This review summarizes the history of ERECTA (ER) research including studies on stomatal development, then introduces ER functions in vascular tissues and discusses its interactions with phytohormones and other receptor pathways.
Keywords: Endodermis, EPFL, ERECTA, ligand, peptide, phloem, receptor, stem, stomata, vasculature
Abstract
Plant cells communicate with each other using a variety of signaling molecules. Recent studies have revealed that various types of secreted peptides, as well as phytohormones known since long ago, mediate cell–cell communication in diverse contexts of plant life. These peptides affect cellular activities, such as proliferation and cell fate decisions, through their perception by cell surface receptors located on the plasma membrane of target cells. ERECTA (ER), an Arabidopsis thaliana receptor kinase gene, was first identified as a stem growth regulator, and since then an increasing number of studies have shown that ER is involved in a wide range of developmental and physiological processes. In particular, molecular functions of ER have been extensively studied in stomatal patterning. Furthermore, the importance of ER signaling in vascular tissues of inflorescence stems, especially in phloem cells, has recently been highlighted. In this review article, first we briefly summarize the history of ER research including studies on stomatal development, then introduce ER functions in vascular tissues, and discuss its interactions with phytohormones and other receptor kinase signaling pathways. Future questions and challenges will also be addressed.
Brief early history of ERECTA research
The erecta (er) mutant of Arabidopsis thaliana was first isolated in the 1950s as a single recessive mutant displaying a compact inflorescence (Rédei, 1962, 1992). The mutant was generated by X-ray radiation of Rédei’s Landsberg seeds. This mutant line was thus called Landsberg erecta (Ler) and since then has been widely used as a ‘wild type’ in a large number of Arabidopsis studies because of its space-saving compact stature for laboratory use. However, although it is believed that the parental Landsberg line was the La-1 accession (Zapata et al., 2016), the authenticated parental line was unfortunately lost.
When the ER gene was cloned, known phenotypes were mostly limited to a compact inflorescence with short internodes, short pedicels, blunt fruits, and also altered organ shape such as round leaves with short petioles (Rédei, 1962; Torii et al., 1996). There were also interesting reports yet to be explained at the molecular level. For instance, er mutation promotes stem growth in the acaulis1 mutant background that displays extremely short stems (Tsukaya et al., 1993), which is in contrast to the original er phenotype of compact stems. Later, a number of other phenotypes caused by attenuation of ER activity were reported: leaf adaxial–abaxial polarity (Qi et al., 2004; Xu et al., 2003), pathogen response (Godiard et al., 2003; Häffner et al., 2014; Llorente et al., 2005; Sánchez-Rodríguez et al., 2009), transpiration efficiency (Masle et al., 2005), flowering time (Hall et al., 2007), shade avoidance (Patel et al., 2013), drought tolerance (Villagarcia et al., 2012; Shen et al., 2015), and thermotolerance (Shen et al., 2015).
Identification of two Arabidopsis genes closely related to ER, ER-LIKE1 (ERL1) and ERL2, has further expanded ER-related research (Shpak et al., 2004). These three genes compose the ER family (ERf), and act redundantly for a variety of developmental processes. The triple mutant er erl1 erl2 confers extreme dwarfism, an enlarged shoot apical meristem (SAM), sterility, and formation of clustered stomata (Shpak et al., 2004, 2005; Pillitteri et al., 2007a; Hord et al., 2008; Chen et al., 2013; Uchida et al., 2013). Increasing evidence highlights the involvement of ER in unusually diverse processes in plant development and physiology.
Ligands and other components acting in the ER-family signaling pathway revealed by research on stomata
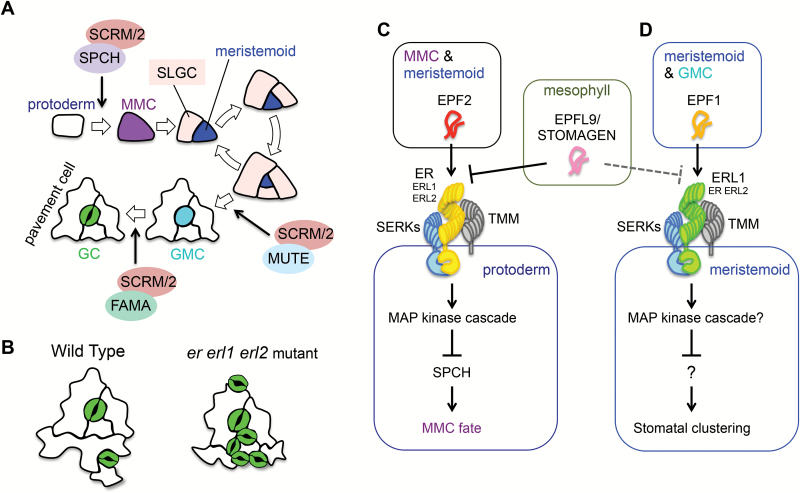
The ER gene encodes a receptor kinase consisting of an extracellular leucine-rich repeat (LRR) domain, a single transmembrane domain, and a cytosolic kinase domain (Torii et al., 1996). Therefore, it was plausible to expect the existence of ligands that would bind to the LRR domain, and the activated ER proteins would in turn trigger downstream signaling. In addition, overexpression of a kinase-truncated version of ER exerted a dominant-negative effect that induced more severe phenotypes than the er single mutant, suggesting that ER would associate with interacting partners (Shpak et al., 2003). Though such ligands and components in all ER-related processes reported have not been fully revealed yet, a number of factors have been identified in the past decade, especially from studies on stomatal patterning. Stomatal development follows a characteristic cell division sequence (Fig. 1A), thus serving as a good model system to study developmental patterning.
Fig. 1.
