Abstract
We report daptomycin minimum inhibitory concentrations for vancomycin-resistant Enterococcus faecium isolated from bloodstream infections over a four-year period. The daptomycin MIC increased over time hospital-wide for initial isolates and increased over time within patients, culminating in 40% of patients having daptomycin non-susceptible isolates in the final year of the study.
Introduction
Vancomycin-resistant Enterococcus faecium (VRE faecium) is a common cause of hospital-acquired bloodstream infections. VRE faecium are frequently resistant to all beta-lactam antibiotics, aminoglycosides, and tetracycline derivatives, resulting in few reliable antibiotic options for the treatment of these infections. Daptomycin is a cyclic lipopeptide with in vitro bactericidal activity against VRE and is often used as first-line therapy for the treatment of invasive infections due to its favorable tolerability and minimal drug-drug interactions. Alarmingly, several institutions have reported the emergence of daptomycin non-susceptibility among Enterococcus spp., defined as a minimum inhibitory concentration (MIC) >4 µg/ml.1–4 Although the Clinical and Laboratory Standards Institute has defined a MIC ≤ 4µg/ml as susceptible, clinical breakpoints for “intermediate susceptibility” and “resistance” have not yet been established.5 In this study, we describe the emergence of daptomycin non-susceptible Enterococcus faecium (DNSE) amongst clinical isolates at a single institution with a focus on evaluating the relationship between the change in daptomycin MIC that occurs across the population over time and the change that occurs within individual patients.
Methods
Research was approved by the University of Michigan Institutional Review Board. All patients admitted to a single tertiary care hospital with at least one blood culture positive for VRE faecium from January 1, 2011 through December 31, 2014 were evaluated. Daptomycin MICs were determined using the E-test method throughout the study period, which was reported by our microbiology laboratory in units of 2-fold concentration. Per protocol, the daptomycin MIC was determined for the initial bloodstream isolate and for subsequent positive blood cultures taken from a patient if susceptibility testing had not occurred in the previous three days. Daptomycin utilization was quantified as the number of patient days of therapy (DOT) per month across the hospital and was extracted from billing data.
Changes in daptomycin MICs over time within host and hospital-wide were analyzed using linear regression of log-transformed MICs for both initial and subsequent isolates per patient. Descriptive statistics were utilized to evaluate hospital MIC trends according to year of initial isolate and initial isolate MIC.
Results
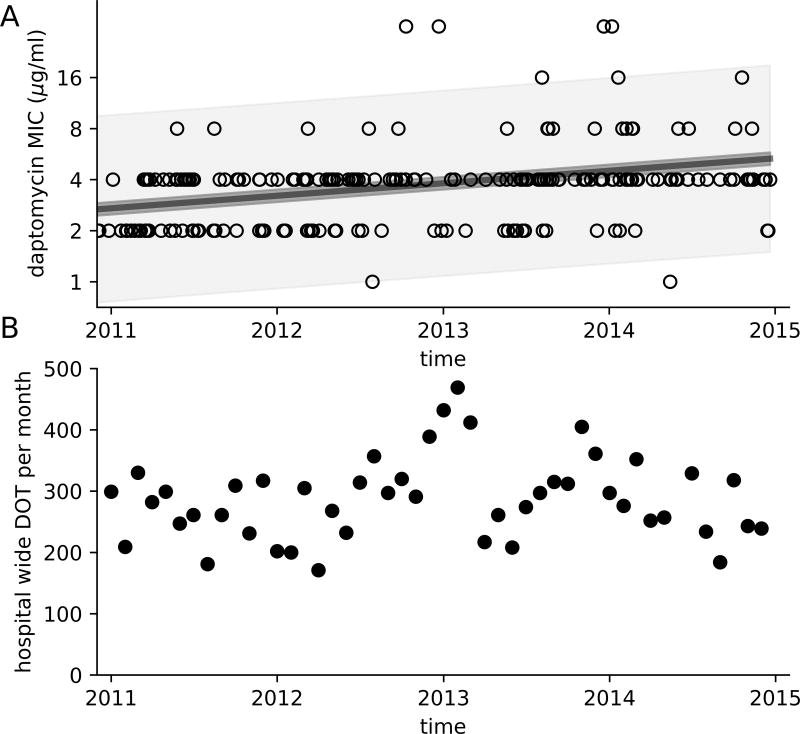
A total of 211 patients were identified with VRE faecium bloodstream infections. Amongst these patients, 371 VRE faecium isolates were identified for which the daptomycin MIC was measured. The daptomycin MIC increased over time for both the initial isolate taken from a patient (p < 0.0001) and for subsequent isolates (p = 0.0002), with a doubling time of 4.1 years (Figure 1A). Additionally, the daptomycin MIC rose significantly within patients over time (p = 0.008), with an average doubling time of 91 days. Total daptomycin utilization across the hospital remained similar through the study period at 228 DOT/month in this approximately 1000 bed hospital (Figure 1B).
Figure 1.
