Abstract
The Janus kinases (JAK) are a family of kinases that play an essential role in cytokine signaling and are implicated in the pathogenesis of autoimmune diseases and hematological malignancies. As a result, the JAKs have become attractive therapeutic targets. The discovery of a JAK2 point mutation (JAK2 V617F) as the main cause of polycythemia vera lead to the development and FDA approval of a JAK1/2 inhibitor, ruxolitinib, in 2011. This review focuses on the various JAK and associated components aberrations implicated in myeloproliferative neoplasms, leukemias, and lymphomas. In addition to ruxolitinib, other JAK inhibitors are currently being evaluated in clinical trials for treating hematological malignancies. The use of JAK inhibitors alone or in combination therapy should be considered as a way to deliver targeted therapy to patients.
Keywords: Janus kinase, ruxolitinib, myeloproliferative neoplasms, leukemia, precision medicine
1. Introduction
The differentiation, proliferation, survival, and immune functions of hematopoietic cells are regulated by cytokines which bind to their appropriate cell surface receptor. Some important cytokine receptors, however, lack intrinsic kinase activity and thus rely on a family of tyrosine kinases called Janus Kinases (comprised of 4 members JAK1, JAK2, JAK3, and TYK2) that associate with the cytoplasmic tail of the receptor [1, 2]. Following the binding of a cytokine to its receptor, JAKs autophosphorylate and transphosphorylate other proteins. JAKs phosphorylate sites on the cytokine receptor cytoplasmic tails, which create docking sites for signaling effectors, principally the signal transducers and activators of transcription (STATs). The STATs are then phosphorylated, resulting in nuclear translocation. The STAT family of proteins play critical roles in regulating gene expression. JAKs play important roles in erythroid, myeloid and lymphoid cells. In the erythroid lineage, JAK2 associates with the erythropoietin receptor (EPOR), and in the myeloid lineage with the thrombopoietin receptor (TPOR) and granulocyte colony stimulating factor receptor (G-CSFR). In lymphoid cells, JAK1 mainly associates with the cytokine chain (IL2, IL4, IL7, IL9, IL15, IL21), and JAK3 associates with the common gamma chain (γc) to result in a fully functional cytokine receptor heterodimer [3].
The significance of JAKs in hematopoietic function is clear when these kinases are deleted. JAK1 and JAK2 deletions have been shown to be embryonic lethal; loss of JAK1 results in defective neural and lymphoid development, while the loss of JAK2 effects erythropoiesis [4]. JAK3 mutations cause severe combined immunodeficiency (SCID), resulting in patients who lack T cells and NK cells, largely due to IL-7 and IL-15 receptor loss of function [2, 5, 6].
The finding that loss of JAK3 results in SCID highlights the necessity of this kinase in immune function. However, while cytokine signaling is critical for immune cell function, their aberrant function is also implicated in the pathogenesis of autoimmune diseases and hematopoietic malignancies. Since JAK3 is immediately downstream of many cytokine receptors, this kinase became an attractive therapeutic target for treating autoimmune and organ transplant patients. Furthermore, since JAK3 is only expressed in a few cell types, inhibiting or downregulating its expression had the potential to be less toxic than other broad immunosuppressants [4]. The interest in using JAK inhibitors to treat hematological malignancies originated with the underlying cause of polycythemia vera in over 95% of patients is due to a single point mutation in JAK2 (JAK2 V617F) which renders the enzyme hyperactive and cytokine-independent. Since then, mutations in components of the JAK/STAT pathway (IL7R, CRLF2, JAK1, JAK2, or JAK3) have been discovered in other hematological malignancies such as acute lymphoblastic leukemia (ALL), acute myeloleukemia (AML), and lymphomas. Due to these discoveries, the idea of using JAK inhibitors as a monotherapy or in combination with other chemotherapies is becoming an attractive option in this era of precision medicine. Using a targeted therapy approach could hopefully cure patients with various mutations that historically have a poor prognosis. This review will aim to highlight common JAK/STAT pathway mutations in hematological malignancies, where a JAK inhibitor may be useful in the treatment regimen.
2. Tofacitinib and Ruxolitinib- two FDA approved JAK inhibitors
The idea of creating JAK inhibitors to treat immune diseases was initiated for rheumatoid arthritis (RA) therapy. RA is generally treated with monoclonal antibodies, particularly anti-tumor necrosis factor (TNFα) antibodies that block cytokine and cytokine receptor activity. The possibility to treat autoimmune diseases with a JAK inhibitor was initially realized in 1995 [5, 7]. The concept of targeting JAKs for the treatment of chronic autoimmune diseases had several advantages over other biologics such as monoclonal antibodies. TNF inhibitors are a popular therapeutic option for rheumatoid arthritis, psoriasis, and inflammatory bowel disease, but patients often need to take drugs for decades to control the disease. Many patients do not want to receive injections or intravenous therapy; research has shown that only 50% of rheumatoid arthritis patients are still receiving monoclonal antibody treatment after two years [8]. JAK inhibitors, on the other hand, are taken orally. Tofacitinib, a JAK1 and JAK3 inhibitor, was FDA approved in 2012 for the treatment of rheumatoid arthritis. Tofacitinib is currently being explored for use in alopecia areata, psoriasis, and ulcerative colitis. The use of tofacitinib to treat autoimmune disorders is outside the scope of this review and will not be discussed here.
The discovery of the JAK2 V617F mutation in myeloproliferative neoplasms (MPN) opened up new roles for JAK inhibitors, especially in the treatment of hematological malignancies. Ruxolitinib, a JAK1 and JAK2 inhibitor, was FDA approved in 2011 for the treatment of myelofibrosis. Ruxolitinib is a competitive inhibitor of the ATP binding site on kinase domain. In a first-in-human pharmacodynamics study, ruxolitinib was shown to inhibit STAT3 phosphorylation in a dose- and time-dependent manner; maximal inhibition of STAT3 occurred within 1–2 hours following a single oral dose of the drug [9]. In a Phase I/II study with JAK2V617F positive and negative myelofibrosis (PMF) patients, the maximum tolerated dose was determined to be 25 mg twice a day or 100 mg once a day; the dose is limited by reversible thrombocytopenia [10]. Following a single oral 24 mg dose, ruxolitinib is rapidly and efficiently absorbed, reaching peak plasma concentrations within 2 hours. The drug has a relatively short half-life of about three hours. Healthy volunteers receiving a single dose of ruxolitinib excrete approximately 70% of metabolized compound within 24 hours, mostly through the urine. After ten days of dosing, it was determined that very little of the parent compound or metabolites accumulate in the body [9, 11]. Clinical efficacy of ruxolitinib in trials with myelofibrosis patients will be discussed below.
3. Myeloproliferative neoplasms (MPN)
MPNs are a group of chronic disorders characterized by a proliferation of abnormal hematopoietic progenitor cells in the bone marrow. MPNs include PMF, polycythemia vera (PV), essential thrombocythemia (ET), and chronic myelogenous leukemia (CML). The underlying pathogenesis of CML is the BCR-ABL1 oncogene, which is managed with the tyrosine kinase inhibitor imatinib. PV is characterized by an overproduction of red blood cells while ET results from an overproduction of platelets due to abnormal proliferation of megakaryocytes [12, 13]. PMF results from abnormal proliferation of hematopoietic stem cells leading to bone marrow fibrosis [14]. As a result of abnormal hematopoietic cell proliferation, MPN patients often have abnormal cytokine production, splenomegaly, extramedullary hematopoiesis, anemia, fatigue, and other symptoms that lead to a poor quality of life [14–16]. Furthermore, 10–20% of patients progress to acute myeloid leukemia (AML) within the first decade following diagnosis. Collectively, these complications severely impact the quality of life and contribute to an abbreviated lifespan of the patients, with an overall survival of 69 months following diagnosis [17].
