Abstract
Objective
Low vitamin D levels have been associated with and could play a role in the pathogenesis of idiopathic benign paroxysmal positional vertigo (iBPPV). Since otoconia degeneration contributes to iBPPV and a lack of vitamin D may impact otoconia structure and integrity, we proposed a negative association between vitamin D levels and levels of a proposed circulatory biomarker for otolithic degeneration, otolin-1.
Study design
Cross-sectional clinical study.
Setting
Clinical research center.
Patients
Seventy-nine men and women ranging in age from 22 to 95 years old without known vertigo.
Interventions
Diagnostic.
Main outcome measures
Blood levels of 25-OH vitamin D and otolin-1.
Results
Previously, we had reported higher otolin-1 levels in older age groups. The majority of the subjects (83%) had vitamin D levels that were below 40ng/mL. Vitamin D level was lowest in the young and increased with age before declining in subjects 70 years of age and older (p = 0.005). There was a negative correlation between vitamin D and otolin-1 levels of subjects over 70 (r = −0.36, p = 0.036).
Conclusion
Our results demonstrate a relationship between vitamin D and otolin-1. The majority of our subjects had abnormally low vitamin D levels, but only those over 70 years of age showed a negative correlation with high otolin-1 levels. We postulate that a seasonal drop in vitamin D may not be sufficient for otoconia fragmentation and ultimately iBPPV, rather, chronically low vitamin D maybe required to induce otoconia degeneration.
Keywords: Aging, idiopathic benign paroxysmal positional vertigo, biomarker, otoconia, otolin-1, vitamin D, otolith degeneration
Introduction
Osteoporosis and idiopathic benign paroxysmal positional vertigo (iBPPV) appear to be strongly associated with each other1–4. However, observation of a strong association does not necessarily imply a causal relationship between two conditions5. To better understand etiological factors contributing to the pathogenesis of iBPPV, we have utilized a potential circulatory biomarker for otoconia degeneration, otolin-1, and demonstrated its lack of correlation with biomarkers of osteoporosis, 3, suggesting that these two conditions are unlikely to be directly linked to each other. Instead, it has been proposed that a third disorder, namely vitamin D deficiency, may be contributing to the development of both conditions6,7 and we have proposed a mechanistic conceptual model for this relationship8. In addition to its well-known roles in bone biology and osteoporosis9, decreased vitamin D has also been associated with a variety of other conditions9, most recently idiopathic BPPV4,6–8,10–14, particularly in the recurrent disease6,10,15,16. It has been argued that the prevalence of osteoporosis and vitamin D deficiency in the general population is high and therefore that the coexistence of BPPV with osteoporosis and vitamin D deficiency may be purely coincidental17. Additional evidence is needed to explore the association between low vitamin levels and BPPV. Unfortunately, we currently lack adequate experimental animal models of BPPV to accommodate such studies. As noted above, we have proposed the use of circulatory biomarkers toward better understanding of BPPV. We recently reported otolin-1 levels in healthy subjects as a function of age and noted a small but statistically significant age-related increase, consistent with degeneration of otoconia with age18. Now we hypothesize that if there is causal relationship between BPPV and low vitamin D levels, then there should be a relationship between otolin-1 and vitamin D levels in subjects without known vertigo.
Methods
Subjects
This study was approved by the Institutional Review Board at UConn Health (#12-188-2; 15-006-3). Details of subject recruitment and otolin-1 levels for our subjects were reported elsewhere18. Briefly, after receiving informed consent, blood samples were obtained from 20 young (20–30 years old), 20 middle-aged (50–65 years old), and 20 old (66–80 years old) individuals, as well as 19 subjects in the oldest-old group (81–95 years old) using services of the UConn Center on Aging Recruitment and Community Outreach Research Cores (http://health.uconn.edu/aging/research/research-cores/). Recruitment criteria were selected to identify individuals representative of the mean or typical normal health status of the local population within the corresponding age groups, thus increasing the likelihood that findings can be translated to the general population19. Therefore, for the first three age groups, subjects were excluded if they were frail or reported history of a major systemic illness, were undergoing active cancer treatment, took prednisone above 10 mg daily, took other immunosuppressive drugs, took any medications for rheumatoid arthritis other than NSAIDs, or had received antibiotics in the previous 6 months. In contrast, given the high prevalence of frailty and multi-morbidity in the oldest group, there were no exclusion criteria for this group other than an inability to consent.
