Summary
Objective
The outcomes of patients with metastatic phaeochromocytoma (PHEO) and paraganglioma (PGL) are unclear. We performed a systematic review and meta-analysis of baseline characteristics and mortality rates of patients with metastatic PHEO and PGL (PPGL).
Design
Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Scopus, and Web of Science and references of key articles were searched from inception to 2016.
Patients
Selected studies comprised at least 20 patients with metastatic PPGL and reported baseline characteristics and follow-up data.
Measurements
Reviewers extracted standardized data and assessed risk of bias of each study using a modified Newcastle-Ottawa tool. Random-effects meta-analysis was used to pool event rates across studies.
Results
Twenty retrospective noncomparative studies reported on 1338 patients with metastatic PHEO (685, 52.9%) and PGL (611, 47.1%), diagnosed at a mean age of 43.9±5.2 years. Mean follow-up was 6.3±3.2 years. Of 532 patients with reported data, 40.4% had synchronous metastases. Five-year (7 studies, n=738) and 10-year (2 studies, n=55) mortality rates for patients with metastatic PPGL were 37% (95% CI, 24–51%) and 29% (95% CI, 17–42%), respectively. Higher mortality was associated with male sex (RR 1.50) and synchronous metastases (RR 2.43).
Conclusions
Available low quality evidence from heterogeneous studies suggests low mortality rates of patients with metastatic PPGL. Male sex and synchronous metastases correlated with increased mortality. The assessment of outcomes of patients with metastatic PPGL has been inadequate, indicating the need for carefully planned prospective studies.
Keywords: phaeochromocytoma, paraganglioma, succinate dehydrogenase, mortality, neoplasm metastasis
1. Introduction
Phaeochromocytoma (PHEO) and paraganglioma (PGL) are rare neuroendocrine tumours derived from chromaffin tissues of the adrenal medulla and extra-adrenal paraganglia, respectively. PHEO and PGL (PPGL) can occur sporadically or as inherited genetic syndromes, primarily multiple endocrine neoplasia type 2 (MEN2), von Hippel-Lindau (VHL) disease, neurofibromatosis type 1 (NF1), and mutations in succinate dehydrogenase subunits (SDHx)1–4.
Approximately 2–13% of patients with PHEO and 2.4–50% with PGL have been reported to have metastatic disease with differences mainly due to selection bias5–7. Reported survival rates of patients with metastatic PPGL vary considerably between studies, with 5-year survival rates ranging from 12% to 84%2,8–12. Due to the rarity of this disease, variability of malignancy definitions, fragmented nature of studies, and small numbers of patients, there is no clear and consistent documentation of the outcomes in patients with metastatic PPGL. Therefore, a more accurate survival estimation of patients with metastatic PPGL is warranted.
The aim of this study was to perform a systematic review and meta-analysis to evaluate clinical characteristics of patients with metastatic PPGL and reported mortality rates. We also sought to identify predictive features correlating with shorter survival in patients with this rare disease.
2. Materials and Methods
A. Eligibility criteria
This study was performed based on a protocol that was designed in advance. The results of this review are reported according to PRISMA statement (preferred reporting items for systematic reviews and meta-analysis)13. We searched for original prospective and retrospective comparative and noncomparative studies that enrolled patients with metastatic PPGL (as defined by authors of original studies).
B. Inclusion and exclusion criteria
We included studies that described at least 20 patients with metastatic PPGL and reported baseline characteristics and follow-up data. Eligible studies were not restricted to any language. In case of multiple studies describing an overlapping cohort of patients, only the study which comprised the largest number of subjects and/or the longest duration of follow-up was included for this review. We included studies regardless their publication status. We excluded all non-original research studies and case reports. Because highly selected cohorts can overestimate or underestimate mortality rate, we also excluded studies reporting on a selected group of patients with metastatic PPGL (e.g., only patients with bone metastases or only those undergoing chemotherapy)
C. Data Sources and Search Strategies
A comprehensive search of several databases from each database’s inception to December 9, 2016, any language, was conducted (Supporting Information). The databases included Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Scopus, and Web of Science. The search strategy was designed and conducted by an experienced librarian with input from the study’s principal investigator. Controlled vocabulary supplemented with keywords was used to search for studies evaluating selected outcomes in patients with metastatic PHEO and PGL. Experts in the field were consulted and the references from primary studies were scanned to identify studies missed by the search strategy. The actual strategy is available in the appendix.
D. Selection of studies
All studies obtained from the search were entered into reference manager software (EndNote). Reviewers (O.H, L.G., J.S.) working independently and in duplicate reviewed all titles and abstracts of the identified studies and selected full text manuscripts for eligibility. Many of the identified studies retrieved by our search were non-relevant or non-original and were excluded at this phase. Full text screening was then performed in duplicate to assess eligibility for final inclusion. Disagreements at full text screening were resolved by consensus or referral to the full text of the study.
E. Data extraction and management
Potentially relevant studies were retrieved for detailed assessment. Working independently and in duplicate four reviewers (O.H., L.G., J.S, and Q.Y.) used standardized data collection form that was developed based on the protocol to extract information from each eligible study. Disagreements between reviewers were resolved by consensus or referral to the full text of the study.
