INTRODUCTION
The video head impulse test (vHIT) is an objective test of the vestibulo-ocular reflex (VOR). The vHIT outcome parameter receiving most attention to-date has been vHIT gain, the ratio of eye movement to head movement. In normal controls, age has little impact on vHIT gain up to the 8th or 9th decades (1-3). In patients, vHIT gain cut-offs of 0.68 and 0.8 and below have been recommended for diagnosing vestibular loss (4;5).
In spite of the stability of gain across the age spectrum, the correlation between the caloric test and vHIT is less than 100%. While there is better agreement between these measures for caloric weaknesses greater than 40% (6), these two clinical tests are not consistently in agreement. Several factors are proposed to account for the difference between vHIT and calorics for diagnosing vestibular loss. These factors include type of pathology (7;8), the frequencies tested with each exam (high frequency with vHIT; low frequency with calorics), and ensuring adequate head velocity above 150°/s during head impulses (1).
Another outcome of vHIT is the presence of corrective saccades (CS). With vHIT, both gain and CS can be objectively measured. CS can be characterized by their amplitude (velocity), latency and frequency. The amplitude of CS increases with decreasing vHIT gain (9;10); in the horizontal canal, patients with gain below 0.8 generate CS that are greater than 110 degrees/s in amplitude (11). CS are affected by age, whereas normal adults over 75.9 years demonstrate significantly larger CS amplitudes (12). Similarly, in a patient group with complaint of dizziness, but with vHIT gain above 0.8 (n = 899), the frequency of CS was shown to increase with age (11).
These findings demonstrate that higher amplitude and greater CS frequency are indicators of vestibular loss. While age related changes occur with respect to vHIT gain and CS, age has not been shown to significantly affect interpretation (11). What is unknown, and what has been speculated by others (13;14), is whether repeatable CS indicate small VOR deficits even with normal gain, suggesting that vHIT could be considered abnormal when coupled with a repeatable CS regardless of the gain value. We hypothesize that interpreting vHIT using gain and/or the presence of a CS could increase the sensitivity of vHIT as a tool for identifying vestibular loss. Therefore, the purpose of the present study was to characterize CS in a control group and then compare this data to individuals with vestibular loss, examining the sensitivity of vHIT for identifying vestibular loss using both gain and/or CS.
MATERIALS AND METHODS
Study population
Control Sample
Seventy subjects with normal vestibular function served as the control group (mean age: 44.1 years, range 10-78, 33 males). All control subjects had a case history denying significant hearing loss or history of dizziness, imbalance, or other neurologic complaints. To assess for the effects of age, control subjects were classified into the following age groups:
10 – 19 years: n = 10 (mean 12.9; range 10 – 17)
20 – 29 years: n = 10 (mean 23.5; range 20 – 28)
30 – 39 years: n = 10 (mean 35.6; range 31 – 39)
40 – 49 years: n = 10 (mean 44.3; range 40 – 48)
50 – 59 years: n = 10 (mean 55.9; range 52 – 59)
60 – 69 years: n = 10 (mean 63.2; range 60 – 67)
70 – 79 years: n = 10 (mean 73.8; range 70 – 78)
Data from all control subjects with the exception of 10 control subjects between 10 – 19 years have been reported previously (15). Informed consent was obtained from all control subjects for testing approved by the Institutional Review Board at Boys Town National Research Hospital and/or the University of Nebraska-Lincoln.
Patient Sample
Data from 49 patients with vestibular loss was retrospectively reviewed for comparison to the control group (mean age: 50, range 7 – 81, 19 males). Thirty-two patients had unilateral vestibular loss (UVL) diagnosed by bithermal water caloric weakness > 25% (mean caloric weakness: 64%, range 26 – 100%). Healthy ears from the UVL group were not included in analyses. Seventeen patients had bilateral vestibular loss (BVL) diagnosed by either bilaterally reduced calorics (total response ≤ 20, n = 1), reduced sinusoidal harmonic acceleration rotary chair gains and abnormal phase lead across the frequency spectrum up to 0.16 Hz (n = 11), or both (n = 5), for a total of 66 ears affected with vestibular loss.