Data Collection
Non-fasting blood samples were collected from all subjects. The blood samples were collected between March and May 2016 with all of those for the oldest group being collected in March 2016. Collected specimens were spun at 1,300 G for 10 minutes. Plasma was then removed, divided into equal aliquots for subsequent analyses, and frozen at −80° C until time of assay. All the samples were thawed at the same time. Otolin-1 measurements for these subjects have been previously described18. We measured 25-hydroxy vitamin D levels using an enzyme-linked immunosorbent assay (ELISA) kit (detection range 2.5 – 150 ng/mL, sensitivity 1 ng/mL) (MyBiosource.com, San Diego, Ca) as described in the manufacturer’s instruction manual. A 1:5 dilution was prepared. The optical density in the wells of the ELISA microplate was measured at 450 nm using a BioTek ELx808 plate reader, and data were compiled using the KCJunior software package (BioTek Instruments, Winooski, Vermont). According to convention, undetectable values were defined as being one-half the minimal detectable concentration for the assay.
Statistics
SPSS v 22 was used for statistical analyses. Normality of distributions was verified with the Shapiro-Wilk test. Analyses of variance (ANOVA) were carried out and main effects were followed up with Tukey HSD tests. Correlation analysis was carried out using Pearson Correlation and one-tailed p values were used to determine statistical significance. Level of significance was set at p < 0.05.
Results
Vitamin D
Figure 1 shows vitamin D level in the four age groups. Interestingly, the young group (20–30 years old) had the lowest vitamin D level and the old group (66–80 years old) had the highest. Mean vitamin D level increased with age through the old group and then declined in the oldest old (81–95 year old) group. Shapiro-Wilk tests showed that there were no significant differences between vitamin D distributions of the four age groups and that of a normal distribution. ANOVA showed a main effect of age (p = 0.005). Follow up Tukey HSD showed that this was mainly due to the difference between the young and the old groups (p = 0.002).
Figure 1.
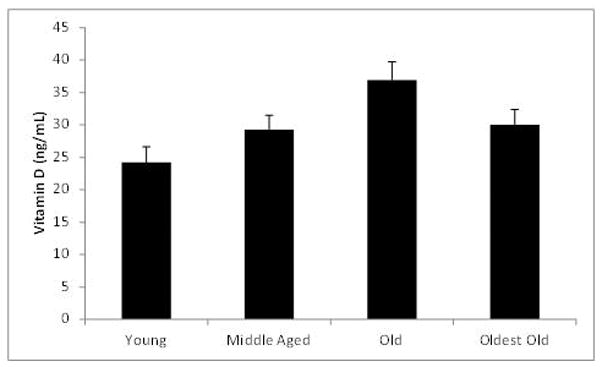
Mean vitamin D levels for the four age groups after removal of outliers. The number of subjects in each group was: young (20–30 years) = 20, middle aged (50–65 years) = 20, old (66–80 years) = 20 and oldest old (81–95 years) = 19. Error bars represent standard error of the mean (SEM).
Table 1 shows the vitamin D levels in the four age groups as a function of sex. Statistical comparison as a function of sex was not feasible due to the small number of males in each group. The trends shown in Figure 1 are seen in both sexes. Therefore, due to unequal and smaller male subject numbers, for the remainder of the analyses, sex is excluded as a variable. It is interesting to note, however, that males tended to have lower vitamin D levels than females at each age group, except 50–65.
Table 1.
