Abstract
Myeloid-derived suppressor cells (MDSCs) are a population of myeloid cells generated during a large array of pathologic conditions ranging from cancer to obesity. These cells represent a pathologic state of activation of monocytes and relatively immature neutrophils. MDSCs are characterized by a distinct set of genomic and biochemical features, and can, with recent findings, be distinguished by specific surface molecules. The salient feature of these cells is their ability to inhibit T cell function and thus contribute to the pathogenesis of various diseases. In this review, we discuss the origin and nature of these cells, their distinctive features and biological roles in cancer, infectious diseases, autoimmunity, obesity and pregnancy.
The name myeloid-derived suppressor cells (MDSCs) was introduced to scientific literature 10 years ago1 describing initially a loosely defined group of myeloid cells with potent immune regulatory activity. In recent years, the nature and biological role of MDSC became clearer and MDSC emerged as a universal regulator of immune function in many pathologic conditions. MDSCs consist of two large groups of cells: granulocytic or polymorphonuclear (PMN-MDSC) and monocytic (M-MDSC). PMN-MDSC are phenotypically and morphologically similar to neutrophils, whereas M-MDSC are more similar to monocytes 2. Studies in humans demonstrated the existence of a third small population of MDSCs that are represented by cells with colony forming activity and other myeloid precursors. These cells are currently termed early-stage MDSC (eMDSC) 3 and have yet to be defined in mice.
Intensive clinical studies identified MDSC as a valuable predictive marker in cancer and extensive efforts in MDSC targeting is ongoing. However, despite such advances, the nature of MDSCs still raises questions and skepticism. This review is not a comprehensive analysis of MDSC phenotype or function (these topics were addressed in many reviews in recent years4, 5), but is our attempt to address the most controversial issues pertinent to these cells. We discuss new information regarding the development, activation status, phenotype and function that allow for a better discrimination of MDSCs from other myeloid cells. We also discuss their role in regulation of different pathologic conditions.
What are these cells?
The main controversial issue associated with MDSCs since initial discovery is their nature. Morphologically and phenotypically MDSCs are similar to neutrophils and monocytes. What is so special about these cells that would justify a separate name? What makes these cells different? Below, we will present our view on why MDSC are indeed a very special group of cells with unique features and biological roles.
The major populations of bone marrow (BM)-derived myeloid cells include granulocytes (with their most abundant representative – neutrophils) and mononuclear cells: monocytes and terminally differentiated macrophages (MΦ) and dendritic cells (DC). In contrast to experiments in vitro, where both MΦ and DCs can be easily differentiated from monocytes, in tissues under steady state conditions, MΦ expand largely in situ and most DCs differentiate from their specific BM precursors6. However, during inflammation and cancer, BM-derived monocytes are the primary precursors of MΦ, especially tumor associated macrophages (TAM) and a population of inflammatory DCs7.
Myeloid cells have emerged in evolution as one of the major protective mechanisms against pathogens and are an important element of tissue remodeling. Under physiological conditions, GM-CSF drives myelopoiesis and G-CSF and M-CSF induce the differentiation of granulocytes and macrophages, respectively8. In cancer and in other pathological conditions, these factors are overproduced and favor the generation of MDSC2, 9. Thus, accumulation of MDSC takes place alongside the same differentiation pathways as neutrophils and monocytes.
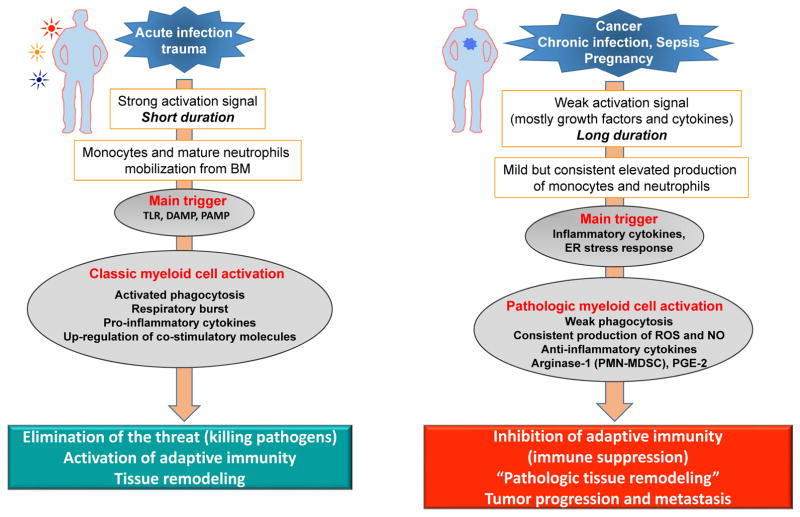
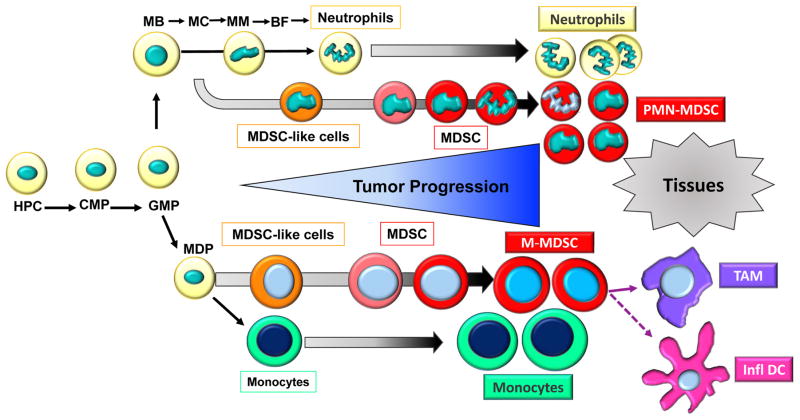
Classical activation of myeloid cells takes place in response to relatively strong signals coming from pathogens primarily in form of toll-like receptor (TLR) ligands, various damage associated molecular patterns (DAMP) and pathogen-associated molecular pattern (PAMP) molecules10. This results in rapid mobilization of monocytes and neutrophils from the BM, a dramatic increase in phagocytosis, respiratory burst, production of pro-inflammatory cytokines, as well as up-regulation of major histocompatibility complex (MHC) class II and co-stimulatory molecules11, 12. This response is usually of short duration and ends in elimination of the threat. During unresolved inflammation such as persistent infection, cancer, and other chronic conditions, the nature of signals activating myeloid cells differs13, 14. These signals are relatively weak and of a long duration, often in the form of growth factors and inflammatory mediators as described in detail below. Neutrophils and monocytes generated under these conditions display an immature phenotype and morphology, relatively weak phagocytic activity, increased background levels of reactive oxygen species (ROS) and nitric oxide (NO) production, high expression of arginase, PGE2, and a number of anti-inflammatory cytokines15, 16. Most of these features are absent in classically activated neutrophils and monocytes. Therefore, this state of activation can be characterized as pathologic (Fig. 1). This state of activation leads not to the elimination of the threat or activation of immunity, but to the inhibition of adaptive immunity (immune suppression) and support of tumor progression and metastases. Cells in this pathologic state of activation can be identified functionally, biochemically, and to some extent, phenotypically, and are now termed MDSC. The longer the myeloid compartment is exposed to the effects of factors described above, the more potent the pathologic activation of these MDSCs detected in patients and mice. Therefore, at any given moment, there is a heterogeneous population of cells in tissues represented by classically activated neutrophils, monocytes, and pathologically activated MDSCs (Fig. 2). For instance, at early stages of cancer, bona-fide immune suppressive MDSCs are rarely detected. However, there are cells with some biochemical and genomic characteristics of MDSC 17, 18, 19 probably representing an intrinsic part of MDSC development. They could be called MDSC-like cells (MDSC-LC) (Fig. 2). It could be possible that pathologic activation of MDSC can be transferred via hematopoietic progenitor cells, as a sort of innate immune memory triggered by chronic inflammatory conditions through signals interfering with transcription factors and epigenetic reprogramming20. Memory or trained immunity can rely on an altered functional state of immune cells that persists for weeks to months after the elimination of the initial stimulus. The true nature of this process in MDSC needs to be elucidated.
Figure 1.
Pathologic activation of neutrophils and monocytes. (a) In the presence of strong activation signals coming from pathogens in the form of toll-like receptors ligands (TLRL), damage associated molecular pattern (DAMP), pathogen-associated molecular patterns (PAMP) molecules monocytes and neutrophils are mobilized from the BM. This response results in classic myeloid cell activation. (b) In the presence of weak activation signal mediated mostly by growth factors and cytokines, myeloid cells undergo modest but continuous expansion. Pro-inflammatory cytokines and ER stress responses contribute to pathologic myeloid cells activation that manifests in weak phagocytic activity, increased production of reactive oxygen species (ROS), nitric oxide (NO), arginase 1 (not expressed in human monocytes and M-MDSC) and prostaglandin-E2 (PGE2). This results in immune suppression.
Figure 2.
MDSC differentiation and accumulation. Neutrophils and monocytes are differentiated in bone marrow from hematopoietic progenitor cells (HPC) via common myeloid progenitors (CMP) and granulocyte-macrophage progenitors (GMP). Neutrophils differentiation progress through several progenitor and precursors stages. Among them are myeloblasts (MB), myelocytes (MC), metamyelocyte (MM), band forms (BF). Monocytes originate from monocyte/macrophages and dendritic cell (MDP) precursors. Under pathologic conditions, immature myeloid cells are expanded and converted to immunosuppressive MDSC. In early stages cells with some biochemical features of MDSC do not have suppressive activity and can be called MDSC-like cells. In cancer patients, in any given moment neutrophils and monocytes and pathologically activated MDSC co-exist, with accumulation of more MDSC during tumor progression. In tumors, M-MDSC rapidly differentiate in tumor associated macrophages (TAM) and inflammatory dendritic cells (infl DC).