The Video Head Impulse Test (vHIT)
vHIT was administered using an Otometrics Impulse (Schaumberg, IL) device. During vHIT subjects visualized an eye level target on the wall at a distance of 1 meter. The examiner stood behind the participant with their hands placed on the participant’s chin. Head impulses (100 to 250°/s peak head velocity) were randomized (for timing and direction) in the plane of the horizontal semicircular canals. Testing continued until 20 head impulses were acceptable to each right and left. All head impulses were completed by experienced practitioners. The outcome parameters were gain and CS frequency, peak velocity, and latency.
Analysis of corrective saccades
Individual head impulse data was analyzed in MATLAB (version 2014a), shown in figure 1: 1) Head velocity (top), 2) eye velocity (bottom), and 3) peak velocity of the first CS. All data was cleaned based on the classification scheme reported by Mantokoudis et al (2014). The following outcomes were calculated on the first CS: 1) frequency, 2) peak velocity, and 3) latency, where CS frequency is the rate of occurrence (i.e., 100% indicates a CS for each individual head impulse), peak velocity is the average amplitude of the CS (in degrees/sec), and latency is the average time of the peak velocity (ms). Covert and overt saccades were analyzed together.
Figure 1.
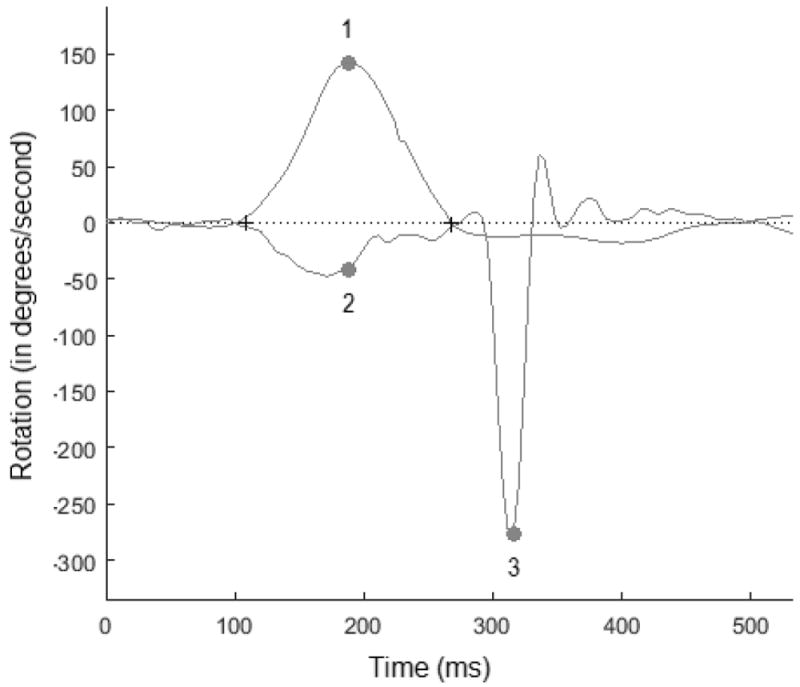
Individual head impulse data was analyzed in Matlab. The following were measured for each head impulse: 1) Peak head velocity (top), 2) eye velocity (bottom), and 3) frequency, peak velocity, and latency, of the first corrective saccade.
Statistical Analyses
Across the control subjects (n = 70), a mixed effects ANOVA was completed to examine the effects of impulse side (within-subjects) and age group (between-subjects, 7 age groups) for each vHIT parameter (gain and CS frequency, peak velocity, and latency). Correlations were calculated to investigate the relationship between vHIT parameters and individual subject age. To determine whether there were mean differences between the control group and ears affected with vestibular loss, a one-way ANOVA was completed for each vHIT parameter. Lastly, logistic regression and receiver operating characteristic curve analysis was completed to determine the sensitivity and specificity of using gain and/or CS to identify ears affected with vestibular loss, using calorics and rotary chair as the gold standard.
