistula or graft was present before the first dialysis in 22.3%. Compared to the untreated group (representing those without depression and those with prior diagnoses of depression who lacked antidepressant drug use at baseline), the antidepressant group was more likely to have diabetes (64.3% vs. 32.4%), coronary artery disease (28.6% vs. 11.8%), and heart failure (32.1% vs. 14.7%), P<.05 for all.
Table 1.
Baseline characteristics of young adult incident dialysis patients either receiving antidepressants or untreated at time of dialysis initiation
| No depression/Depression untreated (N=102) |
Antidepressant (N=28) |
Total (N=130) |
p-value | |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 33.7 (8.4) | 34.4 (8.3) | 33.8 (8.3) | .67 |
| Median (IQR) | 35.9 (25.8, 41.7) | 35.4 (27, 42.5) | 35.9 (25.9, 42) | |
| 18 to 24 | 24 (23.5%) | 4 (14.3%) | 28 (21.5%) | |
| 25 to 29 | 10 (9.8%) | 4 (14.3%) | 14 (10.8%) | |
| 30 to 34 | 15 (14.7%) | 6 (21.4%) | 21 (16.2%) | |
| 35 to 39 | 21 (20.6%) | 4 (14.3%) | 25 (19.2%) | |
| 40 to 45 | 32 (31.4%) | 10 (35.7%) | 42 (32.3%) | |
| Male Gender | 60 (58.8%) | 13 (46.4%) | 73 (56.2%) | .24 |
| Race | .46 | |||
| White | 76 (74.5%) | 24 (85.7%) | 100 (76.9%) | |
| Black | 12 (11.8%) | 2 (7.1%) | 14 (10.8%) | |
| Other/Unknown | 14 (13.7%) | 2 (7.1%) | 16 (12.3%) | |
| Comorbidity | ||||
| Diabetes mellitus | 33 (32.4%) | 18 (64.3%) | 51 (39.2%) | .002 |
| Coronary artery disease | 12 (11.8%) | 8 (28.6%) | 20 (15.4%) | .03 |
| Heart failure | 15 (14.7%) | 9 (32.1%) | 24 (18.5%) | .04 |
| Kidney failure cause | ||||
| Diabetes | 23 (22.5%) | 11 (39.3%) | 34 (26.2%) | .07 |
| Glomerular/tubulointerstitial disease | 22 (21.6%) | 3 (10.7%) | 25 (19.2%) | .20 |
| Failing kidney transplant | 21 (20.6%) | 7 (25.0%) | 28 (21.5%) | .62 |
| Other/Unknown | 36 (35.3%) | 7 (25.0%) | 43 (33.1%) | .31 |
| Year of hemodialysis initiation | .64 | |||
| 2001 to 2004 | 35 (34.3%) | 7 (25.0%) | 42 (32.3%) | |
| 2005 to 2008 | 36 (35.3%) | 11 (39.3%) | 47 (36.2%) | |
| 2009 to 2013 | 31 (30.4%) | 10 (35.7%) | 41 (31.5%) | |
| Prior outpatient nephrology evaluation | 74 (72.5%) | 23 (82.1%) | 97 (74.6%) | .30 |
| First dialysis location | .33 | |||
| Hospital | 55 (53.9%) | 18 (64.3%) | 73 (56.2%) | |
| Outpatient | 47 (46.1%) | 10 (35.7%) | 57 (43.8%) | |
| Reason HD discontinued in 1st year | Untreated (N=102) | Antidepressant (N=28) | Total (N=130) | |
| Died | 6 (5.9%) | 1 (3.6%) | 7 (5.4%) | >.99 |
| Withdrew and died | 3 (2.9%) | 0 (0.0%) | 3 (2.3%) | >.99 |
| Kidney transplant | 33 (32.4%) | 4 (14.3%) | 37 (28.5%) | .06 |
| Recovered kidney function | 4 (3.9%) | 1 (3.6%) | 5 (3.8%) | >.99 |
| Transferred to non-Mayo center | 13 (12.7%) | 3 (10.7%) | 16 (12.3%) | .77 |
| Home dialysis or peritoneal dialysis | 1 (1.0%) | 1 (3.6%) | 2 (1.5%) | .39 |
| Continued HD beyond 1 year | 42 (41.2%) | 18 (64.3%) | 60 (46.2%) | .03 |
Substance use and psychiatric disorders in the cohort are shown in Table 2. Overall, tobacco and/or alcohol abuse were common (47.7%) compared to any illicit substance use within 6 months of dialysis start (7.7%). Cannabis was the most commonly used illicit substance (15.4%). Other than depression (36.2%), the most common comorbid psychiatric conditions were adjustment disorders (10.8%) and anxiety disorders (10.0%). At least one prior suicide attempt was found in 6.9%. Although not on antidepressants at the time of dialysis initiation, 19/102 (18.6%) had a history of a depressive disorder. Of these, 9.8% began antidepressant medication during the 1-year follow-up period. Of the 28 patients on antidepressants at dialysis initiation, 21 (75%) were on an SSRI, 1 (3.6%) on an SNRI, 4 (14%) on a TCA, 4 (14%) on a bupropion, and 1 (3.6%) on another medication (trazodone). Four patients (14%) were on more than one antidepressant.