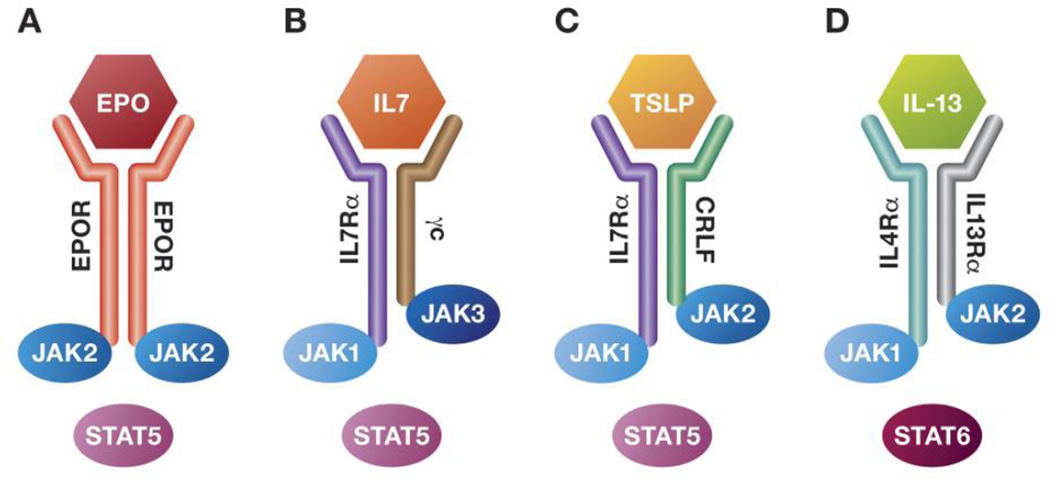
In myeloid lineage cells, JAK2 associates with EPOR, TPOR, or G-CSF. Downstream, JAK2 activates STAT5 and STAT3, which in turn translocate to the nucleus and activate transcription of genes involved in proliferation, survival, and differentiation (Fig 1A) [18, 19]. Aberrant JAK2 signaling plays a key role in the pathogenesis of MPNs. Notably, 95% of PV patients and 50–60% of MF and ET patients have a single point mutation in exon 14 of JAK2 replacing valine 617 with phenylalanine (JAK2V617F). This mutation is in the pseudokinase domain, resulting in cytokine-independent constitutive activation of the JH1 kinase domain and JAK/STAT, PI3K, RAS, and MAPK pathways [20–23].
Figure 1. Receptors involved in hematological malignancies and their associated Janus kinases.
(A) Erythropoietin receptor on red blood cell progenitors; (B) IL-7 receptor on T cells; (C) TSLP receptor on B cells; (D) IL-13 receptor on B cells. Binding of the cytokine induces dimerization of receptor subunits, auto- and trans-phosphorylation of the JAKs and recruitment, phosphorylation, and activation of STATs. Aberrant expression or activation of various subunits of the receptor complexes leads to constitutive activation of the pathways, resulting in malignancy.
In mouse models, oral ruxolitinib reduces splenomegaly and inflammatory cytokines, eliminates JAK2V617F cells, and increases survival [24, 25]. The Phase I/II study showed that ruxolitinib at 25 mg twice daily resulted in reductions in splenomegaly and pro-inflammatory cytokines as well as resolution of constitutional symptoms [10]. Multiple Phase III trials served to corroborate the results in the Phase I/II trial. COMFORT-1 was a randomized, double-blind Phase III trial comparing ruxolitinib to placebo in intermediate- or high-risk MF. Ruxolitinib was given orally at 15 mg twice a day (patients with platelet counts between 100 and 200 × 109/L) or 20 mg twice a day for patients with platelet counts above 200 × 109/L. After 24 weeks, 41.9% of patients treated with ruxolitinib saw a 35% or greater reduction in spleen volume, and 45.9% of patients saw an improvement in constitutional symptoms [26]. COMFORT-II was a randomized Phase III trial comparing ruxolitinib with best available therapy (hydroxyurea, steroids, or supportive therapy) in patients with intermediate or high risk MF. After 48 weeks, 28.5% of patients treated with ruxolitinib saw a 35% or greater reduction in spleen volume [27]. Both studies reported similar side effects of anemia, thrombocytopenia, neutropenia, and diarrhea, concluding that this is a generally well tolerated drug.
Recently, new JAK2 inhibitors have entered clinical trials for MPN with variable success. Fedratinib is a selective JAK2 inhibitor with activity also against FLT3 and RET. This drug demonstrated dose dependent reductions in spleen volume and STAT3 activity. Fedratinib reached phase III clinical trials, but the study and clinical development of this drug was terminated when cases of encephalopathy were reported [15, 18]. Momelotinib is a JAK1/2 inhibitor that has shown to reduce spleen volume in 48% of patients [28]. A Phase III trial comparing momelotinib to ruxolitinib in intermediate or high risk MF patients is ongoing [29]. Pacritinib is a JAK2 and FLT3 inhibitor that binds to “activated” JAK2 and inhibits phosphorylation at Y221, thereby inhibiting STAT3 and STAT5 activation and inducing apoptosis [18, 30]. In 2014, pacritinib received Fast Track Designation by the FDA for the treatment of MF [31]. Clinical trials studying the safety profile of pacritinib have shown that this drug is well tolerated, with minimal myelotoxicity and the major adverse effects being gastrointestinal [18, 32]. Preclinical studies in mice and Phase I/II clinical trials have shown parcritinib to be effective in MF, based on spleen reductions of 35% or greater, and has now progressed into Phase III trials [18, 33, 34]. Finally, lestaurtinib is a multikinase inhibitor that has been shown to improve symptoms in JAKV617F positive ET and PV patients with 15% of patients demonstrating a 15% or greater reduction in JAK2V617F allele burden [35–37].
4. Acute Lymphoblastic Leukemia (ALL)
ALL results from the transformation of either immature B or T cells and is the most common childhood cancer (25% of cases). B-ALL is more common (85% of cases), and has a more favorable prognosis than T-ALL [38]. In the 1940’s, the first effective chemotherapeutic agent was developed to treat ALL, and since then, the cure rate has increased from 10% to 80–90%. However, ALL remains the second leading cause of cancer deaths in children. In adults, ALL has a less favorable outcome with a cure rate of 30–40% and about 50% of patients relapsing after achieving complete remission [39]. The first indication that JAK aberrations could play a role in hematological malignancies was observed in a pediatric ALL patient with a translocation event between JAK2 and the transcription factor TEL. While TEL-JAK2 appears to be a rare event in human leukemia, the TEL-JAK2 transgenic mouse model results in T-ALL and this model has been useful in revealing the relationship between JAK/STAT aberrations and leukemogenesis [40, 41]. In order to improve the prognosis for certain subtypes of ALL, it is important to understand the underlying molecular mechanisms of this heterogeneous disease to develop more targeted and less toxic therapies.
4.1 T cell Acute Lymphoblastic Leukemia (T-ALL)
IL7Rα mutations are seen in approximately 9% of pediatric T-ALL patients. These mutations are gain of function amino acid insertions that induce ligand-independent activation of the IL7 pathway, including constitutive JAK1 and STAT5 phosphorylation. Almost all of these patients (82%) have insertional sequences that contain an unpaired cysteine residue in the extracellular juxtamembrane-transmembrane region in the IL7Rα chain. This results in an intermolecular disulfide bond dimerizing two IL7Rα chains, allowing for constitutive activation of JAK1 in the absence of JAK3 and γc (Fig 1B). Remarkably, no two patients are reported to have the same exact insertional mutation, despite each one containing a cysteine and often a proline [42, 43]. In vitro studies showed that both tofacitinib and ruxolitinib were effective at inhibiting JAK1 phosphorylation and inducing cell death in a thymocyte cell line engineered to express mutant IL7Rα [42, 44]. Non-cysteine mutations in IL7Rα have also been reported; however, when BaF3 cells were transduced with the these IL7Rα mutated constructs, only 2 of the 3 resulted in cytokine independent growth and leukemia in Balb/c mice [45].
Early T Cell Precursor (ETP) is a subset of T-ALL in which the leukemia cells express myeloid and early progenitor stem cell markers, as well as T cell lineage markers. These patients in particular have a high risk of treatment failure and a poor prognosis. IL7Rα mutations are also seen in ETP ALL, along with mutations in JAK1 and JAK3 [46]. In mouse xenograft models of ETP-ALL, JAK/STAT inhibition was effective in reducing leukemic burden [47].