The accumulation of MDSC is a complex and gradual phenomenon governed by multiple factors. We have previously suggested that accumulation of MDSC depends on two groups of interconnected signals. The first group of signals is important for the expansion of immature myeloid cells, whereas the second group is responsible for their pathologic activation21. The first group of signals is driven by factors produced by tumors or BM stroma in response to chronic infection and inflammation and includes: GM-CSF, G-CSF, M-CSF, S-SCF, VEGF, and polyunsaturated fatty acids (PUFAs). 22, 23, 24, 25. The transcriptional factors/regulators STAT3, STAT5, IRF8, C/EBPβ, NOTCH play a major role in this process26. Other factors involved in this process include adenosine receptors A2b, NLRP3, retinoblastoma protein 1 (RB1), and alarmins S100A9 and A8. Furthermore, a recent study showed that the antiapoptotic molecules c-FLIP and MCL-1 are involved in the development of M-MDSC and PMN-MDSC in cancer, respectively27. The second group of signals is mediated by inflammatory cytokines and DAMP, which include interferon (IFN)-γ, IL-1β, IL-4, IL-6, IL-13, TNF and the, TLR ligand, HMGB1. These factors mainly signal via NF-κB, STAT1, and STAT6 28. A recent study provided a direct experimental demonstration of this concept showing that the licensing of monocytes with GM-CSF was required for subsequent IFN-γ-mediated conversion of these cells to immune suppressive M-MDSC 29.
Phenotypic and molecular features of MDSC
Another controversial issue that was apparent from the beginning is how to identify these cells. The field of MDSC research is still immature and it is difficult to distinguish these cells from neutrophils and monocytes. In mice, PMN-MDSC can be defined as CD11b+Ly6G+Ly6Clo with high side scatter (SSC)30. However, this phenotype is typical for neutrophils but in some experimental models, PMN-MDSC can express markers not normally present on neutrophils like CD115 and CD24416. The direct phenotypic distinction between murine PMN-MDSC and tumor associated neutrophils (TANs) is even more difficult. TANs are a heterogeneous population of cells, including neutrophils (N1) with anti-tumor properties and neutrophils (N2) with potent suppressive functions31. Based on these functional characteristics, it is likely that N2 neutrophils are in fact PMN-MDSC 32, 33, 34. However, this question cannot be resolved until specific markers of mouse PMN-MDSC are identified.
M-MDSCs are defined as CD11b+Ly6G−Ly6Chi with low SSC30. This is the classic phenotype of inflammatory monocytes present in healthy mice. Other typical markers shared by these cells include CD115, CCR2 and CD49d (VLA4)35. However, M-MDSC usually lack surface markers of monocytes like CD11c and MHC class II23, 36. Phenotypically, M-MDSC can be readily separated from TAMs2, 37 since TAMs have high expression of F4/80, low-to-intermediate expression of Ly6C and low or undetectable expression of S100A9 protein (Table 1).
Table 1.
Phenotypical, molecular and functional properties of neutrophils, monocytes and MDSC
| Human | ||||||
|---|---|---|---|---|---|---|
| Neutrophils | PMN-MDSC | Monocytes | M-MDSC | e-MDSC | TAM | |
| Surface Phenotype | CD11b+CD14−CD15+CD66b+ LOX-1− | CD11b+CD14−CD15+CD66b+ LOX-1+ | CD14+CD15− HLA-DR+ | CD14+CD15− HLA-DR−/lo | CD3−CD14−CD15−CD19−CD56−HLA-DR−CD33+ | CD206+CD163+CD204+CD45+ |
| Density | High | Low | Low | Low | Low | Not applicable |
| Immune suppression | − | + | − | ++ | ++ | +++ |
| ROS | + | +++ | −/+ | −/+ | ++ | ++ |
| NO | − | + | + | +++ | ++ | +++ |
| Arginase-1 | + | ++ | − | − | −? | − |
| PGE-2 | − | ++ | − | + | N/A | − |
| S100A8/A9 | + | ++ | −/+ | + | N/A | − |
| ER Stress | −/+ | ++ | −/+ | ++ | N/A | N/A |
| STAT3 | −/+ | ++ | −/+ | ++ | N/A | N/A |
| Mouse | ||||||
| Neutrophils | PMN-MDSC | Monocytes | M-MDSC | TAM | ||
| Minimal surface phenotype | CD11b+Ly6GhiLy6Clo | CD11b+Ly6G−Ly6Chi | CD11b+F4/80hiLy6CloLy6G−CD115hi | |||
| Immune Suppression | − | + | − | ++ | +++ | |
| ROS | −/+ | ++ | −/+ | −/+ | ++ | |
| NO | − | + | + | ++ | ++ | |
| Arginase-1 | − | ++ | + | ++ | ++ | |
| PGE-2 | − | ++ | − | + | N/A | |
| S100A8/A9 | + | ++ | −/+ | + | − | |
| ER Stress | −/+ | ++ | −/+ | ++ | N/A | |
| STAT3 | −/+ | ++ | −/+ | ++ | −/+ | |
| IRF8 | + | −/+ | + | N/A | ++ | |
| C/EBPβ | −/+ | ++ | −/+ | + | N/A | |
| RB1 | + | −/+ | + | −/+ | N/A | |
N/A=not available
Comparisons are shown between cells for each factor separately. Therefore, different factors should not be compared with each other.
In humans, the equivalent of murine PMN-MDSC and M-MDSC has been found in the low-density Ficoll gradient fraction of peripheral blood mononuclear cell (PBMC). In contrast, neutrophils are isolated from the high-density fraction. PMN-MDSC and neutrophils share similar phenotype CD11b+CD14−CD15+ (or CD66b+) CD33+. However, different density allowed for distinction of these cells (Table 1). In healthy individuals, PMN-MDSC are practically undetectable. Recently identified lectin-type oxidized LDL receptor 1 (LOX-1) allows for better distinction between human neutrophils and PMN-MDSC without use of a gradient38. Immune suppressive LOX-1+ cells, with features defining them as PMN-MDSC, represent 4–15% of all neutrophils in blood of cancer patients and up to 40% in tumor tissues. In healthy individuals, these cells represent less than 1% of neutrophils38.
Monocytes and M-MDSC can be separated based of expression of MHC class II molecules. M-MDSC have a phenotype CD11b+CD14+CD15−CD33+HLA-DR−/lo, whereas monocytes are HLA-DR+19. M-MDSC represents a very small fraction of PBMC39. eMDSC are defined as Lin− (CD3, CD14, CD15, CD19, CD56) HLA-DR−CD33+ 19, 39.
Thus, in humans, MDSC can be separated from neutrophils and monocytes based on phenotypic markers and density gradient, whereas in mice such distinction is much more challenging. This is probably due to differences between mouse models and human diseases. Most of the cancer models utilize transplantation of tumor cells, which is associated with inflammation and rapid tumor progression. This leads to a dramatic expansion of MDSC that may replace most of the neutrophils and monocytes. This is not the case in human disease40. More detailed studies including single cells sequencing may help to address this question.
Human PMN-MDSC have a gene expression profile that distinguishes them from neutrophils in cancer patients, and from healthy donors38. They include eukaryotic translation initiation factors 2 and 4 (eIF2 and eIF4) associated with ER stress (discussed below), up-regulation of mTOR signaling, the MAPK pathway, CSF1, and the IFN-γ regulated pathways38. It is important to point out that none of these pathways by themselves can define MDSC. cDNA array analyses of sorted mouse PMN-MDSC and neutrophils revealed that PMN-MDSC had a higher expression of genes associated with cell cycle, autophagy, G-protein signaling, and the CREB pathway16. Neutrophils, on the other hand, had higher expression of genes associated with NF-κB signaling via CD40, IL-1, IL-6, TLR, and TNF pathways, as well as lymphotoxin-β receptor signaling. Substantial differences between PMN-MDSC from tumor-bearing and neutrophils from tumor -free mice were identified using whole transcriptomic analysis 41. Quantitative proteomics of murine MDSC determined that these cells constitute a distinct myeloid population characterized by a “kinase signature” and well-defined interactomes42, 43. Thus, it appears that distinct genomic and proteomic signatures of MDSC can be developed based on available information but more formal validation studies are needed to establish their value.
In addition to gene and protein expression profiles, MDSC are distinguished from neutrophils and monocytes by the activity and the expression of specific molecules. Up-regulation of STAT3 is a hallmark of MDSC as this transcriptional factor is directly implicated in the accumulation of MDSC in human and mice37, 44, 45, 46. Interestingly, although STAT3 activity is critical for MDSC expansion in bone marrow and spleen, inside the tumor, MDSC seem to down-regulate STAT3 activity by a mechanism involving the hypoxia-inducible activation of CD45 phosphatase47. This promotes rapid differentiation of M-MDSC to TAMs. Down-regulation of IRF8, a member of the interferon related factor (IRF) family is closely associated with PMN-MDSC expansion in mice30, 31, 32. A very recent study showed that growth of 4T1mammary adenocarcinoma was associated with a selective expansion of IRF8lo granulocyte progenitors. These progenitors had an increased ability to form PMN-MDSC48. Up-regulation of C/EBPβ, a member of a family of basic-region-leucine zipper transcriptional factors, is also associated with MDSC expansion 49. C/EBPβ regulates the expression of arginase (ARG1) and inducible nitric oxide synthase (NOS2), which are required for the suppressive functions of MDSC50. RB1 (p105) is a member of RB family, that also includes RB2 (130) and p107, which repress E2F and block cell proliferation. Low expression of RB1 in M-MDSC was associated with these cells ability to differentiate to PMN-MDSC, whereas RB1hi M-MDSC give rise to MΦ and DCs51. The accumulation of RB1lo PMN-MDSC has also been described in the PyMT transgenic model of breast cancer52.
