Abstract
Objective
Mindfulness training has been shown to improve psychological well-being and physical health. One proposed pathway for the positive effects of mindfulness training is through the development of new emotion regulation strategies, such as the ability to experience emotions by observing and accepting them without judgment. Theoretically, this should facilitate recovery from negative emotional states; however, this has rarely been examined empirically. The goal of the current study was to determine whether mindfulness training is associated with more efficient emotional and cardiovascular recovery from induced negative affect.
Methods
The current study tested emotional and cardiovascular recovery from induced negative affect during a personal recall task in women randomly assigned to 6-weeks of mindfulness training (n=39) compared to women assigned to a wait-list control condition (n=32). During baseline, task, and post-task rest, blood pressure and heart rate were monitored at fixed intervals and heart rate variability (HRV) and pre-ejection period (PEP) were monitored continuously. This study was embedded within a randomized trial that evaluated the effects of mindfulness training in a sample of younger breast cancer survivors, a group in need of access to effective psychosocial intervention as they can experience high stress, anxiety, and physical symptoms for many years in to survivorship.
Results
In response to the personal recall task, women in both the intervention and control groups showed significant increases in sadness, anxiety, and anger, with the intervention group reaching higher levels of sadness and anger than controls. Further, the intervention group showed a significantly steeper decline in sadness and anger, as well as steeper initial decline in diastolic blood pressure compared to women in the wait list control condition. Groups did not differ in their self-reported feelings of anxiety, or in blood pressure, heart rate, or pre-ejection period (PEP) responses to the task. The control group demonstrated an increase in heart rate variability (HRV) during the task (indexed by the root mean square of successive differences in heart rate; RMSSD) while the intervention group remained flat throughout the task.
Conclusion
Compared to the control group, women in the intervention group experienced greater negative emotions when recalling a difficult experience related to their breast cancer, and demonstrated an efficient emotional and blood pressure recovery from the experience. This suggests that mindfulness training may lead to an enhanced emotional experience coupled with the ability to recovery quickly from negative emotional states.
1. Introduction
Being mindful is the act of “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” (Kabat-Zinn, 1994, p. 4). Mindfulness is a way of relating to the present moment that is thought to reduce overall suffering and increase well-being (Ie, Ngnoumen, Langer, 2014). Indeed, randomized controlled trials have shown that mindfulness-based psychological interventions can improve stress-related mental health outcomes including anxiety, depressive symptoms, and perceived stress (for reviews see Keng, Smoski, & Robins, 2011, and Goyal et al., 2014).
One proposed pathway for the positive effects of mindfulness training is its facilitation of new strategies for adaptive emotion regulation (e.g. Bishop et al., 2004; Kabat-Zinn, 1994; Teper, Segal, & Inzlicht, 2013). Emotion regulation has been defined as “the process by which individuals influence which emotions they have, when they have them, and how they experience and express these emotions” (Gross, 1998). Mindfulness training theoretically alters how one experiences emotions because the training exercises teach participants to non-judgmentally observe and accept, rather than react to, their thoughts and feelings (Kabat-Zinn, 1994). Therefore, we might expect mindfulness training to improve responses to and recovery from negative emotions by increasing acceptance of the emotion and decreasing negative thinking about it (Bishop et al., 2004). Indeed, several studies have found that mindfulness-based interventions lead to self-reported improvements in emotion regulation, including reduced rumination (for discussions of this literature see Chambers, Gullone, Allen, 2009; Chiesa & Serretti, 2009; Roemer, Williston, & Rollins, 2015).
Studies examining the effects of mindfulness on responses to negative emotions are primarily experimental studies in which mindfulness is induced via a short guided meditation immediately prior to showing participants emotionally provocative stimuli such as emotional film clips (e.g. Arch & Craske, 2006; Erisman & Roemer, 2010). These laboratory studies suggest that brief inductions of mindfulness can decrease reactivity and speed recovery from aversive stimuli (Keng, Smoski, & Robins, 2011). However, it remains unknown whether mindfulness training over several weeks (consistent with mindfulness-based psychological interventions) alters responses to negative emotions without inducing states of mindfulness, and whether responses are altered in situations that trigger a complex set of emotions, not just single emotions as is the case when using emotional film clips as the stimuli.
Few studies have examined whether mindfulness training alters emotional responses to induced negative affect in healthy adults. Previous studies have shown that mindfulness training can decrease perceptions of how stressful the Trier Social Stress Test (TSST) is, a commonly used acute stress paradigm that uses social evaluate threat to induce negative affect, including anxiety (Britton, Shahar, Szepsenwol, and Jacobs, 2012; Creswell et al., 2014; Nyklíček et al., 2013). However, only one of these studies reports on group differences in emotions post-task, which is important in the evaluation of whether mindfulness improves the ability to recovery quickly from an emotionally arousing event. This study found that in comparison to the control group, participants who received 8-weeks of mindfulness-based cognitive training (MBCT) reported significantly lower state anxiety after the TSST (Britton et al., 2012). No studies reported on treatment group differences in emotional recovery trajectories (change in emotion ratings from during the task to the post-task rest period). These studies are limited in that they do not examine whether mindfulness training specifically improves recovery from negative affect inducing situations, or whether mindfulness training alters responses to tasks other than the TSST.
Coupled with improving emotional recovery, mindfulness training may also enhance physiologic recovery from experiences of strong emotion (Kabat-Zinn, 1994), though few studies have examined this hypothesis in healthy adults. A study by Nyklíček et al. (2013) reported that eight weeks of mindfulness-based stress reduction (MBSR) led to a significant decrease in blood pressure reactivity in response to the TSST from pre- to post-intervention compared to an active health education control condition, though the authors did not report tests of group differences in blood pressure recovery. In a study of a brief mindfulness intervention, participants who engaged in three days (25 minutes per day) of mindfulness training compared to three days of cognitive training did not differ in the overall pattern of blood pressure response to the TSST (Creswell et al., 2014). Examining the effect of mindfulness training on recovery from emotional arousal is an important area of research given the proposed role of physiological recovery in healthy biological functioning. Specifically, prolonged physiologic activation, indicating inefficient recovery, is thought to damage the cardiovascular, immune, and endocrine systems over time, acting as a key mediator in stress-disease models (Linden et al., 1997; McEwen, 1998). Longitudinal studies have demonstrated that slower blood pressure recovery from stressful behavioral tasks (e.g. mirror tracing) predicts cardiovascular disease events years later (Pieper and Brosschot, 2005; Steptoe and Marmot, 2005). Thus, faster, more efficient recovery from arousal may protect health.
In addition to interest in the role of cardiovascular responses to emotional arousal, a growing number of studies have examined the relationship between the autonomic nervous system and emotional responses and regulation (Appelhans & Luecken, 2006). Theoretical models, and some empirical evidence, point to an association between parasympathetically mediated heart rate variability (HRV) and various measures and outcomes relevant to regulated emotional responding (Appelhans & Luecken, 2006; Porges, 2007; Thayer & Lange, 2000). Experimental studies of HRV responses to acute induction of discrete emotions have shown that some emotions increase HRV (e.g. sadness, amusement) and some decrease HRV (e.g. anger, anxiety; for a review see Kreibig, 2010). HRV responses to more complex emotional experiences, such as those induced by personal recall paradigms, have rarely been explored. Additionally, what constitutes a ‘good’ HRV response to emotional arousal, and how psychosocial interventions may module HRV responses, remains unknown.