Vitamin D levels as a function of age groups and sex.
| Gender | Age (years) Mean ± SEM (n) |
|||
|---|---|---|---|---|
| 20 – 30 | 50 – 65 | 66 – 80 | 81 – 95 | |
| Female | 25.9 ± 2.9 (15) | 29.0 ± 2.7 (16) | 39.6 ± 4 (12) | 33.0 ± 2.4 (14) |
| Male | 19.1 ± 3.3 (5) | 30.3 ± 3.3 (4) | 32.9 ± 3.5 (8) | 21.6 ± 4.1 (5) |
Vitamin D - Otolin-1 Relationship
Figure 2 shows the distribution of vitamin D levels as a function of age. There appeared to be a trend toward increased vitamin D levels with age up to about 70 years of age and declined thereafter. A 2nd order polynomial trendline confirmed that the reversal of the trend occurred around 70 years of age. Based on the age at which the reversal of the trend occurred, to investigate the relationship between age, otolin-1 and vitamin D levels, we divided our subjects into those younger than 70 years of age and those 70 and older. Six subjects had exceedingly high otolin-1 levels. Analysis using a conservative index for outliers (g=2.2)20,21 suggested that all values greater than 354 pg/mL were considered statistical outliers. The relationship between vitamin D and otolin-1 levels in this group was considered separately.
Figure 2.
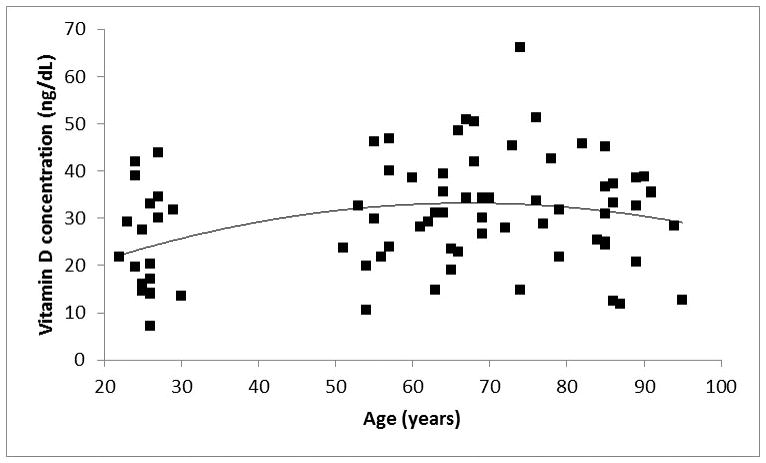
Scatter plot of vitamin D levels as a function of age. A second degree regression line demonstrates the age-related trends.
In examining the majority group (n=73), there was a positive relationship between age and vitamin D level up to 70 years of age (r = 0.38, p = 0.0015) and for those 70 years of age and older, there was a negative relationship between age and vitamin D level (r = −0.31, p = 0.024). In other words, up to 70 years of age, vitamin D levels and age increased together, however, after that age, vitamin D level decreased as subjects grew older.
There was no significant correlation between the otolin-1 and vitamin D in those under 70 years of age. Figure 3 shows the relationship between otolin-1 and vitamin D levels in those 70 and older. There was a negative relationship between vitamin D and otolin-1 level (r = −0.36, p = 0.036). With the outliers included, the strength of this relationship decreased (r = −0.26). When all outliers are considered separately, there was a positive relationship between vitamin D and otolin-1 (r = 0.57), but this relationship was not statistically significant (p = 0.12), due to the small number of subjects (n=6).
Figure 3.