Immune suppressive activity of MDSC
Another controversial issue in MDSC biology is whether the functional activity of these cells is uniquely associated with a specific set of biochemical events. Immune suppression is the main feature of MDSC that allows MDSC to be distinguished from monocytes and neutrophils in peripheral blood in humans and in spleens of mice. Splenic monocytes in mice and monocytes isolated from peripheral blood of humans can acquire immune suppressive features upon culture for several days on plastic. This approach is used for generation of MDSC in vitro. However, although suppressive activity of these in vitro-derived cells and some suppressive mechanisms (like NO) are shared with M-MDSC, at this moment, it is not clear whether these cells have similar biochemical and genomic profiles. MDSC generated from hematopoietic progenitors also have the same issue53, 54. Generation of suppressive neutrophils in vitro is a more difficult task probably due to their nature as terminally differentiated cells and their very short survival in culture. However, neutrophils isolated from healthy donor’s blood acquire potent suppressive activity upon treatment with ER stress inducers38.
M-MDSC are more suppressive than PMN-MDSC when assessed on a per cell basis 23, 36. PMN-MDSC and M-MDSC use different mechanisms to suppress immune responses and some of the mediators of suppression can be used to distinguish MDSC from neutrophils and monocytes. The most prominent factors implicated in MDSC suppressive activity includes arginase, NO, up-regulation of ROS and the production of prostaglandin E2 (PGE2)55, 56, 57. Changes in oxidative phosphorylation and glycolysis in tumors has also been associated with the function of MDSC. In mice, glycolysis is increased concurrently with increased ARG1 activity in MDSC. Interestingly, AMP-activated protein kinase (AMPK) is also activated and normally drives metabolism towards oxidative phosphorylation58. A very recent study showed that tumor-infiltrating MDSC preferentially use fatty acid β oxidation (FAO) as a primary source of energy. Tumor-infiltrating MDSC show increased mitochondrial mass, key FAO-associated genes, and oxygen consumption rate59. The inhibition of FAO affects the suppressive functions of MDSC and enhances the efficacy of cancer immune therapy.
Endoplasmic reticulum (ER) stress response has emerged in recent years as an important mechanism regulating pathologic activation of MDSC and thus critical for their functions. The ER stress response is an evolutionary conserved mechanism used by cells for protection from dysregulated proliferation, oxidative stress, nutrient deprivation, hypoxia and acidic extracellular pH. Three major sensors of ER stress are currently described: protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6). The transcription factor CCAAT-enhancer-binding protein homologous protein (CHOP) is a critical mediator of the PERK pathway, whereas spliced X-box binding protein-1 (sXBP1) is a mediator of the IRE1 pathway60, 61. In tumor DCs, ER stress leads to increased lipid peroxidation and was directly implicated in their defective function 62. MDSC from tumor-bearing mice and cancer patients demonstrate a much higher ER stress response than neutrophils and monocytes from tumor-free hosts63. Up-regulation of PERK and IRE1 pathways was observed. The level of ER stress response at the tumor site was substantially higher than in peripheral lymphoid organs in mice 63. The direct cause of ER stress induction in MDSC is not clear. However, the functional consequences have been identified. Experimental induction of ER stress enhanced the immunosuppressive capacity of tumor-infiltrating MDSC by increasing expression of ARG1, NOS2, and NOX264. Tumor MDSC from mice deficient for CHOP showed low expression of pSTAT3, decreased production of IL-6 and ARG1, which are directly involved in immune suppression. CHOP-deficient MDSC isolated from tumor-bearing mice had reduced immunosuppressive activity65. Increased sXBP1 was observed in human LOX-1+ PMN-MDSC as compared to LOX-1− neutrophils38. Moreover, the induction of ER stress in neutrophils isolated from healthy donors converts them to potent suppressive cells38. ER stress also controls MDSC survival in tumors and favors apoptosis through tumor necrosis factor (TNF)-related apoptosis-induced ligand receptor 2 (DR5) and caspase-8 activation63. In fact, targeting TRAIL-R2 results in elimination of different populations of MDSC without affecting mature myeloid or lymphoid cells in cancer patients66. Another study showed that trabectedin, an approved chemotherapeutic agent, induces apoptosis of monocytes and macrophages by activating the extrinsic apoptotic pathway downstream of TRAIL receptors67. Consistent with these observations, the absence of CHOP was shown to delay apoptosis and prolonged survival of MDSC in cancer65. Other mechanisms of MDSC-mediated immune suppression include up-regulation of regulatory T cells and immune suppressive cytokines. They are reviewed in detail elsewhere2. Altogether, these unique features of MDSC allow for identification of these cells and provide an insight into their biological activity.
How important is the immature state for MDSC biology?
One of the controversial questions of MDSC biology is whether all MDSC are immature cells. Large number of studies in mice and humans demonstrated that most of PMN-MDSC have a morphology similar to immature granulocytes and in some in vitro studies PMN-MDSCs are able to acquire characteristic of mature neutrophils upon short term culture16. However, a recent report showed that granulocytic MDSC in patients with Hodgkin’s lymphoma were mostly composed of mature low-density suppressive neutrophils in an activated state68. Another recent study confirms that activated mature neutrophils expressing CD10, isolated from G-CSF–treated donors, systemic lupus erythematous and cancer patients, also have suppressive properties69. Although more studies are needed to clarify this issue, it is likely that pathologic activation of PMN-MDSC would include mature cells.
In tumor tissues, M-MDSC rapidly differentiate into TAMs and inflammatory DCs29, 63. These terminally differentiated myeloid cells can persist in tissues for a long time. TAMs have a long-established role as inhibitors of immune responses and promoters of tumor progression70, 71. Some studies have shown that inflammatory DCs can promote anti-tumor T cell responses72, 73. However, it has been also shown that they can contribute to immune suppression in tumor-bearing hosts74.
Do MDSC have similar role in different pathologic conditions?
Cancer was historically the first condition where MDSCs were described. We will discuss below the data directly implicating MDSC in clinical outcome and response to therapy. In recent years, a large body of evidence demonstrated the role of MDSC in regulation of immune responses and pathogenesis of many pathologic conditions. The question is whether these MDSC have similar origin and function. No direct side-by-side comparison have been performed so far. However, analysis of available data (below) may clarify this question.
Cancer
MDSC role in mouse tumor models is well established15. In most of the studies PMN-MDSC cells expanded the most. In recent years, the clinical role of MDSC has emerged. Initial studies monitored MDSC in cancer patients, analyzing total MDSC population (PMN-and M-MDSC together). Results showed a positive correlation of MDSC numbers in peripheral blood with cancer stage and tumor burden in colorectal carcinomas, breast, bladder thyroid and NSCLC75, 76, 77, 78, 79, 80, 81. In melanoma and breast cancer, both PMN- and M-MDSC numbers correlate with stage and metastasis82. In a meta-analysis, elevated numbers of MDSC in the circulation was found to be an independent indicator of poor outcomes in patients with solid tumors83. Accumulation of M-MDSC in peripheral blood was associated with shorter progression-free interval (PFI) and/or overall survival (OS) in NSCLC, colorectal, bladder, thyroid and uterine cervical cancer81, 84, 79, 85, 80, 86. In melanoma and hepatocellular carcinoma, both PMN- and M-MDSC correlated with poorer outcomes82, 87. In non-solid tumors, M-MDSC numbers correlated with reduced survival in multiple myeloma, Hodgkin and non-Hodgkin lymphoma and diffuse large B cell lymphoma88, 89, 68. Notably, most of the studies consider only circulating MDSC, but some attention should also be paid to tumor infiltrating cells. In one report, neutrophil (CD66b+) infiltration in colorectal cancer tissue was associated with good prognosis90. TANs can also contribute to tumor progression, upregulating tumor proliferation pathways, promoting angiogenesis and supporting tumor extravasation by disrupting the extracellular matrix (reviewed in 91). Until recently, histological studies presented technical challenges since multiple markers were required to identify MDSC. Introduction of multiplex immunohistochemistry and new markers of MDSC (like LOX-1) should help address this problem.
Recent studies demonstrated the value of MDSC in predicting the response to various cancer therapies. The frequency of M-MDSC numbers negatively correlated with the response to chemotherapy in breast, cervical, prostate and colorectal cancer 78, 86, 92, 84 squamous cell carcinoma, multiple myeloma and Hodgkin lymphoma93, 94, 95 ; similarly, PMN-MDSC numbers negatively correlated with chemotherapy response in colorectal cancer 84. M-MDSC numbers were a predictor of radiotherapy failure in hepatocellular carcinoma77, 96. High numbers of circulating MDSC have been associated with vaccine failure in melanoma, NSCLC and colon adenocarcinoma97, 98. The percentage of circulating M-MDSC and PMN-MDSC negatively correlated with objective clinical response to Ipilimumab (CTLA-4 antibody) in patients with unresectable melanoma 99, 100, 101. Moreover, in melanoma, M-MDSC frequency could predict the failure of the second line immunotherapy using anti-PD1 antibody (Nivolumab) after failure of first line Ipilimumab treatment102. Recent studies in mouse tumor models showed that MDSC inhibition during immunotherapy increases its therapeutic effect103, 104, 105, 106, 107, 108.
