Abstract
Depression is a common problem affecting millions, usually treated with selective serotonin uptake inhibitors. Interest in vitamin D as a co-therapy was stimulated by some association studies that correlated depression with low serum 25OHD levels. There are few longitudinal studies of vitamin D and depression and most are single doses of vitamin D. In this study we examined the effect of one-year treatment with several doses of vitamin D on the Geriatric depression score (GDS) in older Caucasian and African American women. The clinical trial was a study of seven daily oral doses of vitamin D (400-4800IU/d) in Black and White older women. The trial was a double blind, randomized and placebo controlled lasting 12-months. The main inclusion criterion was serum 25 hydroxyvitamin D (25OHD) ≤ 20ng/ml (50nmo/L). Calcium supplements were given to maintain calcium intake 1000 mg in young people and 1200–1400 mg/day in older women. Data on Geriatric depression (GDS) was collected using the validated long form at baseline and 12-months. The change in serum 25OHD was the primary outcome and GDS was one of the secondary outcomes. Adjustments were made for relevant covariates. Analysis of vitamin D effect was by dose low, medium and high compared to placebo or by quintiles. Serum 25OHD increased as a quadratic curve function to a mean of 46 ng/ml (115nmol/l) in white women and 49ng/ml (122.5nmol/L) in black women on the highest dose of 4800 IU. In older women mean GDS scores changed from 3.8 (SD±4.2) at baseline to 3.6 (SD±4.1) at 12 months in whites and from 3.0 (SD±3.7) to 3.02 (SD±4.2) in Blacks. (p = 0.790 in whites; p=0.958 in blacks). After 12-months there was no effect of dose on change in GDS score in women treated with different doses of vitamin D (p=0.507 in whites and p=0.340 in blacks). When both Caucasians and African Americans were divided into 3 dose groups, low (400-800 IU), medium (1600-3200 IU) and high (4000-4800 IU) doses, the change in score was 0.8 on low dose, −0.30 on medium dose and −0.31 on high dose compared to 0.11 on placebo (p=0.546). In summary, there was no improvement in GDS scores in Caucasians or African Americans on either increasing doses of vitamin D or quintiles of achieved response in serum 25OHD. The changes were small and not significant perhaps because of the relatively lower numbers of depressed women in the groups. Further studies should recruit larger numbers, 3 dose groups covering a serum25OHD range of 20–60ng/ml and more subjects with clinical depression in order to fully address the question of vitamin D effects on depression.
1 INTRODUCTION
The discovery of the vitamin D receptor (VDR) in various human tissues led to increasing interest in exploring the extra-skeletal benefits of vitamin D. Vitamin D treatment has been shown to have an effect on mental health (1) and the relationship between vitamin D and mental health from association studies has raised awareness of possible benefits in the effect of vitamin D treatment. Epidemiological studies have shown that Vitamin D deficiency is associated with an 8% 14% increase in depression (2–6). A recent study from Holland showed decreasing serum 25OHD levels were associated with lower GDS scores particularly in subjects with serum 25OHD, < 10 ng/ml (7).
The occurrence of depression could be explained by the fact that VDR is expressed in the many regions of brain (8) and vitamin D can cross blood brain barrier and regulate gene expression of serotonin (9). The pathophysiology of depression includes an alteration in the neurotransmitters of serotonergic system although other neurotransmitters are also involved (10). Few longitudinal trials (11, 12, and 13) have tried to explore the supplementation of vitamin D in depressive subjects but the results are equivocal and Institute of Medicine (IOM) has concluded that evidence is inconclusive. On the other hand, depression by itself can cause low serum 25OHD levels as these people have less sun exposure due to low physical activity and poor dietary habits. In the Women’s Health Initiative study (11), although low levels of serum 25OHD were associated with depressive symptoms, treatment with vitamin D did not improve depression, however the dose was only 400 IU daily. In a study of obese subjects, symptoms of depression improved with vitamin D treatment (13). Thus, at this time a definitive cause-effect relationship has not been established.