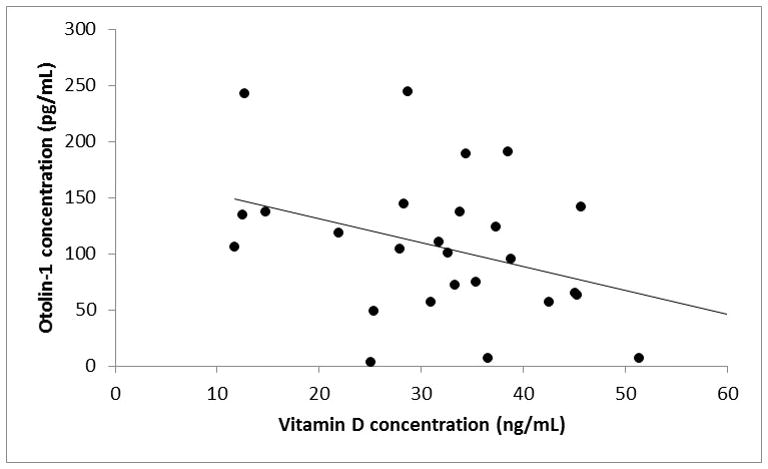
Scatter plot of the relationship between vitamin D and otolin-1 levels in subjects 70 years of age and older. A linear trendline demonstrates graphically illustrates the relationship between the two variables. Subjects with otolin-1 levels > 350 ng/ml were determined to be statistical outliers are not represented in this figure.
Discussion
We had previously reported that circulating levels of otolin-1 were significantly higher in subjects 65 years and older as compared to younger controls18. In extending our earlier findings, we now report that levels of 25-OH vitamin D declined with age for subjects 70 years and older, with evidence of a negative correlation between vitamin D and otolin-1 levels. This latter finding has potential significant implications for inner ear pathology and iBPPV.
We have proposed otolin-1 as an otolithic degeneration marker18,22,23. Otolin-1 is a secreted glycoprotein whose mRNA expression is restricted to the inner ear, specifically the support cells of the vestibular maculae, semicircular canal cristae, organ of Corti and marginal cells of the stria vascularis24. Its functions include interaction with other specialized inner ear proteins, such as otoconin 90, to form and maintain otoconia24–26. The age-related increase in otolin-1 in healthy individuals is consistent with the age-related increase in prevalence of BPPV8 and previous research showing degenerative changes in otoconia with age26,27.
A strong association exists between BPPV and osteopenia/osteoporosis 1–3,7,10,12,28. However, although otolin-1 level correlates negatively with T-score, it demonstrates no correlation with biomarkers of bone turnover23. Therefore, the relationship between disorders of bone turnover and BPPV is not likely to be causal. We have argued that the strong association between iBPPV and disorders of bone turnover implies otoconia are subject to the same systemic regulatory mechanisms whose disruption leads to osteopenia/osteoporosis3. One potential candidate is vitamin D. We have proposed a conceptual model whereby low vitamin D levels result in disruption of the calcium absorptive system in the inner ear, increasing receptor activator of nuclear factor-kappa B ligand (RANKL) with decreased osteoprotegrin in the bone, causing resorption of calcium carbonate in the otoconia and increased osteoclast differentiation and calcium phosphate resorption, respectively8. Resorption of calcium (demineralization) results in fragmentation of otoconia and increased vulnerability to BPPV, as well as, osteomalacia and decreased bone density. If we accept otolin-1 as a biomarker29 or surrogate for otolithic degeneration, then the negative correlation between otolin-1 and vitamin D levels in those over 70 (Figure 3), supports our proposed conceptual model and further implicates low vitamin D levels in the pathophysiology of iBPPV1–9. A role for vitamin D in maintaining stability of otoconia may also account for the findings of decreased falls in some studies of elderly subjects receiving vitamin D supplementation and is perhaps a more biologically plausible explanation than improved muscle strength30.
Otolin-1 as a biomarker for otolithic degeneration has the potential to greatly facilitate our ability to better understand BPPV, which is typically thought to be episodic. Since most patients are relatively asymptomatic in between episodes, standard clinical assessments during these periods do not yield significant insights. The use a circulatory biomarker may transform BPPV from an episodic disorder to a progressive disease of otolithic degeneration with episodic manifestations of vertigo. Our results highlight the need for a prospective, controlled investigation of otolin-1 in BPPV and its relationship with possible etiological factors such as vitamin D. Biomarkers may also have a role in clinical management of BPPV through identification of patients, particularly older patients, at risk of BPPV and potential candidates for vitamin D supplementation.
