Abstract
Background
In patients with resectable synchronous colorectal liver metastases (CRLM), either two-staged or simultaneous resections of the primary tumor and liver metastases are performed. Data on radiofrequency ablation (RFA) for the treatment of CRLM during a simultaneous procedure is lacking. The primary aim was to analyze short-term and long-term outcome of RFA in simultaneous treatment. A secondary aim was to compare simultaneous resection with the colorectal-first approach.
Methods
Retrospective analysis of 241 patients with colorectal cancer and synchronous CRLM between 2000–2016. Median follow-up was 36.1 months (IQR 18.2–58.8 months). A multivariable analysis was performed to analyze the postoperative morbidity, using the comprehensive complication index. A propensity matched analysis was performed to compare survival rates.
Results
In multivariable analysis, the best predictor of lower complication severity was treatment with RFA (p = 0.040). Higher complication rates were encountered in patients who underwent an abdominoperineal resection (p = 0.027) or age > 60 years (p = 0.022). The matched analysis showed comparable overall survival in RFA treated patients versus patients undergoing a liver resection with a five year overall survival of 39.4% and 37.5%, respectively (p = 0.782). In a second matched analysis, 5-year overall survival rates in simultaneously treated patients (43.8%) was comparable to patients undergoing the colorectal first approach (43.0%, p = 0.223).
Conclusions
RFA treatment of CRLM in simultaneous procedures is associated with a lower complication severity and non-inferior oncological outcome as compared to partial liver resection. RFA should be considered a useful alternative to liver resection.
Introduction
About 20% of patients with colorectal cancer (CRC) already have liver metastases at the time of presentation of their primary tumor. The most widely used intentionally curative approach for the treatment of both tumor locations is a staged procedure with resection of the primary tumor first, followed by liver surgery at a later stage [1]. As alternative, the liver-first staged procedure was introduced in 2008 and was proposed to prevent progression of the liver metastases during the interval between resection of the primary and the liver metastases [2,3]. The third treatment option is to perform simultaneous surgery, in which the primary colorectal cancer and the liver metastases are resected during the same operative session.
Simultaneous resection of the primary CRC and synchronous CRLM has been shown to be feasible with an acceptable complication rate compared to the colorectal-first approach [4–7]. These studies score complications based on the Clavien-Dindo classification, mainly comparing the incidence of major complications [8]. However, selection of only patients in a good clinical condition contributes to this outcome. Because comorbidity and impact of surgery are predictors for developing complications, the decision to perform two major procedures simultaneously or a staged procedure is important [9–11]. As an alternative for liver resection, radiofrequency ablation (RFA) of CRLM has been shown to be associated with lower morbidity and mortality and comparable survival rates [12–15].
Strikingly, in a recent multidisciplinary international consensus paper about treatment strategy for synchronous liver metastases, RFA as a treatment option of CRLM was not even mentioned [16]. This is due to lack of evidence, and studies in this field are certainly warranted. Therefore, the aim of the present study is to analyze patients undergoing simultaneous treatment of primary CRC and CRLM, focusing on the role of RFA in short-term complications. Furthermore, a matched analysis is performed to study survival rates in RFA-treated patients vs. patients who underwent a liver resection. The last aim is to analyze survival rates in matched patients who underwent a simultaneous procedure vs. colorectal-first procedure.
Patients and methods
The Department of Surgery of the University Medical Centre Groningen (UMCG) is a secondary and tertiary referral centre for patients with advanced colorectal cancer including CRLM in the North-Eastern part of the Netherlands. An analysis was performed using a prospectively maintained database of all patients with CRLM, in which for this study a selection of patients with synchronous liver metastases was used. The procedures, performed between January 2000 and September 2016, consisted of surgery aiming at radical resection of the primary CRC and radical resection and/or ablation of liver metastases. Only surgical resections in patients with microscopic tumor-free margins (R0) were included in the analysis. Each patient was discussed in a tumor board of hepato-pancreato-biliary and colorectal surgeons, gastroenterologists, radiologists, radiation oncologists, pathologists and medical oncologists. Most patients who underwent the colorectal-first procedure are treated for colorectal cancer in a primary hospital. Another reason for not performing simultaneous surgery is comorbidity or large liver resections (>70% of liver volume). In simultaneous procedures, we always performed the liver procedure first and the colorectal surgery second. The total duration of the procedure included anesthesia induction time. A proportion of patients with rectal cancer underwent neoadjuvant chemoradiotherapy, including 5x5 Gy radiotherapy and 6 cycles of capecitabine/oxaliplatin/bevacizumab [17,18]. Patients with more advanced liver metastases, in whom radical liver surgery was questionable, received neoadjuvant chemotherapy. They were evaluated after two or three cycles of chemotherapy, and no further cycles were administered if the computer tomography (CT) scan showed that a R0 resection was possible. Two or three additional cycles were given in patients with insufficient response.