Table 2.
Substance use and psychiatric disorders in young adult incident dialysis patients either receiving antidepressants or untreated at time of dialysis initiation
| No depression/Depression untreated (N=102) |
Antidepressant (N=28) |
Total (N=130) |
p-value | |
|---|---|---|---|---|
| Tobacco and/or alcohol use | 47 | 15 | 62 | – |
| Tobacco use ever | 45 (44.1%) | 14 (50.0%) | 59 (45.4%) | |
| Tobacco use within 6 months | 31 (30.4%) | 5 (17.9%) | 36 (27.7%) | |
| Alcohol abuse ever | 10 (9.8%) | 4 (14.3%) | 14 (10.8%) | |
| Any illicit substance use* | 16 | 10 | 26 | – |
| Cannabis | 13 (12.7%) | 7 (25.0%) | 20 (15.4%) | |
| Heroin/cocaine | 5 (4.9%) | 4 (14.3%) | 9 (6.9%) | |
| Methamphetamines | 4 (3.9%) | 4 (14.3%) | 8 (6.2%) | |
| Prescription pills | 0 (0.0%) | 1 (3.6%) | 1 (0.8%) | |
| Prescription opiates | 1 (1.0%) | 2 (7.1%) | 3 (2.3%) | |
| Other illegal substances | 2 (2.0%) | 1 (3.6%) | 3 (2.3%) | |
| Any illegal substance within 6 months | 6 (5.9%) | 4 (14.3%) | 10 (7.7%) | |
| Psychiatric disorders* | ||||
| Depression disorder | 19 (18.6%) | 28 (100%) | 47 (36.2%) | – |
| Anxiety disorders | 9 (8.8%) | 4 (14.3%) | 13 (10.0%) | .39 |
| Bipolar disorders | 1 (1.0%) | 0 (0.0%) | 1 (0.8%) | >.99 |
| Psychotic disorders | 0 (0.0%) | 1 (3.6%) | 1 (0.8%) | .22 |
| Adjustment disorders | 9 (8.8%) | 5 (17.9%) | 14 (10.8%) | .17 |
| Personality disorders | 3 (2.9%) | 2 (7.1%) | 5 (3.8%) | .29 |
| Other disorders | 2 (2.0%) | 1 (3.6%) | 3 (2.3%) | .52 |
| Suicide attempts | 3 (2.9%) | 6 (21.4%) | 9 (6.9%) | .003 |
Patients could have more than 1 substance use or psychiatric disorder. Totals may not equal 100%.
Follow-up
Overall median study follow-up was 307 days (range 32-366 days). The antidepressant group had a median study follow-up of 365 days (range 32-366 days) while the follow-up in the untreated group was 238 days (range 32-366 days) ]. A higher percentage of patients in the antidepressant group continued on HD at 1 year compared to the nontreated patients (64.3% vs 41.2%, P=.03) The proportion of patients undergoing kidney transplantation during follow-up period was lower in the antidepressant group than the untreated group, but results were not statistically significant (14.3% vs 32.4%; P=.06).