RESULTS
Normal Control Group
For vHIT gain, there was no significant interaction between age group and impulse side (F(6,63) = 1.341, p = 0.252) and no effect of age group (F(6,63) = 1.08, p = 0.384); however, there was a significant effect of impulse side (F (1,63) = 77.639, p < 0.001). Mean [SD] vHIT gain was significantly higher for rightward (0.99 [0.1]) compared to leftward impulses (0.92[0.09]), suggesting impulse side effects vHIT gain. Age was not correlated with vHIT gain (r = 0.071, p = 0.405, n = 140). Analyses were completed to determine if head velocity accounted for the effect of impulse side. There was no significant interaction between age group and impulse side (F(6, 63) = 1.386, p = 0.234) and no effect of age group (F(6, 63) = 1.287, p = 0.276). There was an effect of impulse side (F(1, 63) = 39.36, p < 0.001); however head velocities were higher for leftward (167.1 [18.56]) compared to rightward impulses (157.5 [22.3]), the opposite direction of vHIT gain differences, suggesting head velocity did not account for this effect.
For all CS outcomes, one subject’s right ear data was missing from analysis. For CS frequency, there was no significant interaction between age group and impulse side (F(6,62) = 1.281, p = 0. 279), no effect of age group (F(6,62) = 1.614, p < 0. 158), and no effect of impulse side (F (1,62) = 1.564, p = 0. 216), suggesting neither age group nor impulse side significantly affect CS frequency. While there were no mean differences for age group, individual subject age was weakly correlated with CS frequency (r = 0.203, p = 0.017, n = 139), suggesting CS frequency slowly increases with age.
For CS peak velocity, subjects who did not elicit a CS were given a velocity value of 0 (n = 8). This allowed all subjects to be included in the analysis. There was no significant interaction between age group and impulse side (F(6,62) = 0.508, p = 0.8), no effect of age group (F(6,62) = 1.5481, p = 0.178), and no effect of impulse side (F (1,62) = 0.051, p = 0.22), suggesting neither age nor impulse side significantly affect CS peak velocity. Age was not correlated with CS peak velocity (r = 0.036, p = 0.67, n = 139).
For CS latency, subjects who did not elicit a CS were given a latency value of ‘no response’, which eliminated them from the analysis. There was no significant interaction between age group and impulse side (F(6,56) = 1.123, p = 0.361), and no effect of age group (F(6,56) = 0.554, p = 0.765); however, there was an effect of impulse side (F (1,56) = 4.642, p = 0.036). Mean [SD] CS latency was significantly later for rightward (242.54 [60.5]) compared to leftward impulses (223.9 [54.9]), suggesting impulse side, but not age, significantly affects CS latency. Age was not correlated with CS latency (r = -0.013, p = 0.885, n = 131).
In summary, results demonstrate there is not a substantial effect of age regarding CS peak velocity or latency, and only a weak relationship between CS frequency and age for a control group age 10 – 78 years. Gain and CS latency were the only parameters affected by impulse side, demonstrating higher gain and longer latency for rightward impulses.
Comparison to Vestibular Loss Group
To determine if diagnostic accuracy is affected by the right impulse side effect in normal controls, ROC analysis was completed for all vHIT parameters comparing ears with right UVL to right ear control data and averaged (right and left) control data. Similar analyses were completed for ears with left UVL. Shown in Table 1, diagnostic accuracy was marginally better when right UVL data was compared to right ear control data; however, diagnostic accuracy was better when left UVL data was compared to averaged control data. For this reason, averaged control data was used for all comparisons here on out.
Table 1.
ROC Area Under the Curve (AUC) values comparing unilateral vestibular loss with different normal control data.
| Vestibular Loss | Normal Control | vHIT Parameters | |||
|---|---|---|---|---|---|
| Gain | Latency | Frequency | Velocity | ||
| Right Ear | Right Ear | 0.889 | 0.630 | 0.780 | 0.696 |
| Average | 0.852 | 0.601 | 0.782 | 0.691 | |
| Left Ear | Left Ear | 0.748 | 0.570 | 0.692 | 0.711 |
| Average | 0.779 | 0.601 | 0.704 | 0.712 | |
Shaded regions denote the higher AUC for each ear.