Infectious diseases
Many studies have shown that bacteria (both Gram-positive and Gram-negative) can induce or modulate MDSC in vitro and in vivo (reviewed in109). In some studies, subpopulation of MDSC have not been analyzed in detail, thus we refer to those studies with the broader definition of “MDSC”.
In Staphylococcus aureus infection models, M-MDSC and PMN-MDSC numbers expand and suppress T cells110, contributing to the aggravation of infection111. Mycobacterium tuberculosis induced expansion of PMN-and M-MDSC suppressive activity in mice112. Patients with sepsis exhibited expansion of MDSC numbers. PMN-MDSC were expanded mainly in sepsis caused by gram-positive pathogens, while M-MDSC expanded in response to gram-positive or gram-negative pathogens113. Sepsis-associated MDSC have upregulated ARG1 expression and are associated with adverse outcome114. MDSC expansion after bacterial infection does not always translate to worse outcome. In Pseudomonas.aeruginosa and Klebsiella pneumoniae, MDSC expansion is associated with host protection and better outcome115. It appears that in early stages of sepsis (as probably in other infectious as well) accumulation of neutrophils and monocytes with potent anti-pathogen activity protect the host. MDSC are probably either not present or present at very low frequency. However, if infection is not resolved, the frequency of MDSC increases gradually and these cells exert an immune suppressive effect on adaptive immunity, which is very similar to the situation observed in cancer
Human pathogenic fungi could regulate the immune system and directly induce MDSC accumulation. In these studies, MDSC were monitored as Gr1+CD11b+ cells without further distinction between PMN- and M-MDSC. Albicans fumigatus and Candida albicans induced MDSC through the recognition of the receptor Dectin-1. Interestingly, MDSC were protective against C.albicans infections, but not A.fumigatus infection116. In a follow up study, the same group demonstrated that MDSC induction and suppressive activity on T cells were dependent on the Candida species117. MDSC expanded in mouse and rat models of P. pneumonia infection where MDSC exert their function through ARG1 and NOS2, and contribute to a more severe infection118.
M-MDSC are expanded in patients with chronic hepatitis C virus (HCV) infection. This accumulation correlated with the disease progression and response to antiviral therapy119. Different studies report ROS production120 or ARG1119 as the main mechanisms for MDSC mediated T cell suppression. HCV-induced MDSC could directly inhibit NK cell activities via ARG1-independent mechanisms121, 122. In vitro studies show that HCV directly induces M-MDSC through TLR2-STAT3 signaling. These MDSC stimulate Treg cell accumulation and inhibit CD4+ T cell proliferation123.
M-MDSC are also expanded in HIV-1-infected individuals and suppress T cell function via ARG1124, 125. Similarly to HCV, HIV directly induced expantion of M-MDSC126 that promoted differentiation of Tregs cell127. PMN-MDSC may induce T-cell anergy by suppressing CD3ζ expression and inhibiting CD8+ T cells through PD-L1-PD1 interaction125, 128. PMN-MDSC accumulation in patients with primary HIV-1 infection may be regulated by TRAIL and GM-CSF levels and positively correlates with disease progression128, 129. Thus, in infectious diseases, accumulation of MDSC, their functional activity, as well as major biochemical features closely resemble cancer-associated MDSC.
Autoimmune disorders
If in cancer and infectious disease MDSC activity is deleterious for patients, the role of MDSC in autoimmune disease is more complex. It has been shown that MDSC are expanded in murine models and patients with autoimmune diseases130. Nevertheless, MDSC role in this process is not established, with contrasting studies showing positive and negative roles for MDSC in regulation of disease progression. Systemic lupus erythematosus (SLE) is a systemic autoimmune disorder, with high cellular infiltration of organs. In mouse models, MDSC suppressed CD4+ T cell proliferation via ARG1 and were expanded in peripheral blood and kidney during disease progression131. The ability of MDSC to expand regulatory B cells has also been demonstrated132. However, in lupus-prone mice MDSC function was found to be impaired suggesting that SLE development is associated with a defect in MDSC133. A recent study demonstrated that M-MDSC and PMN-MDSC are both expanded in peripheral blood of SLE patients and their frequency positively correlates with serum ARG1 concentration, TH17 cell responses and lupus severity134.
Rheumatoid arthritis (RA) is a chronic inflammatory disease that leads to inflammation of multiple joints, progressing to cartilage destruction and bone erosion. Initial studies in a mouse collagen induced arthritis (CIA) model showed that suppressive MDSC accumulate in the spleen and adoptive transfer of MDSC reduced the severity of RA by blocking the CD4+ T cell pro-inflammatory immune response135. MDSC lost suppressive activity during arthritis development, thus failing to control the disease135. M-MDSC isolated from BM of CIA mice were also able to inhibit B cell proliferation and function, improving CIA outcome136. In support of a beneficial role of MDSC in RA models, synovial fluid of RA patients contained PMN-MDSC, that were able to suppress T cell activity in vitro137, similarly to what was previously shown in mice138. Moreover, circulating MDSC in patients with RA negatively correlates with TH17 cells and plasma arginin concentrations139. Recently, it has been reported that MDSC could have the opposite role by promoting RA onset in mice by sustaining TH17 cell differentiation. MDSC infiltration in arthritic joints positively correlated with high disease activity, but MDSC frequency in peripheral blood negatively correlated with TH17 cell numbers136. Overall, RA studies consistently report beneficial effects of MDSC adoptive transfer for inhibiting RA progression in mouse models consistent with the suppressive activity of these cells, but MDSC recruitment shows inconsistency with TH17 and Treg cell expansion and RA onset. This inconsistency could be due to the heterogeneity of the myeloid cell population discussed above, with variable frequencies of MDSC present in the total population of myeloid cells. Several studies have also associated MDSC with inflammatory bowel disease (IBD). MDSC reduced severity of experimentally-induced colitis in mice140. In IBD patients, M-MDSC were expanded and their frequency was associated with disease activity141.
Thus, the importance of MDSC in autoimmune diseases is evident. However, in contrast to cancer and infectious diseases, the expansion of MDSC is less prominent, which results in a larger heterogeneity of the myeloid population and variable frequency of MDSC among myeloid cells, which may result in contradictory results. This heterogeneity is apparently due to the different severity of autoimmune diseases and specifics of the microenvironment.
Obesity and pregnancy
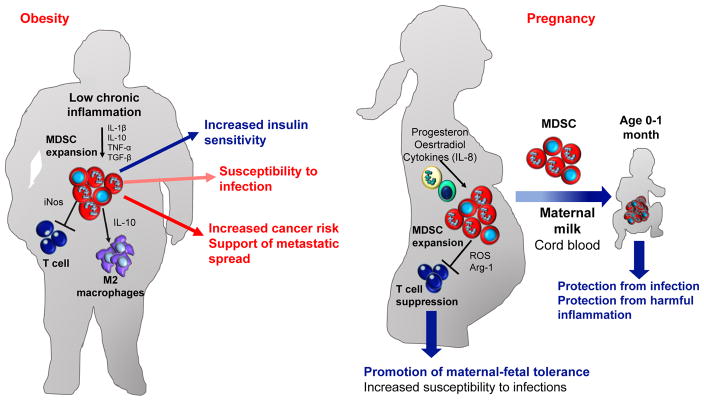
Obesity is associated with chronic inflammation and linked to increased risk of breast, prostate, and colorectal cancer, as well as cardiovascular metabolic disorders and type 2 diabetes mellitus142,143. The metabolic changes in the microenvironment associated with obesity and the associated chronic inflammation led to the hypothesis that MDSC could play a role in maintaining immune homeostasis in obese subjects. In a diabetes mouse model, MDSC were able to down-regulate immune responses and prevent diabetes onset144. Tissue-infiltrating MDSC were crucial in controlling obesity-associated inflammation and increasing insulin sensitivity. MDSC suppressed CD8+ T cells by NOS2 and IFN-γ-dependent mechanisms. MDSC also induced M2 MΦ polarization, probably through IL-10145. Obese subjects are often susceptible to infections and poorly respond to vaccines. This observation could be explained by MDSC expansion and consequent suppression of T and B cell functions as was demonstrated in obese mice146. There are few human studies in the field, but it was reported that M-MDSC numbers were increased in the peripheral blood of obese subjects147. Recent work showed that obesity caused expansion of neutrophils in mouse lungs, enhancing breast cancer metastasis through IL-5 and GM-CSF148 (Fig. 3a).
Figure 3.
MDSC role in obesity and pregnancy. (a) MDSC are expanded in obese subjects due to chronic inflammation and contribute to increase insulin sensitivity. MDSC in obese subjects directly inhibit T cells via NO secretion and induce macrophage differentiation to the M2 phenotype via IL-10 secretion, leading to increased susceptibility to infections. Moreover, increased MDSC increases cancer risk and favors metastatic spread of early stage cancers. (b) During pregnancy MDSC are expanded and are crucial for successful pregnancy. In pregnant women MDSC suppress T cells via ROS and Arg-1 thus sustaining maternal-fetal tolerance. Moreover, MDSC are expanded also in cord blood (CB) and neonates protecting newborns from infections and harmful inflammation.