The purpose of the current study was to experimentally probe whether mindfulness training improves the ability to recover from negative affect and associated physiological arousal. This study was embedded within a randomized trial that evaluated the effects of 6-weeks of mindfulness training in a sample of younger breast cancer survivors (Bower et al., 2015). Approximately 20% of breast cancers occur among women younger than 50 (American Cancer Society, 2014). Younger women with breast cancer report higher levels of stress and depression and more physical symptoms such as fatigue and sleep disturbance compared to older women with breast cancer and healthy age-matched controls (Howard-Anderson, Ganz, Bower, & Stanton, 2012). These women are diagnosed with a life-threatening illness at a time that is out of sync with expectations of health problems. Many feel isolated from both their peers and from the breast cancer community (Thewes, Butos, Girgis, & Pendelbury, 2004), where the median age of diagnosis in the U.S. is 62 (National Cancer Institute, 2016). Their need makes them an important group to target for intervention, and more broadly, they present a good model for examining interventions that aim to decrease stress and improve physiological health in high-risk samples. Results of the parent trial indicated significant a decrease in perceived stress and marginal decrease in depressive symptoms among women randomized to mindfulness training compared to women in the wait-list control group post-intervention, and a decrease in inflammation-related gene expression (Bower et al., 2015).
In order to probe recovery from experiencing acute negative affect using an ecologically valid approach, we adapted a previously validated personal recall paradigm (Gerin et al., 2006; Salas et al., 2012). In this version of the task, participants re-lived an anxiety-provoking experience related to their diagnosis and treatment of cancer. We chose this task to approximate the real-world emotional experiences of young breast cancer survivors. Cancer-related anxiety is particularly relevant for our sample of young breast cancer survivors, as they can struggle with fears of recurrence and cancer-related intrusive thoughts for years after diagnosis (Bloom et al., 2001; Dupont, Bower, Stanton, and Ganz, 2014; Koch et al., 2012).
We hypothesized that women in the mindfulness group would recover more quickly on outcomes of state negative affect, blood pressure, and heart rate in response to recalling a negative emotional experience compared to the control group, given that mindfulness training emphasizes observing and accepting emotions without efforts to alter them. We also examined the effects of the intervention on parasympathetic nervous system (via a measure of heart rate variability; HRV) and sympathetic (via measurement of pre-ejection period; PEP) responses. We did not have directional hypotheses about these outcomes given the limited research on autonomic nervous system responses to tasks that evoke a complex set of emotions, such as the personal recall task we used.
2. Methods
2.1 Study Design
Data came from a study of 71 breast cancer survivors who participated in a single-center, two-armed RCT of mindfulness meditation at the UCLA Medical Center, Los Angeles, CA between March 2011 and October 2012. Study recruitment and randomization are described in detail in Bower et al. (2015). Briefly, participants were randomly assigned to a six-week mindfulness intervention or wait-list control. They completed an in-person assessment pre- and post-intervention, and questionnaires at a 3-month follow-up. The UCLA Institutional Review Board approved study procedures, and written informed consent was obtained from participants.
2.2. Participants
Women were eligible for the study if they had been diagnosed with Stage 0 – III breast cancer at or before age 50. Participants were required to have completed local and/or adjuvant cancer therapy (except hormonal therapy) at least three months prior to study entry, and were eligible for up to 10 years post-cancer treatment. Women were excluded if they had a breast cancer recurrence, metastasis, or another cancer diagnosis (excluding non-melanoma skin cancer), had an active, uncontrolled medical illness, or were unable to commit to the intervention schedule.
2.3. Intervention
The mindfulness intervention was based on the Mindful Awareness Practices (MAPs) program developed by the UCLA Mindful Awareness Research Center (MARC; http://marc.ucla.edu). The intervention was tailored for younger breast cancer survivors by focusing on issues known to be relevant for them (e.g. fears of recurrence, staying healthy in survivorship). Women assigned to the intervention group met for six weekly two-hour group sessions. These sessions included presentation of theory and research on mindfulness and relaxation, experiential practice of mindfulness meditation, and a psycho-educational component for cancer survivors. Diana Winston, the Director of Mindfulness Education at MARC, with over 20 years of experience teaching mindfulness meditation, taught the course. The wait-list condition controlled for levels of interest in mindfulness training, as well as naturally occurring changes in stress and other outcomes over the assessment period. Participants in the waitlist control condition were offered the intervention at the end of the study.
Key areas of focus in the mindfulness program were paying attention to the present moment, managing pain, working with negative emotions, and implementing mindfulness practices in daily life. Techniques for working with negative emotions included cultivating meta-awareness to learn to let go of difficult thoughts and feelings rather than being caught in them, and cultivating positive emotions to counteract a negative orientation. For example, one exercise led participants through a meditation in which they used imagery to visualize their thoughts as “floating down a river while you are seated on the riverbank.” Theoretically, this meta-cognitive approach allows women to experience negative thoughts and emotions with distance, thereby avoiding the feed-forward loop of perseverative cognition (i.e. rumination, worry) and associated physiologic arousal that is thought to prevent efficient recovery (Brosschot, Pieper, and Thayer, 2005).
2.4. Procedures for Post-Intervention Assessment
Participants completed an in-person clinic visit before and after the intervention (within two weeks of the final class). During the visit participants completed psychosocial questionnaires and provided blood samples for immune evaluation. Effects of the intervention on these outcomes are described in Bower et al. (2015).
At the post-intervention assessment, participants also completed a personal recall task designed to induce negative emotions. To begin this task, participants were first connected to physiological monitoring equipment (described below) and asked to rest quietly for 10 minutes while baseline physiological measures were collected. Women were then told they were going to engage in a brief conversation about something related to their breast cancer experience. Specifically, women were asked to identify a time within the last year that they experienced anxiety related to their breast cancer experience. Anxiety was chosen given that more than half of younger breast cancer survivors report high levels of anxiety, even five years after diagnosis (Bloom et al., 2001). To ensure the chosen topic was potent enough to elicit arousal, participants were asked to rate from 1 to 10 how anxious they were at the time of the original event. If they reported a score less than 7, they were asked to think of another experience that provoked more anxiety. Common experiences described were: yearly surveillance mammograms, a friend’s cancer diagnosis, and fearing a perceived lump may indicate cancer recurrence.
After identifying the personal experience, participants spent five minutes telling the story of that experience, and were asked to connect with their original emotions as best they could by describing the feelings, thoughts, and sensations they had during that time. The research staff asked follow-up questions to prompt participants (e.g., “Where did you feel that emotion in your body?”, “Tell me more about what you were thinking in that moment.”). After completing the task, participants were thanked by the research assistant for sharing their experience and asked to sit quietly for a 12-minute period of rest. Participants were not cued to use mindfulness or any other skills during or after the task. A trained graduate-level research assistant who was unaware of group assignment administered this task. The task was conducted only at post-intervention to avoid habituation.