For all included studies, the following data were extracted: first author, year of publication, study design, setting (country, referral centre, database), population studied, number of subjects with metastatic PHEO and PGL, genetic mutation status, duration of follow-up, age at diagnosis of primary tumour and metastatic spread, functional status (defined by elevated urine or plasma catecholamines and/or metanephrines), size of primary tumour, location of metastases, systemic and regional treatments, overall survival, and 5- and 10-year survival rates. The outcomes of interest were focused on factors associated with shorter survival and higher mortality risk.
F. Methodological quality and risk of bias assessment
The present meta-analysis is based on observational studies. Risk of bias and quality of each study were assessed by reviewers working independently and in duplicate using a modified Newcastle-Ottawa tool, which included assessment of the following: a) how the sample represented the population of interest, b) how the data on metastatic PPGL was collected, c) sufficient follow-up period for the outcomes to occur (at least 5 years of follow-up); d) adequacy of follow-up (the proportion of patients assessed at the completion of the study); and e) how the outcome was assessed. Elements of risk of bias assessment were used to explore potential heterogeneity. Disagreements were resolved by consensus or by referring to the full text of the study. Determination of exposure (baseline characteristics and survival outcomes) was adequately reported in thirteen studies.
G. Statistical analysis: summary measures and synthesis of results
We conducted a meta-analysis using the random-effects model to pool estimates from the included studies. We calculated the cumulative incidence of mortality at 5, 10 and overall (longest follow-up) years for patients with: PHEO, PGL, skull base and neck PGL, and SDHB-positive tumours. We also assessed the effects of synchronous vs metachronous metastasis and male vs female sex on mortality risk. All statistical analyses were performed using Stata v14.0 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
We used the I2 statistic to assess for heterogeneity across individual studies and to estimate the percentage of total between-study variation (ranging from 0 to 100%)14. I2 values of 25, 50 and 75% respectively indicate low, moderate, and high inconsistency across studies (heterogeneity) not explained by chance. Given the expected clinical heterogeneity and within-study variability, a random effects model was used.
3. Results
A. Study selection
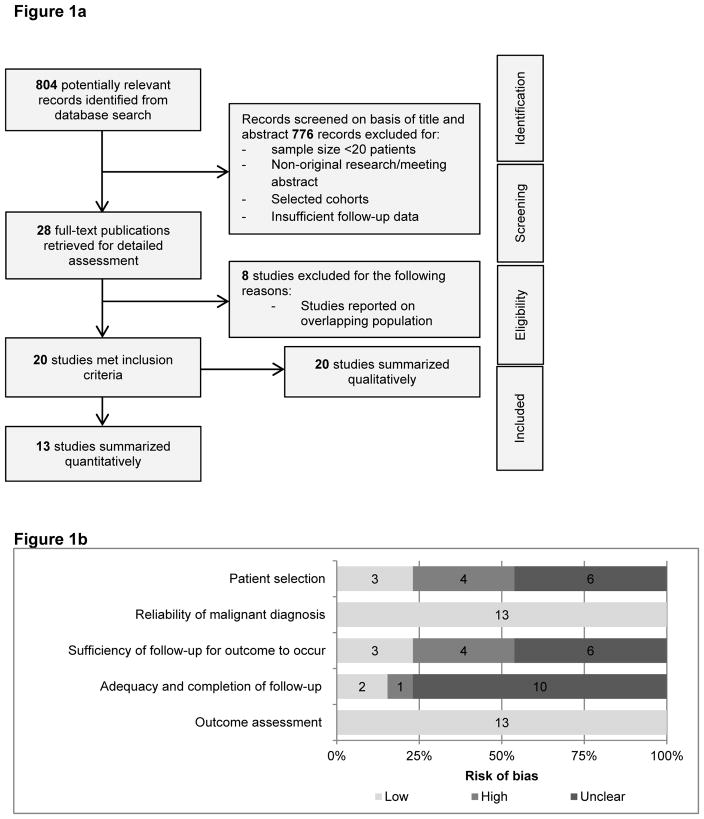
The initial search yielded 804 unique records for title and abstract screening; of these, 28 studies were selected for detailed assessment (Figure 1a). After full text assessment, 8 studies comprised a population already described in the publications included in our review; they were excluded because they described the smaller sample sizes and/or shorted duration of follow-up. Finally, a total of 20 studies were included in the present analysis. Eighteen studies were written in English, one in French, and one in Chinese (all familiar to authors). Of these, only 13 studies contained sufficient data to be included in the quantitative meta-analysis.
Figure 1.
Figure 1a PRISMA flow diagram of study selection.
Figure 1b Summary risk of bias based on the modified Newcastle-Ottawa tool.
B. Risk of bias assessment
Characteristics of the risk of bias assessment are shown in Figure 1b. All studies were observational and were of moderate-to-high risk of bias. Samples were not sufficiently representative in most studies (10 out of 13). Most of the included studies had insufficient or unclear duration of follow-up. Outcomes were adequately reported in all 13 studies. Clear description of how metastatic PPGL was defined and diagnosed was used in all articles.