Insulin resistance promotes metabolic syndrome development, characterized by elevated serum inflammatory cytokines and high macrophage infiltration in adipose tissue. Macrophages accumulate in adipose tissue as obesity progresses and in late obesity M2 macrophages are induced in parallel with MDSC infiltration of adipose tissue (reviewed in 149). Insulin resistance could be the result of adaptation to bacterial infection in order to provide glucose to M1 macrophages, which rely on glycolysis. M2 macrophages, instead, rely on oxidative phosphorylation (reviewed in 150). Increased concentrations of IGF-1 and estrogens associated with insulin resistance and obesity can directly polarize myeloid cells in adipose tissues to the M2 phenotype 151, 152. The obesity microenvironment can also induce differentiation of M2 macrophages to M1 macrophages, that then recruit T cells153 and induce monocytes migration via CCL2154. In vitro, macrophages coming from obese subjects or exposed to conditions mimicking the obese microenvironment showed increases in migration and upregulation of markers of TAM. In the same study, it was also demonstrated that obesity promoted macrophages infiltration in the prostate tumor microenvironment, and induced TAM polarization through the COX2-PGE2 pathway155.
Maternal-fetal tolerance is critically important for normal pregnancy. Pregnancy failure (miscarriage, implantation failure, preterm birth, preeclampsia) is associated with dysregulation of the immune system (reviewed in156). Early observations in mice showed that MDSC accumulate in mouse placenta157 and their level decreased towards the time of delivery. Subsequently, MDSC expansion has been observed in peripheral blood and the uterus of pregnant mice, in association with anti-inflammatory functions158. MDSC recruitment was driven by CXCR2159, and progesterone supported MDSC differentiation and activation via STAT3 signaling160. MDSC suppressed T cells either via ARG1161 or ROS production160 or by preventing T cell activation by down modulating L-selectin expression on naïve T cells162.
During human pregnancy, MDSC are expanded in peripheral blood163 and decidua164 of healthy pregnant women and rapidly drop to normal levels after birth165. Reduction of MDSC in peripheral blood, endometrium and placenta is associated with early miscarriage166, and low level of arginine and reduced NOS2 expression in placental tissues are found in women with preeclampsia167,168. A study demonstrated that MDSC are expanded during the first trimester, with a decrease towards the third166. Conversely, another study observed that MDSC are equally expanded during the entire gestation period. In this study, the main population of MDSC expanded in peripheral blood was PMN-MDSC, expressing ARG1 and NOS2 and producing high ROS levels165.
During pregnancy, estradiol expand M-MDSC in the circulation via STAT3 activation that suppressed T cells in a ROS-dependent fashion160. Conversely, in placental tissue, M-MDSC were expanded by CCL2 and overexpressed IDO1, ARG1 and COX2168. PMN-MDSC in the placenta could also interact with other immune system cell populations, directly expanding Tregs cells via the TGFβ-β-catenin pathway161 (Fig. 3b).
MDSC expansion is rapidly canceled in post-partum women, but MDSC are found expanded in neonates during the first month of life. In neonates, the expanded MDSC are mainly PMN-MDSC that suppress T cells in a contact-depended manner and reduce IFN-γ production. PMN-MDSC decrease rapidly in the first 6 week of life and reach adult levels by 6 months of age169. In the same studies, PMN-MDSC were found expanded in the cord blood (CB), where their frequency correlated with the proliferative capacity of T cells upon stimulation in vitro. M-MDSC and PMN-MDSC expansion in CB modulated the adaptive immune response170. PMN-MDSC from CB directly inhibited TH1 cell responses and induced TH2 responses and Treg cells. In this setting PMN-MDSC mediates suppression via expression of ARG1 and NOS2, production of ROS, and degradation of tryptophan by IDO expression171. Neonatal PMN-MDSC show reduced apoptosis and immunosuppressive activity upon infection with Escherichia Coli172. A recent study shows that M-MDSC were expanded in neonates and respond to microbial stimulation173. Mouse studies from the same group showed that S100A8/A9 prevented expansion of these cells and prevented death from septic shock173. Studies in humans showed that S100A9 secretion protected neonates from sepsis by regulating MyD88-dependend gene programs174. Thus, MDSC in pregnancy mainly follows the pattern observed in cancer and is apparently one of the important regulators of fetal-maternal tolerance. Because of limited information, at this time the biological role of MDSC in newborns is not clear. It is possible that MDSC has evolved as a protective mechanism limiting inflammation associated with bacterial colonization of the gut.
Conclusions
MDSC are now recognized as one of the major negative regulators of immune responses in many pathologic conditions. The challenge is to identify specific markers of these cells that allow for easy phenotypical distinction of MDSC from neutrophils and monocytes in mice and to expand the already existing panel of markers in humans. This would allow for better understanding of the biology of these cells. It appears that in contrast to Treg cells or checkpoint molecules, MDSC are not present in steady state condition. This opens a unique opportunity to target these cells without possible side effects. Understanding the molecular mechanisms regulating the accumulation and function of these cells allow for more precise targeted therapy. The clinical significance of MDSC in cancer and in some infectious diseases is now established. The next step is to determine whether targeting MDSC may provide tangible clinical benefits. The next several years will provide an answer to this question.
Acknowledgments
This work was supported by the National Institutes of Health CA084488 and CA100062 to DIG. We thank Ms. Rina Kim for help in preparation of the manuscript.
Footnotes
Authors declare no competing financial interests
References
- 1.Gabrilovich D, Bronte V, Chen S-H, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61(8):1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann NY Acad Sci. 2014;1319(1):47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 5.Bronte V, Brandau S, Chen S-H, Colombo M, Frey A, Greten T, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28(5):509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harbor perspectives in biology. 2012;4(3) doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11(3):e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 14.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umansky V, Blattner C, Gebhardt C, Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines (Basel) 2016;4(4) doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91(1):167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz ML, Lu L, Ramachandran I, Gabrilovich DI. Myeloid-derived suppressor cells in the development of lung cancer. Cancer Immunol Res. 2014;2(1):50–58. doi: 10.1158/2326-6066.CIR-13-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nature communications. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. Journal of immunology. 1999;162(10):5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 23.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40(1):22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 24.Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer microenvironment : official journal of the International Cancer Microenvironment Society. 2013;6(2):169–177. doi: 10.1007/s12307-012-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, et al. Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol. 2013;43(11):2943–2955. doi: 10.1002/eji.201343472. [DOI] [PubMed] [Google Scholar]
- 26.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. Journal of leukocyte biology. 2015;98(6):913–922. doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haverkamp JM, Smith AM, Weinlich R, Dillon CP, Qualls JE, Neale G, et al. Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity. 2014;41(6):947–959. doi: 10.1016/j.immuni.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015 doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Ribechini E, Hutchinson JA, Hergovits S, Heuer M, Lucas J, Schleicher U, et al. Novel GM-CSF signals via IFN-γR/IRF-1 and AKT/mTOR license monocytes for suppressor function. Blood advances. 2017;1:947–960. doi: 10.1182/bloodadvances.2017006858. This study demonstrates actual example of two-phase process in generation of MDSC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, et al. Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry Part B, Clinical cytometry. 2015;88(2):77–91. doi: 10.1002/cyto.b.21206. Detailed description of phenotypic characterization of MDSC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cimen Bozkus C, Elzey BD, Crist SA, Ellies LG, Ratliff TL. Expression of Cationic Amino Acid Transporter 2 Is Required for Myeloid-Derived Suppressor Cell-Mediated Control of T Cell Immunity. J Immunol. 2015;195(11):5237–5250. doi: 10.4049/jimmunol.1500959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mairhofer DG, Ortner D, Tripp CH, Schaffenrath S, Fleming V, Heger L, et al. Impaired gp100-Specific CD8(+) T-Cell Responses in the Presence of Myeloid-Derived Suppressor Cells in a Spontaneous Mouse Melanoma Model. J Invest Dermatol. 2015;135(11):2785–2793. doi: 10.1038/jid.2015.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. 2014;134(12):2853–2864. doi: 10.1002/ijc.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185(1):203–210. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 36.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 37.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2) doi: 10.1126/sciimmunol.aaf8943. Identification of specific marker of human PMN-MDSC and the possibility of converting of neutrophils to PMN-MDSC by inducing ER stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer immunology, immunotherapy : CII. 2016;65(2):161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eruslanov EB, Singhal S, Albelda SM. Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap. Trends in cancer. 2017;3(2):149–160. doi: 10.1016/j.trecan.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PloS one. 2012;7(2):e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gato M, Blanco-Luquin I, Zudaire M, de Morentin XM, Perez-Valderrama E, Zabaleta A, et al. Drafting the proteome landscape of myeloid-derived suppressor cells. Proteomics. 2016;16(2):367–378. doi: 10.1002/pmic.201500229. [DOI] [PubMed] [Google Scholar]