Daptomycin MIC of VRE faecium isoaltes over time as determined by E-test for the first blood stream isolate from 211 patients with VRE faecium bloodstream infection between 2011 and 2014 (A). Simple linear regression was fit to the log-transformed MIC values of first isolate per patient (solid line, p < 0.0001, r2=0.08), the 95% confidence interval for the regression is indicated in dark shade. The 95% prediction inteval (i.e. range in which 95% of the values are expected to fall) is represented in light shade, demonstrating a diversity of MIC measurement, despite a doubling of the average MIC. The number of days of therapy (DOT) of daptomycin per month (B).
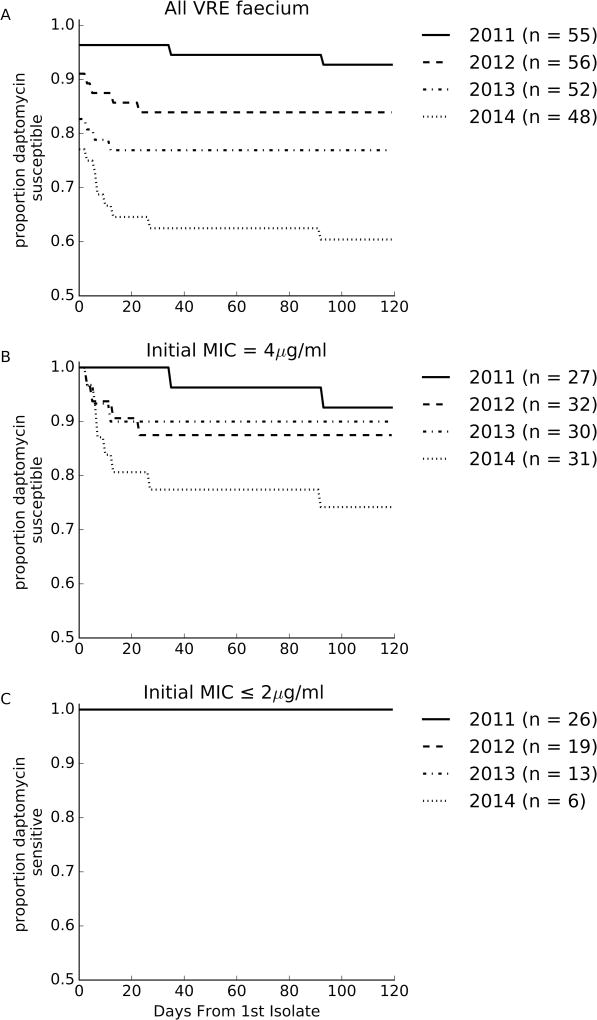
The proportion of patients with a first isolate susceptible to daptomycin (MIC ≤ 4 µg/ml) decreased each year over the study period (Figure 2A, y-intercepts, 96%, 91%, 82% and 77% respectively, p = 0.016). The proportion of patients whose first isolate was susceptible but had at least one subsequent isolate that was daptomycin non-susceptible (MIC >4 µg/ml) increased from 3.7% (2/53) in 2011 to 27.6% (8/37) in 2014 (p=0.029) (Figure 2A).
Figure 2.
Evolution of daptomycin MICs. The proportion of patients that had no daptomycin non-susceptible VRE faecium isolated as a function of time since the first positive blood culture for all patient with VRE faecium blood stream infections (A), just patients with an initial MIC of 4 µg/ml (B) and just patients with an initial MIC less than or equal to 2 µg/ml (C).
The evolution of DNSE in patients with initially susceptible isolates occurred only in patients with an initial isolate MIC of 4 µg/ml (Figure 2B). Of the 64 patients with an initial isolate with MIC ≤2 µg/ml, none were later identified to have a non-susceptible isolate (Figure 2C). However, increasing daptomycin MICs were observed within patients, irrespective of initial isolate MIC (MIC ≤2 µg/ml: 9/64 (14.1%) vs. MIC = 4 µg/ml: 12/120 (10%), p=0.6).
Discussion
The daptomycin MIC of the first isolate per patient and the propensity for DNSE to emerge within patients both increased over the four-year study period. Over this study period, the daptomycin MIC of the first isolate identified per patient nearly doubled, a difference that can largely be explained by a loss of VRE faecium isolates with a daptomycin MIC ≤2 µg/ml and a relative increase in those with an MIC ≥ 4 µg/ml in this population (Figure 1A). This hospital-wide trend is concerning for transmission of strains with elevated daptomycin MICs. Daptomycin was the preferred antimicrobial agent for the treatment of invasive VRE infections at our institution during this time period, resulting in significant exposures amongst patients (Figure 1B). Proper infection prevention practices and antimicrobial stewardship efforts may be critical in preventing widespread daptomycin resistance.
DNSE also commonly emerged within patients, and the propensity to become non-susceptible over time increased during the study period. This trend is likely to be related to the increasing daptomycin MIC of first isolates. More patients were infected with strains just below the clinical breakpoint (MIC 4 µg/ml) later in the study and only patients with an initial MIC of 4 µg/ml were at risk of developing DNSE (Figure 2B,C). Given this risk, caution should be used when treating VRE bloodstream infections with isolates with daptomycin MIC 4 µg/ml.