To assess emotional and physiological responses to the task, data were captured at set intervals or continuously (for certain physiological measures) throughout a 10-minute baseline period, during the task (for physiological assessment only), immediately post-task, and during a 12-minute period of rest.
2.5. Emotional Response Measures
Emotional responses were captured with three items from the Positive and Negative Affect Schedule (PANAS; Watson and Clark, 1994) at baseline, immediately after the task, and a mid-way through the 12-minute rest period. Women reported how anxious, sad, and angry they felt on a scale from 1 (not at all) to 4 (extremely). These emotions were chosen a priori as these were the emotions we anticipated to the task would induce.
2.6. Physiologic Response Measures
Physiological responses to the task were assessed via periodic blood pressure and heart rate monitoring and continuous collection of electrocardiography (ECG) and impedance cardiography (ICG) data throughout the task. Participants were asked to sit comfortably in a chair with both legs on the floor and arms in their lap or on the armrest as the data were collected. They were not given reading materials or other distractions, and were asked to minimize moving and speaking during the baseline and rest periods.
2.6.1. Blood pressure and heart rate data collection
Cardiovascular data were captured with heart rate and blood pressure monitoring at set intervals throughout the personal recall task via an automated blood pressure cuff (Dinamap Pro100V2; GE Medical Systems): during the 10-minute baseline (readings at minutes 5, 7, and 9), during the 5-minute recall task (readings at minutes 2 and 4), immediately post-task (reading within 3 minutes of the task completing), and in the second half of the 12-minute rest (at minutes 7, 10, and 12). Values were averaged across each time period (Christenfeld, Glynn, & Gerin, 2000).
2.6.2. Heart rate variability (HRV) and pre-ejection period (PEP) data collection
Autonomic nervous system (ANS) activity was monitored with electrocardiography (ECG) and impedance cardiography (ICG). Three general purpose electrodes (Biopac Systems, Inc., USA) were placed on the participants’ inner forearms and on the collar bone to form a modified lead II electrode placement. Four bioimpedance strip transducers (Biopac Systems, Inc., USA) were placed on the back of the neck and lower back. Electrodes were attached to the processor (MP150; Biopac Systems, Inc., USA), which was connected to a local computer via ethernet. Data were captured continuously and processed off-line in 2–3 minute bins of data using AcqKnowledge 4.2 (Biopac Systems Inc., USA) and Kubios software programs (University of Kuopio, Finland). Data from baseline, during task, and the rest period were averaged to calculate the final values used in analyses. Since we anticipated arousal levels to differ in the beginning versus the end of the rest period, we calculated averaged values from the first three minutes post-task and from the second half of the rest period (minutes 6–12).
To examine parasympathetic nervous system activity, we used an index of HRV, the root mean square of successive differences in R-R intervals (RMSSD). RMSSD is computed from continuous ECG recordings by analyzing the variability in the intervals between heart beats (R-R intervals; Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology, 1996). RMSSD is reported in milliseconds (ms), with higher RMSSD indicating increased parasympathetic activation (Berntson et al., 1994; Task Force, 1996).
PEP is a systolic time interval measure that has been used as an estimate of beta adrenergic sympathetic nervous system activity (Berntson et al., 1994; Berntson, Lozano, Chen, and Cacioppo, 2004). PEP is measured by estimating the time interval from the beginning of the QRS complex (i.e. the onset of the Q wave) in the ECG to the B point in the dZ/dt waveform in the ICG recordings. Shorter PEP times reflect increased β-adrenergic sympathetic nervous system drive on the heart.
2.7. Statistical Analyses
Emotional and physiologic responses to the personal recall task were tested using multi-level modeling, which is appropriate for analyzing data with more than two time points nested within individuals. Multi-level modeling allows for the inclusion of all available data, even when outcome data are missing. Intervention group was included as a predictor at the level of the individual, with the control group coded as ‘0’ and the intervention group coded as ‘1’. Separate analyses were conducted for each outcome. Each model first estimated the effects of group, time, and the group by time interaction (group difference in overall response pattern), then tested specific phases of the recovery pattern with simple effects estimates. An independent errors variance component structure was used.
We examined group differences in emotional recovery by testing differences in mean change from immediately post-task (our attempt to capture peak emotional experience since emotion ratings were not captured during the task) to the rest period. We examined blood pressure and heart rate recovery by testing the mean change from during the task to the end of the rest period (recovery A). To test whether group differences in recovery were more or less pronounced at the beginning or end of the recovery trajectory, we also examined two pieces of the recovery trajectory separately: change from during the task to the first post-task blood pressure reading (recovery B) and change from the first post-task blood pressure reading to the end of the rest period (recovery C). Thus, recovery A allows for the examination of the overall recovery pattern, and recovery B and C allows us to test whether group differences in recovery happened at the beginning or end of the recovery process. We followed the same analysis procedure for RMSSD and PEP responses. Results for reactivity (mean change from baseline to during task) are reported when appropriate though we had no specific hypotheses about group differences in reactivity. We also tested group differences in peak outcome scores (physiologic arousal during the task and emotion ratings immediately after the task) using follow-up contrasts.
3. Results
3.1. Sample Characteristics
Detailed participant characteristics and intervention effects on primary psychological and immune outcomes have been reported (Bower et al., 2015). Briefly, 71 women were randomized to the 6-week Mindful Awareness Practices (MAPs; n=39) intervention or to a wait-list control condition (n=32). Women were on average 47 years old, 4 years since diagnosis, White (76%), employed at least part-time (75%), and had a family income over $100k (60%). Participants self-reported their cancer stage: 11% reported stage 0 (n=8), 35% stage 1 (n=25), 44% stage 2 (n=31), and 10% stage 3 (n=7). The majority of women had received chemotherapy (73%) and were currently on endocrine therapy (64%). The intervention and control groups were comparable at baseline on most demographic and cancer-related variables, though women in the intervention group were slightly less likely to be married, more likely to have received radiation, and more likely to have a history of smoking than women in the control group (p’s ≤ .05). These group differences were included as co-variates in the multi-level models, and in the models with physiologic outcomes, body mass index (BMI) and age were also included as these characteristics can influence biological outcomes.