- 43.Gato-Canas M, Martinez de Morentin X, Blanco-Luquin I, Fernandez-Irigoyen J, Zudaire I, Liechtenstein T, et al. A core of kinase-regulated interactomes defines the neoplastic MDSC lineage. Oncotarget. 2015;6(29):27160–27175. doi: 10.18632/oncotarget.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172(1):464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 45.Rebe C, Vegran F, Berger H, Ghiringhelli F. STAT3 activation: A key factor in tumor immunoescape. Jak-Stat. 2013;2(1):e23010. doi: 10.4161/jkst.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44(2):303–315. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Netherby CS, Messmer MN, Burkard-Mandel L, Colligan S, Miller A, Cortes Gomez E, et al. The Granulocyte Progenitor Stage Is a Key Target of IRF8-Mediated Regulation of Myeloid-Derived Suppressor Cell Production. Journal of immunology. 2017;198(10):4129–4139. doi: 10.4049/jimmunol.1601722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365(Pt 3):561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbasi K, Fadaei Araghi M, Zafarghandi M, Karimi A, Ahmadi H, Marzban M, et al. Concomitant carotid endarterectomy and coronary artery bypass grafting versus staged carotid stenting followed by coronary artery bypass grafting. The Journal of cardiovascular surgery. 2008;49(2):285–288. [PubMed] [Google Scholar]
- 51.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14(3):211–220. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015;112(6):E566–575. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dufait I, Schwarze JK, Liechtenstein T, Leonard W, Jiang H, Escors D, et al. Ex vivo generation of myeloid-derived suppressor cells that model the tumor immunosuppressive environment in colorectal cancer. Oncotarget. 2015;6(14):12369–12382. doi: 10.18632/oncotarget.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casacuberta-Serra S, Pares M, Golbano A, Coves E, Espejo C, Barquinero J. Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes. Immunol Cell Biol. 2017;95(6):538–548. doi: 10.1038/icb.2017.4. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, et al. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol. 2009;9(7–8):937–948. doi: 10.1016/j.intimp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 57.Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73(13):3877–3887. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 58.Hammami I, Chen J, Murschel F, Bronte V, De Crescenzo G, Jolicoeur M. Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC cell biology. 2012;13:18. doi: 10.1186/1471-2121-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res. 2015;3(11):1236–1247. doi: 10.1158/2326-6066.CIR-15-0036. This paper shows the involvement of lipid metabolism in the functionality of MDSC in cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nature reviews Immunology. 2008;8(9):663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 61.Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nature reviews Immunology. 2016;16(8):469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cubillos-Ruiz Juan R, Silberman Pedro C, Rutkowski Melanie R, Chopra S, Perales-Puchalt A, Song M, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161(7):1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124(6):2626–2639. doi: 10.1172/JCI74056. Together with reference 65 this study demonstrates the role of ER stress in MDSC function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee BR, Chang SY, Hong EH, Kwon BE, Kim HM, Kim YJ, et al. Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget. 2014;2:2. doi: 10.18632/oncotarget.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65*.Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41(3):389–401. doi: 10.1016/j.immuni.2014.08.015. Together with reference 63 this study demonstrates the role of ER stress in MDSC function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, et al. Selective Targeting of Myeloid-Derived Suppressor Cells in Cancer Patients Using DS-8273a, an Agonistic TRAIL-R2 Antibody. Clin Cancer Res. 2017;23(12):2942–2950. doi: 10.1158/1078-0432.CCR-16-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23(2):249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 68*.Marini O, Spina C, Mimiola E, Cassaro A, Malerba G, Todeschini G, et al. Identification of granulocytic myeloid-derived suppressor cells (G-MDSC) in the peripheral blood of Hodgkin and non-Hodgkin lymphoma patients. Oncotarget. 2016;7(19):27676–27688. doi: 10.18632/oncotarget.8507. This paper reports that mature and activated neutrophils isolated from cancer patients have suppressive functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marini O, Costa S, Bevilacqua D, Calzetti F, Tamassia N, Spina C, et al. Mature CD10+ and immature CD10- neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood. 2017;129(10):1343–1356. doi: 10.1182/blood-2016-04-713206. [DOI] [PubMed] [Google Scholar]
- 70.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cellular & molecular immunology. 2015;12(1):1–4. doi: 10.1038/cmi.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 73.Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AH, Ugel S, et al. T Cell Cancer Therapy Requires CD40-CD40L Activation of Tumor Necrosis Factor and Inducible Nitric-Oxide-Synthase-Producing Dendritic Cells. Cancer Cell. 2016;30(4):651. doi: 10.1016/j.ccell.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Tesone AJ, Rutkowski MR, Brencicova E, Svoronos N, Perales-Puchalt A, Stephen TL, et al. Satb1 Overexpression Drives Tumor-Promoting Activities in Cancer-Associated Dendritic Cells. Cell reports. 2016;14(7):1774–1786. doi: 10.1016/j.celrep.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun HL, Zhou X, Xue YF, Wang K, Shen YF, Mao JJ, et al. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J Gastroenterol. 2012;18(25):3303–3309. doi: 10.3748/wjg.v18.i25.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PloS one. 2013;8(2):e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, et al. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62(8):1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang G, Shen W, Zhang Y, Liu M, Zhang L, Liu Q, et al. Accumulation of myeloid-derived suppressor cells (MDSC) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget. 2017;8(24):38378–38388. doi: 10.18632/oncotarget.16386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Angell TE, Lechner MG, Smith AM, Martin SE, Groshen SG, Maceri DR, et al. Circulating Myeloid-Derived Suppressor Cells Predict Differentiated Thyroid Cancer Diagnosis and Extent. Thyroid. 2016;26(3):381–389. doi: 10.1089/thy.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62(9):1439–1451. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, et al. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother. 2013;62(11):1711–1722. doi: 10.1007/s00262-013-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83*.Zhang S, Ma X, Zhu C, Liu L, Wang G, Yuan X. The Role of Myeloid-Derived Suppressor Cells in Patients with Solid Tumors: A Meta-Analysis. PloS one. 2016;11(10):e0164514. doi: 10.1371/journal.pone.0164514. Meta-analysis of association between MDSC accumulation and clinical outocme in cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tada K, Kitano S, Shoji H, Nishimura T, Shimada Y, Nagashima K, et al. Pretreatment Immune Status Correlates with Progression-Free Survival in Chemotherapy-Treated Metastatic Colorectal Cancer Patients. Cancer Immunol Res. 2016;4(7):592–599. doi: 10.1158/2326-6066.CIR-15-0298. [DOI] [PubMed] [Google Scholar]
- 85.Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. 2017;36(15):2095–2104. doi: 10.1038/onc.2016.367. [DOI] [PubMed] [Google Scholar]
- 86.Kawano M, Mabuchi S, Matsumoto Y, Sasano T, Takahashi R, Kuroda H, et al. The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Scientific reports. 2015;5:18217. doi: 10.1038/srep18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X, Xing YF, Lei AH, Xiao Q, Lin ZH, Hong YF, et al. Neutrophil count is associated with myeloid derived suppressor cell level and presents prognostic value of for hepatocellular carcinoma patients. Oncotarget. 2017;8(15):24380–24388. doi: 10.18632/oncotarget.15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Z, Zhang L, Wang H, Xiong S, Li Y, Tao Q, et al. Tumor-induced CD14+HLA-DR (-/low) myeloid-derived suppressor cells correlate with tumor progression and outcome of therapy in multiple myeloma patients. Cancer Immunol Immunother. 2015;64(3):389–399. doi: 10.1007/s00262-014-1646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu C, Wu X, Zhang X, Chai Y, Guo Q, Li L, et al. Prognostic significance of peripheral monocytic myeloid-derived suppressor cells and monocytes in patients newly diagnosed with diffuse large b-cell lymphoma. International journal of clinical and experimental medicine. 2015;8(9):15173–15181. [PMC free article] [PubMed] [Google Scholar]
- 90.Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139(2):446–456. doi: 10.1002/ijc.30076. [DOI] [PubMed] [Google Scholar]
- 91.Hurt B, Schulick R, Edil B, El Kasmi KC, Barnett C., Jr Cancer-promoting mechanisms of tumor-associated neutrophils. Am J Surg. 2017 doi: 10.1016/j.amjsurg.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Wang J, Yang J. Identification of CD4+CD25+CD127- regulatory T cells and CD14+HLA-DR-/low myeloid-derived suppressor cells and their roles in the prognosis of breast cancer. Biomed Rep. 2016;5(2):208–212. doi: 10.3892/br.2016.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH, et al. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5(18):8716–8728. doi: 10.18632/oncotarget.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SE, Lim JY, Ryu DB, Kim TW, Yoon JH, Cho BS, et al. Circulating immune cell phenotype can predict the outcome of lenalidomide plus low-dose dexamethasone treatment in patients with refractory/relapsed multiple myeloma. Cancer Immunol Immunother. 2016;65(8):983–994. doi: 10.1007/s00262-016-1861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Romano A, Parrinello NL, Vetro C, Forte S, Chiarenza A, Figuera A, et al. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin Lymphoma patients treated up-front with a risk-adapted strategy. Br J Haematol. 2015;168(5):689–700. doi: 10.1111/bjh.13198. [DOI] [PubMed] [Google Scholar]