In a vitamin D dose ranging trial we evaluated the effect of vitamin D on serum 25OHD levels. One of the secondary outcomes was the Geriatric Depression Scores (GDS) and in this analysis we were able to analyze the longitudinal effects of various doses of vitamin D3 plus total calcium intake on depression scores in older Caucasian and African-American women.
2 METHODS
2.1 Study design and subject population: VIDOS Study
This clinical trial was a one-year randomized, double-blind, multi-dose, placebo controlled study (ViDOS-Vitamin D supplementation in Older Subjects) to determine the effects of increasing doses of vitamin D3 in older women. The primary outcomes were serum 25OHD and serum PTH. A secondary outcome was measuring geriatric depression scores.
163 Caucasian women and 110 African-American elderly women at least seven years post menopause, ranging from age 57–90 years were recruited from general population with the help of advertising in local newspapers, churches and direct mailings. The main inclusion criterion was vitamin D insufficiency defined as serum 25OHD ≤ 20 ng/ml (50 nmol/L). Participants were enrolled in winter and spring over 2 years, April – May 2007 and January –May 2008. The essential details of the trial are summarized in the following paragraphs but detailed methodology is given in the primary outcome paper (14, 15).
2.2 Randomization and Treatment
In VIDOS study elderly Caucasian women were randomly assigned to one of 8 groups - 400, 800, 1600, 2400, 3200, 4000, 4800 IU of vitamin D3 per day or matching placebo (14). Calcium tablets contained 200mg elemental calcium (Citracal) were taken twice daily to maintain a total calcium intake between 1000–1400 mg/d based on a baseline 7-day food diary. The statistician provided the randomization schedule using computer-generated codes with SAS software (SAS Institute Inc., Cary, NC). Patients, providers, researchers, and persons who assessed outcomes were blinded to treatment assignment. African American women were randomly assigned to one of 8 groups -, 400, 800, 1600, 2400, 3200, 4000 or 4800 IU of vitamin D3 per day or matching placebo (15). However, African Americans were stratified for potential effects of weight on baseline serum 25OHD. The randomization method was randomized blocks, stratified by screening serum 25OHD level less than 15 vs 15 ng/mL or greater (37.4 vs 37.4 nmol/L)(15).
Adherence was measured at 3, 6, 9 and 12 months by counting the number of vitamin D and calcium pills returned. New bottles of vitamin D and calcium were re dispensed every 3 months. Every 3 months, information was collected on new medications and supplements. Subjects were not allowed to take other vitamin D supplements while on study, those who wanted to take vitamin supplements were provided free with multivitamins without vitamin D (Kirkman multivitamin w/o vitamins A&D,) The Institutional Review Board at Creighton University approved the study protocol and all subjects were enrolled after signing an informed consent.
2.3 Food Diary
Dietary intake of calcium and vitamin D was calculated by a dietician from 7-day food diaries using the Food processor II Plus nutrition and diet analysis system (version 5.1, ESHA Research, Salem, OR). These were done at baseline and at the end of the study. Plastic food models (NASCO, Fort Atkinson, WI) were used to help participants’ better estimate the quantities consumed.
2.4 Biochemical measurements
Serum 25OHD and serum PTH levels were measured at baseline, 6 and 12 months. Serum 25OHD and 1,25(OH)2D was measured by radioimmunoassay (RIA) in the Bone Metabolism laboratory using kits manufactured by Diasorin, Inc. (Stillwater, MN). The National Institute of Standards and Technology standards (NIST) were also used in the assays. The interassay variations for NIST standards were as follows: level 1; 8.4%; level 2; 9%; level 3; 7.7%; and level 4; 10.5%.