C. Study characteristics
Individual study characteristics are shown in Table 1. Included studies were published between 1992 and 2016. Nineteen studies were classified as retrospective cohort studies, and 1 study was classified as an inception cohort study. The studies were mostly conducted in Europe (n=9) and Asia (n=6), and the U.S. (n=5). In 2 manuscripts, only metastatic PPGL originating from skull base and neck were studied; in 3 studies, only metastatic PHEOs were assessed; in 2 studies, only patients with genetic mutation testing were studied.
Table 1.
Characteristics of included studies
| Study description | Malignant PHEO/PGL | Mutation analysis | Metastatic diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Country | Study design | Data collection | Population studied | N (women) | PHEO | PGL |
SDHB- negative |
SDHB- positive |
Age at diagnosis (years) |
Age at metastases (years) |
Synchronous | Duration of follow- up |
| Proye, 1992 | France | Retrospective (single centre) | 1971–1991 | Surgically managed benign and metastatic PPGL | 26 (NR) | 13 | 13 | 51.3 | 49.8 months (0–86) | ||||
| Schlumberger, 1992 | France | Retrospective (single centre) | 1985–1990 | Metastatic PPGL | 20 (4) | 16 | 4 | 40 (11–76) | 11 | 28 months (3–352) | |||
| Glodny, 2001 | Germany | Retrospective (single centre) | 1967–1998 | Benign and metastatic PPGL | 29 (11) | 22 | 7 | 39.2±21.9 | NR | ||||
| Lee, 2002 | USA | Retrospective (national databasemulticentre) | 1985–1996 | Metastatic skull base and neck PGL | 59 (30) | -- | 59 | 44 | 44 | 59 | 65.5 months | ||
| Mignon, 2002 | France | Retrospective (multicentre) | 1992–2002 | Benign and metastatic PHEO | 27 (17) | 27 | 47 (13–74) | NR | |||||
| Thompson, 2002 | USA | Retrospective (single centre) | 1970–1997 | Surgically managed benign and metastatic PHEO | 33 (17) | 33 | 48.3(9–80) | NR | |||||
| Dannenberg, 2003 | Netherlands Frances Switzerland |
Retrospective (multicentre) | 1973–1997 | Benign and metastatic PPGL | 24 (12) | 18 | 6 | NR | |||||
| Amar, 2007 | France | Retrospective (multicentre) | NR | Metastatic PHEO and thoraco-abdominal PGL with genetic testing | 54 (25) | 29 | 25 | 49 | 23 | 37.9±13.9 | 42.0±13.8 | 24 | 79 months (IQR 24–190) |
| Nomura, 2009 | Japan | Inception cohort (single centre) | 1985–2006 | Metastatic PPGL | 32 (14) | 12 | 20 | 10.7±9.4 years (range 0.5–39.7) | |||||
| Ricketts, 2010 | UK | Retrospective (unclear) | NR | Benign and metastatic PPGL with SDHB and SDHD mutations | 42 (NR) | ? | ? | 40 | NR | ||||
| Ayala-Ramirez, 2011 | USA | Retrospective (single centre) | 1960–2009 | Benign and metastatic PHEO & sympathetic PGL | 131 (NR) | 68 | 63 | 9 | 67 | 5.8 years (range 0.01–57.3) | |||
| Feng, 2011 | China | Retrospective (single centre) | 1999–2008 | Surgically managed benign and metastatic PPGL | 31 (13) | 17 | 14 | 43.1±17.5 | 13 | 110 months (range 6–246) | |||
| Zheng, 2012 | China | Retrospective (single centre) | 1975–2010 | Benign and metastatic skull base and neck PGL | 25 (13) | -- | 25 | 4 | 40±5 (range17–71) | 13.7±8.3 years | |||
| Goffredo, 2013 | USA | Retrospective (national database/multicentre) | 1988–2009 | Metastatic PPGL in adults | 508 (244) | 287 | 221 | 53.5±15. PHEO 51.2 ±16.1 PGL |
NR | ||||
| Hescot, 2013 | France | Retrospective (multicentre) | 2001–2011 | Therapy-naïve metastatic PPGL | 57 (24) | 27 | 30 | 29 | 20 | 49±15 | 25 | 27 months (range 6–62) | |
| Cao, 2015 | China | Retrospective (single centre) | 2003–2012 | Metastatic PHEO | 32 (15) | 32 | -- | 40.12±12.18 synchronous; 47.13±11.29 metachronous | 17 | 51 months In synchronous 41 months in metachronous |
|||
| Choi, 2015 | Korea | Retrospective | 1997–2013 | Benign and metastatic PPGL | 33 (20) | 27 | 6 | 43.6±17.4 PHEO 40.2±9.7 PGL |
7.2 years (2.2–19.9) | ||||
| Ezzat Abdel-Aziz, 2015 | US | Retrospective (multicentre) | 1983–2012 | Benign and metastatic PPGL | 23 (9) | 9 | 14 | 1 | 45 (5–71) | 8 | 80 months (5–300) | ||
| Khadilkar, 2016 | India | Retrospective (multicentre) | 2000–2015 | Benign and metastatic PPGL | 20 (10) | 10 | 10 | 2 | 39.3±13.4 | 13 | NR | ||
| Turkova, 2016 | USA | Retrospective (single centre) | 2000–2014 | Metastatic PPGL | 132 (55) | 38 | 94 | 59 | 73 | 35±16 | 41±16 | 23 | NR |
As expected, studies used different definitions of metastatic PPGL. In 11 studies, diagnosis was made when metastases were present in nonchromaffin sites in accordance with the World Health Organization (WHO) classification criteria of endocrine tumours 15,16. In 2 studies, metastatic PPGL was defined as the presence of distant metastases, local invasion17 or histologic features (Phaeochromocytoma of the Adrenal gland Scaled Score)18. We were not able to exclude data of patients with local invasion or histologic features from the study cohort. However, since the presence of metastases met our inclusion criteria for defining metastatic PPGL, we decided to include these studies for our review.