In adults, T-ALL has a poor prognosis. Adult T-ALL is classified into three subtypes based on immunophenotype: early immature TALL, mature TALL, and thymic T-ALL, with thymic T-ALL having the best prognosis of the three. IL-7Rα mutations have not yet been reported in adult T-ALL patients [48]. However, JAK mutations are common in adult T-ALL; 13.6% of adults with T-ALL have mutations in JAK3, and JAK1 mutations have been detected in 4–18% of adult T-ALLs [49–51]. These mutations are seen in high risk patients with an immature T-ALL phenotype.
4.2 B Cell ALL (B-ALL)
Aberrations in components of the JAK/STAT pathway are common in B-ALL that are described as “Philadelphia chromosome (Ph)-like”. These leukemias have a poor prognosis and a transcriptional signature similar to the Philadelphia chromosome positive leukemias but do not have the BCR-ABL rearrangement [52, 53]. In normal B cells, the IL7Rα dimerizes with CRLF2 to form the TSLP receptor. CRLF2 directly interacts with both wild type and aberrant JAK2 (Fig 1C). Chromosomal translocations, rearrangements, or gene duplications in CRLF2 have been identified in 5–10% of both pediatric and adult B-ALL, and are associated with poor prognosis [54]. A translocation event leads to an immunoglobulin heavy chain (IgH)-CRLF2 fusion, placing CRLF2 under alternate transcriptional control and resulting in overexpression of CRLF2. Alternatively, a deletion results in a P2RY8-CRLF2 fusion [55, 56]. However, CRLF aberrations alone are not sufficient to induce leukemogenesis. The majority of these cells also contain mutations in other components of the TSLP signaling pathway, such as JAK2 (in 30–50% of cases), cysteine insertions in IL7Rα, or an F232C substitution in CRLF2 resulting in constitutive homodimerization via disulfide bond (similar to IL7Rα mutations) [57–59]. Patients with CRLF2 aberrations also commonly have deletions or mutations in the lymphoid transcription factor IKAROS (IKZF1). These patients are also described as “Philadelphia chromosome-like” and patients with IKZF1/CRLF2 mutations have a poor prognosis [52].
JAK mutations are common in high risk B-ALL. These mutations occur independently of CRLF2 [44]. Out of 187 patients sequenced, 20 (10.7%) patients had mutations in JAK1, JAK2, or JAK3. Sixteen patients had JAK2 mutations, with 13 in the pseudokinase domain (R683G, R683S, I682F, QGinsR683), and 3 within the kinase domain (R687Q, D873N, and P933R). Three patients had mutations in the pseudokinase domain of JAK1 (L624_R629>W, S646F, and V658F), and one patient had a JAK3 mutation (S789P). Two of the patients with JAK2 mutations had were DS-ALL cases (discussed below). These JAK mutations are highly associated with mutations in the lymphoid transcription factor IKAROS (IKZF1) (70% of all JAK cases) and deletion of CDKN2A (70%). JAK mutations with IZKF1 alterations have a very poor outcome; 78% of patients with JAK and IKZF1 mutations will relapse, die, or have a second malignancy within 4 years [52].
Children with Down Syndrome have a high risk of leukemia. The risk of ALL (DS-ALL) is 33 times higher for Down syndrome patients compared to children without Down syndrome [58]. They are almost exclusively B-ALL, and have a poor prognosis compared to non-DS ALL patients [60]. Approximately 60% of DS-ALLs have aberrant expression of CRLF2 that is associated with other mutations in the JAK-STAT pathway [43, 55, 58]. JAK2 mutations are also common in DS-ALL patients, particular involving the JAK2 R682 residue. One study reported 16 out of 88 DS-ALL patients with R683 mutations [61], while another reported a 20% incidence of JAK2 R683 mutations [62], suggesting this residue is a “hotspot” in DS-ALL patients [63].
Clinical trials are ongoing for the use of ruxolitinib and other JAK inhibitors in the treatment of ALL. Recently, MD Anderson Cancer Center has completed a Phase I/II trial to assess the efficacy of ruxolitinib in relapsed or refractory AML or ALL patients (ages 14 and older) [64]. Incyte has recently initiated a Phase II trial to assess the efficacy of combining ruxolitinib with the standard chemotherapy regimen for B-ALL patients with CRLF2 or JAK/STAT pathway aberrations [65].
5. Acute Myeloid Leukemia (AML)
AML is due to aberrant growth of myeloid cells that accumulate in the bone marrow. Pediatric AML accounts for 15–20% of pediatric leukemia, with survival rates of approximately 70% [66]. In adults, AML is the most common type of leukemia, but only 10–20% of patients older than 60 will be cured [67, 68]. Furthermore, about 5–10% of patients will die from side effects of the treatment protocol, with additional patients suffering long-term side effects such as anthracycline-induced cardiomyopathy [69]. Like ALL, advances have been made in understanding the molecular mechanisms underlying AML, which may improve treatments. JAK2 mutations are rare in AML, but the majority of AMLs have activated STAT3 and/or STAT5, which may be due to aberrations in signaling molecules upstream of STAT, such as FLT3 [70]. However, AML is a common complication stemming from MPNs [71]. The efficacy of ruxolitinib in treating relapsed/refractory AML and ALL was studied in a Phase II trial. After 28 days of treatment, 8 of the 23 enrolled patients demonstrated a complete remission, partial remission or stable disease; 5 of these 8 patients had the JAKV617F mutation. A follow up to this study demonstrated 15 of 38 patients had a response to ruxolitinib with 2 of the 15 patients positive for JAK2V617F [68]. Lestaurtinib has also shown efficacy in treating adult and pediatric AML [72, 73].
Down Syndrome patients are also at extremely high risk for AML. The incidence of AML (ML-DS) is about 150 times higher than children without Down syndrome [58]. ML-DS manifests as erythromegakaryoblastic leukemias that often present with thrombocytopenia and/or myelodysplasia [58, 74], and usually responds well to therapy due to its sensitivity to chemotherapeutics, particularly cytosine arabinoside [75]. The underlying genetic mechanism of ML-DS is an acquired mutation in GATA1, but this mutation alone is insufficient for the development of ML-DS. Common mutations that promote the growth and proliferation of the cells do include mutations in the JAK-STAT pathway, especially JAK2 [58, 75, 76].
6. Lymphoma
Lymphoma is a lymphoid malignancy arising from mature B or T cells. Lymphoma has a diverse molecular etiology; however, aberrations in the JAK/STAT pathway are common. To date, only JAK3 and JAK2 mutations have been described in lymphoma patients. 32% of Natural Killer/T cell lymphoma patients are reported to have JAK3 point mutations (A572V, A573V) [77]. Enhanced JAK/STAT activity has been observed in Primary Mediastinal B cell lymphoma (PBML) and Hodgkin’s Lymphoma (HL). In particular, 55% of PMBL patients and 35% of HL patients share a common genetic signature of an amplification of chromosome 9p24, which involves 21 genes including JAK2 [78, 79]. Data suggest that the amplified JAK2 works in an autocrine feedback loop with the IL13 receptor; JAK2 is activated by IL13 and the signal is amplified by the 9p24 amplicon. The JAK2 activation in turn phosphorylates STAT6 which translocates to the nucleus and results in the transcription of the Il13 gene and production of IL-13 protein. The IL-13 is secreted from the cell and binds to the IL-13 receptor, thereby continuing to enhance the JAK2/STAT6 pathway (Fig 1D) [80].
A few clinical trials have focused on JAK inhibitors in the treatment of lymphoma. Fedratinib, a JAK2 inhibitor, has been shown to block phospho-STAT3, cell viability, and proliferation in 9p24-amplicon positive HL and PMBL cell lines in vivo and in vitro [81]. Pacritinib is the first JAK2 inhibitor to enter Phase I clinical trials for lymphoma patients. Pacritinib showed therapeutic benefit in 55% of patients as evidenced by reduction in tumor size [82]. The effects of ruxolitinib on primary HL or PMBL have not been reported yet [80].