Roles for ER family receptors and EPF peptides in stomatal patterning. (A) Schematic illustration of stomatal development. A part of the protodermal cell population acquires the character of a meristemoid mother cell (MMC). The MMC executes an asymmetric cell division, giving rise to two daughter cells with different characters: a meristemoid and a stomatal lineage ground cell (SLGC). A meristeoid cell undergoes stereotypical asymmetric divisions repeatedly for self-renewal and amplifying SLGC. When a meristemoid is committed to differentiation, its character changes to a guard mother cell (GMC), and the cell shape is deformed from triangular to circular. Lastly, the GMC undergoes a symmetric division to form a pair of guard cells (GCs), forming a stoma. SLGCs differentiate into pavement cells. The transcription factors SPEECHLESS (SPCH), MUTE, and FAMA form heterodimers with SCREAM (SCRM) or SCRM2, playing essential roles in the respective key steps as indicated by arrows (Ohashi-Ito and Bergmann, 2006; MacAlister et al., 2007; Pillitteri et al., 2007b; Kanaoka et al., 2008). (B) The phenotype of the er erl1 erl2 mutant on epidermal differentiation. The mutant shows increased and clustered formation of stomata. (C) EPF2 peptides secreted from early stomatal lineage cells (MMCs and meristemoids) are perceived by a receptor complex consisting of ER, TMM, and SERKs in neighboring protoderm cells, which prevents the protoderm from entering into the stomatal lineage. ERL1 and ERL2 also participate in this step as minor but significant factors, playing a redundant role with ER. EPFL9/STOMAGEN peptides secreted from mesophyll cells bind to ERf competitively against EPF2, blocking EPF2 signaling. The MAP kinase cascade activated by the EPF2–receptor complex destabilizes the SPCH protein, preventing the cell from acquiring the cell fate of a MMC. (D) EPF1 peptides secreted from later stomatal lineage cells (late meristemoids and GMCs) are perceived by a receptor complex consisting of ERL1, TMM, and SERKs in neighboring merostemoids, which prevents the merostemoid from producing stomata next to the existing stoma. ER and ERL2 also participate in this step as minor but significant factors, playing a redundant role with ERL1. EPFL9/STOMAGEN may interfere the EPF1 signaling.
The triple mutant er erl1 erl2 forms clusters of stomata (Fig. 1B) (Shpak et al., 2005). Such clusters are hardly observed in wild-type plants as stomata are usually formed with at least one non-stomatal cell between stomata according to the ‘one-cell-spacing rule’ (Fig. 1B). This observation suggests that ERf and its hypothetical ligand(s) are involved in the cell–cell communication that prevents the formation of clustered stomata. Meanwhile, from a reverse-genetic screen in which 153 genes encoding small secreted peptides were overexpressed in the wild type, EPIDERMAL PATTERNING FACTOR1 (EPF1) and EPF2 were identified as secreted peptides regulating stomatal development (Hara et al., 2007, 2009; Hunt and Gray, 2009). Both EPF1 and EPF2 are closely related cysteine-rich peptides and prevented stomata formation when overexpressed. Importantly, these effects depended on ERf, suggesting that EPF1 and EPF2 peptides might act as ligands which physically bind to ERf receptor kinases (Hara et al., 2007, 2009). This idea was later proved by biochemical experiments (Lee et al., 2012). These studies also demonstrated that EPF2 peptides are primarily perceived by ER (Fig. 1C; EPF2–ER module) in an early step of formation of stomata, preventing protodermal cells from entering the stomatal lineage. On the other hand, EPF1 peptides mainly act on ERL1 (Fig. 1D; EPF1–ERL1 module) in a later step, inhibiting formation of stomata next to the existing stoma. In the Arabidopsis genome, nine genes encoding EPF-LIKE (EPFL) peptides were identified, and the 11 factors including EPF1 and EPF2 compose the EPF/EPFL family (Hara et al., 2009). Of these gene products, EPFL9/STOMAGEN was shown to act as a positive regulator of formation of stomata, behaving antagonistically to EPF2 and possibly also ERF1 (Fig. 1C, D) (Hunt et al., 2010; Kondo et al., 2010; Sugano et al., 2010; Lee et al., 2015).
TOO MANY MOUTHS (TMM) was identified as the gene responsible for enforcing the one-cell-spacing rule (Yang and Sack, 1995; Nadeau and Sack, 2002). TMM encodes a receptor-like protein with an extracellular LRR domain and a single transmembrane domain but no kinase domain, implying that TMM requires an interacting partner to trigger its downstream signaling. Interestingly, the tmm mutant not only forms clusters of stomata but also is insensitive to EPF2, like the er erl1 erl2 mutant (Hara et al., 2009). Later, it was shown that TMM associates with ERf, acting as a co-receptor (Lee et al., 2012). Recently, SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) family receptor kinases, which act as co-receptors for many other LRR-type receptor kinases, including the brassinosteroid (BR) receptor BRASSINOSTEROID INSENSITIVE 1 (Li et al., 2002; Sun et al., 2013a), bacterial peptide receptor FLAGELLIN-SENSITIVE 2 (Chinchilla et al., 2007; Sun et al., 2013b), abscission-regulating receptor HAESA (Meng et al., 2016), and PHYTOSULFOKINE RECEPTOR (Wang et al., 2015; Santiago et al., 2016), were also shown to interact with ERf (Meng et al., 2015; Jordá et al., 2016). Accordingly, a multiple mutant of SERK family genes exhibits the stomata cluster phenotype (Meng et al., 2015), showing that, in addition to TMM, SERK family proteins also function as co-receptors in ERf signaling (Fig. 1C, D).
ERf activates the mitogen-activated protein (MAP) kinase cascade to regulate stomatal patterning. This cascade consists of YODA (YDA) as a MAP kinase kinase kinase, MKK4/5 as MAP kinase kinases, and MPK3/6 as MAP kinases (Bergmann et al., 2004; Wang et al., 2007; Lampard et al., 2009). The EPF2–ER signaling destabilizes the transcription factor SPEECHLESS (SPCH) that specifies the initiation and proliferation of stomatal lineage cells (Fig. 1C) (MacAlister et al., 2007; Pillitteri et al., 2007b; Lampard et al., 2008; Jewaria et al., 2013). On the other hand, the transcription factor that acts as a substrate of MPK3/6 downstream of the EPF1–ERL1 signaling remains unknown (Fig. 1D).
Thus, studies on stomatal patterning have led to the identification and dissection of ligands and other components of ERf signaling (Fig. 1C, D). It will be important to investigate what parts of the framework drawn by research on stomata are conserved in other ERf-related developmental and physiological processes and what parts are different. Below we summarize functions of ERf in vascular tissues and also discuss questions and challenges to be addressed in the future.