D. Descriptive analysis of baseline characteristics
Twenty studies were qualitatively analysed for baseline characteristics of patients with metastatic PPGL. A total of 1338 patients were described (Table 2). Of these, distinction between PHEO and PGL was available in 1139 patients with 685 (56.5%) metastatic PHEO and 611 (46.7%) metastatic PGL. The patients were followed for a mean of 6.3±3.2 years (range of means, 2.2–13.7 years).
Table 2.
Description of included patients
| Value | Total no. patients in cited studies | No. of studies (citations) | |
|---|---|---|---|
| Baseline characteristics | |||
| Total N | 1338 | 20 9,10,12,17–29,39–42 | |
| Women | 533 (46.7%) | 1139 | 17 9,10,17–19,21–28,39–42 |
| PHEO | 685 (52.9%) | 1212 | 17 9,12,17–19,22–29,39–42 |
| PGL | 611 (47.1%) | 1204 | 16 9,10,12,17,19,21–29,41,42 |
| SDHB mutation | 172 (35.5%) | 484 | 8 12,19–25 |
| Adrenergic signs and symptoms | 176 (66.2%) | 266 | 9 18,22–24,26,29,39,41,42 |
| Functional | 308 (83.9%) | 367 | 8 19,22,24–26,28,29,39 |
| Surgical resection of primary tumours | 698 (84.8%) | 823 | 10 9,10,17,18,22,23,26,28,29,40 |
| Synchronous | 215 (40.4%) | 532 | 10 12,17,19,22–26,40,41 |
| Time to metastases, yrs | 3.6±1.9 | 339 | 7 19,22–26,28 |
| Duration of follow-up, yrs | 6.3±3.2 Range of means, 2.2–13.7 |
523 | 12 10,12,17,19,21–23,26,28,29,40,41 |
| Age at primary tumour diagnosis | |||
| Overall, yrs | 43.9±5.2 | 1109 | 16 9,10,18,19,21–26,28,29,39–42 |
| PHEO, yrs | 46.5±5.0 | 406a | 5 9,18,28,39,40 |
| PGL, yrs | 43.85±5.2 | 311a | 4 9,10,21,28 |
| SDHB-positive, yrs | 34.9±4.6 | 105b | 3 12,19,25 |
| SDHB-negative, yrs | 40.5±0.7 | 186 | 2 19,25 |
| Tumour size | |||
| Overall, cm | 7.5±1.3 | 995 | 10 9,12,18,19,21,24,25,40–42 |
| PHEO, cm | 8.3±0.5 | 420a | 4 9,12,18,40 |
| PGL, cm | 6.2±1.3 | 309a | 3 9,12,21 |
| SDHB-positive, cm | 6.4±0.4 | 96b | 2 19,25 |
| SDHB-negative, cm | 7.7±0.4 | 186 | 2 19,25 |
Categorical data presented as number (percentages).
Continuous data presented as mean±SD.
Total number of patients with PHEO and PGL in cited studies, respectively.
Total number of patients with SDHB mutation in cited studies.
Abbreviations used: yrs, years.
Eight studies reported on genetic testing of patients with metastatic PPGL12,19–25. In 3 studies, absence of SDHB mutation was confirmed by genetic testing in all patients with metastatic PPGL19,20,25. In the other 5 studies, SDHB-negative patients were those who a) tested negative for SDHB mutation, b) tested positive for other mutations, c) met clinical criteria for NF1, or d) did not undergo genetic testing. Of 484 patients reported in these studies, 172 (35.5%) patients with metastatic PPGL had a SDHB mutation. Other reported mutations included: 12 patients with VHL12,19,22,26–28, 9 MEN2A12,18,24,28,29, 5 NF119,22,26, 5 SDHD20,22,23, 2 SDHC12,22, 2 familial/syndromic24, 1 Turner syndrome18, and 1 MEN112.