Hospitalizations and Emergency Department visits within the first year
Hospitalizations
Hospitalization events are shown in Table 3. While the overall hospitalization rate was 4.73 per 1000 person-days (95% CI 4.00, 5.56), the rate was higher in the antidepressant group compared to the non-treated group (8.17 (95% CI 6.26, 10.47) vs 3.63 (95% CI 2.91, 4.49) per 1000 person-days, respectively, P<.001). Less than half (49%) of the untreated group had a hospitalization during the 1-year follow-up period, while 64.3% of the antidepressant group had at least one hospitalization during this time. Notably, the antidepressant group had a higher proportion of patients with ≥3 hospital admissions (35.7% vs 8.8%, P=.001). Kaplan-Meier plots showed first hospitalization occurring earlier in patients on antidepressants compared to the non-treated group, though this was not statistically significant (median 55 vs 213 days, Figure 1, Log Rank test, P=.09). Andersen-Gill models were used to calculate hazard ratios for multiple hospitalizations per patient. The proportional hazards (PH) assumption was satisfied for the hospitalization outcome. Risk of hospitalization was higher in the antidepressant group (Hazard Ratio (HR) 2.35; 95% CI 1.39, 3.96; P=.001); this finding persisted after controlling for comorbidities (diabetes, coronary artery disease, and heart failure) in a multiple-regression model, (HR 1.94; CI 1.22, 3.1; P=.006). In an Andersen-Gill model with a time-varying covariate for antidepressant use, hospitalization rate adjusted for comorbidities was still higher among patients on antidepressants (HR 2.19, 95% CI 1.39, 3.45, P<.001).
Table 3.
Hospitalizations within the first year of hemodialysis initiation among young adult incident dialysis patients either receiving antidepressants or untreated at time of dialysis initiation
| No depression/Depression untreated (N=102) | Antidepressant (N=28) | Total (N=130) | |
|---|---|---|---|
| Number hospitalizations* | |||
| 0 | 52 (51.0%) | 10 (35.7%) | 62 (47.7%) |
| 1 | 28 (27.5%) | 6 (21.4%) | 34 (26.2%) |
| 2 | 13 (12.7%) | 2 (7.1%) | 15 (11.5%) |
| 3 | 4 (3.9%) | 2 (7.1%) | 6 (4.6%) |
| 4 | 5 (4.9%) | 5 (17.9%) | 10 (7.7%) |
| 5+ | 0 (0.0%) | 3 (10.7%) | 3 (2.3%) |
| Total | 86 | 62 | 148 |
| Time at risk (days) | 23672 | 7589 | 31261 |
| Admissions per 1000 person-days | 3.63 (95% CI 2.91, 4.49) | 8.17 (95% CI 6.26, 10.47) | 4.73 (95% CI 4.00, 5.56) |
| Median days to 1st Hospitalization | 213 | 55 | 171 |
| Mean LOS (days)** | 5.7 (95% CI 4.3, 7.7) | 5.9 (95% CI 3.8, 9.2) | 5.8 (95% CI 4.6, 7.4) |
P=.001is the resultant p-value from a Fisher’s exact test with 1 degree of freedom comparing patients with ≥3 hospital admissions to those with <3 hospital admissions in the antidepressant group and the untreated group.
P=.91is the resultant p-value from a model using generalized estimating equations with the log link function to assess average length of hospital stay between the antidepressant and untreated groups.
CI: 95% Confidence Interval; LOS: Length of hospital stay
Figure 1.
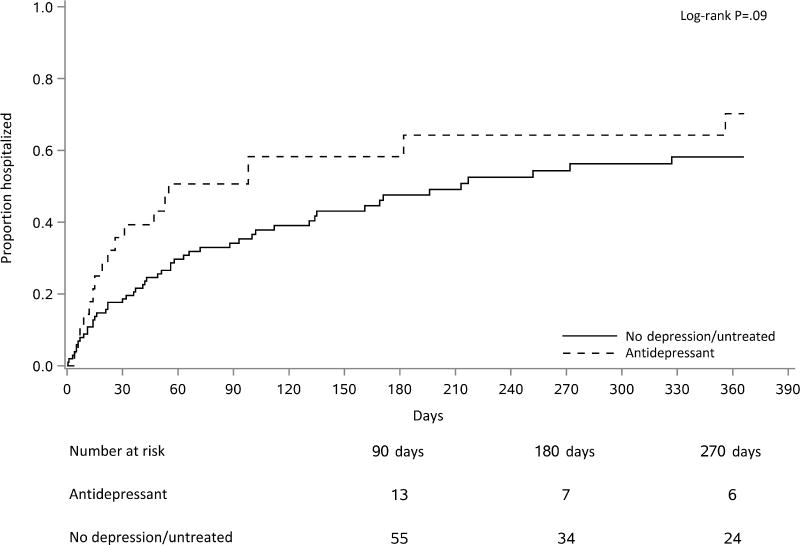
Hospitalization events within the first year among young, adult incident patients, according to antidepressant use at hemodialysis initiation.