Regulation of inflorescence architecture through endodermis–phloem communication
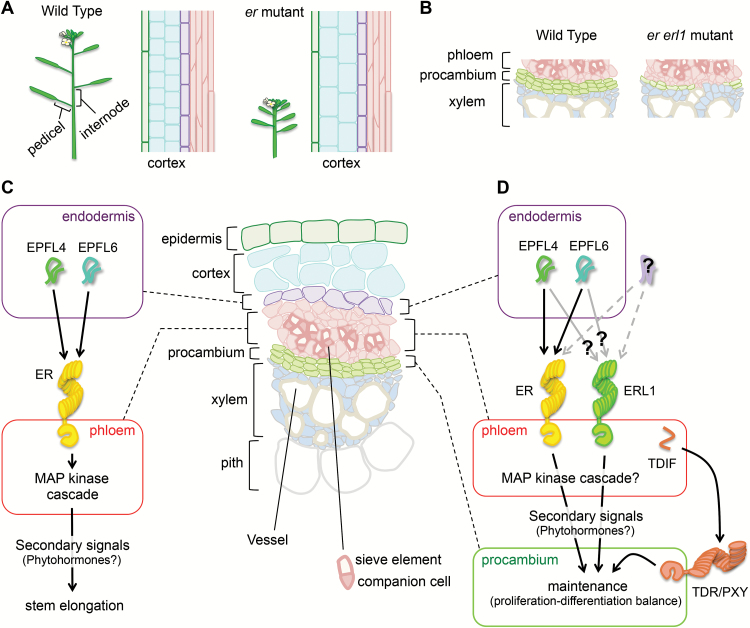
Loss-of-function er mutation confers a compact inflorescence, pedicels, and siliques due to reduced cell numbers (Fig. 2A) (Torii et al., 2003). Yet, the molecular nature of the ligand perceived by ER to regulate inflorescence architecture had long been unclear since the ER gene was first cloned in 1996 (Torii et al., 1996). Identification of EPF1/EPF2/EPFL9 as the ligands for ERf in stomatal patterning led to the idea that a ligand(s) acting in ER-dependent inflorescence development might also be one or some of the EPFL-family peptides.
Fig. 2.
Roles for ER family receptors and EPFL peptides in inflorescence development. (A) The phenotype of the er mutant on stem elongation. Inflorescence architectures and vertical stem sections are illustrated. The mutant shows short internodes and pedicels with a decreased number of cortex cells (cyan). Cortex cells in the mutant also show abnormal enlargement. (B) The phenotype of the er erl1 mutant on procambial maintenance. The mutant has fewer procambial cells than the wild type. Occasionally, some phloem cells directly contact xylem cells without intervening procambial cells. (C) Current model for action of the ER receptor and EPFL peptides in stem elongation. EPFL4 and EPFL6 peptides secreted from the endodermis of stems are perceived by ER in phloem, which activates stem elongation accompanied by cortex cell proliferation through unknown secondary cell–cell communication. In the schematic illustration of a vascular bundle, epidermis, cortex, endodermis, phloem, procambium (or cambium), xylem, and pith tissues are arranged from the outside to the inside. Sieve elements and companion cells are formed in phloem. Vessels are produced in the xylem. (D) Current model for action of ER-family (ERf) receptors and EPFL peptides in procambial maintenance. Endodermis-derived EPFL4 and EPFL6 are perceived either by only ER or by both ER and ERL1 in the phloem. Another unknown ligand may also activate both ER and ERL1 or it may activate only ERL1. The molecular nature of phloem-derived secondary signals downstream of ERf signaling is unknown. TDIF peptides secreted from phloem are perceived by TDR/PXY in the procambium. ER and TDR signaling pathways act in parallel for procambial maintenance.
EPFL-family genes are found in a wide range of land plant species, and the EPFL peptides are classified into four subgroups based on their amino acid sequences (Takata et al., 2013). EPF1, EPF2, and EPFL7 are included in the same subgroup which is closely related to another subgroup including EPFL9. The remaining two subgroups are one including EPFL1, EPFL2, and EPFL3, and the other including EPFL4, EPFL5, EPFL6, and EPFL8, though EPFL8 may constitute an additional subgroup (Bessho-Uehara et al., 2016).
Recent studies elucidated that EPFL4 [known also as CHALLAH-LIKE2 (CLL2)] and EPFL6 [CHALLAH (CHAL)] redundantly regulate inflorescence growth as the epfl4 epfl6 double mutant showed a compact inflorescence strikingly similar to the er mutant (Abrash et al., 2011; Uchida et al., 2012). Furthermore, it was shown that EPFL4/6 peptides physically interact with ER (Fig. 2C; Abrash et al., 2011; Uchida et al., 2012). Moreover, the MAP kinase cascade consisting of YDA, MKK4/5, and MPK3/6 acts as downstream signaling of ER in inflorescence regulation as well as stomatal patterning (Fig. 2C; Meng et al., 2012), suggesting that the common signaling module is adopted for these biological processes. Substrates of MPK3/6 in regulating inflorescence architecture are still unknown. Such substrates may be transcription factors such as SPCH in stomatal development. It is noteworthy that dependence on TMM differs between EPF1/2 and EPFL4/6 (Abrash et al., 2011). EPF1/2 require TMM to activate ERf, while EPFL4/6 do not. Rather, in the presence of TMM, EPFL4/6 do not efficiently stimulate the ER signaling. This situation implies that TMM, which is expressed in stomatal lineage cells, functions to minimize a signal bleed-through from internal tissues of stems, where EPFL4/6, but not EPF1/2, are expressed (see the following paragraph) (Abrash et al., 2011; Torii, 2012). It is interesting to note further that the serk family mutant resembles er inflorescence phenotypes (Meng et al., 2015), although the requirement of SERKs for EPFL4/6 perception by ER has not been tested.
Identification of EPFL4/6 and analysis of their expression patterns led to the discovery of a novel cell–cell communication promoting inflorescence growth (Uchida et al., 2012). In developing inflorescence stems, EPFL4/6 are specifically expressed in the endodermis (Fig. 2C). On the other hand, the ER expression is detected in phloem and xylem tissues as well as the epidermis in stems. Since the specific expression of ER in phloem companion cells but neither its xylem- nor epidermis-specific expression rescued the inflorescence phenotypes of er, ER activity in the phloem is the key to regulate the inflorescence architecture. Taken together, it is proposed that EPFL4/6 peptides secreted from the endodermis are perceived by ER in phloem companion cells, and the activated ER signaling in the phloem cells promotes growth of inflorescence tissues (Fig. 2C).
In inflorescences of er mutants, cell numbers in the cortex are reduced compared with those of the wild type, while the mutant cells are elongated possibly due to compensatory cell growth (Fig. 2A) (Shpak et al., 2003, 2004; Bundy et al., 2012; Uchida et al., 2012). Interestingly, this phenomenon was rescued by the phloem-specific expression of ER (Uchida et al., 2012), suggesting that an unknown secondary signal derived from the phloem affects cell proliferation in surrounding stem tissues (Fig. 2C). Given that phloem expresses groups of genes for specific metabolic pathways, some of which can affect stem growth (e.g. polyamines) (Kakehi et al., 2008; Pommerrenig et al., 2011), such phloem-derived metabolites may mediate the inflorescence growth by ER.