Mean age at primary tumour diagnosis was 43.9±5.2 years for the entire population, 46.5±5.0 years in patients with PHEO, and 43.85±5.2 years in patients with PGL. SDHB-positive patients were diagnosed with primary tumour at a mean age of 34.9±4.6 years and SDHB-negative patients were diagnosed at a mean age of 40.5±0.7 years. Mean time to development of metastases was 3.6±1.9 years. In studies where onset of metastases was reported, synchronous metastases were noted in 215 out of 532 patients (40.4%). Similarly, in studies where presenting symptoms were described, adrenergic symptoms were present in 176 out of 266 patients (66.2%). Of 367 tumours, 308 (83.9%) were functional. Mean primary tumour size was 7.5±1.3 cm. PHEOs were 8.3±0.5 cm and PGLs were 6.2±1.3 cm. In patients with a SDHB mutation, mean tumour size was 6.4±0.4 cm; and in SDHB-negative patients, mean tumour size was 7.7±0.4 cm. Surgical resection of primary tumours was reported in 308 out of 367 patients (83.9%).
Distribution of metastases in patients with metastatic PPGL in reported studies was: 53.5% (211/394) bone18,19,21–23,25–27,29, 41.6% (155/371) lymph nodes18,19,21,22,25–27,29, 38.0% (150/394) mediastinum and lungs18,19,21–23,25–27,29, 31.1% (122/394) liver18,19,21–23,25–27,29, 17.4% (34/195) abdomen and pelvis18,19,21,22,29, and 3.8% (3/80) brain19,29.
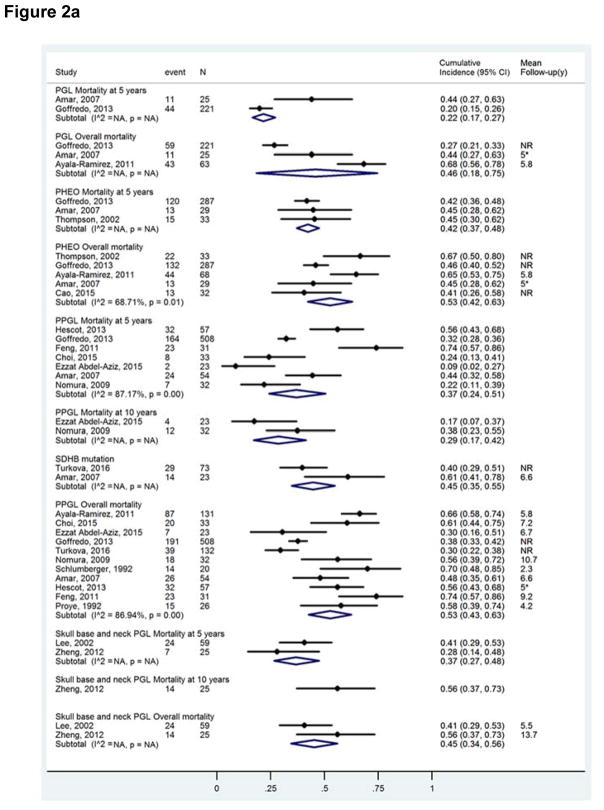
Mortality of patients with metastatic PPGL: meta-analysis
Results of the meta-analysis, stratified by the type of population, are shown in Figure 2a. All analyses were performed with a random effects model. Overall, 5- and 10-year mortality rates of patients with metastatic PPGL ranged 43%–63% (11 studies; n=1047), 24%–51% (7 studies; n=738), and 17–42% (2 studies; n=55), respectively. Overall and 5-year mortality rates in patients with PGL were 46% (95% CI, 18–75%; 3 studies; n=309) and 22% (95% CI, 17–27%; 2 studies, n=246), respectively. Overall and 5-year mortality rates in patients with metastatic PHEO were 53% (95% CI, 42–63%; 5 studies; n=449) and 42% (95% CI, 37–48%; 3 studies; n=349), respectively. Overall and 5-year mortality rates in patients with metastatic skull base and neck PGL reported in studies ranged 34%–56% (2 studies; n=84) and 27%–48% (2 studies; n=84), respectively. For patients with a SDHB-mutation, overall mortality ranged 35%–55% (2 studies; n=96). There was substantial heterogeneity (I2 values over 50%) in analyses of overall and 5-year mortality.
Figure 2.
Figure 2a Meta-analysis of mortality in patients with metastatic PPGL.
Footnote: The size of the boxes is proportional to the weight of each study.
Abbreviations used: NR, not reported.
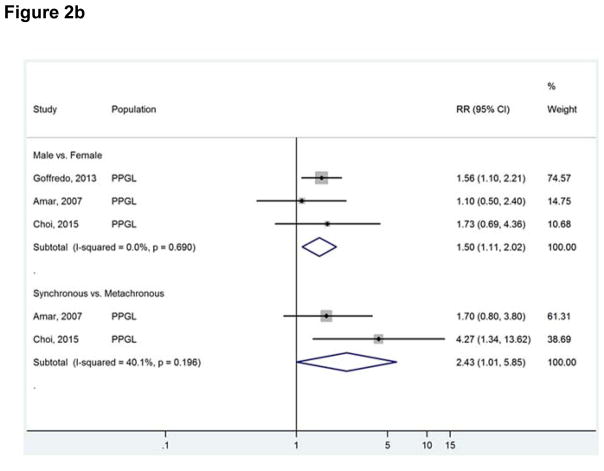
Figure 2b Factors associated with higher mortality risk.
Footnote: Abbreviations used: RR, relative risk.