Descriptive data for the vestibular and control groups are shown in Table 2. Both vestibular loss groups had significantly lower mean vHIT gain (F(2,135) = 64.84, p < 0.001), higher mean CS frequency (F(2,135) = 27.85, p < 0.001), higher mean CS peak velocity (F(2,135) = 31.21, p < 0.001), and earlier CS latency (F(2, 129) = 5.796, p = 0.017) compared to the control group. Compared to the UVL group, the BVL group had significantly lower mean vHIT gain (p < 0.001) and higher CS velocities (p = 0.015). When further analyzing CS latency it was noted that the vestibular groups had significantly smaller mean standard deviations (SD) of the CS latency (UVL = 37.16, BVL = 37.82) compared to the control group (74.32, F (2, 125) = 28.53, p < 0.001) suggesting their CS occur more consistently, and time-locked.
Table 2.
Means and 95% CI for vHIT gain and corrective saccade frequency, velocity, and latency. The p-value denotes a significant mean difference between the groups.
| Control Group | Unilateral Vestibular Loss | Bilateral Vestibular Loss | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| MEAN | 95% CI | MEAN | 95% CI | MEAN | 95% CI | |||
| vHIT gain | 0.95 | 0.93 – 0.97 | 0.71 | 0.61 – 0.81 | 0.5 | 0.41 – 0.6 | < 0.001* | |
| Corrective Saccade | Frequency (%) | 45 | 38.6 – 51.6 | 73.2 | 59.6 – 86.8 | 88.8 | 80.3 – 97.2 | < 0.001+ |
| Velocity (degrees) | 81.2 | 72.7 – 89.7 | 140.8 | 108.9 – 172.6 | 186.3 | 156.4 – 216.3 | < 0.001 | |
| Latency (ms) | 229.73 | 217.7 – 241.7 | 218.5 | 190.3 – 246.7 | 192 | 169.3 – 214.6 | < 0.013* | |
= significant differences between all groups,
= significant differences between both vestibular loss groups and control group
For the UVL group, lower vHIT gain was associated with a higher caloric weakness (r = -0.6, p < .001). Higher CS frequency (r = -0.684, p < 0.001, Figure 2A), higher CS peak velocity (r = 0.741, p < .001, Figure 2B), shorter CS latency (r = 0.44, p < 0.001), and higher SDs of the CS latency (r = 0.446, p < 0.001) were associated with lower vHIT gain.
Figure 2.
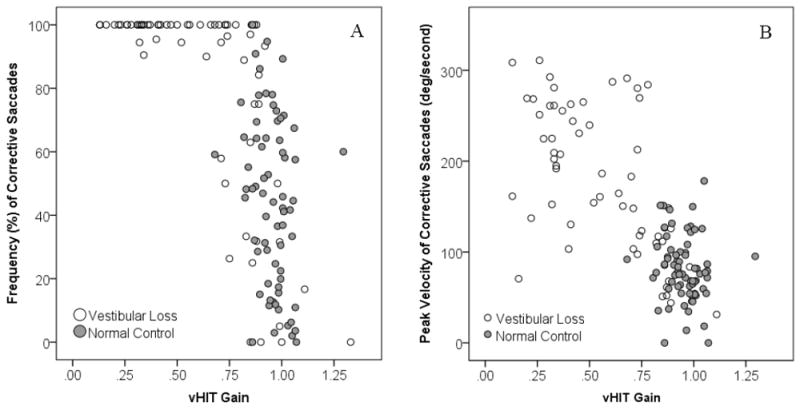
Corrective saccade frequency (A) and velocity (B) plotted as a function of vHIT gain
Logistic regression and ROC analysis were then completed to determine how well each of these factors (vHIT gain, and CS frequency, peak velocity, latency, and SD of the latency) identified vestibular loss. All ears with vestibular loss were combined; results can be found in Table 2. For each factor, the cut point was chosen where the overall correct classification was maximized. The cut point was then calculated by back transformation using the following formula: ln(p/(1-p) – intercept]/slope, where p = cut point probability. These cut points suggest that gain < 0.78, CS frequency > 81.89%, CS peak velocity > 135.8, CS latency < 192.7 ms, and SD of the CS latency < 41.68 are all associated with vestibular loss. When the factors were analyzed separately, vHIT gain provided the best classification (overall classification = 83.8%; AUC: 0.895), closely followed by CS frequency (overall classification = 83.1%; AUC: 0.819). While specificity was highest using gain alone, sensitivity was highest using CS frequency alone. When combining all variables sensitivity improved (overall classification = 90.5%; AUC 0.983). However, using step-wise selection, the best model included gain and SD of the CS latency. This 2-factor model performed better than the model using vHIT gain alone (overall classification = 92.1%; AUC: 0.979).