3.2. Emotional Response to the Personal Recall Task
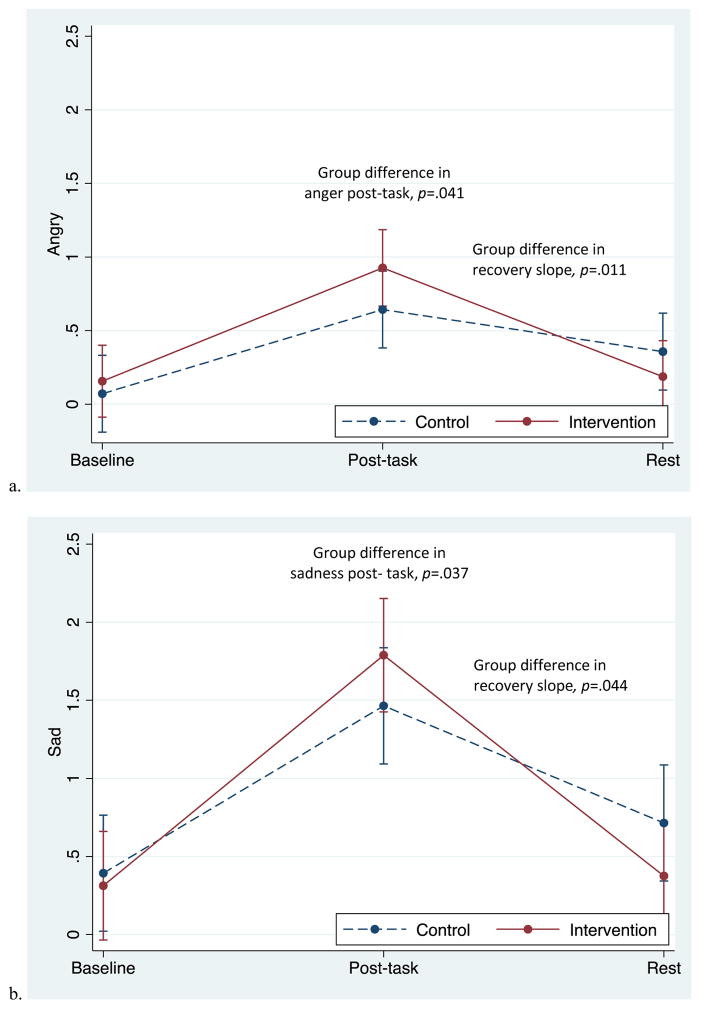
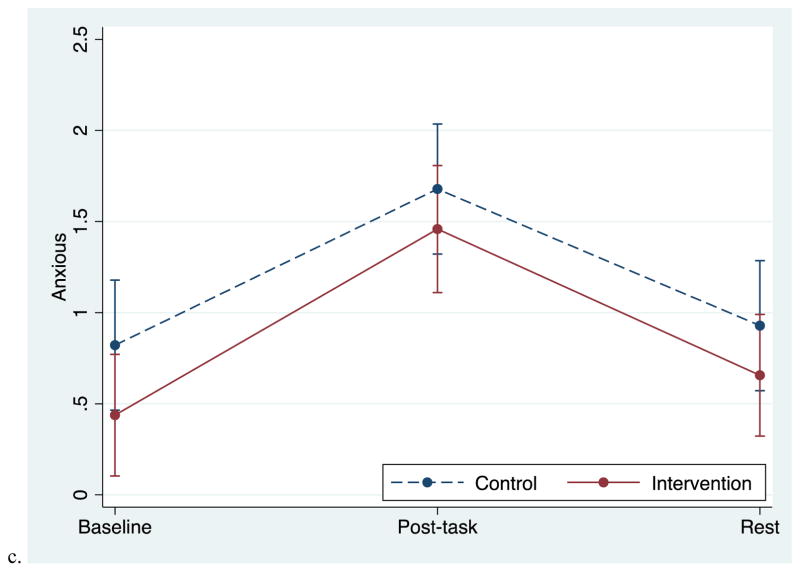
Both groups reported increased anxiety, anger, and sadness in response to the task, followed by significant decreases during the recovery period (Figures 1a – 1c). Table 1 presents unadjusted mean values at each time point and p values for group differences in reactivity and recovery. The slope of recovery differed between groups, with the intervention group showing a steeper decline for anger (p=.044) and sadness (p=.011) from post-task to rest than controls. Groups did not differ in the recovery of anxiety (p=.83). Reactivity slopes did not differ between groups, though the intervention group did report greater sadness (p=.041) and greater anger (p=.037) immediately post-task compared to the control group in adjusted analyses.
Figure 1.
Adjusted mean responses to the personal recall task in the two groups. Data from multilevel models. Error bars reflect +-1 standard error.
Table 1.
Unadjusted means at each time point and results from planned contrasts comparing the intervention and control groups on emotional responses to the personal recall task.
| Unadjusted means (SD) | Group differences | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-task | Rest | Reactivitya | Recoveryb | ||||
|
| ||||||||
| Intervention | Control | Intervention | Control | Intervention | Control | p | p | |
| Angry | .16 (.45) | .07 (.26) | .93 (1.15) | .64 (.87) | .19 (.47) | .36 (.78) | .372 | .044 |
| Sad | .31 (.69) | .39 (.83) | 1.79 (1.37) | 1.46 (1.32) | .38 (.66) | .71 (1.12) | .122 | .011 |
| Anxious | .44 (.62) | .82 (.86) | 1.5 (1.23) | 1.68 (1.16) | .66 (.90) | .93 (1.05) | .507 | .83 |
Reactivity was calculated as the change from baseline to post-task.
Recovery was calculated as the change from post-task to the rest period.
Note. Unadjusted means are presented here. P values for reactivity and recovery are based on adjusted models. Emotion ratings were given 6 minutes in to the 12-minute rest period.
Emotional intensity increased on average from “not at all” to “a little bit” in response to the task. These responses indicate that the participants were experiencing the negative emotions at relatively low intensity at the time points they were measured. However, participants also reported that they were engaged with the task and able to re-experience their negative emotions in this setting, reporting on average that they felt extremely able to connect with their original emotions during the task (x̄= 7.6, SD=2.15, scale of 1 at the lowest to 10 at the highest).
3.3. Blood Pressure and Heart Rate Response to the Personal Recall Task
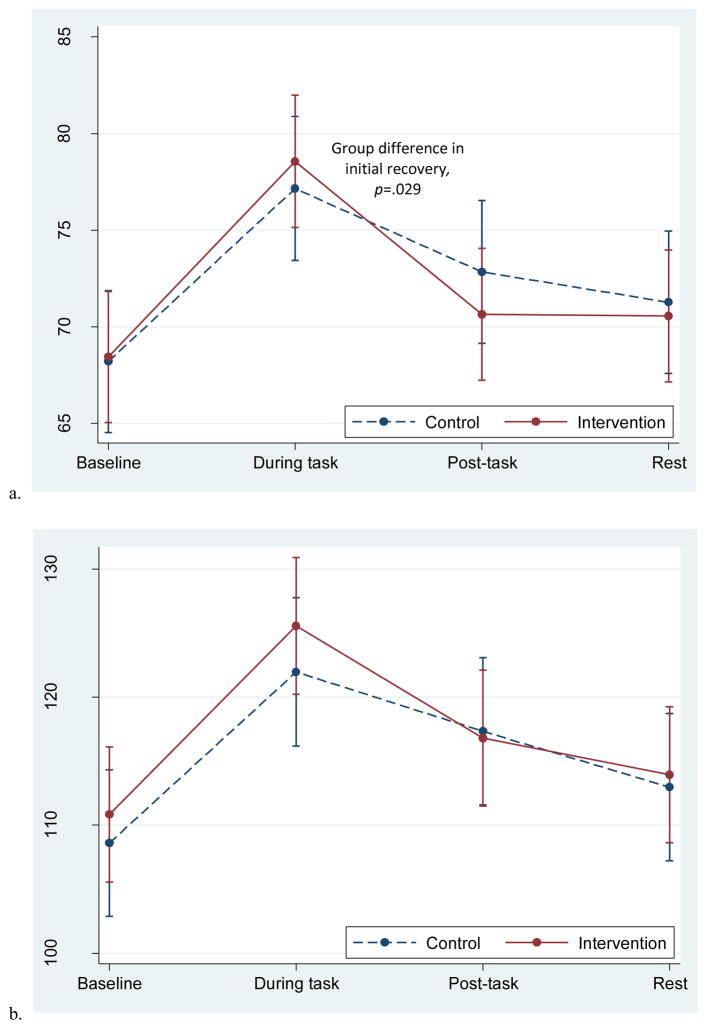
Blood pressure and heart rate increased in response to the task and then decreased during the rest period for both groups (Figure 2a–c). The recovery slope for DBP differed by group; the intervention group demonstrated a steeper initial decline of DBP from during the task to post-task (recovery B), χ2 (1, N=60)=4.77, p=.029, indicating a more pronounced recovery in the beginning of the rest period compared to the control group. A similar pattern was seen for SBP, with the intervention group showing a steeper initial decline, though this did not reach statistical significance (recovery B; p=.104). Groups did not differ in heart rate recovery speed, or in DBP, SBP, or HR reactivity. The unadjusted means at each time point by group are presented in Table 2a and the results of the tests of reactivity and recovery are presented in Table 2b.