2.5 Geriatric Depression Scale (GDS) score
The Long Form 30(GDS-LF30) was used to collect data on depressive symptoms, consisting of 30 depression related self-rating questions with simple ‘yes’ or ‘no’ options and a score ranging from 0 to 30. This scale was exclusively developed for screening and rating severity of depression in elderly and has shown very good criterion validity. When a cutoff score of 11 is used, it has a sensitivity of 84% and specificity is 95% for detecting depression (16). We used the same cutoff value to differentiate between non-depressed and depressed (0–10=Not depressed, 11–30=Depressed). GDS scores were collected at baseline and at the end of the study. At baseline we also collected information about other associated variables such as level of education, previous history of depression, physical activity, history of hypothyroidism, living status, smoking habits and alcohol consumption.
2.6 Statistical Methods and Analyses
All analyses were performed using SPSS Version 21 (IBM SPSS Inc). Baseline characteristics were compared among the vitamin D dose groups by means of one-way ANOVA for continuous variables and by chi-square test for categorical variables. Since the data was skewed and there was some within-group variability we conducted ANOVA analysis without assuming equal variance. We used Pearson’s correlation to find a relationship between baseline 25OHD levels with depression scores. We also compared GDS scores based on severe vitamin D deficiency with a serum 25OHD cutoff point of 10 ng/mL. We did not divide our study population into depressed and non-depressed because our group had less number of depressed people. We used continuous scale of GDS scores to find an effect of vitamin D on risk of depression score. For the longitudinal analyses we used multiple regression analysis to determine the effect of vitamin D dose and serum 25OHD on depression scores adjusting for BMI, antidepressant use, smoking status, alcohol consumption, caffeine use, thyroid disease, educational status, living status and physical activity. We also explored the relationship between improvement in GDS score (Baseline GDS minus 12 month GDS) and serum 25OHD and vitamin D dose. The significance level was set to 0.05 for all analyses in our study. We performed the per protocol analyses on 147 Caucasian women in VIDOS and 91 African American women who came in for final visit in VIDOS study.
3 RESULTS
A total of 163 white women and 110 black women (79 from Indiana and 31 from Omaha) were enrolled in this study. All the participants completed the GDS questionnaire at baseline (n=163). At the end of the study 147 white and 91 black participants (~85%) completed the GDS questionnaire and a total of 35 women dropped out of study. There were no significant differences in the baseline characteristics among the races (summarized in Table 1).
Table 1.
Baseline characteristics comparison between Caucasians and African Americans
| Caucasians N=163 Mean (SD) |
African Americans N=110 Mean (SD) |
|
|---|---|---|
| Age, years | 66.3 (7.3) | 66.6 (7.5) |
| BMI, kg/m2 | 30.2 (5.7) | 33.7 (7.0) |
| Serum Calcium (mg/dl) | 9.5 (0.3) | 9.4 (0.4) |
| Serum 25OHD, screening (ng/dl) | 15.3 (3.9) | 13.2(4.3) |
| Serum 1,25(OH)2D (pg/ml) | 43.2 (14.1) | 42.4 (12.8) |
| Geriatric Depression Scale Score | 3.8(4.2) | 3.0(3.7) |
3.1 Caucasians
The mean age was 66.3(SD±7.3). Twenty (12%) women were taking antidepressants for various reasons at baseline but 10 (6%) women had depression based on baseline GDS scores. There were 31 (19%) women who had a diagnosis of hypothyroidism and 54 (33%) were living alone. There were 5 (3%) participants who were minimally educated. Compliance was 94% for vitamin D and 91% for calcium supplementation. Mean GDS score at baseline was 3.8 (SD±4.2) and at 12-months was 3.6 (SD±4.1). Mean baseline serum 25(OH) D was 15.6 ng/ml (SD±3.7) and overall 12 months’ mean was 36 (SD±12) ng/ml. There were very low numbers of depressed people in our study. There were 10 women with depression at baseline and 12 women at the end of the study based on GDS scores.