- 96.Wang D, An G, Xie S, Yao Y, Feng G. The clinical and prognostic significance of CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol. 2016;37(8):10427–10433. doi: 10.1007/s13277-016-4916-2. [DOI] [PubMed] [Google Scholar]
- 97.Butterfield LH, Zhao F, Lee S, Tarhini AA, Margolin KA, White RL, et al. Immune Correlates of GM-CSF and Melanoma Peptide Vaccination in a Randomized Trial for the Adjuvant Therapy of Resected High-Risk Melanoma (E4697) Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer prevention research. 2013;6(1):18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Coana YP, Wolodarski M, Poschke I, Yoshimoto Y, Yang Y, Nystrom M, et al. Ipilimumab treatment decreases monocytic MDSC and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget. 2017;8(13):21539–21553. doi: 10.18632/oncotarget.15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, et al. Clinical Significance of Circulating CD33+CD11b+HLA-DR- Myeloid Cells in Patients with Stage IV Melanoma Treated with Ipilimumab. Clin Cancer Res. 2016;22(23):5661–5672. doi: 10.1158/1078-0432.CCR-15-3104. [DOI] [PubMed] [Google Scholar]
- 101.Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin Cancer Res. 2016;22(12):2908–2918. doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102*.Weber J, Gibney G, Kudchadkar R, Yu B, Cheng P, Martinez AJ, et al. Phase I/II Study of Metastatic Melanoma Patients Treated with Nivolumab Who Had Progressed after Ipilimumab. Cancer Immunol Res. 2016;4(4):345–353. doi: 10.1158/2326-6066.CIR-15-0193. Association of high number of MDSC with response and survival in patients treated with PD-1 antibody who progressed on CTLA4 antibody therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iida Y, Harashima N, Motoshima T, Komohara Y, Eto M, Harada M. Contrasting effects of cyclophosphamide on anti-CTL-associated protein 4 blockade therapy in two mouse tumor models. Cancer science. 2017 doi: 10.1111/cas.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104*.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Science translational medicine. 2014;6(237):237ra267. doi: 10.1126/scitranslmed.3007974. Demonstration of theraputic effect of blocking of MDSC trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Du Four S, Maenhout SK, Niclou SP, Thielemans K, Neyns B, Aerts JL. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSC. Am J Cancer Res. 2016;6(11):2514–2531. [PMC free article] [PubMed] [Google Scholar]
- 106*.Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash H, Chen Z, et al. Anti-PD-L1 Efficacy Can Be Enhanced by Inhibition of Myeloid-Derived Suppressor Cells with a Selective Inhibitor of PI3Kdelta/gamma. Cancer Res. 2017;77(10):2607–2619. doi: 10.1158/0008-5472.CAN-16-2534. Therapeutic effect in mice after down-regulation of MDSC with PI3K inhibitor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107*.Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543(7647):728–732. doi: 10.1038/nature21676. Demonstration of important role of MDSC in prostate cancer model and potential theraputic benefit of their targeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kamran N, Kadiyala P, Saxena M, Candolfi M, Li Y, Moreno-Ayala MA, et al. Immunosuppressive Myeloid Cells’ Blockade in the Glioma Microenvironment Enhances the Efficacy of Immune-Stimulatory Gene Therapy. Mol Ther. 2017;25(1):232–248. doi: 10.1016/j.ymthe.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ost M, Singh A, Peschel A, Mehling R, Rieber N, Hartl D. Myeloid-Derived Suppressor Cells in Bacterial Infections. Front Cell Infect Microbiol. 2016;6:37. doi: 10.3389/fcimb.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tebartz C, Horst SA, Sparwasser T, Huehn J, Beineke A, Peters G, et al. A major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during Staphylococcus aureus infection. J Immunol. 2015;194(3):1100–1111. doi: 10.4049/jimmunol.1400196. [DOI] [PubMed] [Google Scholar]
- 111.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, et al. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol. 2014;192(8):3778–3792. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dietlin TA, Hofman FM, Lund BT, Gilmore W, Stohlman SA, van der Veen RC. Mycobacteria-induced Gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J Leukoc Biol. 2007;81(5):1205–1212. doi: 10.1189/jlb.1006640. [DOI] [PubMed] [Google Scholar]
- 113.Janols H, Bergenfelz C, Allaoui R, Larsson AM, Ryden L, Bjornsson S, et al. A high frequency of MDSC in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J Leukoc Biol. 2014;96(5):685–693. doi: 10.1189/jlb.5HI0214-074R. [DOI] [PubMed] [Google Scholar]
- 114.Uhel F, Azzaoui I, Gregoire M, Pangault C, Dulong J, Tadie JM, et al. Early Expansion of Circulating Granulocytic Myeloid-derived Suppressor Cells Predicts Development of Nosocomial Infections in Patients with Sepsis. American journal of respiratory and critical care medicine. 2017;196(3):315–327. doi: 10.1164/rccm.201606-1143OC. [DOI] [PubMed] [Google Scholar]
- 115.Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal immunology. 2013;6(1):189–199. doi: 10.1038/mi.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rieber N, Singh A, Oz H, Carevic M, Bouzani M, Amich J, et al. Pathogenic fungi regulate immunity by inducing neutrophilic myeloid-derived suppressor cells. Cell host & microbe. 2015;17(4):507–514. doi: 10.1016/j.chom.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh A, Lelis F, Braig S, Schafer I, Hartl D, Rieber N. Differential Regulation of Myeloid-Derived Suppressor Cells by Candida Species. Frontiers in microbiology. 2016;7:1624. doi: 10.3389/fmicb.2016.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang C, Lei GS, Shao S, Jung HW, Durant PJ, Lee CH. Accumulation of myeloid-derived suppressor cells in the lungs during Pneumocystis pneumonia. Infect Immun. 2012;80(10):3634–3641. doi: 10.1128/IAI.00668-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cai W, Qin A, Guo P, Yan D, Hu F, Yang Q, et al. Clinical significance and functional studies of myeloid-derived suppressor cells in chronic hepatitis C patients. J Clin Immunol. 2013;33(4):798–808. doi: 10.1007/s10875-012-9861-2. [DOI] [PubMed] [Google Scholar]
- 120.Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, et al. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology. 2012;55(2):343–353. doi: 10.1002/hep.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goh CC, Roggerson KM, Lee HC, Golden-Mason L, Rosen HR, Hahn YS. Hepatitis C Virus-Induced Myeloid-Derived Suppressor Cells Suppress NK Cell IFN-gamma Production by Altering Cellular Metabolism via Arginase-1. J Immunol. 2016;196(5):2283–2292. doi: 10.4049/jimmunol.1501881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goh YS, Necchi F, O’Shaughnessy CM, Micoli F, Gavini M, Young SP, et al. Bactericidal Immunity to Salmonella in Africans and Mechanisms Causing Its Failure in HIV Infection. PLoS Negl Trop Dis. 2016;10(4):e0004604. doi: 10.1371/journal.pntd.0004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhai N, Li H, Song H, Yang Y, Cui A, Li T, et al. Hepatitis C Virus Induces MDSC-Like Monocytes through TLR2/PI3K/AKT/STAT3 Signaling. PloS one. 2017;12(1):e0170516. doi: 10.1371/journal.pone.0170516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol. 2013;87(3):1477–1490. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tumino N, Turchi F, Meschi S, Lalle E, Bordoni V, Casetti R, et al. In HIV-positive patients, myeloid-derived suppressor cells induce T-cell anergy by suppressing CD3zeta expression through ELF-1 inhibition. AIDS. 2015;29(18):2397–2407. doi: 10.1097/QAD.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 126.Garg A, Spector SA. HIV type 1 gp120-induced expansion of myeloid derived suppressor cells is dependent on interleukin 6 and suppresses immunity. The Journal of infectious diseases. 2014;209(3):441–451. doi: 10.1093/infdis/jit469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang L, Zhao J, Ren JP, Wu XY, Morrison ZD, Elgazzar MA, et al. Expansion of myeloid-derived suppressor cells promotes differentiation of regulatory T cells in HIV-1+ individuals. AIDS. 2016;30(10):1521–1531. doi: 10.1097/QAD.0000000000001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang ZN, Yi N, Zhang TW, Zhang LL, Wu X, Liu M, et al. Myeloid-Derived Suppressor Cells Associated with Disease Progression in Primary HIV Infection: PD-L1 Blockade Attenuates Inhibition. J Acquir Immune Defic Syndr. 2017 doi: 10.1097/QAI.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 129.Tumino N, Bilotta MT, Pinnetti C, Ammassari A, Antinori A, Turchi F, et al. Granulocytic Myeloid-Derived Suppressor Cells Increased in Early Phases of Primary HIV Infection Depending on TRAIL Plasma Level. J Acquir Immune Defic Syndr. 2017;74(5):575–582. doi: 10.1097/QAI.0000000000001283. [DOI] [PubMed] [Google Scholar]
- 130.Nicholson LB, Raveney BJ, Munder M. Monocyte dependent regulation of autoimmune inflammation. Current molecular medicine. 2009;9(1):23–29. doi: 10.2174/156652409787314499. [DOI] [PubMed] [Google Scholar]
- 131.Iwata Y, Furuichi K, Kitagawa K, Hara A, Okumura T, Kokubo S, et al. Involvement of CD11b+ GR-1 low cells in autoimmune disorder in MRL-Fas lpr mouse. Clin Exp Nephrol. 2010;14(5):411–417. doi: 10.1007/s10157-010-0309-9. [DOI] [PubMed] [Google Scholar]
- 132.Park MJ, Lee SH, Kim EK, Lee EJ, Park SH, Kwok SK, et al. Myeloid-Derived Suppressor Cells Induce the Expansion of Regulatory B Cells and Ameliorate Autoimmunity in the Sanroque Mouse Model of Systemic Lupus Erythematosus. Arthritis Rheumatol. 2016;68(11):2717–2727. doi: 10.1002/art.39767. [DOI] [PubMed] [Google Scholar]