Factors associated with higher relative risk (RR) of mortality were: male sex (RR 1.50; 95% CI, 1.11–2.02) and synchronous metastases (RR 2.43; 95% CI, 1.01–5.85) (Figure 2b). These analyses were homogeneous.
4. Discussion
A. Principal findings
The present systematic review and meta-analysis aimed to assess baseline characteristics and outcomes of patients with metastatic PPGL. Over a third of the patients had a SDHB germline mutation and were younger at primary tumour diagnosis, compared with SDHB-negative patients. The majority of metastatic PPGL tumours were functional and were associated with adrenergic signs and symptoms. More than 40% of patients presented with synchronous metastases.
We found that the 5- and 10-year mortality rates for patients with metastatic PPGL were 37% and 29%, respectively. We also showed that male sex and synchronous metastases were associated with shorter survival and worse outcomes. These data indicate that outcomes of patients with metastatic PPGL are heterogeneous and widely variable, possibly related to the referral bias and duration of follow-up.
SDHB mutations are thought to be highly associated with metastatic PPGL, with rates of developing metastases up to 50%- 90% in some studies1,12,19,30–32. Previous studies have also reported that SDHB mutation was associated with shorter survival rates than other PPGL types1,2,12,19,30–32. However, in many studies the outcomes of metastatic PPGL are unreliable based on small sample size, inadequate follow-up time, evaluation of a specific selected cohort, and referral bias. Although we were not able to analyse the risk of mortality in patients with SDHB mutations, overall mortality of this cohort was 45%. By excluding studies that sampled less than 20 patients with metastatic PPGL and seeking to analyse consecutive patients, the results from our meta-analysis showed that the pooled survival estimation in SDHB-mutation carries is higher than previously appreciated. Therefore, with the results of this study, it is possible to inform patients with metastatic PPGL more adequately concerning their prognosis.
B. Limitations
The 20 studies showed a high risk of bias and were inadequate for assessing outcomes of patients with metastatic PPGLs. The first important limitation is inconsistent definition of metastatic PPGL. In 2004, the WHO stated that metastatic PHEO are diagnosed only by the documented presence of metastases, and less emphasis was placed on local invasion15. However, in 2007 the Armed Forces Institute of Pathology Fascicle Tumours of the Adrenal Glands and Extra-adrenal Paraganglia defined malignancy as “extensive local invasion or documentation of metastases”33. Although some studies determined metastatic diagnosis based on local invasion, the extent of local invasion to adjacent tissues does not necessarily indicate higher risk towards development of metastases2. Use of various definitions of metastatic PPGL might have led to wide ranges of mortality rates. This limitation indicates that the studies were prone to selection bias, thereby potentially falsely lowering the mortality risk.
Mortality of patients with metastatic PPGL could have been misrepresented because of insufficient follow-up in some studies. Since most studies included patients with manifested disease, of whom a substantial proportion were in tertiary care centres, we assume that most included index cases with a higher than average risk of harbouring aggressive metastatic PPGL. Hence, this referral bias might also overestimate the mortality risk of metastatic PPGL. Although our results suggest that SDHB-negative tumours were larger than SDHB-positive tumours, the results of such descriptive statistics can be due to chance.
It was unclear in the majority of studies how the metastatic PPGL cases were selected. Most studies reported on a selected non-consecutive group of patients with metastatic PHEO and/or PGL, had unclear number of patients lost to follow-up, and allowed insufficient duration of follow-up for outcomes to occur. Additionally, 2 studies recruited subjects from the national and international registries 9,10. Although these databases are typically well validated for epidemiologic and clinical studies on cancers 34–36, some data are inherently limited, such as patient comorbidities, sites of metastases, and pertinent biochemical and genetic studies (not collected in SEER).
We were able to quantitatively analyse 13 cohort studies, with follow-up durations ranging from 2.2 and 13.7 years. The paucity of cohort studies with a long-term follow-up duration poses a risk of overrepresentation of patients with rapidly progressive disease and thus overestimation of mortality from metastatic PPGL. Of note, the 10-year mortality rate appeared lower than the 5-year mortality rate, signifying a substantial bias in patient selection and lack of sufficient follow-up. The paucity of genetic information is probably related to the fact that many genetic mutations (including SDHx gene mutations) have only been described quite recently. However, it is reported that metastatic disease can occur more than 20 years after initial diagnosis of primary tumour 37,38. The lack of long-term follow-up in the 2 included cohort studies [metastatic PPGL with overall survival 1.5 years26; therapy naïve metastatic PPGL with 5 year survival 44% (95%CI: 31–59%)22] may have resulted in the selection of rapidly progressive disease and thus overestimation of the mortality of metastatic PPGL.
Two studies exclusively examined patients with skull base and neck PGL10,21 and 3 studies only included patients with metastatic PHEO18,39,40. Inclusion of these studies into computation of pooled survival could have skewed our results, since it is postulated that patients with metastatic PPGL originating from skull base or neck have better survival compared to metastatic disease originating from PHEO or PGL from other sites.
Another important limitation is a variety of metastatic assessments, thereby increasing heterogeneity of the included studies. This could have biased the risk estimates. For example, in some studies, the diagnostic evaluation relied also on clinical information reported by other care givers. We aimed to use elements of risk of bias assessment to explore potential heterogeneity between studies, but due to the small number of included studies, performing a sensitivity analysis was not feasible.