Using vHIT gain < 0.78 and SD of the CS latency < 41.68 resulted in a misclassification of only 6 subjects with vestibular loss and 4 normal control subjects. However, this model was optimal because 7 subjects with vestibular loss and 3 normal controls subjects did not generate greater than 1 CS and thus did not have a SD of the CS latency and were not included in the analysis. Removal of these subjects understandably leads to an improvement in subject classification. Therefore, we re-analyzed vHIT using a 2-variable approach with CS frequency and gain, as every subject would have a value for these variables and these variables generated the highest AUC. Using these 2 variables (gain < 0.78 or CS frequency > 81.89%) resulted in 90% specificity, 78.8% sensitivity, and an overall correct classification rate 84.6%, a marginal improvement.
Depending on the predictor variable, the logistic regression models classified between 18 – 30 ears in the vestibular loss group as having normal vHIT. Therefore, head impulses were further analyzed by removing impulses where head velocity was < 150 degrees/s to determine if accuracy improved. Mean head velocity significantly increased from 166 to 178 degrees/s (t = -10.535, p = 0.001). Logistic regression was then repeated for all CS variables. Logistic regression could unfortunately not be repeated for gain because gain was calculated by the Otometrics Impulse software, while CS data was calculated in an excel file via matlab. Five subjects with vestibular loss were dropped from the analysis as none of the impulses were > 150 degrees/s. As shown in Table 3, classification for identifying vestibular loss did not improve substantially for any of the CS variables, suggesting that when using CS, results are not more accurate when isolating interpretation to impulses > 150 degrees/s.
Table 3.
Logistic regression results using vHIT factors (gain and correct saccade frequency, velocity, latency, and standard deviation of the latency) for identifying vestibular loss.
| Factor (s) | Chi-Sq | β | p-value | Correct Classification | Sensitivity | Specificity | Cut Point | AUC |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gain | 24.5 | -11.2 | < 0.001 | 83.8% | 68.2% | 98.6% | 0.78 | 0.895 |
|
| ||||||||
| Frequency | 30.15 | 0.367 | < 0.001 | 83.1% | 74.2% | 91.4% | 81.89% | 0.819 |
|
| ||||||||
| Velocity | 26.43 | 0.019 | < 0.001 | 76.5% | 59.1% | 92.9% | 135.8 | 0.780 |
|
| ||||||||
| Latency | 5.4 | -0.007 | 0.02 | 63.8% | 51.6% | 75.0% | 192.71 | 0.662 |
|
| ||||||||
| Std Dev | 30.9 | -0.047 | 0.008 | 81.0% | 69.5% | 91.0% | 41.68 | 0.826 |
|
| ||||||||
| Gain | 11.44 | -28.39 | < 0.001 | 90.5% | 91.5% | 89.6% | CND* | 0.983 |
| Frequency | 0.920 | 0.015 | 0.338 | |||||
| Velocity | 1.092 | 0.014 | 0.296 | |||||
| Latency | 4.544 | 0.019 | 0.033 | |||||
| StDev | 10.28 | -0.072 | 0.001 | |||||
|
| ||||||||
| Gain | 16.83 | -24.64 | <0.001 | 92.1% | 89.8% | 94.0% | CND* | 0.979 |
| StDev | 12.73 | -0.067 | <0.001 | |||||
could not determine: the cut-point could not be calculated for individual variables in models that included more than one variable
DISCUSSION
The purpose of the present study was to characterize CS in a control group, extending to the pediatric population, and examine sensitivity of vHIT for identifying vestibular loss using gain and/or CS. In the control group (10 – 78 years), results demonstrate there is not a substantial effect of age for CS peak velocity or latency. The only CS parameter demonstrating a weak relationship with age was CS frequency, which increased with age. A similar increase in CS frequency with age has been reported by others (10;11). In healthy older adults, an increase in CS frequency of 4.5% for every 0.1 decrease in VOR gain has been reported, suggesting the increase in CS frequency is tied to a deficient VOR (10). Additional factors have been speculated to increase CS frequency with age such as inattention, refractive errors, calibration artifact, and failed saccadic inhibition (11). In our investigation, the increase in CS frequency is speculated to be influenced by several factors. CS frequency was negatively correlated with vHIT gain (r = -0.276, p = 0.021) suggesting a deficient VOR could be affecting the increased CS; however, the presence of refractive errors and failed saccadic inhibition could also be contributing. Since data were cleaned according to Mantokoudis et al. (16), we do not speculate artifact was an issue.