None of the subjects in this study were being prescribed vitamin D at the time of this study. However, one weakness of this study is that it is not known if any were taking vitamin D supplements. Vitamin D Council conservatively defines vitamin D deficiency, insufficiency and sufficiency as levels <30 ng/mL, 30–40 ng/mL and > 40 ng/mL, respectively31. Using these standards, 83% of all of our subjects and 95% of the oldest old subjects were vitamin D insufficient or deficient. There is debate on what levels should be used to define abnormal vitamin D levels32. If a systematic investigation of vitamin D, otolin-1 and BPPV verify our findings, then the present results would support setting the boundary at 40 ng/mL, consistent with Vitamin D Council definitions.
Vitamin D levels fluctuate as a function of seasons. In the Northeast USA, vitamin D levels fall during winter and reach a low point during early Spring (March – May)33. A strength of our study is that, to minimize the influence of seasonal variation on our analyses, all of our samples were collected in the early Spring time frame. Given that our data and those of others implicate vitamin D in the pathogenesis of iBPPV, it is not surprising that BPPV presentations are greatest when serum vitamin D level is the lowest in the Northeast USA11. Since iBPPV presentation is not restricted to early Spring, we believe that the timing of an assessment is a crucial factor in evaluating the relationship between vitamin D status and iBPPV. Specifically, in the clinical realm, a recent history of vitamin D insufficiency or deficiency in the weeks prior to presentation with new or recurrent iBPPV, rather than at the time of presentation, should be considered the significant predisposing factor. Since seasonal vitamin D levels are not routinely checked and therefore, vitamin D status before presentation would not be known, caution is needed in interpretation if blood tests reveal sufficient vitamin D levels in a newly diagnosed iBPPV. For example, a low-normal vitamin D level in early summer does not exclude low vitamin D as an etiologic factor. Whether an otolithic degeneration marker, such as otolin-1, can be used to close a potential temporal gap between history of vitamin D and iBPPV presentation remains to be determined.
A key question is why do low vitamin D levels correlate with high otolin-1 levels only in the elderly? We suspect the likely explanation is that the older subjects had chronic changes in vitamin D levels. Elderly subjects are subject to chronic vitamin D deficiency which is not limited to early Spring time frame. This is in part due to decreased production of vitamin D in the skin of older subjects34 and partly due to decreased amount of outdoor activities on a year round basis35. Therefore, we hypothesize that it is not just low vitamin D levels that is a risk factor for otoconia dimeneralization and otolithic degeneration, rather chronically low vitamin D levels that precipitate these abnormalities. Moreover, it is well known that geriatric diseases, conditions and syndromes are highly multifactorial from the perspective of risk factors and pathophysiology, even when similar conditions tend to have less complex presentations at younger ages36. Also, aging and associated biological pathways are thought to contribute to the fact that aging represents a major risk factor for most chronic diseases in adults37. With these considerations in mind, it is possible that low vitamin D predisposes to the onset and progression of iBPPV to a far greater extent in the elderly who are subject to the effects of not only vitamin D deficiency but also aging and associated chronic diseases. Finally, how long vitamin D has to be low for otoconia demineralization to occur and, to extend this concept to therapeutic application, for vitamin D supplementation to stabilize otoconia structure also remains to be determined. Future BPPV studies will need to assess vitamin D status and otolin-1 levels over the course of a year, rather than a single time point.
In conclusion, our results demonstrate a relationship between vitamin D and otolin-1 in subjects over 70 with statistical outliers excluded. Since otolin-1 is a key component of otoconia and degeneration of otoconia is central to the pathophysiology of iBPPV, the present results are consistent with a potential role for vitamin D deficiency in the pathogenesis of iBPPVs.