The most common causes of hospitalizations were infection (20.3%) and gastrointestinal disorders (11.5%) Figure 2. There was only one hospitalization for a primary psychiatric etiology which occurred in the antidepressant group. The average length of hospital stay was similar between the groups (5.9 (95% CI 3.8, 9.2) in the antidepressant group vs 5.7 (95% CI 4.3, 7.7) in the untreated group, P=.91)
Figure 2.
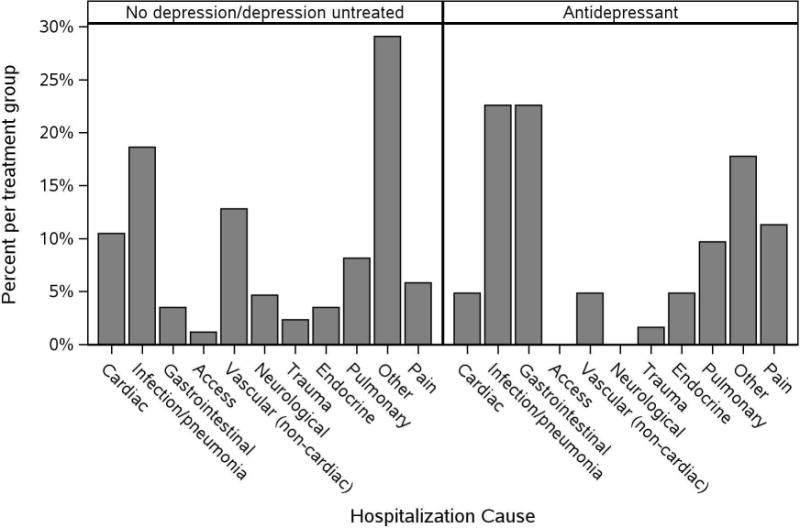
Causes of hospitalizations within the first year by antidepressant use at hemodialysis initiation.
Diabetes effect
Diabetes was prevalent in 64.3% of the antidepressant group and 32.4% of the nontreated group. Among those with diabetes, there were 9.3 (95% CI 6.9, 12.3) hospitalizations per 1000 person-days in the antidepressant group compared to 3.8 (95% CI 2.6, 5.4) per 1000 person-days in the untreated group during the one year of follow-up. Among those without diabetes, there were 5.6 (95% CI 3.0, 9.6) hospitalizations per 1000 person-days in the antidepressant group versus 3.6 (95% CI 2.7, 4.6) in the untreated group. We fit a model to include antidepressant use, diabetes, and the interaction between them to see if the effect of antidepressants differed among those with and without diabetes. The interaction was not statistically significant (P=.35).
Emergency Department visits
ED visits without subsequent hospitalization were common (25.4%), Table 4. However, among patients with at least 1 ED visit (n=33) during the 1-year follow-up period, most (n=22; 66%) had only 1 ED visit. There was no difference in time to first ED visit between antidepressant and untreated groups (Log Rank P=.65). There was no difference in ED visits across depression groups in the first 4 months (HR 0.86 (95% CI 0.34, 2.2, P=.75)) and there was no difference in ED visits across depression groups after 4 months (HR 2.58 (95% CI 0.77, 8.7, P=.12); in addition adjusted models did not show statistically significant differences. Adjusted models yielded similar results.
Table 4.
Emergency Department visits without hospitalization within the first year of hemodialysis initiation among young adult incident dialysis patients either receiving antidepressants or untreated at time of dialysis initiation
| No depression/Depression untreated (N=102) | Antidepressant (N=28) | Total (N=130) | |
|---|---|---|---|
| Number ED visits | |||
| 0 | 78 (76.5%) | 19 (67.9%) | 97 (74.6%) |
| 1 | 17 (16.7%) | 5 (17.9%) | 22 (16.9%) |
| 2 | 5 (4.9%) | 1 (3.6%) | 6 (4.6%) |
| 3 | 0 (0.0%) | 2 (7.1%) | 2 (1.5%) |