Figure 2.
Adjusted mean blood pressure and heart rate responses to the personal recall task in the two groups. Data from multilevel models. Error bars reflect +-1 standard error.
Table 2a.
Unadjusted means for physiologic responses to the personal recall task.
|
Unadjusted means (SD)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | During Task | Post-Task | Rest | |||||
|
| ||||||||
| Variable | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control |
| DBP | 67.9 (7.2) | 68.9 (9.9) | 78.0 (11.4) | 76.7 (10.4) | 70.1 (10.8) | 73.9 (10.7) | 70.0 (9.1) | 71.5 (9.8) |
| SBP | 109.9 (13.0) | 109.8 (13.2) | 123.7 (19.9) | 120.7 (13.8) | 115.2 (18.5) | 119.2 (12.5) | 112.4 (16.4) | 113.1 (11.3) |
| HR | 70.1 (10.7) | 66.3 (8.4) | 76.9 (10.4) | 72.8 (10.6) | 70.7 (10.2) | 68.4 (10.9) | 72.3 (10.3) | 69.0 (8.9) |
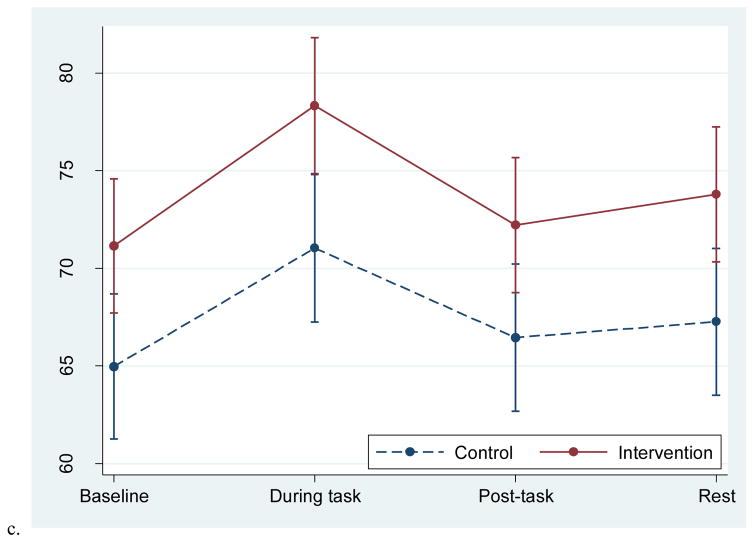
| RMSSD | 28.9 (12.8) | 30.9 (16.2) | 31.9 (17.8) | 37.8 (21.6) | 32.8 (17.5) | 29.8 (13.1) | 27.9 (14.1) | 29.3 (14.8) |
| PEP | 110.6 (16.8) | 113.4 (17.6) | 108.2 (16.7) | 109.5 (14.8) | 110.4 (17.6) | 113.1 (14.9) | 112.4 (18.6) | 114.0 (14.4) |
Note. SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = heart rate; SD = standard deviation; RMSSD = root mean square of successes differences; PEP = pre-ejection period.
Table 2b.
Results from planned contrasts comparing physiologic reactivity to and recovery from the personal recall task in intervention and control groups.
| Group response differences | ||||
|---|---|---|---|---|
| Reactivityb | Recovery Ac | Recovery Bc | Recovery Cc | |
|
| ||||
| Variable | pd | pd | pd | pd |
| DBP | .472 | .196 | .029* | .361 |
| SBP | .587 | .299 | .104 | .546 |
| HR | .480 | .629 | .340 | .632 |
| RMSSD | .229 | .167 | .006** | .164 |
| PEP | .550 | .889 | .538 | .634 |
p<.05.
p<.001
Reactivity was calculated as the change from baseline to during the task.
Recovery A was calculated as the change from during the task to the end of the rest period (minutes 6–12); Recovery B was calculated as the change from during the task to immediately after the task (first 3 minutes of the rest period); Recovery C was calculated as the change from immediately after the task to the end of the rest period.
P value for group difference in reactivity or recovery.
Note. SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = heart rate; RMSSD = root mean square of successes differences; PEP = pre-ejection period; SE = standard error. Analyses control for baseline group differences.
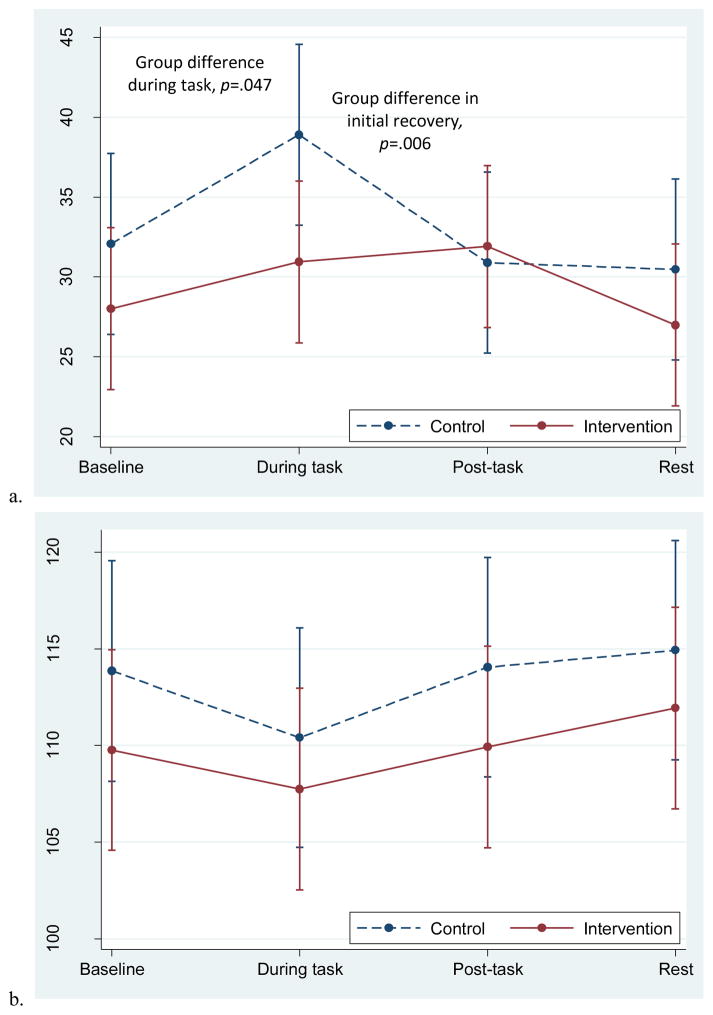
3.4. Autonomic Nervous System Response to the Personal Recall Task
Groups differed significantly in the overall response pattern of RMSSD; the intervention group remained relatively flat throughout the task while the control group had an increase in RMSSD during the task and a return to baseline post-task (Figure 3a). The initial recovery slope differed by group (recovery B), χ2 (1, N=58)=7.70, p=.006. During the task, the groups had significantly different RMSSD values, with the control group having a higher RMSSD compared to the intervention group, p=.047.