7. Potential Obstacles with JAK inhibitor therapy and suggestions
Though FDA approval of Ruxolitinib has been a breakthrough therapy for patients with MPN, it is not a perfect treatment, nor is it curative. In a long-term follow up study of 236 MPN patients receiving ruxolitinib in the COMFORT-1 trial, only 20 achieved a partial response and 6 achieved complete response, as measured by allele burden over 216 weeks [83]. For most patients, chronic therapy with ruxolitinib has not led to molecular or pathological remission. This is not due to acquiring secondary mutations, but rather due to a phenomenon termed “persistence” [84]. Persistence is considered an adaptive form of resistance in which JAK/STAT signaling is reactivated due to heterodimerization between JAK2 and JAK1/TYK2. A potential solution to this is the development of a “Type II” JAK inhibitor called CHZ868. CHZ868 and other Type II inhibitors bind JAK2 in the inactive conformation and occupy the ATP binding site and an induced hydrophobic compartment [85]. CHZ868 was found to potently inhibit CRLF-2 rearranged B-ALL patient derived xenograft (PDX) cells in mice after being treated for only 6 days; furthermore, in in vitro models, CHZ868 synergizes with dexamethasone suggesting that Type II JAK2 inhibitors can be combined with standard chemotherapeutic agents [85]. However, cells can become resistant to CHZ868 and other Type II JAK inhibitors. The mechanism for this has not been fully elucidated but may be related to a mutation in JAK2 L884P which may change the shape of the kinase domain [85]. Additionally, a new mechanism of resistance has been reported by which of cultured cells with a JAK1 mutation acquiring a secondary mutation in JAK3 during the course of ruxolitinib treatment. This mechanism has yet to be reported in humans, but the potential should not be ignored [86].
Rather than monotherapy, a potential solution to reducing resistance or persistence is to combine multiple targeted therapies to inhibit more than one signaling pathway [87, 88]. Ruxolitinib can be combined with mTOR inhibitors, particularly in CRLF2-overexpressed leukemias that often have aberrant PI3k/mTOR activity [88]. In a FLT3-positive AML model, JAK inhibitors were shown to synergize with FLT3 inhibitors to overcome drug resistance in the bone marrow microenvironment [89, 90]. A promising strategy is to combine ruxolitinib with a BCL-2 inhibitor, such as navitoclax or venetoclax. In clinical trials, navitoclax was found to induce dose dependent thrombocytopenia. Therefore, navitoclax was re-engineered as venetoclax, which is more specific for BCL-2 and eliminates the negative effects on platelets. Venetoclax has strong activity against CLL and AML cell lines, as well as subsets of non-Hodgkin’s lymphoma and multiple myeloma [91]. Venetoclax is currently in multiple stages of clinical trials to treat subsets of leukemia and lymphoma, either as a single agent or in combination with other conventional chemotherapies [92].
Combining a JAK inhibitor with a BCL2/BCLxl inhibitor was effective in xenotransplanted human pre-B ALL cells expressing either JAK2 R683G or JAK2 T875N, by inhibiting STAT5 activation and inducing cell death [87]. Additionally, this combination was sufficient to overcome persistence to JAK2 inhibition. After inducing persistence in the megakaryblastic SET-2 cell line (with a JAK2 V617F mutation), these cells were highly sensitive to treatment with a JAK inhibitor plus ABT737. Bcl2 inhibitors such as venetoclax have shown promise in treating T-ALL as well [93]. One particular study focused on Adult T cell leukemia (ATL), which actually does not have any JAK aberrations [94], but rather an upregulation of Bcl-xl which is upregulated by the IL-2 and IL-15 pathways. In this model, there was an additive or synergistic effect with ruxolitinib and either navitoclax, panobinstat, and mTOR, PI3K, or NFkB inhibitors. Combining ruxolitinib and navitoclax resulted a significant decrease in tumor burden and in prolonged survival in an ATL mouse model [95].
8. Implementing precision medicine with the use of JAK inhibitors
Despite the progress in treating hematological malignancies, including leukemia, there is a need for less toxic and better targeted drugs. Current chemotherapy regimens include at least 2–3 years of intense treatment, often with long lasting side effects. Conventional chemotherapy may result in remission, but leave the patient with both acute and chronic side effects, including hair loss, infections, thrombosis, pancreatitis, peripheral neurotoxicity, osteonecrosis, neurocognitive defects, and secondary malignancies [44, 96]. The last ten years have been productive in characterizing aberrations and molecular signatures of hematological malignancies, especially in pediatric leukemia. The current challenge is to determine when it is appropriate to replace or supplement non-specific chemotherapeutic drugs with a targeted drug in specific subtypes of malignancies. One example of this approach is the use of imatinib to treat BCR-ABL1 positive ALL. Though this aberration is a rare event in ALL, it is associated with a poor prognosis and the use of imatinib has improved the prognosis in these patients. For children with BCR-ABL1 positive ALL, the event free survival after imatinib therapy is now 72%, compared to 27% for children treated before the approval of imatinib. [97–101]. Leukemia is a heterogeneous disease, and in many subsets, pediatric and adult patients have unique mutations. Fortunately, many of those mutations affect common pathways, with JAK/STAT being one of them. In addition to the aberrations discussed in this review, there are numerous case studies describing JAK/STAT aberrations in single patients.
The future of oncology should include genomic analysis of each patient to determine if any targeted drugs may be useful in their treatment. In 2010, St Jude Children’s Research Hospital partnered with The Genome Institute at Washington University to establish the Pediatric Cancer Genome Project to identify the mutations that drive cancer and potentially identify novel therapeutic targets [102]. Additionally, centers of precision medicine are being established, including The National Institutes of Health, St. Jude Children’s Research Hospital, University of Pennsylvania, Vanderbilt, Columbia, and Dana-Farber. Another approach is the use of PDX models, in which patient tumors are transferred into immunodeficient mice, while retaining the original characteristics of the tumor including histology and genomic profile. PDX models are used extensively in the research setting, allowing investigators to use clinical relevant samples to study the efficacy of novel therapeutics. In the future, PDX models may allow investigators to develop personalized drug regimens for patients; various drug combinations could be optimized in the mouse before treating the patient, potentially improving clinical outcomes [103, 104].
9. Conclusions
Mutations in the JAK/STAT pathway are common in a spectrum of hematological malignancies. The discovery of JAKV617F in MPNs resulted in FDA approval of ruxolitinib. Ruxolitinib has shown efficacy in treating other hematological malignancies such as ALL, AML, and lymphoma in preclinical and clinical studies. Though persistence or resistance to ruxolitinib occurs in some models, there is potential to combine ruxolitinib with other drugs, for example a Bcl2 inhibitor such as venetoclax. Additionally, more JAK inhibitors are in development, particularly Type II JAK inhibitors, which may turn out to be more effective than ruxolitinib. As we head into an era of precision medicine, targeting the JAK/STAT pathway is a promising therapeutic avenue in hematological malignancies.
Table 1.
Genetic Aberrations affecting the JAK/STAT pathway in hematological malignancies
| Gene | Aberration | Disease | Refs |
|---|---|---|---|
| IL7Rα | Gain-of-function amino acid insertions (containing cysteine and proline) |
Pediatric T-ALL ETP B-ALL |
42, 43, 46 |
| IL7Rα | Gain-of-function mutations not containing cysteine |
Pediatric ALL | 45 |
| CRLF2 | IgH-CRLF2 translocation | B-ALL | 55, 56, 57, 59 |
| CRLF2 | P2RY8-CRLF2 translocation | B-ALL DS-ALL |
55, 56, 59 |
| CRLF2 | F232C | B-ALL | 57, 59 |
| JAK1 | S703I, I631>RGI | ETP | 46 |
| JAK1 | S512L, A634N, R724H, R879S, R879C, R879H |
Adult T-ALL | 49 |
| JAK1 | L624_R629>W, S646F, and V658F |
B-ALL | 52 |
| JAK2 | V617F | Polycythemia vera Essential thrombocythemia Primary myelofibrosis |
20, 21, 22, 23, |
| JAK2 | TEL-JAK2 translocation | Pediatric T-ALL | 41 |
| JAK2 | R683G, R683S, I682F, QGinsR683 R683K, L681-I682ins, I628del |
B-ALL DS-ALL |
52, 61, 62, 63 |
| JAK2 | V617F, L611S, R683S, R867Q | ML-DS | 76 |
| JAK2 | Amplification of chromosome 9p24 |
Primary Mediastinal B cell lymphoma Hodgkin’s Lymphoma |
78, 79 |
| JAK3 | M511I, A573V | ETP | 46 |
| JAK3 | S197N, M511I, R657Q, P664L, L857P, K885E, E960A Frame shift deletion at 958 |
Adult T-ALL | 51 |
| JAK3 | S789P | B-ALL | 52 |
| JAK3 | A572V, A573V | Natural Killer/ T cell lymphoma |
77 |
Acknowledgments
Funding:
This work has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This work was also supported by grants from the Children’s Cancer Foundation, Inc.