| 4 | 1 (1.0%) | 1 (3.6%) | 2 (1.5%) |
| 5+ | 1 (1.0%) | 0 (0.0%) | 1 (0.8%) |
| Total | 37 | 17 | 54 |
| Time at risk (days) | 23672 | 7589 | 31261 |
| Admissions per 1000 person-days | 1.56 (95% CI 1.10, 2.15) | 2.24 (95% CI 1.31, 3.59) | 1.73 (95% CI 1.30, 2.25) |
| Median days to 1st ED visit | – | 198 | – |
CI: 95% Confidence Interval; ED: Emergency department
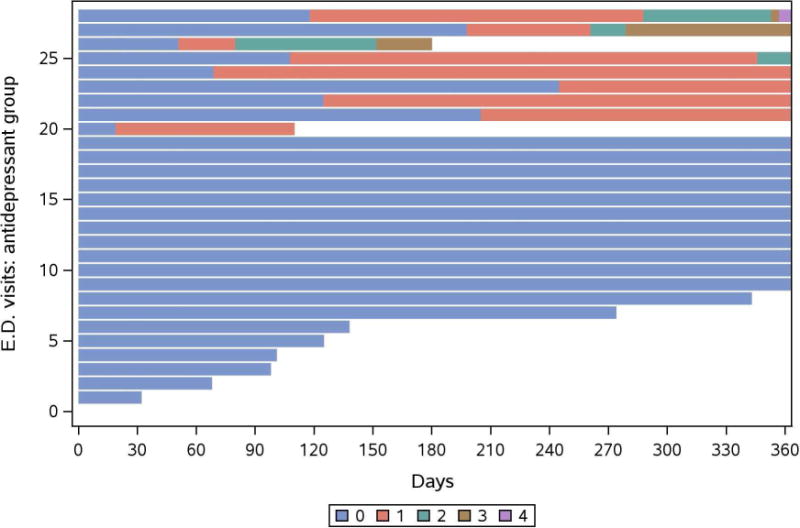
Individualized events in the antidepressant group
We created mosaic plots to graphically illustrate follow-up time combined with individual hospitalizations (Figure 3a) and ED visits (Figure 3b) for each antidepressant patient during the first year. As shown, few patients experienced multiple events, while some were event-free during the 1-year follow-up period. Hospitalization and ED visits included: cardiac, endocrine, gastrointestinal, and infectious etiologies. In a sensitivity analysis, one patient with 13 hospitalizations was excluded from the study cohort, and hospitalizations were reanalyzed with Andersen-Gill models. Results were similar to the main findings and showed an association with antidepressant use and hospitalizations (unadjusted HR 1.98 (CI 1.21, 3.25; P=.007)) even after adjustment for diabetes, coronary artery disease, and heart failure, (HR 1.79 (1.09, 2.92; P=.02)).
Figure 3.
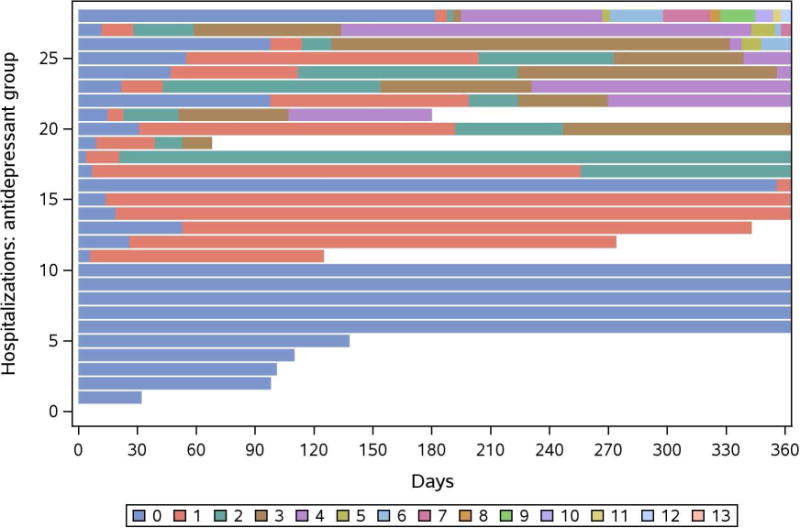
a. Mosaic plot of hospitalizations within the first year after dialysis start in patients receiving antidepressant therapy at hemodialysis initiation. Units represent individual events per patient.
b. Mosaic plot of Emergency Department visits within the first year after dialysis start in patients receiving antidepressant therapy at hemodialysis initiation. Units represent individual events per patient.
“Diagnosis of depression” as a marker for healthcare use
Further sensitivity analyses examined the relationship between healthcare utilization and depression, defined as a diagnosis of depression prior to dialysis start (n=47 (36.2% of total cohort)). Using this definition of exposure, individuals with a depression had a higher risk of hospitalization (HR 1.80; 95% CI 1.10, 2.95; P=.02) within the 1-year follow-up period. However, this association was attenuated after adjustment for diabetes, coronary artery disease, and heart failure (HR 1.51; CI 0.96, 2.39; P=.08). There was no difference observed between ED utilization in those with depression and those without (unadjusted HR 1.96 (CI 0.98, 3.91; P=.06) and adjusted HR 1.50 (CI 0.74, 3.04; P=.30).