During all simultaneous procedures, intraoperative RFA was performed under ultrasound guidance, using the RF 3000 TM Radio Frequency Ablation System (Boston Scientific, Marlborough, MA, USA) according to the manufacturers’ instructions. Depending on the size of the CRLM, the 2.0, 3.5, 4.0 or 5.0 cm diameter LeVeen electrodes were inserted. Percutaneous RFA, which was only applied in the staged approaches, was performed CT guided. In general, RFA was contraindicated in CRLMs with a diameter > 5 cm. Ablation site recurrences were defined as described earlier [19].
The number of CRLM and the size of the largest CRLM are based on pathological findings of resection specimens and on CT scans in case of RFA treatment. Follow-up consisted of a 3–4 monthly survey in the first 2 years after surgery, and 6 monthly thereafter. Follow-up consisted of serum carcinoembryonic antigen-level (CEA), liver ultrasound and thoracic X-ray, or a multiphase contrast enhanced CT-scan or MRI scan. If equivocal results of CT/MRI scan were obtained, positron emission tomography with [F-18]-fluorodeoxyglucose CT (FDG-PET-CT) was performed.
Postoperative complications were scored in the first 90 days after surgery and were categorized into general complications (for instance urinary tract or pulmonary complications), bowel-related complications (anastomotic leakage, hematoma or abscess) and liver-related complications (biloma, liver abscess). Since more than one complication can occur in the postoperative period, we applied the comprehensive complication index (CCI) [20]. This index integrates all complications and, on top of that, includes a grading of severity of all complications. This is especially relevant because apart from general complications (not related to the surgical procedure itself), both the liver procedure and the colorectal procedure can have its associated complications with variable severity.
Ethics statement
This study was approved by the local Medical Ethical committee (METc2015/343), and was judged not to be within the scope of the Medical Research in Human Subjects Act (WMO).
In our retrospective study concerning oncological disease, it is impossible to obtain written consent of all patients, since about 50% of patients already died of the disease at the time of writing the manuscript.
We are, as medical doctors, bound by the law of confidentiality to keep all patient information fully secret. This implies all authors, as researchers, are subject to this law. The local ethics committee approved our method of including patients in our observational, retrospective study, provided that we obey the Dutch law regulations. Because we cannot obtain patient consent because of the aforementioned reasons, we cannot share the data because of patient confidentiality.
The ethics committee that approved our study:
Medical Ethical Review Board, PO Box 30.001, 9700 RB Groningen, The Netherlands, E-mail: metc@umcg.nl.
Statistical analysis
Summary statistics are presented as percentages, median (interquartile range, IQR) or mean (± standard deviation, SD). Non-parametrical tests (chi-square test, Mann-Whitney test and Kruskall-Wallis H and paired equivalents) were applied when appropriate. Regression analyses were performed to determine the risk factors for developing any complication (complication rate) and for developing a higher comprehensive complication index (CCI) [20]. Factors with a p-value < 0.17 in univariable analysis were entered into the multivariable model. Hazard ratios and 95% CI are reported.
Survival rates were estimated using the Kaplan-Meier method with the stratified log-rank test for matched comparisons. In order to compare survival, a propensity score matching was used to reduce the influence of selection bias. A binary logistic regression was performed to predict the probability of belonging to the RFA or non-RFA treatment group, and colorectal first vs. simultaneous group. Covariates used for matching were location of the primary tumor, type of colorectal surgery, major/minor liver surgery, type of liver procedure, sex, age, neoadjuvant chemotherapy and clinical risk score [21]. We used nearest-neighbour matching, using a 1:1 ratio, with a caliper fixed to 0.2.
In all analyses, a p-value < 0.05 was considered significant. Statistical analyses were performed with IBM SPSS Statistics V22 (IBM, Armonk, New York, USA) and R software [22] using the MatchIt package [23].