Abbreviations
- JAK
Janus kinase
- STAT
signal transducers and activators of transcription
- EPOR
erythropoietin receptor
- TPOR
thrombopoietin receptor
- G-CSFR
granulocyte colony stimulating factor receptor
- γc
common gamma chain
- SCID
severe combined immunodeficiency
- ALL
acute lymphoblastic leukemia
- AML
acute myeloleukemia
- RA
rheumatoid arthritis
- TNFα
tumor necrosis factor alpha
- MPN
myeloproliferative neoplasm
- PV
polycythemia vera
- PMF
primary myelofibrosis
- ET
essential thrombocythemia
- CML
chronic myelogenous leukemia
- ETP
early T cell precursor ALL
- Ph
Philadelphia chromosome
- CRLF2
cytokine receptor-like factor 2
- TSLP
thymic stromal lymphopoietin
- IgH
immunoglobulin heavy chain
- DS-ALL
Down’s Syndrome ALL
- ML-DS
Down’s Syndrome ALL
- PMBL
primary mediastinal B-cell lymphoma
- HL
Hodgkin’s Lymphoma
- PDX
patient derived xenograft
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. The Biochemical journal. 2014;462(1):1–13. doi: 10.1042/BJ20140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. The New England journal of medicine. 2013;368(2):161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–2613. doi: 10.1038/onc.2012.347. [DOI] [PubMed] [Google Scholar]
- 4.Khwaja A. The role of Janus kinases in haemopoiesis and haematological malignancy. Br J Haematol. 2006;134(4):366–384. doi: 10.1111/j.1365-2141.2006.06206.x. [DOI] [PubMed] [Google Scholar]
- 5.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, Migone TS, Noguchi M, Markert ML, Buckley RH, O'Shea JJ, Leonard WJ. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science (New York, N.Y.) 1995;270(5237):797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 6.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36(4):515–528. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz DM, Bonelli M, Gadina M, O'Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases, Nature reviews. Rheumatology. 2016;12(1):25–36. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111–ii115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi JG, Chen X, McGee RF, Landman RR, Emm T, Lo Y, Scherle PA, Punwani NG, Williams WV, Yeleswaram S. The pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol. 2011;51(12):1644–1654. doi: 10.1177/0091270010389469. [DOI] [PubMed] [Google Scholar]
- 10.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. The New England journal of medicine. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shilling AD, Nedza FM, Emm T, Diamond S, McKeever E, Punwani N, Williams W, Arvanitis A, Galya LG, Li M, Shepard S, Rodgers J, Yue TY, Yeleswaram S. Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans. Drug Metab Dispos. 2010;38(11):2023–2031. doi: 10.1124/dmd.110.033787. [DOI] [PubMed] [Google Scholar]
- 12.Stein BL, Oh ST, Berenzon D, Hobbs GS, Kremyanskaya M, Rampal RK, Abboud CN, Adler K, Heaney ML, Jabbour EJ, Komrokji RS, Moliterno AR, Ritchie EK, Rice L, Mascarenhas J, Hoffman R. Polycythemia Vera: An Appraisal of the Biology and Management 10 Years After the Discovery of JAK2 V617F. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(33):3953–3960. doi: 10.1200/JCO.2015.61.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beer PA, Green AR. Pathogenesis and management of essential thrombocythemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2009:621–628. doi: 10.1182/asheducation-2009.1.621. [DOI] [PubMed] [Google Scholar]
- 14.Tefferi A. Primary myelofibrosis: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(9):915–925. doi: 10.1002/ajh.23703. [DOI] [PubMed] [Google Scholar]
- 15.Mascarenhas JO, Cross NC, Mesa RA. The future of JAK inhibition in myelofibrosis and beyond. Blood reviews. 2014;28(5):189–196. doi: 10.1016/j.blre.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 17.Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18(11):3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 18.Duenas-Perez AB, Mead AJ. Clinical potential of pacritinib in the treatment of myelofibrosis. Therapeutic advances in hematology. 2015;6(4):186–201. doi: 10.1177/2040620715586527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathur A, Mo JR, Kraus M, O'Hare E, Sinclair P, Young J, Zhao S, Wang Y, Kopinja J, Qu X, Reilly J, Walker D, Xu L, Aleksandrowicz D, Marshall G, Scott ML, Kohl NE, Bachman E. An inhibitor of Janus kinase 2 prevents polycythemia in mice. Biochem Pharmacol. 2009;78(4):382–389. doi: 10.1016/j.bcp.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Goldman JM. A unifying mutation in chronic myeloproliferative disorders. The New England journal of medicine. 2005;352(17):1744–1746. doi: 10.1056/NEJMp058083. [DOI] [PubMed] [Google Scholar]
- 21.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England journal of medicine. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 22.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR P. Cancer Genome. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 23.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 24.Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P, Rupar M, Burn T, Lo Y, Kelley J, Covington M, Shepard S, Rodgers JD, Haley P, Kantarjian H, Fridman JS, Verstovsek S. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesa RA, Yasothan U, Kirkpatrick P. Ruxolitinib. Nature reviews. Drug discovery. 2012;11(2):103–104. doi: 10.1038/nrd3652. [DOI] [PubMed] [Google Scholar]
- 26.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH, Jr, Arcasoy MO, Hexner E, Lyons RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. The New England journal of medicine. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. The New England journal of medicine. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 28.Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A, Hogan WJ, Litzow MR, Leontovich A, Kowalski M, Tefferi A. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27(6):1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilead Sciences. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–2016. Momelotinib Versus Ruxolitinib in Subjects With Myelofibrosis. Available from: https://clinicaltrials.gov/ct2/show/NCT01969838 NLM Identifier: NCT01969838. [Google Scholar]
- 30.Hart S, Goh KC, Novotny-Diermayr V, Hu CY, Hentze H, Tan YC, Madan B, Amalini C, Loh YK, Ong LC, William AD, Lee A, Poulsen A, Jayaraman R, Ong KH, Ethirajulu K, Dymock BW, Wood JW. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia. 2011;25(11):1751–1759. doi: 10.1038/leu.2011.148. [DOI] [PubMed] [Google Scholar]
- 31.Chow V, Weissman A, O'Connell CL, Mehrvar A, Akhtari M. Emerging treatment options for myelofibrosis: focus on pacritinib. Onco Targets Ther. 2016;9:2655–2665. doi: 10.2147/OTT.S93875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzimichael E, Tsolas E, Briasoulis E. Profile of pacritinib and its potential in the treatment of hematologic disorders. J Blood Med. 2014;5:143–152. doi: 10.2147/JBM.S51253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CTI BioPharma. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–2016. Oral Pacritinib Versus Best Available Therapy to Treat Myelofibrosis. Available from: https://clinicaltrials.gov/ct2/show/NCT01773187 NLM Identifier: NCT01773187. [Google Scholar]