In the current study there were no age related changes in CS peak velocity or latency. This is in contrast to Anson et al (2016), who observed an increase in CS amplitude with aging (12), which they attribute partially to mild VOR gain reductions. Additionally, a breakdown in gaze stability mechanisms (i.e., increased levels of cerebellar disinhibition) is speculated to contribute to this finding. This finding was not replicated, likely because the age range in the current study was younger (10 – 78, mean 44.1 years) compared to the population reported by Anson et al. (12) (27 – 93, mean 72 years). For CS latency, others have similarly not documented a significant relationship with age (12).
While gain and CS latency were unaffected by age, both variables were affected by impulse side, demonstrating higher gain and longer CS latency in response to rightward head impulses. A similar finding has been reported by others, where gain is significantly greater in the direction of the ipsilateral recorded eye (1;2;11;17;18). This pattern of gain findings has been attributed to the “demand” placed on the eyes (i.e., larger demand on the right eye for rightward impulses), and a longer neural pathway for the adducting medial rectus (1;17). With respect to CS latencies, we speculate the longer CS latencies in response to rightward impulses could also be due to a longer neural pathway. While not a central focus of this study, findings suggest this could affect sensitivity for diagnosing right UVL.
The vestibular loss groups had significantly lower mean vHIT gain, higher mean CS frequency, higher mean CS peak velocity, earlier CS latency, and smaller mean SDs of the CS latency compared to the control group. While a variety of investigations have consistently noted lower vHIT gain (11;19) and both higher CS frequency and peak velocity (11) with vestibular loss, CS have not been used to interpret vHIT as commonly as gain. Rambold (2015) found that both CS frequency and velocity differentiated patients with and without vestibular loss on vHIT. Those with abnormal vHIT had CS peak velocities greater than 110 degrees/s and CS frequencies above 149%. While this data set consisted of patients and not normal controls, the cutoff values are similar to those noted for the present study (11). We noted peak CS velocities greater than 135.8 degrees/s differentiated normal from vestibular loss. Because we measured only the initial CS, our CS frequency is lower at 81.89%. However, in spite of this difference, CS frequency did demonstrate good separation between normal and vestibular patients.
We hypothesized that interpreting vHIT using both gain and CS would increase the sensitivity of vHIT for identifying vestibular loss. While none of the CS variables performed better than gain alone, incorporating CS did improve diagnostic accuracy. Specifically, we found that using gain and CS frequency improved diagnostic accuracy, yielding 90% specificity, 78.8% sensitivity, and an overall correct classification rate 84.6%, a marginal improvement over using gain alone.
Observation of the raw data suggested that when gain was < 0.68 or > 0.93, both gain and CS classified subjects the same. When gain was < 0.68, all subjects (n = 35) generated CS frequencies above 81.89% (range 95 – 100%); all 35 subjects were in the group with vestibular loss. When gain was > 0.93, all subjects (n = 52) generated CS frequencies below 81.89% (range 0 – 50%); of the 52 ears, 6 were in the group with vestibular loss. The main discrepancy was for gain between 0.68 and 0.93. In this range, there were 25 ears with vestibular loss and 24 normal controls. Of the 25 ears with vestibular loss, 7/25 had both abnormal gain (<0.78) and abnormal CS frequency (> 81.89 %), 3/25 had isolated abnormal gain, 7/25 had isolated abnormal CS frequency, and 8/25 had normal gain with normal CS frequency. In contrast, of the 24 normal controls, 1/24 had isolated abnormal gain, 5/24 had isolated abnormal CS frequency, and 19/24 had normal gain with normal CS frequency. The authors suggest the following for vHIT interpretation:
When low gain (< 0.78) is paired with high CS frequency (>81.89%), vestibular loss is diagnosed. This pattern represents 42/66 ears with vestibular loss and 0/70 normal controls.