Although some phytohormones promote stem elongation, relationships between ER signaling and such phytohormones largely remain to be understood. A suppressor screening of the er mutant demonstrated that overaccumulation of auxin by YUCCA5 overexpression compensates for the reduced growth of stems and pedicels in er (Woodward et al., 2005). This compensation is not simply due to the rescue of cell proliferation in er, but rather the enhancement of cell elongation. On the other hand, the effect of YUCCA5 overexpression on organ growth is enhanced by the er mutant, suggesting that ER signaling buffers auxin action (Woodward et al., 2005). Indeed, auxin response patterns are altered in the SAM and embryos in er erl1 erl2 (Chen et al., 2013; Chen and Shpak, 2014). Very recently, it has been demonstrated that previously uncharacterized EPFL2 peptides play a role in shaping the auxin response pattern via ERf in leaf serration development (Tameshige et al., 2016). Furthermore, the involvement of auxin in stomatal development has also been reported (Balcerowicz et al., 2014; Le et al., 2014; Zhang et al., 2014). Collectively, accumulating evidence emphasizes co-ordinated action between auxin and ERf signaling.
The cytokinin (CK)-accumulating ckx3 ckx5 mutant forms longer pedicels and fruits than the wild type, which is the opposite of the er phenotypes (Bartrina et al., 2011). On the other hand, ckx3 ckx5 also shows thickened stems and an enlarged SAM (Bartrina et al., 2011), which are similar to phenotypes of er and er erl1 erl2, respectively (Torii et al., 1996; Chen et al., 2013; Uchida et al., 2013). It was also reported that the SAM of er erl1 erl2 exhibits enhanced responses to CK (Uchida et al., 2013). Although these observations appear partly controversial, a possible hypothesis may be that the ER signaling buffers CK responses in both way; it may prevent CK response from diminishing and also may suppress excess CK response depending on developmental contexts.
Gibberellin (GA) has long been known for its stem-elongating activity in many plants (Sachs et al., 1959; Arney and Mancinelli, 1966). Consistently, a microarray analysis revealed that er and epfl4 epfl6 mutants commonly show changes in expression of some GA metabolic genes (Uchida et al., 2012). It was also reported that the short stem phenotype of the GA-insensitive mutant short internode (shi) is enhanced by the er mutation (Fridborg et al., 2001). Furthermore, the stem elongation phenotype of the GA-hyper-responsive mutant spindly (spy) is suppressed by er (Swain et al., 2001). However, recent studies on SHI and SPY imply their involvement also in auxin biosynthesis and CK responsiveness, respectively (Steiner et al., 2012; Baylis et al., 2013). Therefore, ER might modify the shi and spy phenotypes through modulating auxin and/or CK actions.
It is still not clear how the ER signaling is integrated with phytohormone actions for stem growth. Yet, it would be intriguing to examine whether some phytohormones mediate cell–cell signaling from phloem to surrounding cells, in light of the non-cell-autonomous nature of ER-mediated stem growth (Fig. 2C) (Uchida et al., 2012). It would also be important to investigate which phytohormone could be metabolized in phloem upon the activation of ER signaling and which cell type responds to such a phytohormone(s).
Roles for EPFL–ER family signaling in vascular development
In the previous section, we described that ER acts in stem elongation. In addition to ER, ERL1 is also expressed in inflorescence stems (Abrash et al., 2011; Uchida et al., 2012), while the erl1 single mutant does not show an obvious stem phenotype (Shpak et al., 2004). However, interestingly, the double mutant er erl1 exhibits a defect in vascular tissues of stems. In er erl1 stems, phloem cells are frequently located adjacent to xylem cells without intervening procambial cells, which is not observed in the wild type, or in single er or erl1 mutants (Fig. 2B) (Uchida and Tasaka, 2013). The phenotype of direct contact of xylem and phloem also occurs in a mutant of TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR RECEPTOR/ PHLOEM INTERCALATED WITH XYLEM (TDR/PXY), which encodes an LRR-type receptor kinase expressed in the procambium (Fisher and Turner, 2007; Hirakawa et al., 2008). Therefore, both ERf and TDR proteins function in procambial maintenance by promoting cell proliferation and/or suppressing cell differentiation. Genetic analyses suggested that these two pathways act in parallel as almost all procambial cells are consumed in tdr er double mutant stems, which is an even more severe defect than er erl1 and tdr phenotypes (Etchells et al., 2013; Uchida and Tasaka, 2013). Like ER, ERL1 expression is also detected in the epidermis, phloem, and xylem (Uchida and Tasaka, 2013), and, interestingly, the procambium defect in er erl1 stems was rescued by phloem-specific activity of ER (Uchida and Tasaka, 2013) as in the case of inflorescence phenotypes in er (Uchida et al., 2012). The phloem-specific ER activity also recovered the severe phenotype of tdr er to the level of the tdr single mutant. Therefore, the ER function in the phloem cells accounts for procambial maintenance independently of TDR. It is suggested that these two receptor systems differentially affect proliferation of procambial cells and their spatial differentiation pattern into xylem and phloem (Fig. 2D) (Etchells et al., 2013). Interestingly, although tdr er shows more severe defects in procambial maintenance and vascular organization than tdr (Etchells et al., 2013; Uchida and Tasaka, 2013), tdr er has similar numbers of vascular cells per bundle compared with tdr (Etchells et al., 2013). This implies that compensational cell proliferation occurs in vascular bundles of tdr er.
ER and TDR receptors perceive their respective ligands. The ligand of TDR is TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR (TDIF) peptides encoded by CLV3/ESR1-LIKE41 (CLE41) and CLE44 (Ito et al., 2006; Hirakawa et al., 2008). TDIF is secreted from the phloem and perceived by TDR at the neighboring procambium (Fig. 2D) (Hirakawa et al., 2008; Etchells and Turner, 2010). On the other hand, EPFL4 and EPFL6 act as ligands of ERf for procambial maintenance of stems as well as for stem elongation, as the severe vascular disorganization of tdr er was phenocopied by the triple mutant tdr epfl4 epfl6 (Uchida and Tasaka, 2013). As mentioned above, EPFL4 and EPFL6 peptides are specifically produced in the endodermis (Uchida and Tasaka, 2013), indicating that the endodermis–phloem communication mediated by the EPFL4/6–ERf module plays an important role in vascular development (Fig. 2D). In summary, the two distinct ligand–receptor systems are required for procambial maintenance, which co-ordinately underlies proper tissue organization in vascular bundles (Fig. 2D) (Etchells et al., 2013; Uchida and Tasaka, 2013).