Acknowledgments
Sources of Support: The UCONN Center on Aging, The Connecticut Institute on Clinical and Translational Science (Parham), NIH R01 AG048023 (Kuchel, McElhaney) and P01 AG021600 (Haynes, McElhaney, Kuchel)
References
- 1.Vibert D, Kompis M, Hausler R. Benign paroxysmal positional vertigo in older women may be related to osteoporosis and osteopenia. The Annals of otology, rhinology, and laryngology. 2003;112:885–9. doi: 10.1177/000348940311201010. [DOI] [PubMed] [Google Scholar]
- 2.Jang YS, Kang MK. Relationship between bone mineral density and clinical features in women with idiopathic benign paroxysmal positional vertigo. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2009;30:95–100. doi: 10.1097/MAO.0b013e31818f5777. [DOI] [PubMed] [Google Scholar]
- 3.Parham K, Leonard G, Feinn RS, Lafreniere D, Kenny AM. Prospective clinical investigation of the relationship between idiopathic benign paroxysmal positional vertigo and bone turnover: a pilot study. The Laryngoscope. 2013;123:2834–9. doi: 10.1002/lary.24162. [DOI] [PubMed] [Google Scholar]
- 4.Lee SB, Lee CH, Kim YJ, Kim HM. Biochemical markers of bone turnover in benign paroxysmal positional vertigo. PLoS One. 2017;12:e0176011. doi: 10.1371/journal.pone.0176011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothman KJ, Greenland S. Causation and causal inference in epidemiology. American journal of public health. 2005;95(Suppl 1):S144–50. doi: 10.2105/AJPH.2004.059204. [DOI] [PubMed] [Google Scholar]
- 6.Buki B, Ecker M, Junger H, Lundberg YW. Vitamin D deficiency and benign paroxysmal positioning vertigo. Medical hypotheses. 2013;80:201–4. doi: 10.1016/j.mehy.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong SH, Kim JS, Shin JW, et al. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. Journal of neurology. 2013;260:832–8. doi: 10.1007/s00415-012-6712-2. [DOI] [PubMed] [Google Scholar]
- 8.Parham K, Kuchel GA. A Geriatric Perspective on Benign Paroxysmal Positional Vertigo. J Am Geriatr Soc. 2016;64:378–85. doi: 10.1111/jgs.13926. [DOI] [PubMed] [Google Scholar]
- 9.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941–55. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 10.Talaat HS, Abuhadied G, Talaat AS, Abdelaal MS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2015;272:2249–53. doi: 10.1007/s00405-014-3175-3. [DOI] [PubMed] [Google Scholar]
- 11.Whitman GT, Baloh RW. Seasonality of benign paroxysmal positional vertigo. JAMA otolaryngology-- head & neck surgery. 2015;141:188–9. doi: 10.1001/jamaoto.2014.2941. [DOI] [PubMed] [Google Scholar]
- 12.Sheikhzadeh M, Lotfi Y, Mousavi A, Heidari B, Monadi M, Bakhshi E. Influence of supplemental vitamin D on intensity of benign paroxysmal positional vertigo: A longitudinal clinical study. Caspian J Intern Med. 2016;7:93–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Kahraman SS, Ozcan O, Arli C, et al. Calcium Homeostasis During Attack and Remission in Patients With Idiopathic Benign Paroxysmal Positional Vertigo. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2016;37:1388–92. doi: 10.1097/MAO.0000000000001167. [DOI] [PubMed] [Google Scholar]
- 14.Maslovara S, Butkovic Soldo S, Sestak A, Milinkovic K, Rogic-Namacinski J, Soldo A. 25 (OH) D3 levels, incidence and recurrence of different clinical forms of BPPV. Brazilian journal of otorhinolaryngology. 2017 doi: 10.1016/j.bjorl.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talaat HS, Kabel AM, Khaliel LH, Abuhadied G, El-Naga HA, Talaat AS. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris, nasus, larynx. 2016;43:237–41. doi: 10.1016/j.anl.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Sheikhzadeh M, Lotfi Y, Mousavi A, Heidari B, Bakhshi E. The effect of serum vitamin D normalization in preventing recurrences of benign paroxysmal positional vertigo: A case-control study. Caspian J Intern Med. 2016;7:173–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Karatas A, Acar Yuceant G, Yuce T, Haci C, Cebi IT, Salviz M. Association of Benign Paroxysmal Positional Vertigo with Osteoporosis and Vitamin D Deficiency: A Case Controlled Study. J Int Adv Otol. 2017 doi: 10.5152/iao.2016.2640. [DOI] [PubMed] [Google Scholar]