Figure 3.
Adjusted mean RMSSD and PEP responses to the personal recall task in the two groups. Data from multilevel models. Error bars reflect +-1 standard error.
For both groups, PEP decreased slightly during the task and increased during the rest period; for the main effect of time, χ2 (3, N=61) =13.9, p=.003 (Figure 3b). This decrease in PEP is consistent with sympathetic nervous system activation and is expected during active tasks. There were no group differences in the pattern of PEP response.
4.0 Discussion
This study assessed whether mindfulness training in younger breast cancer survivors improved emotional and physiologic recovery in response to recalling a negative cancer-related emotional experience. Hypotheses that women randomly assigned to mindfulness training would demonstrate faster recovery of negative affect, blood pressure, and heart rate compared to controls were partially supported. The two groups differed in emotional recovery (from the emotions sad and angry), with the intervention group demonstrating a more efficient recovery pattern. The group differences in recovery slope may be related in part to higher levels of negative emotions experienced by the intervention group. Specifically, women in the intervention group reported experiencing more sadness and anger immediately after the personal recall, although there were no significant group differences in emotional reactivity. Blood pressure response patterns were similar, with the intervention group showing a steeper decline in DBP compared to the control group in the first three minutes after the task, indicating efficient initial recovery. Experiences of anxiety and other cardiovascular indices (SBP or HR) were similar across the two groups.
Our findings demonstrate that mindfulness training may increase the ability to experience heightened emotions, and to recovery efficiently from negative emotions. The intervention group may have experienced more intense negative emotions and recovered from the emotional arousal efficiently because the mindfulness training taught them to non-judgmentally experience, observe, and not attempt to control negative thoughts and emotions (Kabat-Zinn, 1994). This is consistent with evidence that mindfulness-based psychological interventions improve the management of perseverative cognitions that often accompany and prolong negative emotions, such as rumination (Campbell et al., 2012; Jain et al., 2007; Goldin & Gross, 2010; Labelle, Campbell, & Carlson, 2010). Rumination is a form of repetitive thinking about negative experiences that is difficult to disengage from, can lead to continued distress and physiological activation, and is indicative of maladaptive emotion regulation (Ehring et al., 2011; Gerin et al., 2006). Several studies have found that decreases in rumination or related constructs mediated the effect of a mindfulness-based intervention on improvements in psychological well-being (e.g. Shahar, Britton, Sbarra, Figueredo, & Bootzin, 2010; Lengacher et al., 2014). Analyses from the parent study indicate that decreases in self-reported rumination mediated the effect of the intervention on depressive symptoms (Boyle et al., 2017).
Women in the intervention group also demonstrated faster initial decline in DBP after the personal recall task compared to women in the control group. This finding is consistent with models of mindfulness that propose that mindfulness training may exert its health benefits through speeding recovery from emotion-induced physiologic arousal (e.g. Creswell, 2015; Loucks et al., 2015). The efficient recovery may have been driven by decreased rumination on the induced negative emotion and related thoughts, as empirical work has demonstrated that ruminating after an emotion induction is associated with higher DBP in the recovery period as compared to mind wandering and problem solving (Ottaviani et al., 2016). There were no group differences in recovery for other cardiovascular measures. The lack of uniformity in results for DBP, SBP, and heart rate is common in studies examining cardiovascular responses to challenge as these indexes are influenced by different physiological processes and combinations of mood states (Shapiro, Jamner, Goldstein, and Delfino, 2001). Furthermore, the finding of efficient DBP may have greater health relevance for our sample compared to changes in SBP or HR responses as resting DBP is the strongest predictor of cardiovascular risk in adults younger than 50 years of age (Wang, Staessen, Franklin, Fagard, and Gueyffier, 2005). Importantly, the group difference in DBP were small and do not imply differences in immediate disease risk, though small differences in recovery may add up over time to confer protection from prolonged activation thought to be associated with physiological degradation over time (McEwen, 1998).
We found that the control group demonstrated increased RMSSD in response to the personal recall task while the intervention group remained flat throughout the task. This is somewhat surprising given that initial evidence has linked greater resting HRV to the use of adaptive emotion regulation and coping strategies, and lower HRV with outcomes indicative of emotional dysregulation such as anxiety, depression, and rigid attention processing of threat (Appelhans & Luecken, 2006). However, most research on HRV has focused on resting/ baseline levels, whereas our study explored HRV responses to an active task. Researchers have hypothesized that greater increases in HRV during a task that requires emotion regulation is indicative of an adaptive response (e.g. Mendes, 2011), although few studies have tested this. Based on these hypotheses, it would be expected that participants who received the mindfulness training would demonstrate increased RMSSD during the personal recall task as they were taught tools for effectively handling difficult emotions. Instead, intervention participants’ RMSSD remained stable throughout the task, while increases were observed in the control group. This may indicate that mindfulness training somehow reduced the theoretically adaptive HRV response.
Another way to interpret these findings is through theoretical models of self-regulation and parasympathetic functioning which suggest that vagal activity increases while exerting self-regulatory efforts, leading to increases in HRV (Segerstrom and Nes, 2007). Women in the control group may have been using emotion regulation techniques that require more cognitive effort, such as reappraisal (Keng, Robins, Smoski, Dagenbach, & Leary, 2013), which led to the increases in HRV during the task. This possibility is consistent with a handful of studies that have shown that efforts to modify emotional responses are associated with increased vagal activity. For example, women who suppressed their emotions or reappraised them in response to an upsetting film clip showed significant increases in vagal activity from baseline compared to women who were not told to regulate their emotions (Butler, Wilhelm, & Gross; 2006). Future research should explore whether alterations in vagal activity are associated with different types of emotion regulation strategies during affective states, and whether mindfulness training alters use of specific emotion regulation strategies. It is also possible that the two groups differed in their reactivity and recovery at baseline as we did not perform the task prior to the intervention.