C. Generalizability of study results
Studies assessing the survival of patients with metastatic PPGL were included in the meta-analysis. However, most studies included patients from international registries, while the accurate outcomes and survival rates may vary in different countries. Furthermore, these registries mostly comprised symptomatic patients treated in tertiary care centres, were largely retrospective in nature, with registration of patients at a single point in time and little to no entry of follow-up data. These selection mechanisms prohibit simple generalizations of the study results.
D. Recommendations for future research
This meta-analysis assessed the overall, 5- and 10-year mortality rates of patients with metastatic PPGL carrying specific risk factors (male sex, synchronous metastases). However, only one study was conducted in a prospective manner. Heterogeneous reporting of variables and length of follow-up in the available studies on metastatic PPGL do not allow for a more in-depth or additional analysis of variables associated with a more aggressive disease. As metastatic PPGL is a rare disorder, we suggest that it would be of great benefit to perform a large multicentre retrospective study on non-selected patients with metastatic PPGL to include detailed characteristics of clinical, biochemical, imaging, and genetic variables and evaluate outcomes. Multicentre collaboration enables a larger sample size of patients with metastatic PPGL and allows a subgroup analysis in this heterogeneous disorder. Data from a well-designed, large sample size retrospective study will be extremely valuable not only to determine an evidence-based monitoring approach for such patients, but also to design a multicentre longitudinal long-term prospective observational study after agreement on such approach.
5. Conclusion
In conclusion, the present study shows that the mortality of patients with metastatic PPGL is highly variable, depending on the presence of risk factors. Overall pooled 5- and 10-year mortality rates were 37% and 29%. Male sex and synchronous metastases were associated with increased mortality risk. Mortality of patients with metastatic PPGL varied considerably in the included studies due to methodological differences, including selection bias. Past studies have been inadequate for assessing outcomes of patients with metastatic PPGL. Further research is indicated to provide accurate prognostic information for patients with metastatic PPGL,
Footnotes
Disclosure summary. The authors have nothing to disclose.
No funding to report.
(ii) References
- 1.Amar L, Bertherat J, Baudin E, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23(34):8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 2.Jimenez C, Rohren E, Habra MA, et al. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15(4):356–371. doi: 10.1007/s11912-013-0320-x. [DOI] [PubMed] [Google Scholar]
- 3.Dahia PL. Novel hereditary forms of pheochromocytomas and paragangliomas. Front Horm Res. 2013;41:79–91. doi: 10.1159/000345671. [DOI] [PubMed] [Google Scholar]
- 4.Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44(5):328–333. doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- 5.Bravo EL. Pheochromocytoma: new concepts and future trends. Kidney Int. 1991;40(3):544–556. doi: 10.1038/ki.1991.244. [DOI] [PubMed] [Google Scholar]
- 6.Plouin PF, Chatellier G, Fofol I, Corvol P. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension. 1997;29(5):1133–1139. doi: 10.1161/01.hyp.29.5.1133. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein RE, O’Neill JA, Jr, Holcomb GW, 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755–764. doi: 10.1097/00000658-199906000-00001. discussion 764–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moskovic DJ, Smolarz JR, Stanley D, et al. Malignant head and neck paragangliomas: is there an optimal treatment strategy? Head Neck Oncol. 2010;2:23. doi: 10.1186/1758-3284-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goffredo P, Sosa JA, Roman SA. Malignant pheochromocytoma and paraganglioma: a population level analysis of long-term survival over two decades. J Surg Oncol. 2013;107(6):659–664. doi: 10.1002/jso.23297. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Barich F, Karnell LH, et al. National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer. 2002;94(3):730–737. doi: 10.1002/cncr.10252. [DOI] [PubMed] [Google Scholar]
- 11.Gimm O, DeMicco C, Perren A, Giammarile F, Walz MK, Brunaud L. Malignant pheochromocytomas and paragangliomas: a diagnostic challenge. Langenbecks Arch Surg. 2012;397(2):155–177. doi: 10.1007/s00423-011-0880-x. [DOI] [PubMed] [Google Scholar]
- 12.Ayala-Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96(3):717–725. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.DeLellis RALR, Heitz PU, et al. World Health Organization Classification of Tumors. Lyon: IARC; 2004. Tumours of endocrine organs. [Google Scholar]
- 16.Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol. 2017 doi: 10.1007/s12022-017-9484-5. [DOI] [PubMed] [Google Scholar]