DISCUSSION
We observed that antidepressant use for depression is common (21.5%) and a diagnosis of depression, whether treated or not, is highly prevalent (36.2%) in young adult HD patients at dialysis initiation. Moreover, antidepressant use at dialysis start is independently associated with increased healthcare utilization in the first year after dialysis start. Therefore, despite receiving treatment for depressive disorders, young incident HD patients on antidepressant therapy represent a high-risk group and may benefit from interventions to reduce healthcare utilization and costs.
Past studies conducted nearly 10 to 15 years ago focused on depression and hospitalization in the general dialysis population14,15,17,19,24,28. Perhaps the largest studies were conducted by Lopes et al.14,15 using Dialysis Outcomes and Practice Patterns Study (DOPPS) data. In their international study, Lopes et al.14 found an adjusted risk of hospitalization among depressed patients (vs non-depressed) of 1.11 (95% CI 1.01, 1.22, P< .04) which was similar after removing patients on antidepressant therapy (adjusted RR 1.12; 95% CI 1.02, 1.23; P= .024). In a U.S. Veterans Affairs study, Hedayati et al.28 found depression to be associated with an increased number of hospitalizations (adjusted rate ratio, 1.30; 95% CI 1.11, 1.52) and hospital days (adjusted rate ratio, 1.31; 95% CI 1.04, 1.66). Similarly, in our antidepressant-treated patients, the risk of hospitalization adjusted for diabetes, coronary artery disease, and heart failure was nearly 2-fold (HR 1.94; CI 1.22, 3.1; P=.006) suggesting an independent association of depression with healthcare use in the first year after dialysis start.
The prevalence of depression and/or antidepressant use was high in our study, though comparable to previous reports. Watnick et al.29 identified 44% (54 of 123) of incident dialysis patients with positive scores for depressive symptoms meeting the Beck Depression Inventory validated cutoff. Similarly, Palmer et al.13 reported in a systematic review the high prevalence of depressive symptoms (39.3%; 95% CI 36.8-42.0) for dialysis patients; though those with interview-based depression was lower (22.8%; CI 18.6-27.6). While our prevalence (36%) of depression is similar to the general dialysis population, it is concerning to find such high rates of depression and/or antidepressant use in this young dialysis population. Chronic conditions such as diabetes may partly explain the high prevalence of depression in this dialysis population compared to the young, general population30,31. The odds of major depression are higher in adults and pediatric patients with diabetes and concomitant end-organ complications such as kidney failure32–35. Though diabetes was more common in our antidepressant cohort, the difference did not reach statistical significance.
The burden of chronic and/or acute illness in individuals beginning maintenance HD therapy may be overwhelming, particularly in young individuals. Several studies have shown that depression is associated with reduced survival in dialysis patients, while others have not consistently supported this notion. It is unclear whether depression is a marker for or modifier of other aspects of health16,20–24,36. Despite receiving pharmacotherapy, our study patients experienced higher rates of hospitalization than the untreated group. In additional analyses, we examined the association between healthcare use with a diagnosis of depression at baseline, which was not significant after comorbidity adjustment for hospitalizations or ED visits. Therefore, it may be reasonable to use the presence of an antidepressant agent as opposed to a diagnosis of depression as a biomarker for assessing risk of healthcare utilization in young patients initiating HD.
Our study has several limitations. This was not a prospective study and had a relatively small sample size of incident patients over a several-year time-frame during which practice patterns changed. Since young (aged 22-44) patients make up only 11% (n=13,630) of the incident end stage renal disease population in the U.S.1, future studies may require large, multicenter samples of young dialysis patients. Additionally, we did not examine the efficacy of or compliance with antidepressant therapy in the setting of dialysis therapy. Nor, did we examine the impact of psychotherapy or socioeconomic status, both barriers to access to mental health services, or family/community support, which may confound the association between depression, antidepressant use, and healthcare utilization. Each of these factors should be a potential area of focus for future prospective research.
CONCLUSION
In conclusion, a diagnosis of depression and use of antidepressant pharmacotherapy is common in young adult incident HD patients. Antidepressant pharmacotherapy may represent a marker for increased risk of hospitalization during the first year even after controlling for other comorbid medical conditions. However, prospective studies are needed to examine whether modifying depressive symptoms through antidepressant pharmacotherapy, psychotherapy, and other treatment interventions which may involve limiting provider-level obstacles37,38 is of benefit in the reduction of healthcare costs and improvement in quality of life for young patients beginning dialysis.