When isolated low gain with normal CS frequency or when normal gain with isolated abnormal CS frequency occurs, vestibular loss is suspected. This pattern represents 3/66 ears with vestibular loss and 1/70 normal controls, and 7/66 ears with vestibular loss and 5/70 normal controls, respectively.
Normal gain with normal CS frequency does not always suggest normal vestibular function. This pattern represents 14/66 ears with vestibular loss and 64/70 normal controls.
In the 14 ears with vestibular loss and normal gain with normal CS frequency, 9 had UVL with significantly smaller caloric weakness (47.6%) compared to the remaining ears with UVL (70.7%), suggesting that while this 2-variable approach improves the ability to identify mild vestibular loss, it does not identify all cases. This is in agreement with others who demonstrate vHIT to be more sensitive for caloric weaknesses greater than 40% (6) and suggest that that CS follow a similar pattern of increased sensitivity with increased caloric magnitude. Additionally, type of pathology (7;8) and head velocity above 150°/s during head impulses (1) can account for differences between vHIT and calorics. Two subjects were diagnosed with Meniere’s, which could account for their misclassification. While our data demonstrate that controlling for head velocity does not improve accuracy in identifying vestibular loss, mean head velocity was 178 degrees/s. In cases of mild vestibular loss, sensitivity may improve if head velocity is above 200 degrees/s
UVL was diagnosed using caloric testing while BVL was diagnosed using either caloric (n = 1) or rotary chair testing (n = 16). While caloric testing is considered the gold standard for diagnosing UVL and rotary chair is considered the gold standard for diagnosing BVL, use of different vestibular assessments could have influenced the sensitivity and specificity of vHIT outcomes.
Conclusion
We speculated the presence of a repeatable CS could indicate a VOR deficit, suggesting that a repeatable CS, regardless of the gain value, indicates an abnormal vHIT. While the 2-factor model using vHIT gain and standard deviation of the CS latency were the most sensitive combination, subjects have to generate enough CS to make this parameter interpretable. Therefore, we propose that a repeatable CS (> 81.89%) and/or low gain (< 0.78) is a sign of vestibular loss, and improves diagnostic accuracy. Further study is needed to determine whether increasing head velocity above 200 degrees/s results in increased sensitivity.
Table 4.
Adjusted data for head impulses greater than 150 degrees/s
| Factor (s) | Chi-Sq | β | p-value | Correct Classification | Sensitivity | Specificity | Cut Point | AUC |
|---|---|---|---|---|---|---|---|---|
| Frequency | 25.3 | 3.29 | < 0.001 | 83.2% | 73.8% | 91.4% | 88.02 | 0.814 |
| Velocity | 25.8 | 0.0164 | < 0.001 | 77.9% | 59.0% | 94.3% | 153.78 | 0.766 |
| Latency | 5.03 | -0.007 | 0.025 | 64.8% | 61.4% | 67.7% | 203.22 | 0.662 |
| StDev | 26.3 | <0.001 | -0.04 | 82.6% | 80.8% | 84.1% | 49.17 | 0.818 |
Acknowledgments
Source of Funding:
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM109023 and by the National Institute on Deafness and Other Communication Disorders under award numbers R03DC015318 and P30DC004662.
Footnotes
This work was presented at the American Balance Society Meeting in Scottsdale, AZ, March 4, 2015.
Conflicts of Interest:
KLJ provided consulting for Otometrics regarding the clinical use of vestibular evoked myogenic potential testing and video head impulse testing (vHIT) during this time frame.
KB was a consultant to Otometrics during the preparation of this manuscript.