3.2 African-Americans
The mean age was 66.6 (SD±7.5). Twelve (9%) women were taking antidepressants for various reasons at baseline but 6 (5%) women had depression based on baseline GDS scores. There were 15 (14%) women who had a diagnosis of hypothyroidism and 59 (55%) were living alone. There were 15 (14%) participants who were minimally educated. Mean compliance for calcium in Omaha was 79% and for Indiana was 70%. Mean compliance for vitamin D in Omaha was 91% and for Indiana was 81%. Mean GDS score at baseline was 3.0 (SD±3.7) and at 12-months was 3.02 (SD±4.2). Mean baseline serum 25(OH) D was 13.2 ng/ml (SD±4.3) and overall 12 months’ mean was 35 (SD±12) ng/ml. There were very low numbers of depressed people in our study. There were 6 women with depression at baseline and 8 women at the end of the study.
3.3 Baseline analysis of serum 25OHD and depression scores
The serum 25OHD levels at baseline did not correlate significantly with GDS scores at baseline (X2= −0.065, p=0.408 in whites and X2= −0.054, p=0.538 in blacks). To evaluate the differences in GDS scores we also compared GDS scores based on a whether vitamin deficient or not (serum 25OHD level of 10 ng/ml). There was no difference in depression scores between subjects who had serum 25OHD level less than 10 ng/ml compared to those who had greater than 10 ng/ml at baseline (X2= 0.764, p=0.095 in whites) and (X2= 0.01p=0.966 in blacks).
3.4 Longitudinal analysis: Vitamin D dose and depression scores
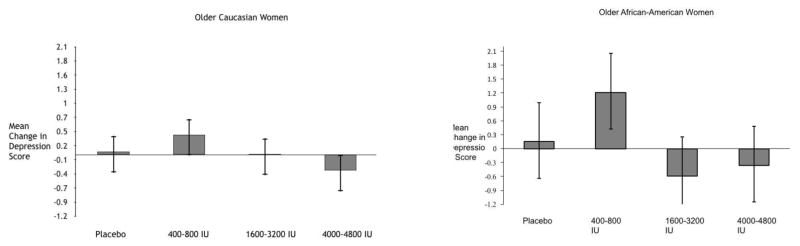
In the longitudinal analysis (n=147 in whites and n=91 in blacks), after 12-months there was no effect of dose on change in GDS score in women treated with different doses of vitamin D (p=0.507 in whites and p=0.340 in blacks). Only the baseline GDS scores were significantly related to 12-month GDS scores (p<0.0001 in both groups). There was no effect of BMI, hypothyroidism, antidepressant use and educational status on GDS (p=0.507 in whites and p=0.340 in blacks). When divided into 3 dose groups, low dose (400-800 IU), medium dose (1600-3200 IU) and high dose (4000-4800 IU) there was no improvement in GDS score in white and blacks (fig 1 and 2). We combined both white and black data and the mean change in GDS score was 0.8 on low dose, −0.30 on medium dose and –0.31 on high dose compared to 0.11 on placebo. (p=0.546).
Fig 1 and 2.
Comparison of mean change (95% CI) in depression scores between placebo, low, medium and high of vitamin D3 in Caucasian and African-American women.
3.5 Serum 25OHD level and depression scores
There was no relationship between 12-month serum 25OHD level and 12-month depression scores (p=0.689) in whites and p=0.127 in blacks). After evaluating whether improvement in the GDS score was related to serum 25(OH) D levels or vitamin D dose, we found no effect of either vitamin D dose or serum 25OHD on the difference in GDS scores. There was no improvement in GDS score with quintiles of 25OHD in both whites (p=0.256) and blacks (p=0.590) (fig 3 and 4).
Fig 3 and 4.
Comparison of mean change (95% CI) in depression scores between quintiles of serum 25OHD in Caucasian and African-American women.
4 DISCUSSION
Our study did not show any significant relation between the risk of depression in older white and black women nor a significant effect with increasing doses of vitamin D3 supplementation. There was no correlation between final serum 25OH vitamin D levels on risk of depression. There was no association between serum 25OHD levels and depression scores at baseline. There were a number of cross-sectional studies on vitamin D status and depression but the results are conflicting due to differences in methodology (2–6).