Results
Demographics
In the study period January 2000—September 2016, a total 574 patients with colorectal liver metastases underwent 904 liver procedures, which consisted of resection, RFA or a combination of both. In the same period, 241 patients presented with synchronous liver metastases which were treated surgically. Median follow-up of all 241 patients with synchronous liver metastases was 36.1 months (IQR 18.2–58.8 months).
Patients who underwent a liver-first approach were excluded from further analyses due to low number of patients (n = 15). In the remaining 226 patients, 106 underwent the simultaneous approach and 120 underwent the colorectal-first approach. First we analyzed the 106 patients receiving the simultaneous approach. Table 1 shows the clinicopathological characteristics of all 106 patients in the simultaneous group. Neoadjuvant chemotherapy consisted in 88.4% (61 out of 69 patients) of the baseline treatment of capecitabine and oxaliplatin, of which 51 patients also received bevacizumab. Major liver resections (≥ 3 liver segments) were performed in 3 of the 24 patients who underwent an APR versus 25 of the 82 patients who underwent non-APR procedures (p = 0.079).
Table 1. Clinicopathological characteristics of the patients undergoing simultaneous treatment of the primary colorectal carcinoma and liver metastases.
| Total | Liver resection | Liver resection + RFA | RFA alone | P value | |
|---|---|---|---|---|---|
| Number of patients | 106 | 59 | 34 | 13 | |
| Patient characteristics | |||||
| Mean age ± SD | 61.3 ± 11.5 | 63.0 ± 11.3 | 58.8 ± 11.0 | 59.8 ± 13.4 | 0.210 |
| Male gender | 59 (55.7%) | 33 (55.9%) | 19 (55.9%) | 7 (53.8%) | 0.990 |
| Comorbidities | |||||
| BMI > 30 | 14 (13.9%) | 6 (10.3%) | 7 (21.9%) | 1 (9.1%) | 0.282 |
| Smoking | 22 (21.2%) | 14 (24.1%) | 5 (15.2%) | 3 (23.1%) | 0.591 |
| ASA score ≥ 3 | 15 (15.0%) | 10 (17.5%) | 5 (15.6%) | 0 (0%) | 0.326 |
| Cardiovascular medication | 47 (44.3%) | 25 (42.4%) | 15 (44.1%) | 7 (53.8%) | 0.752 |
| Diabetic medication | 5 (4.7%) | 1 (1.7%) | 2 (5.9%) | 2 (15.4%) | 0.101 |
| Syst. corticosteroid medication | 6 (5.7%) | 1 (1.7%) | 5 (14.7%) | 0 (0%) | 0.021 |
| Obstructive lung disease | 10 (9.4%) | 6 (10.2%) | 3 (8.8%) | 1 (8.3%) | 0.952 |
| Tumor characteristics | |||||
| Rectal primary | 71 (67.0%) | 36 (61.0%) | 24 (70.6%) | 11 (84.6%) | 0.226 |
| N+ disease | 66 (62.3%) | 40 (67.8%) | 21 (61.8%) | 5 (38.5%) | 0.142 |
| Diameter CRLM in cm (median, IQR) | 2.2 (1.5–3.5) | 2.5 (1.5–4.0) | 2.0 (1.2–3.3) | 1.7 (0.9–2.7) | 0.092 |
| >1 CRLM | 74 (69.8%) | 34 (57.6%) | 34 (100%) | 6 (46.2%) | <0.001 |
| Bilobar disease | 35 (33.0%) | 7 (11.9%) | 26 (76.5%) | 2 (15.4%) | <0.001 |
| Preoperative factors | |||||
| Neoadjuvant chemotherapy | 69 (66.0%) | 34 (57.6%) | 28 (82.4%) | 7 (53.8%) | 0.036 |
| Low clinical risk score (0–2) [21] | 57 (53.8%) | 33 (55.9%) | 15 (44.1%) | 9 (69.2%) | 0.268 |
| Surgery | |||||
| Surgery > 8 hours | 58 (55.2%) | 32 (54.2%) | 20 (58.8%) | 6 (50.0%) | 0.846 |
| Blood loss > 500ml | 49 (48.5%) | 25 (45.5%) | 17 (50.0%) | 5 (41.7%) | 0.705 |
| Extent of liver surgery | <0.001 | ||||
| ≥ 3 segments | 28 (26.4%) | 23 (39.0%) | 5 (14.7%) | - | |
| 1 or 2 segments | 18 (17.0%) | 12 (20.3%) | 6 (17.6%) | - | |
| Local treatment | 60 (56.6%) | 24 (40.7%) | 23 (67.6%) | 13 (100%) | |
| • Wedge resection | 24 | 24 | - | - | |
| • RFA | 13 | - | - | 13 | |
| • RFA + wedge resection | 23 | - | 23 | - | |
| Type of colorectal surgery | 0.460 | ||||
| APR | 24 (22.6%) | 12 (20.3%) | 7 (20.6%) | 5 (38.5%) | |
| LAR | 48 (44.3%) | 25 (42.4%) | 17 (50.0%) | 6 (38.5%) | |
| Colon | 34 (33.0%) | 22 (37.3%) | 10 (29.4%) | 2 (23.1%) |
RFA = radiofrequency ablation, APR = abdominoperineal resection, LAR = low anterior resection, N+ = lymph node positive primary tumor, ASA-score = American Society of Anaesthesiologists physical status classification system.