Reference List
- 1.McGarvie LA, MacDougall HG, Halmagyi GM, et al. The Video Head Impulse Test (vHIT) of Semicircular Canal Function - Age-Dependent Normative Values of VOR Gain in Healthy Subjects. Front Neurol. 2015;6:154. doi: 10.3389/fneur.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matino-Soler E, Esteller-More E, Martin-Sanchez JC, et al. Normative data on angular vestibulo-ocular responses in the yaw axis measured using the video head impulse test. Otol Neurotol. 2015;36:466–471. doi: 10.1097/MAO.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 3.Davalos-Bichara M, Agrawal Y. Normative results of healthy older adults on standard clinical vestibular tests. Otol Neurotol. 2014;35:297–300. doi: 10.1097/MAO.0b013e3182a09ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blodow A, Pannasch S, Walther LE. Detection of isolated covert saccades with the video head impulse test in peripheral vestibular disorders. Auris Nasus Larynx. 2013;40:348–351. doi: 10.1016/j.anl.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 5.MacDougall HG, Weber KP, McGarvie LA, et al. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–1141. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCaslin DL, Jacobson GP, Bennett ML, et al. Predictive properties of the video head impulse test: measures of caloric symmetry and self-report dizziness handicap. Ear Hear. 2014;35:e185–e191. doi: 10.1097/AUD.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 7.McGarvie LA, Curthoys IS, MacDougall HG, et al. What does the head impulse test versus caloric dissociation reveal about vestibular dysfunction in Meniere’s disease? Ann N Y Acad Sci. 2015;1343:58–62. doi: 10.1111/nyas.12687. [DOI] [PubMed] [Google Scholar]
- 8.McCaslin DL, Rivas A, Jacobson GP, et al. The dissociation of video head impulse test (vHIT) and bithermal caloric test results provide topological localization of vestibular system impairment in patients with “definite” Meniere’s disease. Am J Audiol. 2015;24:1–10. doi: 10.1044/2014_AJA-14-0040. [DOI] [PubMed] [Google Scholar]
- 9.Weber KP, Aw ST, Todd MJ, et al. Horizontal head impulse test detects gentamicin vestibulotoxicity. Neurology. 2009;72:1417–1424. doi: 10.1212/WNL.0b013e3181a18652. [DOI] [PubMed] [Google Scholar]
- 10.Anson ER, Bigelow RT, Carey JP, et al. VOR Gain Is Related to Compensatory Saccades in Healthy Older Adults. Front Aging Neurosci. 2016;8:150. doi: 10.3389/fnagi.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rambold HA. Age-related Refixating Saccades in the Three-Dimensional Video-Head-Impulse Test: Source and Dissociation From Unilateral Vestibular Failure. Otol Neurotol. 2016;37:171–178. doi: 10.1097/MAO.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 12.Anson ER, Bigelow RT, Carey JP, et al. Aging Increases Compensatory Saccade Amplitude in the Video Head Impulse Test. Front Neurol. 2016;7:113. doi: 10.3389/fneur.2016.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korsager LE, Faber CE, Schmidt JH, et al. Refixation Saccades with Normal Gain Values: A Diagnostic Problem in the Video Head Impulse Test: A Case Report. Front Neurol. 2017;8:81. doi: 10.3389/fneur.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Fernandez N, Eza-Nunez P. Normal Gain of VOR with Refixation Saccades in Patients with Unilateral Vestibulopathy. J Int Adv Otol. 2015;11:133–137. doi: 10.5152/iao.2015.1087. [DOI] [PubMed] [Google Scholar]
- 15.Janky KL, Patterson JN, Shepard NT, Thomas MLA, Honaker JA. Effects of device on video head impulse test (vHIT) gain. J Am Acad Audiol. 2017;28:778–785. doi: 10.3766/jaaa.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantokoudis G, Saber Tehrani AS, Kattah JC, et al. Quantifying the vestibulo-ocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurootol. 2015;20:39–50. doi: 10.1159/000362780. [DOI] [PubMed] [Google Scholar]
- 17.Weber KP, Aw ST, Todd MJ, et al. Inter-ocular differences of the horizontal vestibulo-ocular reflex during impulsive testing. Prog Brain Res. 2008;171:195–198. doi: 10.1016/S0079-6123(08)00626-2. [DOI] [PubMed] [Google Scholar]
- 18.Yip CW, Glaser M, Frenzel C, et al. Comparison of the Bedside Head-Impulse Test with the Video Head-Impulse Test in a Clinical Practice Setting: A Prospective Study of 500 Outpatients. Front Neurol. 2016;7:58. doi: 10.3389/fneur.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber KP, Aw ST, Todd MJ, et al. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology. 2008;70:454–463. doi: 10.1212/01.wnl.0000299117.48935.2e. [DOI] [PubMed] [Google Scholar]