Findings add to the existing literature in several ways. First, to our knowledge, this is the first trial to examine mindfulness effects on emotional and physiological responses to induced negative affect. One of the key skills taught in mindfulness-based therapies is how to regulate difficult thoughts and emotions, though few previous studies have used experimental inductions to test responses to negative emotional experiences after participating in mindfulness training. Another strength of the study is its use of a personal recall task. Re-experiencing an anxiety-provoking experience related to their cancer is more naturalistic than traditionally used stress paradigms (e.g. TSST) in that young breast cancer survivors experience high levels of anxiety and frequent anxiety-inducing experiences related to their cancer. Indeed, situations women recalled during the experimental task (e.g., finding a breast lump) echoed themes discussed during the mindfulness classes. Furthermore, limited attention has been paid to the recovery phase of the acute stress response in the broader literature and work examining mechanisms of stress-reduction interventions, despite its discussion in models of how stress influences health and well-being (Linden et al., 1997; McEwen, 1998). Finally, examining the influence of mindfulness training on sympathetic and parasympathetic reactivity is novel and provides directions for future research into the physiological mechanisms by which mindfulness might exert its positive influence.
A limitation of this study is the use of a wait-list instead of an active control condition. Future research should employ an active control condition matched on outcome expectancies. Another limitation is the homogeneity of the sample. Participants were all women, majority White, and well educated; research is needed to determine whether mindfulness training effects are similar across different ethnic/racial and socioeconomic groups. Stronger effects may have been seen if the personal recall task had elicited stronger emotions or if a task had been chosen that elicited one single emotion predominantly. Although the personal recall paradigm modeled negative emotions more naturalistically than traditional emotion induction paradigms, a limitation of this task is that experiencing multiple emotions at once or in quick succession makes it difficult to interpret the pattern of HRV response. Future examination of HRV and emotion regulation requires improved granularity in both emotion reporting and parallel HRV values. This can be done by processing data in smaller bin sizes (less than 60 seconds segments of data) and including a greater number of within-person repeated measurements of emotion ratings. Because we asked participants to rate their emotions at the end of the recall task, instead of during the task, we likely missed their peak emotional experience. In addition, stronger effects may have been seen if we had cued women to use their mindfulness tools during or after the task, or if the task had been done both pre and post intervention to examine changes in performance. Future studies could address the concern over habituation to the task by varying the manipulation at the two assessment points (e.g., having participants talk about a different experience at each session).
4.1 Conclusion
Findings suggest that young breast cancer survivors who received six weeks of mindfulness training demonstrated faster recovery of negative affect and DBP compared to women in a wait-list control condition. A better understanding of techniques that improve the efficiency of regulating emotional and physiological responses to emotional arousal may help improve the health and well-being of high-stress, vulnerable groups, such as younger breast cancer survivors. Future studies should explore how mindfulness training can be designed to enhance specific components of emotion regulation. Mindfulness programs could include emotion inductions as part of the therapeutic process, similar to exposure therapy in which anxiety is induced and managed during treatment sessions (for a description see Abramowitz, 2013). Practice using mindfulness tools to cope with negative emotions in the moment might allow participants to use them more readily during daily experiences of negative affect. In addition, future studies could use daily diary and ambulatory monitoring methodologies to probe whether effects on recovery from negative emotional experiences are present in the daily lives of women after mindfulness training. Understanding how mindfulness practice influences daily mood and arousal regulation, especially in the face of challenging daily emotional experiences, could elucidate the pathways by which being more mindful enhances well-being and health.
Highlights.
Mindfulness training improved regulation of responses to acute negative affect.
Mindfulness training led to efficient emotional recovery in breast cancer survivors.
Mindfulness training sped blood pressure recovery in breast cancer survivors.
Acknowledgments
Funding
This work was supported by Susan G. Komen by a Komen Scholar Grant to Dr. Ganz. Dr. Crosswell received fellowship support from the NIGMS (T32GM084903), NIA (T32AG033533; R24AG048024), and the UCLA NCI/NIH Cancer Education and Career Development Program (R25T). Dr. Moreno received fellowship support from the NIGMS (5T32GM084903). Dr. Raposa received fellowship support from NIMH (MH15750). We also acknowledge the Petit Foundation for support of the MARC. The authors do not have any conflicts of interest.
Footnotes
Author Declaration
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all authors included and that there are no persons who satisfied the criteria for authorship but are not listed, and that the order of authors listed has been approved by all of us.
We confirm that our work was conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Sincerely,
Alexandra Crosswell (Corresponding Author)
Patricia Moreno
Elizabeth Raposa
Sarosh Motivala
Annette Stanton
Patricia Ganz
Julie Bower
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowitz JS. The practice of exposure therapy: Relevance of cognitive-behavioral theory and extinction theory. Behavior Therapy. 2013;44(4):548–558. doi: 10.1016/j.beth.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Arch JJ, Craske MG. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behavioral Research and Therapy. 2006;44(12):1849–58. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer treatment and survivorship facts & figures 2014–2015. Atlanta: American Cancer Society; 2014. [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10(3):229–240. [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, Cacioppo JT. Where to Q in PEP. Psychophysiology. 2004;41:333–337. doi: 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, Devins G. Mindfulness: A proposed operational definition. Clinical Psychology: Science and Practice. 2004;11(3):230–241. [Google Scholar]
- Bloom JR, Stewart SL, Johnston M, Banks P, Fobair P. Sources of support and the physical and mental well-being of young women with breast cancer. Social Science & Medicine. 2001;53(11):1513–1524. doi: 10.1016/s0277-9536(00)00440-8. [DOI] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, Ma J, Cole SW, Ganz PA. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer. 2015;121(8):1231–1240. doi: 10.1002/cncr.29194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle CC, Stanton AL, Ganz PA, Crespi CM, Bower JE. Improvements in emotion regulation following mindfulness meditation: Effects on depressive symptoms and perceived stress in younger breast cancer survivors. Journal of Consulting and Clinical Psychology. 2017 doi: 10.1037/ccp0000186. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton WB, Shahar B, Szepsenwol O, Jacobs WJ. Mindfulness-based cognitive therapy improves emotional reactivity to social stress: Results from a randomized controlled trial. Behavior Therapy. 2012;43(2):365–380. doi: 10.1016/j.beth.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology. 2005;30(10):1043–1049. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilheim FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Campbell TS, Labelle LE, Bacon SL, Faris P, Carlson LE. Impact of mindfulness-based stress reduction (MBSR) on attention, rumination and resting blood pressure in women with cancer: A waitlist-controlled study. Journal of Behavioral Medicine. 2012;35(3):262–271. doi: 10.1007/s10865-011-9357-1. [DOI] [PubMed] [Google Scholar]