Combating antibiotic resistance requires identifying the dynamic process through which resistance evolves. This study raise the questions of where selection for daptomycin non-susceptibility is occurring and whether this location is the same for resistance arising in clinical isolates from a single patient and resistant strains potentially transmitted between patients.6 While evolution was observed within individual patients over time, the bloodstream may not be the primary site of selection in transmitted strains. Indeed, the intestinal tract is the more obvious location of selection for transmitted resistance in this organism, since Enterococcus spp. can be a part of normal intestinal microbiota. Furthermore, the evolution of daptomycin non-susceptibility among isolates of VRE causing intestinal colonization has been described.7 Understanding the interaction between drivers of the hospital-wide resistance trend and the resistance evolution that occurs within hosts is important because treatment strategies aimed to slow or prevent the emergence of resistance within patients, such as higher doses or combination therapy, could in principle be at odds with strategies used to slow or prevent the evolution of resistance in the gut, such as preventing intestinal domination of the gut by VRE8 and reducing total exposure to daptomycin.9
Several groups have suggested lowering the daptomycin clinical breakpoint for Enterococcus from 4 µg/ml to 2 µg/ml, because patients who have an initial MIC of 3 µg/ml or 4 µg/ml often have mutations associated with daptomycin non-susceptibility and may have worse clinical outcomes.5,10 In our study only patients whose initial strains had an MIC of 4 µg/ml later developed daptomycin non-susceptibility. However, those with an initial isolate MIC ≤2 µg/ml also experienced increases in MICs of two-fold higher. Thus, lowering the clinical breakpoint would limit the use of daptomycin for the treatment of invasive VRE infections with daptomycin MIC > 2 µg/ml, but it would not be expected to prevent the emergence of DNSE within patients. If the cutoff for daptomycin susceptibility were changed such that 4 µg/ml were defined as intermediate and >4 µg/ml as resistant, only 5/48 blood infections in 2014 would have been fully susceptible throughout their infection, dramatically limiting the use of daptomycin in this hospital. Additional studies that evaluate the clinical impact of daptomycin MICs on treatment outcomes are necessary to determine the optimal clinical breakpoint.
In summary, we report a striking increase in daptomycin MICs amongst VRE isolated from patients with bloodstream infections over a four-year study period. Our data add to the growing body of literature supporting the need for better infection prevention and antimicrobial stewardship practices to prevent the emergence and spread of DNSE.
Acknowledgments
Financial support. R.J.W. received grant support from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (K08AI119182).
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
References
- 1.Kamboj M, Cohen N, Gilhuley K, Babady NE, Seo SK, Sepkowitz KA. Emergence of daptomycin-resistant VRE: experience of a single institution. Infect Control Hosp Epidemiol. 2011;32:391–394. doi: 10.1086/659152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storm JC, Diekema DJ, Kroeger JS, Johnson SJ, Johannsson B. Daptomycin exposure precedes infection and/or colonization with daptomycin non-susceptible enterococcus. Antimicrob Resist Infect Control. 2012;1:19. doi: 10.1186/2047-2994-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang G, Kamalakaran S, Dhand A, et al. Identification of a novel clone, ST736, among Enterococcus faecium clinical isolates and its association with daptomycin nonsusceptibility. Antimicrob Agents Chemother. 2014;58:4848–4854. doi: 10.1128/AAC.02683-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Judge T, Pogue JM, Marchaim D, et al. Epidemiology of vancomycin-resistant enterococci with reduced susceptibility to daptomycin. Infect Control Hosp Epidemiol. 2012;33:1250–1254. doi: 10.1086/668438. [DOI] [PubMed] [Google Scholar]
- 5.Shukla BS, Shelburne S, Reyes K, et al. Influence of Minimum Inhibitory Concentration in Clinical Outcomes of Enterococcus faecium Bacteremia Treated With Daptomycin: Is it Time to Change the Breakpoint? Clin Infect Dis. 2016;62:1514–1520. doi: 10.1093/cid/ciw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipsitch M, Bergstrom CT, Levin BR. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proc Natl Acad Sci U S A. 2000;97:1938–1943. doi: 10.1073/pnas.97.4.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lellek H, Franke GC, Ruckert C, et al. Emergence of daptomycin non-susceptibility in colonizing vancomycin-resistant Enterococcus faecium isolates during daptomycin therapy. Int J Med Microbiol. 2015;305:902–909. doi: 10.1016/j.ijmm.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science. 2016;352:535–538. doi: 10.1126/science.aad9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day T, Read AF. Does High-Dose Antimicrobial Chemotherapy Prevent the Evolution of Resistance? PLoS Comput Biol. 2016;12:e1004689. doi: 10.1371/journal.pcbi.1004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munita JM, Panesso D, Diaz L, et al. Correlation between mutations in liaFSR of Enterococcus faecium and MIC of daptomycin: revisiting daptomycin breakpoints. Antimicrob Agents Chemother. 2012;56:4354–4359. doi: 10.1128/AAC.00509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]