- 34.CTI Biopharma. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–2016. Oral Pacritinib Versus Best Available Therapy to Treat Myelofibrosis With Thrombocytopenia. Available from: https://clinicaltrials.gov/ct2/show/NCT02055781. NLM Identifier: NCT02055781. [Google Scholar]
- 35.Hexner E, Roboz G, Hoffman R, Luger S, Mascarenhas J, Carroll M, Clementi R, Bensen-Kennedy D, Moliterno A. Open-label study of oral CEP-701 (lestaurtinib) in patients with polycythaemia vera or essential thrombocythaemia with JAK2-V617F mutation. Br J Haematol. 2014;164(1):83–93. doi: 10.1111/bjh.12607. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman R. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–2016. CEP-701 (Lestaurtinib) in Myelofibrosis. Available from: https://clinicaltrials.gov/ct2/show/NCT00668421 NLM Identifier: NCT00668421. [Google Scholar]
- 37.Cephalon. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–2016. Open-Label Study of Oral CEP-701 (Lestaurtinib) in Patients With Polycythemia Vera or Essential Thrombocytosis. Available from: https://clinicaltrials.gov/ct2/show/NCT00586651 NLM Identifier: NCT00586651. [Google Scholar]
- 38.Carroll WL, Raetz EA. Clinical and laboratory biology of childhood acute lymphoblastic leukemia. J Pediatr. 2012;160(1):10–18. doi: 10.1016/j.jpeds.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Ronson A, Tvito A, Rowe JM. Treatment of Relapsed/Refractory Acute Lymphoblastic Leukemia in Adults. Current oncology reports. 2016;18(6):39. doi: 10.1007/s11912-016-0519-8. [DOI] [PubMed] [Google Scholar]
- 40.Sayyah J, Sayeski PP. Jak2 inhibitors: rationale and role as therapeutic agents in hematologic malignancies. Current oncology reports. 2009;11(2):117–124. doi: 10.1007/s11912-009-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, Bernard OA. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science (New York, N.Y.) 1997;278(5341):1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 42.Zenatti PP, Ribeiro D, Li W, Zuurbier L, Silva MC, Paganin M, Tritapoe J, Hixon JA, Silveira AB, Cardoso BA, Sarmento LM, Correia N, Toribio ML, Kobarg J, Horstmann M, Pieters R, Brandalise SR, Ferrando AA, Meijerink JP, Durum SK, Yunes JA, Barata JT. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nature genetics. 2011;43(10):932–939. doi: 10.1038/ng.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shochat C, Tal N, Bandapalli OR, Palmi C, Ganmore I, te Kronnie G, Cario G, Cazzaniga G, Kulozik AE, Stanulla M, Schrappe M, Biondi A, Basso G, Bercovich D, Muckenthaler MU, Izraeli S. Gain-of-function mutations in interleukin-7 receptor-alpha (IL7R) in childhood acute lymphoblastic leukemias. The Journal of experimental medicine. 2011;208(5):901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cramer SD, Aplan PD, Durum SK. Therapeutic targeting of IL-7Ralpha signaling pathways in ALL treatment. Blood. 2016 doi: 10.1182/blood-2016-03-679209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shochat C, Tal N, Gryshkova V, Birger Y, Bandapalli OR, Cazzaniga G, Gershman N, Kulozik AE, Biondi A, Mansour MR, Twizere JC, Muckenthaler MU, Ben-Tal N, Constantinescu SN, Bercovich D, Izraeli S. Novel activating mutations lacking cysteine in type I cytokine receptors in acute lymphoblastic leukemia. Blood. 2014;124(1):106–110. doi: 10.1182/blood-2013-10-529685. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M, Lu C, Chen SC, Wei L, Collins-Underwood JR, Ma J, Roberts KG, Pounds SB, Ulyanov A, Becksfort J, Gupta P, Huether R, Kriwacki RW, Parker M, McGoldrick DJ, Zhao D, Alford D, Espy S, Bobba KC, Song G, Pei D, Cheng C, Roberts S, Barbato MI, Campana D, Coustan-Smith E, Shurtleff SA, Raimondi SC, Kleppe M, Cools J, Shimano KA, Hermiston ML, Doulatov S, Eppert K, Laurenti E, Notta F, Dick JE, Basso G, Hunger SP, Loh ML, Devidas M, Wood B, Winter S, Dunsmore KP, Fulton RS, Fulton LL, Hong X, Harris CC, Dooling DJ, Ochoa K, Johnson KJ, Obenauer JC, Evans WE, Pui CH, Naeve CW, Ley TJ, Mardis ER, Wilson RK, Downing JR, Mullighan CG. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maude SL, Dolai S, Delgado-Martin C, Vincent T, Robbins A, Selvanathan A, Ryan T, Hall J, Wood AC, Tasian SK, Hunger SP, Loh ML, Mullighan CG, Wood BL, Hermiston ML, Grupp SA, Lock RB, Teachey DT. Efficacy of JAK/STAT pathway inhibition in murine xenograft models of early T-cell precursor (ETP) acute lymphoblastic leukemia. Blood. 2015;125(11):1759–1767. doi: 10.1182/blood-2014-06-580480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozovski U, Li P, Harris D, Ohanian M, Kantarjian H, Estrov Z. Interleukin-7 receptor-alpha gene mutations are not detected in adult T-cell acute lymphoblastic leukemia. Cancer medicine. 2014;3(3):550–554. doi: 10.1002/cam4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L, Ariola C, Fodale V, Clappier E, Paoloni F, Martinelli S, Fragale A, Sanchez M, Tavolaro S, Messina M, Cazzaniga G, Camera A, Pizzolo G, Tornesello A, Vignetti M, Battistini A, Cave H, Gelb BD, Renauld JC, Biondi A, Constantinescu SN, Foa R, Tartaglia M. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. The Journal of experimental medicine. 2008;205(4):751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asnafi V, Le Noir S, Lhermitte L, Gardin C, Legrand F, Vallantin X, Malfuson JV, Ifrah N, Dombret H, Macintyre E. JAK1 mutations are not frequent events in adult T-ALL: a GRAALL study. Br J Haematol. 2010;148(1):178–179. doi: 10.1111/j.1365-2141.2009.07912.x. [DOI] [PubMed] [Google Scholar]
- 51.Neumann M, Vosberg S, Schlee C, Heesch S, Schwartz S, Gokbuget N, Hoelzer D, Graf A, Krebs S, Bartram I, Blum H, Bruggemann M, Hecht J, Bohlander SK, Greif PA, Baldus CD. Mutational spectrum of adult T-ALL. Oncotarget. 2015;6(5):2754–2766. doi: 10.18632/oncotarget.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, Tasian SK, Loh ML, Su X, Liu W, Devidas M, Atlas SR, Chen IM, Clifford RJ, Gerhard DS, Carroll WL, Reaman GH, Smith M, Downing JR, Hunger SP, Willman CL. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(23):9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X, Chen SC, Payne-Turner D, Churchman ML, Harvey RC, Chen X, Kasap C, Yan C, Becksfort J, Finney RP, Teachey DT, Maude SL, Tse K, Moore R, Jones S, Mungall K, Birol I, Edmonson MN, Hu Y, Buetow KE, Chen IM, Carroll WL, Wei L, Ma J, Kleppe M, Levine RL, Garcia-Manero G, Larsen E, Shah NP, Devidas M, Reaman G, Smith M, Paugh SW, Evans WE, Grupp SA, Jeha S, Pui CH, Gerhard DS, Downing JR, Willman CL, Loh M, Hunger SP, Marra MA, Mullighan CG. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer cell. 2012;22(2):153–166. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Bodegom D, Zhong J, Kopp N, Dutta C, Kim MS, Bird L, Weigert O, Tyner J, Pandey A, Yoda A, Weinstock DM. Differences in signaling through the B-cell leukemia oncoprotein CRLF2 in response to TSLP and through mutant JAK2. Blood. 2012;120(14):2853–2863. doi: 10.1182/blood-2012-02-413252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin MG, Liu W, Zhang J, Ma J, Coustan-Smith E, Harvey RC, Willman CL, Mikhail FM, Meyer J, Carroll AJ, Williams RT, Cheng J, Heerema NA, Basso G, Pession A, Pui CH, Raimondi SC, Hunger SP, Downing JR, Carroll WL, Rabin KR. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nature genetics. 2009;41(11):1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA, Calasanz MJ, Chandrasekaran T, Chapiro E, Gesk S, Griffiths M, Guttery DS, Haferlach C, Harder L, Heidenreich O, Irving J, Kearney L, Nguyen-Khac F, Machado L, Minto L, Majid A, Moorman AV, Morrison H, Rand V, Strefford JC, Schwab C, Tonnies H, Dyer MJ, Siebert R, Harrison CJ. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114(13):2688–2698. doi: 10.1182/blood-2009-03-208397. [DOI] [PubMed] [Google Scholar]