It should be noted that although epfl4 epfl6 causes severe deterioration of the tdr phenotype as in the case of the er mutation, epfl4 epfl6 does not show any obvious vascular phenotype by itself, unlike er erl1 (Uchida and Tasaka, 2013). This implies the existence of an additional ligand(s) that activates the ERf signaling besides EPFL4/6 for vascular development in stems, leading to the following two hypotheses (Fig. 2D). One is that EPFL4/6 and the additional ligand similarly activate both ER and ERL1. The other is that EPFL4/6 activate only ER just as is the case for stem elongation, and the additional ligand activates ERL1. The latter scenario is reminiscent of the stomatal regulation by the EPF2–ER module and the EPF1–ERL1 module (Fig. 1C, D) (Lee et al., 2012). Both hypotheses can explain the genetic redundancy between ER and ERL1 for vascular phenotypes of er erl1.
Outputs of ER signaling activated in the phloem need to be transmitted to the procambium to regulate its maintenance. Since there are several phytohormones involved in vascular development and also in ER or TDR pathways, they might participate in the phloem–procambium communication. Ethylene signaling is up-regulated in the tdr mutant (Etchells et al., 2012), which induces expression of the transcription factors ETHYLENE RESPONSE FACTOR1 (ERF1), ERF109, and ERF018. These ERFs promote procambial proliferation in a TDR-independent manner (Etchells et al., 2012), forming a compensatory loop for procambial maintenance. Given that the ERf pathway also acts as a compensatory mechanism for the TDR pathway (Etchells et al., 2013; Uchida and Tasaka, 2013), ethylene signaling may be closely related to ER signaling. Genetic interaction between ethylene and ER signaling pathways was also reported in leaf hyponastic growth (van Zanten et al., 2010), implying that similar signaling networks may commonly work in distinct events.
Auxin has been recognized to promote cambial activity in stems since as early as the 1930s (Snow, 1935). The auxin-dependent stimulation of cambial activity depends on WUSCHEL-RELATED HOMEOBOX4 (WOX4), a transcription factor downstream of the TDR pathway (Hirakawa et al., 2010; Suer et al., 2011). Auxin up-regulates WOX4 expression, and TDR is required for auxin-dependent WOX4 induction, suggesting a crosstalk between the auxin signaling and TDR pathway to regulate WOX4 expression. On the other hand, it was reported that the wox4 er mutant did not show obvious vascular defects, unlike tdr er that exhibits a severe defect (Uchida and Tasaka, 2013), suggesting that a WOX4-independent mechanism(s) must mediate the co-operative action by ER and TDR. Recently, it has been reported that glycogen synthase kinase 3 proteins (GSK3s) act downstream of TDR but independently of WOX4 (Kondo et al., 2014). Therefore, ER signaling may interact with the GSK3 pathway. Interestingly, BRASSINOSTEROID-INSENSITIVE 2 (BIN2), a member of the GSK3s which was first identified as a component of BR signaling, acts upstream of YDA and downstream of ERf in the regulation of stomatal development (Kim et al., 2012). Because BIN2 is also reported to act in phloem cells (Anne et al., 2015) where ER proteins function for vascular development, it is plausible that the BIN2 pathway modifies ER signaling in the phloem. Furthermore, studies on hypocotyl elongation and lateral root development showed that BIN2 could function as a hub between TDR, BR, and auxin pathways (Vert et al., 2008; Cho et al., 2014). Since ERf modulates auxin responses in some tissues (Chen et al., 2013; Chen and Shpak, 2014; Tameshige et al., 2016), it will be important to examine further relationships between ERf, TDR, auxin, and BR signaling pathways in vascular development.
GA acts as a mobile factor that promotes internode elongation and cambial proliferation in stems (Dayan et al., 2012), and these developmental processes are also regulated by ERf, as described above. GA also induces expansion of the xylem area in the hypocotyl (Ragni et al., 2011). It has recently been reported that ER and ERL1 redundantly suppress the premature expansion of xylem in the hypocotyl (Ikematsu et al., 2017). The increased xylem size in the er erl1 hypocotyl appears to contradict the fact that the er erl1 mutation attenuates procambial proliferation in stems. It is interesting to investigate how the ER-dependent regulatory modules are flipped over between these tissues. The involvement of ER in xylem development in the hypocotyl had also been previously proposed (Ragni et al., 2011). The interpretation was based on the fact that the Ler accession, which carries a loss-of-function allele of the ER gene, shows enhanced enlargement of the hypocotyl xylem, while this phenomenon does not occur in the Lansberg-0 (La-0) accession in which ER is functional (Ragni et al., 2011). However, a recent study characterizing a progeny of Ler×La-0 interaccession crosses revealed that the er mutation is not linked to the xylem expansion phenotype of Ler, suggesting that an as-yet-unknown locus other than the ER locus is responsible for the xylem phenotype of Ler (Ikematsu et al., 2017). This finding may not be surprising given that the parental line of Ler is probably La-1 (Zapata et al., 2016) that is distinct from La-0 based on polymorphism analysis (http://www.lehleseeds.com/cgi-bin/hazel.cgi?action=detail&item=12&template=note1.html, last accessed 24 November 2016). The identification of the locus responsible for the xylem phenotype in Ler may provide novel insight into xylem development in the hypocotyl. ERf also plays a role in a GA-related event of vascular development in the hypocotyl. The hypocotyl xylem produces fiber cells in response to GA (Ragni et al., 2011; Dayan et al., 2012; Ikematsu et al., 2017). Interestingly, ER and ERL1 redundantly interfere the GA-mediated fiber formation (Ikematsu et al., 2017). While it is plausible to expect that some EPF/EPFL peptides act as ligands for ER and ERL1 in vascular development in the hypocotyl, such a ligand(s) remains to be identified.
CK promotes cambial proliferation (Matsumoto-Kitano et al., 2008; Nieminen et al., 2008; Bartrina et al., 2011; Tokunaga et al., 2012). As mentioned in the previous section, it is possible that the ER signaling buffers CK responses in a context-dependent manner, while it is still not clear whether this idea is applicable to vascular regulation. Also, a relationship between the CK pathway and TDR pathway remains to be addressed.
Jasmonate and strigolactone are also reported as regulators of (pro)cambial regulation (Sehr et al., 2010; Agusti et al., 2011), though there is no report which connects the action of these hormones with ER or TDR signaling. One or some of the phytohormones mentioned above may mediate the cell–cell communication from the phloem to the procambium downstream of the activation of ERf.
Concluding thoughts
The same EPFL4/6–ER module regulates two distinct developmental processes, stem elongation and procambial maintenance, raising a hypothesis that common downstream components may be involved in both processes. For example, the MAP kinase cascade and its regulators are candidates for such common components. At the same time, there should also be a mechanism(s) that separates outputs from the common signaling module depending on a developmental context.