In the NHANES survey, Zhao et al (17) found no significant association between serum 25OHD levels and depression after adjusting for multiple known confounders. Similarly in another two studies of 3400 older people no conclusive evidence was shown between serum 25OHD and depressive symptoms after adjusting for multiple confounders (18, 19). Though the above described studies have found a decrease in depressive symptoms when comparing quartiles of serum 25OHD levels, they did not reach a significant level. In contrast, a study of 1200 older adults (20) reported a positive association between low 25OHD levels and depression severity after adjusting for known confounders, in this study only 2% of people were diagnosed with major depressive disorder and the mean 25OHD difference between normal and depressed people was small (22ng/ml vs 19 ng/ml). Similar association was seen in a 2 year case control study (21) involving subjects of age group 18–65 years concluded that low levels of 25(OH) D were associated with the presence and severity of depressive disorder.
There are few longitudinal studies that studied depression and vitamin D levels. The WHI study (WHI) of calcium and 400 IU of vitamin D supplementation is one of the largest studies that evaluated the role of supplementation of vitamin D in improvement of depressive symptoms. The study concluded that there was no relation between supplementation with 400 IU/day vitamin D along with calcium and depression in older women, and recommended evaluation of higher doses. In another randomized placebo controlled trial (22) involving 2317 Australian elderly women, an annual high dose of vitamin D3 (500000 IU) for three years failed to prevent depressive symptoms. Similar negative findings were reported in two other studies (23, 24) that evaluated the vitamin D supplementation in improving the mood during the winter months. Recently, we evaluated the role of calcitriol (biological active form of serum 25 OHD) supplementation for 3 years in improvement of depressive symptoms in 125 postmenopausal women which resulted in similar negative findings (25). In contrary, in a one-year study (13) with 441 overweight and obese men and women, it was shown that participants with serum 25(OH) D levels more than 40 nmol/liter had lower depression scores on the BDI scale but this study was unadjusted for the common confounders and mean depression scores were lower in the subjects suggesting that few participants were clinically depressed. Two meta-analyses (26, 27) that evaluated the potential benefit of vitamin D supplementation in management of depression has left inconclusive evidence and both studies recommended more randomized trials in depressive patients. In another study (28) 109 outpatients with depression and serum 25OHD <16ng/ml were given single dose of 150,000 or 300,000 IU vitamin D3 and placebo and after 3 months depressive symptoms were found to be improved in 300,000 IU group compared to placebo but this needs to be validated in a larger group with conventional doses of vitamin D3.
The direct cause and effect relationship between vitamin D efficiency and depression has not been established. Depression has complex etiology. The relationship with serum 25OHD may be influenced by indirect factors such as age, sex, weight, physical activity, diet and genetic influences. Depressed people tend to have poor dietary habits, reduced sun exposure, less physical activity that makes them vitamin D insufficient or deficient. With the expression of VDR in critical parts of brain that control the gene expression of serotonergic system there is a possibility that very low serum 25OHD levels may play a role in modulating this pathway and leading to depression. In NHANES study (17) it was found that a serum 25OHD level <10 ng/ml was associated with depressive symptoms. It is possible to see depressive symptoms at a level below 10 ng/ml that may cause substrate deficiency of synthesis of 1, 25 (OH)2D because in animal studies it was shown that 1,25(OH)2D through VDR in the brain influence the synthesis and metabolism of neurotransmitters like dopamine and norepinephrine that are involved in the pathophysiology depression (9). It has also been shown that vitamin D regulates gene expression of tyrosine hydroxylase, an enzyme that is involved in the synthesis of norepinephrine and dopamine (10). There is also another hypothesis on genetic basis. The genome-wide association study (GWA study, or GWAS) data of major depressive disorders identified a gene called ADM (adrenomedullin) gene on chromosome 11p15 has been implicated in psychopathology of many psychiatric disorders including depression. Located on the same chromosome is the gene that influences the vitamin D production (CYP2R1). So the variants near this gene may share the same impact i.e. vitamin D insufficiency and mental illness but this may not be generalized to all the population (29).