Acknowledgments
We would like to thank Mrs. Donna K. Lawson for research team overview and coordination of data abstraction.
Funding
L.J.H. was supported by a Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery award; the Extramural Grant Program (EGP) by Satellite Healthcare, a not-for-profit renal care provider; a Mayo Clinic Rochester-Mayo Clinic Health System Integration award; and National Institute of Health (NIH) NIDDK grant K23 DK109134. Additional support was provided by the National Center for Advancing Translational Sciences (NCATS) grant UL1 TR000135. Study’s contents is the sole responsibility of the authors and do not necessarily represent the official views of NIH. Study content was presented in abstract form at the Kidney Week 2016 in Chicago, Illinois.
ABBREVIATIONS
- CI
confidence interval
- ED
emergency department
- HD
hemodialysis
- MCDS
Mayo Clinic Dialysis Services
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
C.L.T. has received grant support from Questcor pharmaceuticals.
The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1–305. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora P, Kausz AT, Obrador GT, et al. Hospital utilization among chronic dialysis patients. J Am Soc Nephrol. 2000;11(4):740–746. doi: 10.1681/ASN.V114740. [DOI] [PubMed] [Google Scholar]
- 3.Quinn MP, Cardwell CR, Rainey A, et al. Patterns of hospitalisation before and following initiation of haemodialysis: a 5 year single centre study. Postgrad Med J. 2011;87(1028):389–393. doi: 10.1136/pgmj.2010.099028. [DOI] [PubMed] [Google Scholar]
- 4.Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S5–11. doi: 10.2215/CJN.05980809. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee MC, D’Angelo L, Harms SR, et al. Enhancing the Role of Internists in the Transition From Pediatric to Adult Health Care. Ann Intern Med. 2016 doi: 10.7326/M16-0514. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 2007;20(4):359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health. 2009;45(3):216–221. doi: 10.1016/j.jadohealth.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller DJ, Duka T, Stimpson CD, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2012;109(41):16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worthy DA, Gorlick MA, Pacheco JL, Schnyer DM, Maddox WT. With age comes wisdom: decision making in younger and older adults. Psychol Sci. 2011;22(11):1375–1380. doi: 10.1177/0956797611420301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Gonzalez de Ferris ME. Adolescents and emerging adults with chronic kidney disease: their unique morbidities and adherence issues. Blood Purif. 2011;31(1–3):203–208. doi: 10.1159/000321854. [DOI] [PubMed] [Google Scholar]
- 12.Fenton N, Ferris M, Ko Z, Javalkar K, Hooper SR. The relationship of health care transition readiness to disease-related characteristics, psychosocial factors, and health care outcomes: preliminary findings in adolescents with chronic kidney disease. J Pediatr Rehabil Med. 2015;8(1):13–22. doi: 10.3233/PRM-150314. [DOI] [PubMed] [Google Scholar]
- 13.Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. doi: 10.1038/ki.2013.77. [DOI] [PubMed] [Google Scholar]
- 14.Lopes AA, Bragg J, Young E, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002;62(1):199–207. doi: 10.1046/j.1523-1755.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 15.Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66(5):2047–2053. doi: 10.1111/j.1523-1755.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 16.Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(4):623–635. doi: 10.1053/j.ajkd.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Abbas Tavallaii S, Ebrahimnia M, Shamspour N, Assari S. Effect of depression on health care utilization in patients with end-stage renal disease treated with hemodialysis. Eur J Intern Med. 2009;20(4):411–414. doi: 10.1016/j.ejim.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Einwohner R, Bernardini J, Fried L, Piraino B. The effect of depressive symptoms on survival in peritoneal dialysis patients. Perit Dial Int. 2004;24(3):256–263. [PubMed] [Google Scholar]