- 18.Tabtabai R, Haynes L, Kuchel GA, Parham K. Age-Related Increase in Blood Levels of Otolin-1 in Humans. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2017;38:865–9. doi: 10.1097/MAO.0000000000001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson D, Williams GH. Clinical and Translational Science: Principles of Human Research. Cambridge, MA: Academic Press; 2009. [Google Scholar]
- 20.Hoaglin DC, Iglewicz B, Tukey JW. Performance of some resistant rues for outlier labeling. Journal of the American Statistical Association. 1986;81:991–9. [Google Scholar]
- 21.Hoaglin DC, Iglewicz B. Fine tuning some resitant rules for outlier labeling. Journal of Am Stat Assoc. 1987;82:1147–9. [Google Scholar]
- 22.Parham K, Sacks D, Bixby C, Fall P. Inner ear protein as a biomarker in circulation? Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2014;151:1038–40. doi: 10.1177/0194599814551127. [DOI] [PubMed] [Google Scholar]
- 23.Sacks D, Parham K. Preliminary Report on the Investigation of the Association Between BPPV and Osteoporosis Using Biomarkers. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2015;36:1532–6. doi: 10.1097/MAO.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 24.Deans MR, Peterson JM, Wong GW. Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1. PLoS One. 2010;5:e12765. doi: 10.1371/journal.pone.0012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Zhao X, Xu Y, Wang L, He Q, Lundberg YW. Matrix recruitment and calcium sequestration for spatial specific otoconia development. PLoS One. 2011;6:e20498. doi: 10.1371/journal.pone.0020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade LR, Lins U, Farina M, Kachar B, Thalmann R. Immunogold TEM of otoconin 90 and otolin - relevance to mineralization of otoconia, and pathogenesis of benign positional vertigo. Hear Res. 2012;292:14–25. doi: 10.1016/j.heares.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walther LE, Wenzel A, Buder J, Bloching MB, Kniep R, Blodow A. Detection of human utricular otoconia degeneration in vital specimen and implications for benign paroxysmal positional vertigo. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2013 doi: 10.1007/s00405-013-2784-6. [DOI] [PubMed] [Google Scholar]
- 28.Yamanaka T, Shirota S, Sawai Y, Murai T, Fujita N, Hosoi H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. The Laryngoscope. 2013;123:2813–6. doi: 10.1002/lary.24099. [DOI] [PubMed] [Google Scholar]
- 29.Micheel CM, Ball JR. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Washington, D.C: National Academies Press; 2010. [PubMed] [Google Scholar]
- 30.Murad MH, Elamin KB, Abu Elnour NO, et al. Clinical review: The effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- 31.I tested my vitamin D level. [Accessed August 12, 2017, 2017];What do my results mean? 2017 at https://www.vitamindcouncil.org/i-tested-my-vitamin-d-level-what-do-my-results-mean/.)
- 32.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 34.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–5. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 35.Baker MR, Peacock M, Nordin BE. The decline in vitamin D status with age. Age and ageing. 1980;9:249–52. doi: 10.1093/ageing/9.4.249. [DOI] [PubMed] [Google Scholar]
- 36.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–91. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