There are other challenges and questions to be addressed. For instance, epfl4 epfl6 exerts milder effects than er on both inflorescence growth and procambial maintenance (Uchida et al., 2012; Uchida and Tasaka, 2013), raising the question of whether there are additional agonistic and/or antagonistic EPF/EPFLs besides EPFL4/6. It was reported that the expression profile of several EPF/EPFL genes changes according to environmental conditions (Richardson and Torii, 2013), implying that ER signaling could be modulated in response to environmental stimuli. Although it is still unknown whether the EPFL4/6 production also responds to environmental changes, it will be interesting to investigate the relationships between ERf-related developmental processes, a variety of environmental cues, and changes in expression profiles of EPF/EPFL genes including EPFL4/6. Furthermore, it was shown that the phloem-specific activity of ER rescued not only phenotypes in inflorescence growth and procambial maintenance but also other phenotypes of er, such as rounder leaves with short petioles and blades, as well as extreme dwarfism of er erl1 erl2 (Uchida et al., 2012). While it is yet to be tested whether these processes could also be regulated by EPFL4/6, it is likely that EPFL4/6 and/or other EPF/EPFLs act on ERf at phloem cells to regulate such diverse developmental processes. These future studies will provide further insights into the molecular nature of EPF/EPFL–ERf signaling that bounces in and out of the phloem.
Acknowledgements
We thank Dr Yuki Hirakawa for critical reading of this manuscript. This work was supported by MEXT/JSPS KAKENHI (grant numbers JP26291057 and JP16H01237 to KUT; JP16H01462 to NU) and the Howard Hughes Medical Institute (HHMI) and the Gordon and Betty Moore Foundation (GBMF) (GBMF3035 to KUT). KUT is an HHMI-GBMF Investigator.
References
- Abrash EB, Davies KA, Bergmann DC. 2011. Generation of signaling specificity in Arabidopsis by spatially restricted buffering of ligand–receptor interactions. The Plant Cell 23, 2864–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J, Herold S, Schwarz M, et al. 2011. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proceedings of the National Academy of Sciences, USA 108, 20242–20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne P, Azzopardi M, Gissot L, Beaubiat S, Hématy K, Palauqui JC. 2015. OCTOPUS negatively regulates BIN2 to control phloem differentiation in Arabidopsis thaliana. Current Biology 25, 2584–2590. [DOI] [PubMed] [Google Scholar]
- Arney SE, Mancinelli P. 1966. The basic action of gibberellic acid in elongation of ‘Meteor’ pea stems. New Phytologist 65, 161–175. [Google Scholar]
- Balcerowicz M, Ranjan A, Rupprecht L, Fiene G, Hoecker U. 2014. Auxin represses stomatal development in dark-grown seedlings via Aux/IAA proteins. Development 141, 3165–3176. [DOI] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. 2011. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. The Plant Cell 23, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis T, Cierlik I, Sundberg E, Mattsson J. 2013. SHORT INTERNODES/STYLISH genes, regulators of auxin biosynthesis, are involved in leaf vein development in Arabidopsis thaliana. New Phytologist 197, 737–750. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. 2004. Stomatal development and pattern controlled by a MAPKK kinase. Science 304, 1494–1497. [DOI] [PubMed] [Google Scholar]
- Bessho-Uehara K, Wang DR, Furuta T, et al. 2016. Loss of function at RAE2, a previously unidentified EPFL, is required for awnlessness in cultivated Asian rice. Proceedings of the National Academy of Sciences, USA 113, 8969–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy MGR, Thompson OA, Sieger MT, Shpak ED. 2012. Patterns of cell division, cell differentiation and cell elongation in epidermis and cortex of Arabidopsis pedicels in the wild type and in erecta. PLoS One 7, e46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Shpak ED. 2014. ERECTA family genes regulate development of cotyledons during embryogenesis. FEBS Letters 588, 3912–3917. [DOI] [PubMed] [Google Scholar]
- Chen MK, Wilson RL, Palme K, Ditengou FA, Shpak ED. 2013. ERECTA family genes regulate auxin transport in the shoot apical meristem and forming leaf primordia. Plant Physiology 162, 1978–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T. 2007. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500. [DOI] [PubMed] [Google Scholar]
- Cho H, Ryu H, Rho S, et al. 2014. A secreted peptide acts on BIN2-mediated phosphorylation of ARFs to potentiate auxin response during lateral root development. Nature Cell Biology 16, 66–76. [DOI] [PubMed] [Google Scholar]
- Dayan J, Voronin N, Gong F, Sun TP, Hedden P, Fromm H, Aloni R. 2012. Leaf-induced gibberellin signaling is essential for internode elongation, cambial activity, and fiber differentiation in tobacco stems. The Plant Cell 24, 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Mishra L, Turner SR. 2013. WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 140, 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Turner SR. 2012. Plant vascular cell division is maintained by an interaction between PXY and ethylene signalling. PLoS Genetics 8, e1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Turner SR. 2010. The PXY–CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 137, 767–774. [DOI] [PubMed] [Google Scholar]
- Fisher K, Turner S. 2007. PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Current Biology 17, 1061–1066. [DOI] [PubMed] [Google Scholar]
- Fridborg I, Kuusk S, Robertson M, Sundberg E. 2001. The Arabidopsis protein SHI represses gibberellin responses in Arabidopsis and barley. Plant Physiology 127, 937–948. [PMC free article] [PubMed] [Google Scholar]
- Godiard L, Sauviac L, Torii KU, Grenon O, Mangin B, Grimsley NH, Marco Y. 2003. ERECTA, an LRR receptor-like kinase protein controlling development pleiotropically affects resistance to bacterial wilt. The Plant Journal 36, 353–365. [DOI] [PubMed] [Google Scholar]
- Häffner E, Karlovsky P, Splivallo R, Traczewska A, Diederichsen E. 2014. ERECTA, salicylic acid, abscisic acid, and jasmonic acid modulate quantitative disease resistance of Arabidopsis thaliana to Verticillium longisporum. BMC Plant Biology 14, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MC, Dworkin I, Ungerer MC, Purugganan M. 2007. Genetics of microenvironmental canalization in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 104, 13717–13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. 2007. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes and Development 21, 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, Torii KU, Kakimoto T. 2009. Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant and Cell Physiology 50, 1019–1031. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H. 2010. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. The Plant Cell 22, 2618–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi-Ito K, Matsubayashi Y, Fukuda H. 2008. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proceedings of the National Academy of Sciences, USA 105, 15208–15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hord CL, Sun YJ, Pillitteri LJ, Torii KU, Wang H, Zhang S, Ma H. 2008. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Molecular Plant 1, 645–658. [DOI] [PubMed] [Google Scholar]