- 19.Hedayati SS, Grambow SC, Szczech LA, Stechuchak KM, Allen AS, Bosworth HB. Physician-diagnosed depression as a correlate of hospitalizations in patients receiving long-term hemodialysis. Am J Kidney Dis. 2005;46(4):642–649. doi: 10.1053/j.ajkd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Kimmel PL, Peterson RA, Weihs KL, et al. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int. 2000;57(5):2093–2098. doi: 10.1046/j.1523-1755.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 21.van Dijk S, van den Beukel TO, Kaptein AA, et al. How baseline, new-onset, and persistent depressive symptoms are associated with cardiovascular and non-cardiovascular mortality in incident patients on chronic dialysis. J Psychosom Res. 2013;74(6):511–517. doi: 10.1016/j.jpsychores.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Lacson E, Jr, Li NC, Guerra-Dean S, Lazarus M, Hakim R, Finkelstein FO. Depressive symptoms associate with high mortality risk and dialysis withdrawal in incident hemodialysis patients. Nephrol Dial Transplant. 2012;27(7):2921–2928. doi: 10.1093/ndt/gfr778. [DOI] [PubMed] [Google Scholar]
- 23.Diefenthaeler EC, Wagner MB, Poli-de-Figueiredo CE, Zimmermann PR, Saitovitch D. Is depression a risk factor for mortality in chronic hemodialysis patients? Rev Bras Psiquiatr. 2008;30(2):99–103. doi: 10.1590/s1516-44462008000200003. [DOI] [PubMed] [Google Scholar]
- 24.Boulware LE, Liu Y, Fink NE, et al. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin J Am Soc Nephrol. 2006;1(3):496–504. doi: 10.2215/CJN.00030505. [DOI] [PubMed] [Google Scholar]
- 25.Schoonover KL, Hickson LJ, Norby SM, et al. Risk factors for hospitalization among older, incident haemodialysis patients. Nephrology (Carlton) 2013;18(11):712–717. doi: 10.1111/nep.12129. [DOI] [PubMed] [Google Scholar]
- 26.Hickson LJ, Chaudhary S, Williams AW, et al. Predictors of outpatient kidney function recovery among patients who initiate hemodialysis in the hospital. Am J Kidney Dis. 2015;65(4):592–602. doi: 10.1053/j.ajkd.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickson LJ, Negrotto SM, Onuigbo M, et al. Echocardiography Criteria for Structural Heart Disease in Patients With End-Stage Renal Disease Initiating Hemodialysis. J Am Coll Cardiol. 2016;67(10):1173–1182. doi: 10.1016/j.jacc.2015.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedayati SS, Jiang W, O’Connor CM, et al. The association between depression and chronic kidney disease and mortality among patients hospitalized with congestive heart failure. Am J Kidney Dis. 2004;44(2):207–215. doi: 10.1053/j.ajkd.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Watnick S, Kirwin P, Mahnensmith R, Concato J. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis. 2003;41(1):105–110. doi: 10.1053/ajkd.2003.50029. [DOI] [PubMed] [Google Scholar]
- 30.Zhao W, Chen Y, Lin M, Sigal RJ. Association between diabetes and depression: Sex and age differences. Public Health. 2006;120(8):696–704. doi: 10.1016/j.puhe.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Olfson M, Blanco C, Marcus SC. Treatment of Adult Depression in the United States. JAMA Intern Med. 2016;176(10):1482–1491. doi: 10.1001/jamainternmed.2016.5057. [DOI] [PubMed] [Google Scholar]
- 32.Egede LE. Effect of comorbid chronic diseases on prevalence and odds of depression in adults with diabetes. Psychosom Med. 2005;67(1):46–51. doi: 10.1097/01.psy.0000149260.82006.fb. [DOI] [PubMed] [Google Scholar]
- 33.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 34.Goldston DB, Kelley AE, Reboussin DM, et al. Suicidal ideation and behavior and noncompliance with the medical regimen among diabetic adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36(11):1528–1536. doi: 10.1016/S0890-8567(09)66561-8. [DOI] [PubMed] [Google Scholar]
- 35.Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology. 2016;70:70–84. doi: 10.1016/j.psyneuen.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Kimmel PL, Peterson RA. Depression in end-stage renal disease patients treated with hemodialysis: tools, correlates, outcomes, and needs. Semin Dial. 2005;18(2):91–97. doi: 10.1111/j.1525-139X.2005.18209.x. [DOI] [PubMed] [Google Scholar]
- 37.Chan R, Dear BF, Titov N, Chow J, Suranyi M. Examining internet-delivered cognitive behaviour therapy for patients with chronic kidney disease on haemodialysis: A feasibility open trial. J Psychosom Res. 2016;89:78–84. doi: 10.1016/j.jpsychores.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Pena-Polanco JE, Mor MK, Tohme FA, Fine MJ, Palevsky PM, Weisbord SD. Acceptance of Antidepressant Treatment by Patients on Hemodialysis and Their Renal Providers. Clin J Am Soc Nephrol. 2017;12(2):298–303. doi: 10.2215/CJN.07720716. [DOI] [PMC free article] [PubMed] [Google Scholar]