- 17.Nomura K, Kimura H, Shimizu S, et al. Survival of patients with metastatic malignant pheochromocytoma and efficacy of combined cyclophosphamide, vincristine, and dacarbazine chemotherapy. J Clin Endocrinol Metab. 2009;94(8):2850–2856. doi: 10.1210/jc.2008-2697. [DOI] [PubMed] [Google Scholar]
- 18.Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26(5):551–566. doi: 10.1097/00000478-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 20.Ricketts CJ, Forman JR, Rattenberry E, et al. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat. 2010;31(1):41–51. doi: 10.1002/humu.21136. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Wei S, Yu Y, et al. Genetic and clinical characteristics of head and neck paragangliomas in a Chinese population. Laryngoscope. 2012;122(8):1761–1766. doi: 10.1002/lary.23360. [DOI] [PubMed] [Google Scholar]
- 22.Hescot S, Leboulleux S, Amar L, et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98(10):4006–4012. doi: 10.1210/jc.2013-1907. [DOI] [PubMed] [Google Scholar]
- 23.Ezzat Abdel-Aziz T, Prete F, Conway G, et al. Phaeochromocytomas and paragangliomas: A difference in disease behaviour and clinical outcomes. Journal of Surgical Oncology. 2015;112(5):486–491. doi: 10.1002/jso.24030. [DOI] [PubMed] [Google Scholar]
- 24.Khadilkar K, Sarathi V, Kasaliwal R, et al. Predictors of malignancy in patients with pheochromocytomas/paragangliomas: Asian Indian experience. Endocr. 2016;5(6):89–97. doi: 10.1530/EC-16-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkova H, Prodanov T, Maly M, et al. Characteristics and Outcomes of Metastatic Sdhb and Sporadic Pheochromocytoma/Paraganglioma: An National Institutes of Health Study. Endocr Pract. 2016;22(3):302–314. doi: 10.4158/EP15725.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlumberger M, Gicquel C, Lumbroso J, et al. Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J Endocrinol Invest. 1992;15(9):631–642. doi: 10.1007/BF03345807. [DOI] [PubMed] [Google Scholar]
- 27.Dannenberg H, De Krijger RR, Van der Harst E, et al. Von Hippel-Lindau gene alterations in sporadic benign and malignant pheochromocytomas. International Journal of Cancer. 2003;105(2):190–195. doi: 10.1002/ijc.11060. [DOI] [PubMed] [Google Scholar]
- 28.Choi YM, Sung T-Y, Kim WG, et al. Clinical course and prognostic factors in patients with malignant pheochromocytoma and paraganglioma: A single institution experience. Journal of Surgical Oncology. 2015;112(8):815–821. doi: 10.1002/jso.24063. [DOI] [PubMed] [Google Scholar]
- 29.Proye C, Vix M, Goropoulos A, Kerlo P, Lecomte-Houcke M. High incidence of malignant pheochromocytoma in a surgical unit. 26 cases out of 100 patients operated from 1971 to 1991. J Endocrinol Invest. 1992;15(9):651–663. doi: 10.1007/BF03345810. [DOI] [PubMed] [Google Scholar]
- 30.Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292(8):943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- 31.King KS, Prodanov T, Kantorovich V, et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29(31):4137–4142. doi: 10.1200/JCO.2011.34.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hulsteijn LT, Dekkers OM, Hes FJ, Smit JW, Corssmit EP. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: a systematic review and meta-analysis. J Med Genet. 2012;49(12):768–776. doi: 10.1136/jmedgenet-2012-101192. [DOI] [PubMed] [Google Scholar]
- 33.EEL . Tumors of the adrenal gland and extraadrenal paraganglia. Washington, DC: American Registry of Pathology; 2007. [Google Scholar]
- 34.National Cancer Institute. SEER: Surveillance, Epidemiology, and End Results Program. SEER Quality Improvement [Google Scholar]
- 35.Harlan LC, Hankey BF. The surveillance, epidemiology, and end-results program database as a resource for conducting descriptive epidemiologic and clinical studies. Journal of Clinical Oncology. 2003;21(12):2232–2233. doi: 10.1200/JCO.2003.94.023. [DOI] [PubMed] [Google Scholar]
- 36.Yu JB, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park) 2009;23(3):288–295. [PubMed] [Google Scholar]
- 37.Baba T, Machida K, Ozaki I, et al. A malignant pheochromocytoma with ileus, polyuria and hypercalcemia: a case of recurrence 17 years after the initial operation. Endocrinol Jpn. 1985;32(2):337–345. doi: 10.1507/endocrj1954.32.337. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka S, Ito T, Tomoda J, Higashi T, Yamada G, Tsuji T. Malignant pheochromocytoma with hepatic metastasis diagnosed 20 years after resection of the primary adrenal lesion. Intern Med. 1993;32(10):789–794. doi: 10.2169/internalmedicine.32.789. [DOI] [PubMed] [Google Scholar]
- 39.Mignon F, Mesurolle B, Laplanche A. Pheochromocytomas and CT: can size predict malignancy? J Radiol. 2002;83(11):1765–1768. [PubMed] [Google Scholar]
- 40.Cao WL, Huang BX, Sun FK, et al. Comparison and analysis of clinical features of primary and recurrent malignant pheochromocytoma. [Chinese] Journal of Shanghai Jiaotong University (Medical Science) 2015;35(8):1169–1173. [Google Scholar]
- 41.Feng F, Zhu Y, Wang X, et al. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. J Urol. 2011;185(5):1583–1590. doi: 10.1016/j.juro.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Glodny B, Winde G, Herwig R, et al. Clinical differences between benign and malignant pheochromocytomas. Endocr J. 2001;48(2):151–159. doi: 10.1507/endocrj.48.151. [DOI] [PubMed] [Google Scholar]