- Hunt L, Bailey KJ, Gray JE. 2010. The signalling peptide EPFL9 is a positive regulator of stomatal development. New Phytologist 186, 609–614. [DOI] [PubMed] [Google Scholar]
- Hunt L, Gray JE. 2009. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Current Biology 19, 864–869. [DOI] [PubMed] [Google Scholar]
- Ikematsu S, Tasaka M, Torii KU, Uchida N. 2017. ERECTA-family receptor kinase genes redundantly prevent premature progression of secondary growth in the Arabidopsis hypocotyl. New Phytologist (in press). doi:10.1111/nph.14335 [DOI] [PubMed] [Google Scholar]
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H. 2006. Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313, 842–845. [DOI] [PubMed] [Google Scholar]
- Jewaria PK, Hara T, Tanaka H, Kondo T, Betsuyaku S, Sawa S, Sakagami Y, Aimoto S, Kakimoto T. 2013. Differential effects of the peptides Stomagen, EPF1 and EPF2 on activation of MAP kinase MPK6 and the SPCH protein level. Plant and Cell Physiology 54, 1253–1262. [DOI] [PubMed] [Google Scholar]
- Jordá L, Sopeña-Torres S, Escudero V, Nuñez-Corcuera B, Delgado-Cerezo M, Torii KU, Molina A. 2016. ERECTA and BAK1 receptor like kinases interact to regulate immune responses in Arabidopsis. Frontiers in Plant Science 7, 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakehi J, Kuwashiro Y, Niitsu M, Takahashi T. 2008. Thermospermine is required for stem elongation in Arabidopsis thaliana. Plant and Cell Physiology 49, 1342–1349. [DOI] [PubMed] [Google Scholar]
- Kanaoka MM, Pillitteri LJ, Fujii H, Yoshida Y, Bogenschutz NL, Takabayashi J, Zhu JK, Torii KU. 2008. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell 20, 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Michniewicz M, Bergmann DC, Wang ZY. 2012. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482, 419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kajita R, Miyazaki A, et al. 2010. Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant and Cell Physiology 51, 1–8. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Ito T, Nakagami H, Hirakawa Y, Saito M, Tamaki T, Shirasu K, Fukuda H. 2014. Plant GSK3 proteins regulate xylem cell differentiation downstream of TDIF–TDR signalling. Nature Communications 5, 3504. [DOI] [PubMed] [Google Scholar]
- Lampard GR, Lukowitz W, Ellis BE, Bergmann DC. 2009. Novel and expanded roles for MAPK signaling in Arabidopsis stomatal cell fate revealed by cell type-specific manipulations. The Plant Cell 21, 3506–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampard GR, Macalister CA, Bergmann DC. 2008. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 322, 1113–1116. [DOI] [PubMed] [Google Scholar]
- Le J, Liu XG, Yang KZ, et al. 2014. Auxin transport and activity regulate stomatal patterning and development. Nature Communications 5, 3090. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hnilova M, Maes M, Lin YC, Putarjunan A, Han SK, Avila J, Torii KU. 2015. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 522, 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, Sarikaya M, Tamerler C, Torii KU. 2012. Direct interaction of ligand–receptor pairs specifying stomatal patterning. Genes and Development 26, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. 2002. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110, 213–222. [DOI] [PubMed] [Google Scholar]
- Llorente F, Alonso-Blanco C, Sánchez-Rodriguez C, Jorda L, Molina A. 2005. ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. The Plant Journal 43, 165–180. [DOI] [PubMed] [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC. 2007. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445, 537–540. [DOI] [PubMed] [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD. 2005. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 436, 866–870. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Vaclavikova K, Miyawaki K, Kakimoto T. 2008. Cytokinins are central regulators of cambial activity. Proceedings of the National Academy of Sciences, USA 105, 20027–20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torii KU, He P, Shan L. 2015. Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Current Biology 25, 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Wang H, He Y, Liu Y, Walker JC, Torii KU, Zhang S. 2012. A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. The Plant Cell 24, 4948–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhou J, Tang J, Li B, de Oliveira MV, Chai J, He P, Shan L. 2016. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Reports 14, 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. 2002. Control of stomatal distribution on the Arabidopsis leaf surface. Science 296, 1697–1700. [DOI] [PubMed] [Google Scholar]
- Nieminen K, Immanen J, Laxell M, et al. 2008. Cytokinin signaling regulates cambial development in poplar. Proceedings of the National Academy of Sciences, USA 105, 20032–20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC. 2006. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 18, 2493–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Basu M, Hayes S, Majláth I, Hetherington FM, Tschaplinski TJ, Franklin KA. 2013. Temperature-dependent shade avoidance involves the receptor-like kinase ERECTA. The Plant Journal 73, 980–992. [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Bemis SM, Shpak ED, Torii KU. 2007a Haploinsufficiency after successive loss of signaling reveals a role for ERECTA-family genes in Arabidopsis ovule development. Development 134, 3099–3109. [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. 2007b Termination of asymmetric cell division and differentiation of stomata. Nature 445, 501–505. [DOI] [PubMed] [Google Scholar]
- Pommerrenig B, Feussner K, Zierer W, Rabinovych V, Klebl F, Feussner I, Sauer N. 2011. Phloem-specific expression of Yang cycle genes and identification of novel Yang cycle enzymes in Plantago and Arabidopsis. The Plant Cell 23, 1904–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Sun Y, Xu L, Xu Y, Huang H. 2004. ERECTA is required for protection against heat-stress in the AS1/AS2 pathway to regulate adaxial–abaxial leaf polarity in Arabidopsis. Planta 219, 270–276. [DOI] [PubMed] [Google Scholar]
- Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke CS. 2011. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. The Plant Cell 23, 1322–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rédei GP. 1962. Single locus heterosis. Zeitschrift für Vererbungslehre 93, 164–170. [Google Scholar]
- Rédei GP. 1992. A heuristic glance at the past of Arabidopsis genetics. In: Koncz C, Chua NH, Schell J, eds. Methods in Arabidopsis research. Singapore: Wold Scientific, 1–15. [Google Scholar]
- Richardson LG, Torii KU. 2013. Take a deep breath: peptide signalling in stomatal patterning and differentiation. Journal of Experimental Botany 64, 5243–5251. [DOI] [PubMed] [Google Scholar]
- Sachs RM, Bretz CF, Lang A. 1959. Shoot histogenenesis: the early effects of gibberellin upon stem elongation in two rosette plants. American Journal of Botany 46, 376–384. [Google Scholar]