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clinical Psychology Review. 2009;29:560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: A review and meta-analysis. Journal of Alternative and Complementary Medicine. 2009;15(5):593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- Christenfeld N, Glynn LM, Gerin W. On the reliable assessment of cardiovascular recovery: An application of curve-fitting techniques. Psychophysiology. 2000;37:543–550. [PubMed] [Google Scholar]
- Creswell JD. Biological pathways linking mindfulness with health. In: Brown KW, Creswell JD, Ryan R, editors. Handbook on Mindfulness Science. Guilford Publications; New York, NY: 2015. [Google Scholar]
- Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1–12. doi: 10.1016/j.psyneuen.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Dupont A, Bower JE, Stanton AL, Ganz PA. Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychology. 2014;33(2):155–163. doi: 10.1037/a0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T, Zetsche U, Weidacker K, Wahl K, Schonfeld S, Ehlers A. The Perseverative Thinking Questionnaire (PTW): Validation of content-independent measure of repetitive negative thinking. Journal of Behavioral Therapy and Experimental Psychiatry. 2011;42(2):225–232. doi: 10.1016/j.jbtep.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman SM, Roemer L. A preliminary investigation of the effects of experimentally induced mindfulness on emotional responding to film clips. Emotion. 2010;10(1):72–82. doi: 10.1037/a0017162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin W, Davidson KW, Christenfeld NJ, Goyal T, Schwartz JE. The role of angry rumination and distraction in blood pressure recovery from emotional arousal. Psychosomatic Medicine. 2006;68(1):64–72. doi: 10.1097/01.psy.0000195747.12404.aa. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, … Ranasinghe PD. Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Internal Medicine. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Levenson R. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. Journal of the National Cancer Institute. 2012;104(5):386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- Ie A, Ngnoumen CT, Langer EJ. Origins and Theory. In: Ie A, Ngnoumen CT, Langer EJ, editors. The Wiley Blackwell Book of Mindfulness. West Sussex, UK: John Wiley & Sons Ltd; 2014. pp. 1–7. [Google Scholar]
- Jain S, Shapiro SL, Swanick S, Roesch SC, Mills PJ, Bell I, Schwartz GE. A randomized controlled trial of mindfulness meditation versus relaxation training: Effects on distress, positive states of mind, rumination, and distraction. Annals of Behavioral Medicine. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Wherever you go, there you are: Mindfulness meditation in everyday life. New York, NY: Hyperion; 1994. [Google Scholar]
- Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: A review of empirical studies. Clinical Psychology Review. 2011;31(6):1041–1056. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keng SL, Robins CJ, Smoski MJ, Dagenbach J, Leary MR. Reappraisal and mindfulness: A comparison of subjective effects and cognitive costs. Behavioral Research and Therapy. 2013;51(12):899–904. doi: 10.1016/j.brat.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long-term (≥5 years) cancer survivors—a systematic review of quantitative studies. Psycho-Oncology. 2012;22(1):1–11. doi: 10.1002/pon.3022. [DOI] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010;84:394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Labelle LE, Campbell TS, Carlson LE. Mindfulness-based stress reduction in oncology: Evaluating mindfulness and rumination as mediators of change in depressive symptoms. Mindfulness. 2010;1(1):28–40. [Google Scholar]
- Lengacher CA, Shelton MM, Reich RR, Barta MK, Johnson-Mallard V, Moscoso MS, Paterson C, Ramesar S, Budhrani P, Carranza I, Lucas J, Jacobsen PB, Goodman MJ, Kip KE. Mindfulness based stress reduction (MBSR(BC)) in breast cancer: Evaluating fear of recurrence (FOR) as a mediator of psychological and physical symptoms in a randomized control trial (RCT) Journal of Behavioral Medicine. 2014;37(2):185–195. doi: 10.1007/s10865-012-9473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden W, Earle TL, Gerin W, Christenfeld N. Physiological stress reactivity and recovery: Conceptual siblings separated at birth? Journal of Psychosomatic Research. 1997;42(2):117–135. doi: 10.1016/s0022-3999(96)00240-1. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Schuman-Oliver Z, Britton WB, Fresco DM, Desbordes G, Brewer JA, Fulwiler C. Mindfulness and cardiovascular disease risk: State of the evidence, plausible mechanisms, and theoretical framework. Current Cardiology Reports. 2015;17(112) doi: 10.1007/s11886-015-0668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mendes WB. Autonomic nervous system: Obtaining, quantifying, and interpreting peripheral physiological responses. In: Blascovich J, Vanman EJ, Mendes WB, Dickerson S, editors. Social Psychophysiology for Social and Personality Psychology. Los Angeles: SAGE Publications Inc; 2011. pp. 10–41. [Google Scholar]
- National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Female Breast Cancer. 2016 Retrieved from https://seer.cancer.gov/statfacts/html/breast.html.
- Nyklíček I, Mommersteeg P, Van Beugen S, Ramakers C, Van Boxtel GJ. Mindfulness-based stress reduction and physiological activity during acute stress: A randomized controlled trial. Health Psychology. 2013;32(10):1110. doi: 10.1037/a0032200. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Brosschot JF, Lonigro A, Medea B, Van Diest I, Thayer JF. Hemodynamic profiles of functional and dysfunctional forms of repetitive thinking. Annals of Behavioral Medicine. 2016 doi: 10.1007/s12160-016-9851-3. in press. [DOI] [PubMed] [Google Scholar]
- Pieper S, Brosschot JF. Prolonged stress-related cardiovascular activation: Is there any? Annals of Behavioral Medicine. 2005;30(2):91–103. doi: 10.1207/s15324796abm3002_1. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer L, Williston SK, Rollins LG. Mindfulness and emotion regulation. Current Opinion in Psychology. 2015;3:52–57. [Google Scholar]
- Salas CE, Radovic D, Turnbull OH. Inside-out: Comparing internally generated and externally generated basic emotions. Emotion. 2012;12(3):568–578. doi: 10.1037/a0025811. [DOI] [PubMed] [Google Scholar]
- Shahar B, Britton WB, Sbarra DA, Figueredo AJ, Bootzin RR. Mechanisms of change in mindfulness-based cognitive therapy for depression: Preliminary evidence from a randomized controlled trial. International Journal of Cognitive Therapy. 2010;3(4):402–418. [Google Scholar]
- Shapiro D, Jamner LD, Goldstein IB, Delfino RJ. Striking a chord: Moods, blood pressure, and heart rate in everyday life. Psychophysiology. 2001;38:197–204. [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological Science. 2007;18(3):275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. Journal of Hypertension. 2005;23(3):529–536. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354–381. [PubMed] [Google Scholar]
- Teper R, Segal ZV, Inzlicht M. Inside the mindful mind: How mindfulness enhances emotion regulation through improvements in executive control. Current Directions in Psychological Science. 2013;22(6):449–454. [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61(3):201–206. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thewes B, Butow P, GIrgis A, Pendlebury S. The psychosocial needs of breast cancer survivors: A qualitative study of the shared and unique needs of younger versus older survivors. Psycho-Oncology. 2004;13:177–189. doi: 10.1002/pon.710. [DOI] [PubMed] [Google Scholar]
- Wang JG, Staessen JA, Franklin SS, Fagard R, Gueyffier F. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension. 2005;45(5):907–913. doi: 10.1161/01.HYP.0000165020.14745.79. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule—Expanded Form. The University of Iowa; 1994. [Google Scholar]