- 57.Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, West N, Xiao Y, Brown JR, Mitsiades C, Sattler M, Kutok JL, DeAngelo DJ, Wadleigh M, Piciocchi A, Dal Cin P, Bradner JE, Griffin JD, Anderson KC, Stone RM, Ritz J, Foa R, Aster JC, Frank DA, Weinstock DM. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(1):252–257. doi: 10.1073/pnas.0911726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts I, Izraeli S. Haematopoietic development and leukaemia in Down syndrome. Br J Haematol. 2014;167(5):587–599. doi: 10.1111/bjh.13096. [DOI] [PubMed] [Google Scholar]
- 59.Chen IM, Harvey RC, Mullighan CG, Gastier-Foster J, Wharton W, Kang H, Borowitz MJ, Camitta BM, Carroll AJ, Devidas M, Pullen DJ, Payne-Turner D, Tasian SK, Reshmi S, Cottrell CE, Reaman GH, Bowman WP, Carroll WL, Loh ML, Winick NJ, Hunger SP, Willman CL. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2012;119(15):3512–3522. doi: 10.1182/blood-2011-11-394221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buitenkamp TD, Pieters R, Gallimore NE, van der Veer A, Meijerink JP, Beverloo HB, Zimmermann M, de Haas V, Richards SM, Vora AJ, Mitchell CD, Russell LJ, Schwab C, Harrison CJ, Moorman AV, van den Heuvel-Eibrink MM, den Boer ML, Zwaan CM. Outcome in children with Down's syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia. 2012;26(10):2204–2211. doi: 10.1038/leu.2012.84. [DOI] [PubMed] [Google Scholar]
- 61.Bercovich D, Ganmore I, Scott LM, Wainreb G, Birger Y, Elimelech A, Shochat C, Cazzaniga G, Biondi A, Basso G, Cario G, Schrappe M, Stanulla M, Strehl S, Haas OA, Mann G, Binder V, Borkhardt A, Kempski H, Trka J, Bielorei B, Avigad S, Stark B, Smith O, Dastugue N, Bourquin JP, Tal NB, Green AR, Izraeli S. Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet. 2008;372(9648):1484–1492. doi: 10.1016/S0140-6736(08)61341-0. [DOI] [PubMed] [Google Scholar]
- 62.Gaikwad A, Rye CL, Devidas M, Heerema NA, Carroll AJ, Izraeli S, Plon SE, Basso G, Pession A, Rabin KR. Prevalence and clinical correlates of JAK2 mutations in Down syndrome acute lymphoblastic leukaemia. Br J Haematol. 2009;144(6):930–932. doi: 10.1111/j.1365-2141.2008.07552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee P, Bhansali R, Izraeli S, Hijiya N, Crispino JD. The biology, pathogenesis and clinical aspects of acute lymphoblastic leukemia in children with Down syndrome. Leukemia. 2016 doi: 10.1038/leu.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MD Anderson Cancer Center. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–2016. Phase l/II Study of Ruxolitinib for Acute Leukemia. Available from: https://clinicaltrials.gov/ct2/show/NCT01251965 NLM Identifier: NCT01251965. [Google Scholar]
- 65.Incyte. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000–2016. A Phase 2 Study of Ruxolitinib With Chemotherapy in Children With Acute Lymphoblastic Leukemia. Available from: https://clinicaltrials.gov/ct2/show/NCT02723994 NLM Identifier: NCT02723994. [Google Scholar]
- 66.Taga T, Tomizawa D, Takahashi H, Adachi S. Acute myeloid leukemia in children: Current status and future directions. Pediatrics international : official journal of the Japan Pediatric Society. 2016;58(2):71–80. doi: 10.1111/ped.12865. [DOI] [PubMed] [Google Scholar]
- 67.Daver N, Cortes J. Molecular targeted therapy in acute myeloid leukemia. Hematology. 2012;17(Suppl 1):S59–S62. doi: 10.1179/102453312X13336169155619. [DOI] [PubMed] [Google Scholar]
- 68.Naqvi K, Verstovsek S, Kantarjian H, Ravandi F. A potential role of ruxolitinib in leukemia. Expert Opin Investig Drugs. 2011;20(8):1159–1166. doi: 10.1517/13543784.2011.589383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Rooij JD, Zwaan CM, van den Heuvel-Eibrink M. Pediatric AML: From Biology to Clinical Management. Journal of clinical medicine. 2015;4(1):127–149. doi: 10.3390/jcm4010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steensma DP, McClure RF, Karp JE, Tefferi A, Lasho TL, Powell HL, DeWald GW, Kaufmann SH. JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia. 2006;20(6):971–978. doi: 10.1038/sj.leu.2404206. [DOI] [PubMed] [Google Scholar]
- 71.Noor SJ, Tan W, Wilding GE, Ford LA, Barcos M, Sait SN, Block AW, Thompson JE, Wang ES, Wetzler M. Myeloid blastic transformation of myeloproliferative neoplasms--a review of 112 cases. Leukemia research. 2011;35(5):608–613. doi: 10.1016/j.leukres.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fathi AT, Levis M. Lestaurtinib: a multi-targeted FLT3 inhibitor. Expert review of hematology. 2009;2(1):17–26. doi: 10.1586/17474086.2.1.17. [DOI] [PubMed] [Google Scholar]
- 73.Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, Clark R, Levis MJ, Small D. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 74.Gruber TA, Downing JR. The biology of pediatric acute megakaryoblastic leukemia. Blood. 2015;126(8):943–949. doi: 10.1182/blood-2015-05-567859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge Y, Stout ML, Tatman DA, Jensen TL, Buck S, Thomas RL, Ravindranath Y, Matherly LH, Taub JW. GATA1, cytidine deaminase, and the high cure rate of Down syndrome children with acute megakaryocytic leukemia. J Natl Cancer Inst. 2005;97(3):226–231. doi: 10.1093/jnci/dji026. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida K, Toki T, Okuno Y, Kanezaki R, Shiraishi Y, Sato-Otsubo A, Sanada M, Park MJ, Terui K, Suzuki H, Kon A, Nagata Y, Sato Y, Wang R, Shiba N, Chiba K, Tanaka H, Hama A, Muramatsu H, Hasegawa D, Nakamura K, Kanegane H, Tsukamoto K, Adachi S, Kawakami K, Kato K, Nishimura R, Izraeli S, Hayashi Y, Miyano S, Kojima S, Ito E, Ogawa S. The landscape of somatic mutations in Down syndrome-related myeloid disorders. Nature genetics. 2013;45(11):1293–1299. doi: 10.1038/ng.2759. [DOI] [PubMed] [Google Scholar]
- 77.Bouchekioua A, Scourzic L, de Wever O, Zhang Y, Cervera P, Aline-Fardin A, Mercher T, Gaulard P, Nyga R, Jeziorowska D, Douay L, Vainchenker W, Louache F, Gespach C, Solary E, Coppo P. JAK3 deregulation by activating mutations confers invasive growth advantage in extranodal nasal-type natural killer cell lymphoma. Leukemia. 2014;28(2):338–348. doi: 10.1038/leu.2013.157. [DOI] [PubMed] [Google Scholar]
- 78.Meier C, Hoeller S, Bourgau C, Hirschmann P, Schwaller J, Went P, Pileri SA, Reiter A, Dirnhofer S, Tzankov A. Recurrent numerical aberrations of JAK2 and deregulation of the JAK2-STAT cascade in lymphomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2009;22(3):476–487. doi: 10.1038/modpathol.2008.207. [DOI] [PubMed] [Google Scholar]