The strengths of our studies are wider age range of women population, very good compliance to study medications, excellent validity of GDS LF 30 scale which is a powerful screening tool covering wide range of depressive symptoms, baseline adjustment to the known potential confounders. The unique strength of this study was the use of different doses of vitamin D3. However, all the data collected on depression were self-reported instead of clinical diagnosis. There are some limitations in our study. The study was not designed for depression and depression scores were only secondary outcome. The GDS scores in our study are low, so they cannot be expected to improve after vitamin D treatment. We had no detailed data on socio-economic status that is one of the known confounders.
5 CONCLUSION
In summary, different doses of vitamin D3 ranging from 400 IU/d through 4800 IU/d given for 1 year did not influence the depression score in vitamin D insufficient older white and black women. The persons with depressive symptoms may be less active outdoors and have poor nutritional status, which might be a reason for observing a low serum 25OHD level in observational studies. None of the longitudinal data support this relationship. Therefore, initiating studies of patients with both clinical depression and vitamin D deficiency (serum 25OHD<10 ng/ml) are needed to find an interaction of vitamin D supplementation with the treatment of depression.
Highlights.
There was no effect of vitamin D supplementation for 1 year on geriatric depression scores in older Caucasian and African American women.
There was no correlation between serum 25OHD level and depression scores.
There was no difference in mean change in depression scores between Caucasians and African Americans after treatment with Vitamin D.
Acknowledgments
Financial Support – This study was supported by National Institute on Aging (RO1-AG28168)
Footnotes
Disclosures: VY has nothing to disclose. JCG has received consulting fees and honoraria from Wyeth-Pfizer and Roche. He has also served on the Advisory Council and as a speaker for Pfizer and Roche.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 2.Ganji V, Milone C, Cody M, McCarty F, Wang YT. Serum Vitamin D concentrations are related to depression in young adult US population: The Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29. doi: 10.1186/1755-7682-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May HT, Bair TL, Lappé DL, Anderson JL, Horne BD, Carlquist JF, Muhlestein JB. Association of Vitamin D levels with incident depression among a general cardiovascular population. Am Heart J. 2010;159:1037–1043. doi: 10.1016/j.ahj.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 4.MT, DeFina LF, Willis BL. Association between low serum 25-hydroxyVitamin D and depression in a large sample of healthy adults: The Cooper Center Longitudinal Study. Mayo Clin Proc. 2011;86:1050–1055. doi: 10.4065/mcp.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjærgaard M, Joakimsen R, Jorde R. Low serum 25-hydroxyVitamin D levels are associated with depression in an adult Norwegian population. Psychiatry Res. 2011;190:221–225. doi: 10.1016/j.psychres.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Stewart R, Hirani V. Relationship Between Vitamin D Levels and Depressive Symptoms in Older Residents from a National Survey Population. Psychosomatic Medicine. 2010;72:608–612. doi: 10.1097/PSY.0b013e3181e9bf15. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer-Brolsma EM1, Dhonukshe-Rutten RA2, van Wijngaarden JP2, van der Zwaluw NL2, Sohl E3, In’t Veld PH2, van Dijk SC4, Swart KM3, Enneman AW4, Ham AC4, van Schoor NM3, van der Velde N45, Uitterlinden AG4, Lips P6, Feskens EJ2, de Groot LC2. Low vitamin D status is associated with more depressive symptoms in Dutch older adults. Eur J Nutr. 2016 Jun;55(4):1525–34. doi: 10.1007/s00394-015-0970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D and brain health: the need for vitamin D supplementation and sensible sun exposure. J Intern Med. 2014 Sep 16; doi: 10.1111/joim.12308. [DOI] [PubMed] [Google Scholar]