- Santiago J, Brandt B, Wildhagen M, Hohmann U, Hothorn LA, Butenko MA, Hothorn M. 2016. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife 5, e15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez C, Estévez JM, Llorente F, Hernández-Blanco C, Jordá L, Pagán I, Berrocal M, Marco Y, Somerville S, Molina A. 2009. The ERECTA receptor-like kinase regulates cell wall-mediated resistance to pathogens in Arabidopsis thaliana. Molecular Plant-Microbe Interactions 22, 953–963. [DOI] [PubMed] [Google Scholar]
- Sehr EM, Agusti J, Lehner R, Farmer EE, Schwarz M, Greb T. 2010. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. The Plant Journal 63, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhong X, Zhao F, et al. 2015. Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nature Biotechnology 33, 996–1003. [DOI] [PubMed] [Google Scholar]
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU. 2004. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131, 1491–1501. [DOI] [PubMed] [Google Scholar]
- Shpak ED, Lakeman MB, Torii KU. 2003. Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. The Plant Cell 15, 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. 2005. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309, 290–293. [DOI] [PubMed] [Google Scholar]
- Snow R. 1935. Activation of cambial growth by pure hormones. New Phytologist 34, 347–360. [Google Scholar]
- Steiner E, Efroni I, Gopalraj M, Saathoff K, Tseng TS, Kieffer M, Eshed Y, Olszewski N, Weiss D. 2012. The Arabidopsis O-linked N-acetylglucosamine transferase SPINDLY interacts with class I TCPs to facilitate cytokinin responses in leaves and flowers. The Plant Cell 24, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suer S, Agusti J, Sanchez P, Schwarz M, Greb T. 2011. WOX4 imparts auxin responsiveness to cambium cells in Arabidopsis. The Plant Cell 23, 3247–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano SS, Shimada T, Imai Y, Okawa K, Tamai A, Mori M, Hara-Nishimura I. 2010. Stomagen positively regulates stomatal density in Arabidopsis. Nature 463, 241–244. [DOI] [PubMed] [Google Scholar]
- Sun Y, Han Z, Tang J, Hu Z, Chai C, Zhou B, Chai J. 2013a Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Research 23, 1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J. 2013b Structural basis for flg22-induced activation of the Arabidopsis FLS2–BAK1 immune complex. Science 342, 624–628. [DOI] [PubMed] [Google Scholar]
- Swain SM, Tseng TS, Olszewski NE. 2001. Altered expression of SPINDLY affects gibberellin response and plant development. Plant Physiology 126, 1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Yokota K, Ohki S, Mori M, Taniguchi T, Kurita M. 2013. Evolutionary relationship and structural characterization of the EPF/EPFL gene family. PLoS One 8, e65183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tameshige T, Okamoto S, Lee JS, Aida M, Tasaka M, Torii KU, Uchida N. 2016. A secreted peptide and its receptors shape the auxin response pattern and leaf margin morphogenesis. Current Biology 26, 2478–2485. [DOI] [PubMed] [Google Scholar]
- Tokunaga H, Kojima M, Kuroha T, Ishida T, Sugimoto K, Kiba T, Sakakibara H. 2012. Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in cytokinin activation. The Plant Journal 69, 355–365. [DOI] [PubMed] [Google Scholar]
- Torii KU. 2012. Mix-and-match: ligand–receptor pairs in stomatal development and beyond. Trends in Plant Science 17, 711–719. [DOI] [PubMed] [Google Scholar]
- Torii KU, Hanson LA, Josefsson CAB, Shpak ED. 2003. Regulation of inflorescence architecture and organ shape by the ERECTA gene in Arabidopsis. In: Sekimura T, Noji S, Ueno N, Maini PK, eds. Morphogenesis and pattern formation in biological systems: experiments and models. Tokyo: Springer Japan, 153–164. [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y. 1996. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. The Plant Cell 8, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya H, Naito S, Rédei GP, Komeda Y. 1993. A new class of mutations in Arabidopsis thaliana, acaulis1, affecting the development of both inflorescences and leaves. Development 118, 751–764. [Google Scholar]
- Uchida N, Lee JS, Horst RJ, Lai HH, Kajita R, Kakimoto T, Tasaka M, Torii KU. 2012. Regulation of inflorescence architecture by intertissue layer ligand–receptor communication between endodermis and phloem. Proceedings of the National Academy of Sciences, USA 109, 6337–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Shimada M, Tasaka M. 2013. ERECTA-family receptor kinases regulate stem cell homeostasis via buffering its cytokinin responsiveness in the shoot apical meristem. Plant and Cell Physiology 54, 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Tasaka M. 2013. Regulation of plant vascular stem cells by endodermis-derived EPFL-family peptide hormones and phloem-expressed ERECTA-family receptor kinases. Journal of Experimental Botany 64, 5335–5343. [DOI] [PubMed] [Google Scholar]
- van Zanten M, Basten Snoek L, van Eck-Stouten E, Proveniers MC, Torii KU, Voesenek LA, Peeters AJ, Millenaar FF. 2010. Ethylene-induced hyponastic growth in Arabidopsis thaliana is controlled by ERECTA. The Plant Journal 61, 83–95. [DOI] [PubMed] [Google Scholar]
- Vert G, Walcher CL, Chory J, Nemhauser JL. 2008. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proceedings of the National Academy of Sciences, USA 105, 9829–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagarcia H, Morin AC, Shpak ED, Khodakovskaya MV. 2012. Modification of tomato growth by expression of truncated ERECTA protein from Arabidopsis thaliana. Journal of Experimental Botany 63, 6493–6504. [DOI] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. 2007. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. The Plant Cell 19, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li H, Han Z, Zhang H, Wang T, Lin G, Chang J, Yang W, Chai J. 2015. Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature 525, 265–268. [DOI] [PubMed] [Google Scholar]
- Woodward C, Bemis SM, Hill EJ, Sawa S, Koshiba T, Torii KU. 2005. Interaction of auxin and ERECTA in elaborating Arabidopsis inflorescence architecture revealed by the activation tagging of a new member of the YUCCA family putative flavin monooxygenases. Plant Physiology 139, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Xu Y, Dong A, Sun Y, Pi L, Xu Y, Huang H. 2003. Novel as1 and as2 defects in leaf adaxial–abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130, 4097–4107. [DOI] [PubMed] [Google Scholar]
- Yang M, Sack FD. 1995. The too many mouths and four lips mutations affect stomatal production in Arabidopsis. The Plant Cell 7, 2227–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata L, Ding J, Willing EM, et al. 2016. Chromosome-level assembly of Arabidopsis thaliana Ler reveals the extent of translocation and inversion polymorphisms. Proceedings of the National Academy of Sciences, USA 113, E4052–E4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, He SB, Li L, Yang HQ. 2014. Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proceedings of the National Academy of Sciences, USA 111, E3015–E3023. [DOI] [PMC free article] [PubMed] [Google Scholar]