- 79.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, Kutok JL, Shipp MA. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scott LM, Gandhi MK. Deregulated JAK/STAT signalling in lymphomagenesis, and its implications for the development of new targeted therapies. Blood reviews. 2015;29(6):405–415. doi: 10.1016/j.blre.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Hao Y, Chapuy B, Monti S, Sun HH, Rodig SJ, Shipp MA. Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and mediastinal large B-cell lymphoma growth in vitro and in vivo. Clin Cancer Res. 2014;20(10):2674–2683. doi: 10.1158/1078-0432.CCR-13-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Younes A, Romaguera J, Fanale M, McLaughlin P, Hagemeister F, Copeland A, Neelapu S, Kwak L, Shah J, de Castro Faria S, Hart S, Wood J, Jayaraman R, Ethirajulu K, Zhu J. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(33):4161–4167. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deininger M, Radich J, Burn TC, Huber R, Paranagama D, Verstovsek S. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015;126(13):1551–1554. doi: 10.1182/blood-2015-03-635235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, Liu F, Saunders LM, Mullally A, Abdel-Wahab O, Leung L, Weinstein A, Marubayashi S, Goel A, Gonen M, Estrov Z, Ebert BL, Chiosis G, Nimer SD, Bernstein BE, Verstovsek S, Levine RL. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489(7414):155–159. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu SC, Li LS, Kopp N, Montero J, Chapuy B, Yoda A, Christie AL, Liu H, Christodoulou A, van Bodegom D, van der Zwet J, Layer JV, Tivey T, Lane AA, Ryan JA, Ng SY, DeAngelo DJ, Stone RM, Steensma D, Wadleigh M, Harris M, Mandon E, Ebel N, Andraos R, Romanet V, Dolemeyer A, Sterker D, Zender M, Rodig SJ, Murakami M, Hofmann F, Kuo F, Eck MJ, Silverman LB, Sallan SE, Letai A, Baffert F, Vangrevelinghe E, Radimerski T, Gaul C, Weinstock DM. Activity of the Type II JAK2 Inhibitor CHZ868 in B Cell Acute Lymphoblastic Leukemia. Cancer cell. 2015;28(1):29–41. doi: 10.1016/j.ccell.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Springuel L, Hornakova T, Losdyck E, Lambert F, Leroy E, Constantinescu SN, Flex E, Tartaglia M, Knoops L, Renauld JC. Cooperating JAK1 and JAK3 mutants increase resistance to JAK inhibitors. Blood. 2014;124(26):3924–3931. doi: 10.1182/blood-2014-05-576652. [DOI] [PubMed] [Google Scholar]
- 87.Waibel M, Solomon VS, Knight DA, Ralli RA, Kim SK, Banks KM, Vidacs E, Virely C, Sia KC, Bracken LS, Collins-Underwood R, Drenberg C, Ramsey LB, Meyer SC, Takiguchi M, Dickins RA, Levine R, Ghysdael J, Dawson MA, Lock RB, Mullighan CG, Johnstone RW. Combined targeting of JAK2 and Bcl-2/Bcl-xL to cure mutant JAK2-driven malignancies and overcome acquired resistance to JAK2 inhibitors. Cell reports. 2013;5(4):1047–1059. doi: 10.1016/j.celrep.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maude SL, Tasian SK, Vincent T, Hall JW, Sheen C, Roberts KG, Seif AE, Barrett DM, Chen IM, Collins JR, Mullighan CG, Hunger SP, Harvey RC, Willman CL, Fridman JS, Loh ML, Grupp SA, Teachey DT. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. 2012;120(17):3510–3518. doi: 10.1182/blood-2012-03-415448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weisberg E, Liu Q, Nelson E, Kung AL, Christie AL, Bronson R, Sattler M, Sanda T, Zhao Z, Hur W, Mitsiades C, Smith R, Daley JF, Stone R, Galinsky I, Griffin JD, Gray N. Using combination therapy to override stromal-mediated chemoresistance in mutant FLT3-positive AML: synergism between FLT3 inhibitors, dasatinib/multi-targeted inhibitors and JAK inhibitors. Leukemia. 2012;26(10):2233–2244. doi: 10.1038/leu.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Annesley CE, Brown P. Novel agents for the treatment of childhood acute leukemia. Therapeutic advances in hematology. 2015;6(2):61–79. doi: 10.1177/2040620714565963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matulis SM, Gupta VA, Nooka AK, Hollen HV, Kaufman JL, Lonial S, Boise LH. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia. 2015 doi: 10.1038/leu.2015.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nature reviews. Cancer. 2016;16(2):99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 93.Peirs S, Matthijssens F, Goossens S, Van de Walle I, Ruggero K, de Bock CE, Degryse S, Cante-Barrett K, Briot D, Clappier E, Lammens T, De Moerloose B, Benoit Y, Poppe B, Meijerink JP, Cools J, Soulier J, Rabbitts TH, Taghon T, Speleman F, Van Vlierberghe P. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124(25):3738–3747. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- 94.Kameda T, Shide K, Shimoda HK, Hidaka T, Kubuki Y, Katayose K, Taniguchi Y, Sekine M, Kamiunntenn A, Maeda K, Nagata K, Matsunaga T, Shimoda K. Absence of gain-of-function JAK1 and JAK3 mutations in adult T cell leukemia/lymphoma. International journal of hematology. 2010;92(2):320–325. doi: 10.1007/s12185-010-0653-2. [DOI] [PubMed] [Google Scholar]
- 95.Zhang M, Mathews Griner LA, Ju W, Duveau DY, Guha R, Petrus MN, Wen B, Maeda M, Shinn P, Ferrer M, Conlon KD, Bamford RN, O'Shea JJ, Thomas CJ, Waldmann TA. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(40):12480–12485. doi: 10.1073/pnas.1516208112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orkin SH, Fisher DA, Ginsburg D, Look AT, Lux SE, Nathan DG. Nathan and Oski's Hematology and Oncology of Infancy and Childhood. Philadelphia: Elsevier Saunders; 2015. [Google Scholar]
- 97.Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, Wang C, Davies SM, Gaynon PS, Trigg M, Rutledge R, Burden L, Jorstad D, Carroll A, Heerema NA, Winick N, Borowitz MJ, Hunger SP, Carroll WL, Camitta B. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(31):5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biondi A, Schrappe M, De Lorenzo P, Castor A, Lucchini G, Gandemer V, Pieters R, Stary J, Escherich G, Campbell M, Li CK, Vora A, Arico M, Rottgers S, Saha V, Valsecchi MG. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9):936–945. doi: 10.1016/S1470-2045(12)70377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, Zheng HW, Davies SM, Gaynon PS, Trigg M, Rutledge R, Jorstad D, Winick N, Borowitz MJ, Hunger SP, Carroll WL, Camitta B, Children's Oncology G. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children's Oncology Group study AALL0031. Leukemia. 2014;28(7):1467–1471. doi: 10.1038/leu.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Izraeli S, Shochat C, Tal N, Geron I. Towards precision medicine in childhood leukemia--insights from mutationally activated cytokine receptor pathways in acute lymphoblastic leukemia. Cancer letters. 2014;352(1):15–20. doi: 10.1016/j.canlet.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 101.Carroll WL, Hunger SP. Therapies on the horizon for childhood acute lymphoblastic leukemia. Current opinion in pediatrics. 2016;28(1):12–18. doi: 10.1097/MOP.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Downing JR, Wilson RK, Zhang J, Mardis ER, Pui CH, Ding L, Ley TJ, Evans WE. The Pediatric Cancer Genome Project. Nature genetics. 2012;44(6):619–622. doi: 10.1038/ng.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cho SY, Kang W, Han JY, Min S, Kang J, Lee A, Kwon JY, Lee C, Park H. An Integrative Approach to Precision Cancer Medicine Using Patient-Derived Xenografts. Molecules and cells. 2016;39(2):77–86. doi: 10.14348/molcells.2016.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nature reviews. Cancer. 2015;15(5):311–316. doi: 10.1038/nrc3944. [DOI] [PubMed] [Google Scholar]