- 9.Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:261–271. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean AJ, Bellgrove MA, Hall T, et al. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults—a randomised controlled trial. PLoS One. 2011;6(11):e259. doi: 10.1371/journal.pone.0025966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elizabeth R, Bertone-Johnson, Sally I. Powers, Leslie Spangler Vitamin D Supplementation and Depression in the Women’s Health Initiative Calcium and Vitamin D Trial. Am J Epidemiol. 2012 Jul 1;176(1):1–13. doi: 10.1093/aje/kwr482. Published online May 9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008 Dec;264(6):599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012 Mar 20;156(6):425–37. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher JC, Peacock M, Yalamanchili V, Smith LM. Effects of Vitamin D Supplementation in Older African American Women. J Clin Endocrinol Metab. 2013 Mar;98(3):1137–1146. doi: 10.1210/jc.2012-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yesavage Jerome A, Brink TL. Development and validation of a geriatric depression screening scale: A preliminary report. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Ford ES, Li C, Balluz LS. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. Br J Nutr. 2010;20:1–7. doi: 10.1017/S0007114510002588. [DOI] [PubMed] [Google Scholar]
- 18.Pan A, Lu L, Franco OH, Yu Z, Li H, Lin X. Association between depressive symptoms and 25-hydroxyvitamin D in middle aged and elderly Chinese. J Affect Disord. 2009;118:240–243. doi: 10.1016/j.jad.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Brouwer-Brolsma EM, Feskens EJ, Steegenga WT, de Groot LC. Associations of 25-hydroxyvitamin D with fasting glucose, fasting insulin, dementia and depression in European elderly: the SENECA study. Eur J Nutr. 2013 Apr;52(3):917–25. doi: 10.1007/s00394-012-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoogendijk WJG, Lips P, Kik MG, Deeg DJH, Beekman ATF, Penninx BWJH. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65:508–12. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- 21.Milaneschi Y, Hoogendijk W, Lips P, et al. The association between low vitamin D and depressive disorders. Mol Psychiatry. 2014 Apr;19(4):444–51. doi: 10.1038/mp.2013.36. [DOI] [PubMed] [Google Scholar]
- 22.Sanders KM, Stuart AL, Williamson EJ, et al. Annual highdose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry. 2011;198(5):357–364. doi: 10.1192/bjp.bp.110.087544. [DOI] [PubMed] [Google Scholar]
- 23.Dumville JC, Miles JN, Porthouse J, Cockayne S, Saxon L, King C. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10:151–153. [PubMed] [Google Scholar]
- 24.Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatr Res. 1993;49:77–87. 50. doi: 10.1016/0165-1781(93)90031-b. [DOI] [PubMed] [Google Scholar]
- 25.Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012;19:697–703. doi: 10.1097/gme.0b013e31823bcec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Mbuagbaw L, Samaan Z. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014 Mar;99(3):757–67. doi: 10.1210/jc.2013-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014 Apr 11;6(4):1501–18. doi: 10.3390/nu6041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozaffari-Khosravi H1, Nabizade L, Yassini-Ardakani SM, Hadinedoushan H, Barzegar K. The effect of 2 different single injections of high dose of vitamin D on improving the depression in depressed patients with vitamin D deficiency: a randomized clinical trial. J Clin Psychopharmacol. 2013 Jun;33(3):378–85. doi: 10.1097/JCP.0b013e31828f619a. [DOI] [PubMed] [Google Scholar]
- 29.Savas HA, Herken H, Yurekli M, Uz E, Tutkun H, Zoroglu SS, Ozen ME, Cengiz B, Akyol O. Possible role of nitric oxide and adrenomedullin in bipolar affective disorder. Neuropsychobiology. 2002;45(2):57–61. doi: 10.1159/000048677. [DOI] [PubMed] [Google Scholar]