- 133.Vlachou K, Mintzas K, Glymenaki M, Ioannou M, Papadaki G, Bertsias GK, et al. Elimination of Granulocytic Myeloid-Derived Suppressor Cells in Lupus-Prone Mice Linked to Reactive Oxygen Species-Dependent Extracellular Trap Formation. Arthritis Rheumatol. 2016;68(2):449–461. doi: 10.1002/art.39441. [DOI] [PubMed] [Google Scholar]
- 134.Wu H, Zhen Y, Ma Z, Li H, Yu J, Xu ZG, et al. Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSC in systemic lupus erythematosus. Science translational medicine. 2016;8(331):331ra340. doi: 10.1126/scitranslmed.aae0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang H, Wang S, Huang Y, Wang H, Zhao J, Gaskin F, et al. Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation. Clin Immunol. 2015;157(2):175–186. doi: 10.1016/j.clim.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo C, Hu F, Yi H, Feng Z, Li C, Shi L, et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann Rheum Dis. 2016;75(1):278–285. doi: 10.1136/annrheumdis-2014-205508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kurko J, Vida A, Glant TT, Scanzello CR, Katz RS, Nair A, et al. Identification of myeloid-derived suppressor cells in the synovial fluid of patients with rheumatoid arthritis: a pilot study. BMC Musculoskelet Disord. 2014;15:281. doi: 10.1186/1471-2474-15-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Egelston C, Kurko J, Besenyei T, Tryniszewska B, Rauch TA, Glant TT, et al. Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum. 2012;64(10):3179–3188. doi: 10.1002/art.34494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiao Z, Hua S, Wang W, Wang H, Gao J, Wang X. Increased circulating myeloid-derived suppressor cells correlated negatively with Th17 cells in patients with rheumatoid arthritis. Scandinavian journal of rheumatology. 2013;42(2):85–90. doi: 10.3109/03009742.2012.716450. [DOI] [PubMed] [Google Scholar]
- 140.Guan Q, Moreno S, Qing G, Weiss CR, Lu L, Bernstein CN, et al. The role and potential therapeutic application of myeloid-derived suppressor cells in TNBS-induced colitis. J Leukoc Biol. 2013;94(4):803–811. doi: 10.1189/jlb.0113050. [DOI] [PubMed] [Google Scholar]
- 141.Kontaki E, Boumpas DT, Tzardi M, Mouzas IA, Papadakis KA, Verginis P. Aberrant function of myeloid-derived suppressor cells (MDSC) in experimental colitis and in inflammatory bowel disease (IBD) immune responses. Autoimmunity. 2017;50(3):170–181. doi: 10.1080/08916934.2017.1283405. [DOI] [PubMed] [Google Scholar]
- 142.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3 Suppl 1):S12–18. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 143.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114(1):71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 144.Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM, et al. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185(10):5828–5834. doi: 10.4049/jimmunol.0903636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286(26):23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen S, Akbar SM, Miyake T, Abe M, Al-Mahtab M, Furukawa S, et al. Diminished immune response to vaccinations in obesity: role of myeloid-derived suppressor and other myeloid cells. Obes Res Clin Pract. 2015;9(1):35–44. doi: 10.1016/j.orcp.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 147.Bao Y, Mo J, Ruan L, Li G. Increased monocytic CD14(+)HLADRlow/- myeloid-derived suppressor cells in obesity. Molecular medicine reports. 2015;11(3):2322–2328. doi: 10.3892/mmr.2014.2927. [DOI] [PubMed] [Google Scholar]
- 148.Quail DF, Olson OC, Bhardwaj P, Walsh LA, Akkari L, Quick ML, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol. 2017;19(8):974–987. doi: 10.1038/ncb3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okwan-Duodu D, Umpierrez GE, Brawley OW, Diaz R. Obesity-driven inflammation and cancer risk: role of myeloid derived suppressor cells and alternately activated macrophages. Am J Cancer Res. 2013;3(1):21–33. [PMC free article] [PubMed] [Google Scholar]
- 150.Thomas D, Apovian C. Macrophage functions in lean and obese adipose tissue. Metabolism. 2017;72:120–143. doi: 10.1016/j.metabol.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25(1):358–369. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11(10):936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 153.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–894. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pirvulescu MM, Gan AM, Stan D, Simion V, Calin M, Butoi E, et al. Subendothelial resistin enhances monocyte transmigration in a co-culture of human endothelial and smooth muscle cells by mechanisms involving fractalkine, MCP-1 and activation of TLR4 and Gi/o proteins signaling. Int J Biochem Cell Biol. 2014;50:29–37. doi: 10.1016/j.biocel.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 155.Galvan GC, Johnson CB, Price RS, Liss MA, Jolly CA, deGraffenried LA. Effects of Obesity on the Regulation of Macrophage Population in the Prostate Tumor Microenvironment. Nutr Cancer. 2017:1–7. doi: 10.1080/01635581.2017.1359320. [DOI] [PubMed] [Google Scholar]
- 156.Ghaebi M, Nouri M, Ghasemzadeh A, Farzadi L, Jadidi-Niaragh F, Ahmadi M, et al. Immune regulatory network in successful pregnancy and reproductive failures. Biomed Pharmacother. 2017;88:61–73. doi: 10.1016/j.biopha.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 157.Fainaru O, Hantisteanu S, Hallak M. Immature myeloid cells accumulate in mouse placenta and promote angiogenesis. Am J Obstet Gynecol. 2011;204(6):544e518–523. doi: 10.1016/j.ajog.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 158*.Pan T, Liu Y, Zhong LM, Shi MH, Duan XB, Wu K, et al. Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice. J Leukoc Biol. 2016;100(3):499–511. doi: 10.1189/jlb.1A1015-481RR. Together with reference 162 this study demonstrate the role of MDSC in maintenance of feto-maternal tolerance. [DOI] [PubMed] [Google Scholar]
- 159.Kang X, Zhang X, Liu Z, Xu H, Wang T, He L, et al. CXCR2-Mediated Granulocytic Myeloid-Derived Suppressor Cells’ Functional Characterization and Their Role in Maternal Fetal Interface. DNA Cell Biol. 2016;35(7):358–365. doi: 10.1089/dna.2015.2962. [DOI] [PubMed] [Google Scholar]
- 160.Pan T, Zhong L, Wu S, Cao Y, Yang Q, Cai Z, et al. 17beta-Oestradiol enhances the expansion and activation of myeloid-derived suppressor cells via signal transducer and activator of transcription (STAT)-3 signalling in human pregnancy. Clin Exp Immunol. 2016;185(1):86–97. doi: 10.1111/cei.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kang X, Zhang X, Liu Z, Xu H, Wang T, He L, et al. Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25-T cells by activation of the TGF-beta/beta-catenin pathway. Molecular human reproduction. 2016;22(7):499–511. doi: 10.1093/molehr/gaw026. [DOI] [PubMed] [Google Scholar]
- 162*.Ostrand-Rosenberg S, Sinha P, Figley C, Long R, Park D, Carter D, et al. Frontline Science: Myeloid-derived suppressor cells (MDSC) facilitate maternal-fetal tolerance in mice. J Leukoc Biol. 2017;101(5):1091–1101. doi: 10.1189/jlb.1HI1016-306RR. Together with reference 158 this study demonstrate the role of MDSC in maintenance of feto-maternal tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gantt S, Gervassi A, Jaspan H, Horton H. The role of myeloid-derived suppressor cells in immune ontogeny. Frontiers in immunology. 2014;5:387. doi: 10.3389/fimmu.2014.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bartmann C, Junker M, Segerer SE, Hausler SF, Krockenberger M, Kammerer U. CD33(+) /HLA-DR(neg) and CD33(+) /HLA-DR(+/−) Cells: Rare Populations in the Human Decidua with Characteristics of MDSC. Am J Reprod Immunol. 2016;75(5):539–556. doi: 10.1111/aji.12492. [DOI] [PubMed] [Google Scholar]
- 165.Kostlin N, Kugel H, Spring B, Leiber A, Marme A, Henes M, et al. Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses. Eur J Immunol. 2014;44(9):2582–2591. doi: 10.1002/eji.201344200. [DOI] [PubMed] [Google Scholar]
- 166.Nair RR, Sinha P, Khanna A, Singh K. Reduced Myeloid-derived Suppressor Cells in the Blood and Endometrium is Associated with Early Miscarriage. Am J Reprod Immunol. 2015;73(6):479–486. doi: 10.1111/aji.12351. [DOI] [PubMed] [Google Scholar]
- 167.Kim YJ, Park HS, Lee HY, Ha EH, Suh SH, Oh SK, et al. Reduced L-arginine level and decreased placental eNOS activity in preeclampsia. Placenta. 2006;27(4–5):438–444. doi: 10.1016/j.placenta.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y, Qu D, Sun J, Zhao L, Wang Q, Shao Q, et al. Human trophoblast cells induced MDSC from peripheral blood CD14(+) myelomonocytic cells via elevated levels of CCL2. Cellular & molecular immunology. 2016;13(5):615–627. doi: 10.1038/cmi.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gervassi A, Lejarcegui N, Dross S, Jacobson A, Itaya G, Kidzeru E, et al. Myeloid derived suppressor cells are present at high frequency in neonates and suppress in vitro T cell responses. PloS one. 2014;9(9):e107816. doi: 10.1371/journal.pone.0107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rieber N, Gille C, Kostlin N, Schafer I, Spring B, Ost M, et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin Exp Immunol. 2013;174(1):45–52. doi: 10.1111/cei.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kostlin N, Vogelmann M, Spring B, Schwarz J, Feucht J, Hartel C, et al. Granulocytic myeloid-derived suppressor cells from human cord blood modulate T-helper cell response towards an anti-inflammatory phenotype. Immunology. 2017;152(1):89–101. doi: 10.1111/imm.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Leiber A, Schwarz J, Kostlin N, Spring B, Fehrenbach B, Katava N, et al. Neonatal myeloid derived suppressor cells show reduced apoptosis and immunosuppressive activity upon infection with Escherichia coli. Eur J Immunol. 2017;47(6):1009–1021. doi: 10.1002/eji.201646621. [DOI] [PubMed] [Google Scholar]
- 173.Heinemann AS, Pirr S, Fehlhaber B, Mellinger L, Burgmann J, Busse M, et al. In neonates S100A8/S100A9 alarmins prevent the expansion of a specific inflammatory monocyte population promoting septic shock. FASEB J. 2017;31(3):1153–1164. doi: 10.1096/fj.201601083R. [DOI] [PubMed] [Google Scholar]
- 174.Ulas T, Pirr S, Fehlhaber B, Bickes MS, Loof TG, Vogl T, et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat Immunol. 2017;18(6):622–632. doi: 10.1038/ni.3745. [DOI] [PubMed] [Google Scholar]