Cardiovascular medication includes: regulators of blood pressure and anticoagulants.
Diabetic medication includes: insulin derivatives and DM type 2 variants (e.g. metformin, tolbutamide).
Obstructive lung disease is defined as COPD and/or asthma.
RFA was performed in 47 patients if partial liver resection was not able to render the liver tumor-free. More specifically, RFA was performed because of bilobar metastases (n = 28), risk of insufficient liver function after resection (comorbidity, age or chemotherapy induced liver parenchymal damage, n = 11) or tiny remnant metastases after neoadjuvant chemotherapy for which resection was considered target overshoot (n = 8). The average number of ablated lesions per patient was 1.81 (SD 1.26, range 1–7) and the average size of the largest RFA-treated lesion was 16.4 mm (SD 9.5 mm).
The median follow-up in the patients who underwent simultaneous treatment was 25.5 (IQR = 9.43–49.53) months. At the end of the follow-up period, 47/106 (44.3%) were alive without recurrent disease, 26/106 (24.5%) were alive with recurrent disease and 33/106 (31.1%) patients were deceased. The in-hospital mortality was 3/106 (2.8%), all in the liver resection group. Two out of 47 patients (4.3%) treated by RFA developed an ablation site recurrence after 8 and 23 months, which were re-treated by liver resection or re-ablation, both with curative intent. The five-year overall survival of all 106 patients undergoing simultaneous treatment was 54.3% with a median overall survival of 70.2 months (95%CI = 43.0–97.5). S1 Table shows all complications registered and categorized in liver-related, bowel-related and general complications stratified by type of liver treatment.
Multivariate analysis of complication rate and severity in simultaneous treatment
In this study, we separately analyzed the complication rate and the complication severity in patients undergoing simultaneous treatment (Tables 2 and 3). To this end we performed a regression analysis to determine the risk factors for developing any complication (complication rate, Table 2). Secondly, we performed a regression analysis to determine the risk factors for developing a high CCI only in the group of patients who developed complications (complication severity, Table 3). In total, 63 of 106 patients (59.4%) suffered from complications. Table 2 shows that patients undergoing an APR (p = 0.027) and patients older than 60 years (p = 0.022) have a higher complication rate. With respect to the treatment of the primary tumor, patients who underwent an APR (20/24) more often suffered complications compared to low anterior resection (LAR) (25/48; p = 0.01) or colon treatment (18/34; p = 0.016). Of note, in univariate analysis of patients that underwent RFA, the diameter of the ablated metastases was larger in patients with complications (18.6mm ± 10.5) versus those without complications (12.8mm ± 6.4, p = 0.023).
Table 2. Regression analysis of complication rate.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Factors | P value | HR | 95% CI | P value | HR | 95% CI |
| Patient characteristics | ||||||
| Female sex | 0.244 | 0.628 | 0.287–1.373 | |||
| Age > 60 years | 0.068 | 2.095 | 0.946–4.639 | 0.022 | 3.118 | 1.176–8.262 |
| BMI > 30 | 0.638 | 0.977 | 0.885–1.078 | |||
| Current smoking | 0.741 | 0.848 | 0.320–2.248 | |||
| Comorbidity | ||||||
| ASA score ≥ 3 | 0.260 | 2.020 | 0.595–6.861 | |||
| Cardiovascular medication | 0.224 | 1.635 | 0.741–3.607 | |||
| Diabetic medication | 0.979 | 1.025 | 0.164–6.407 | |||
| Syst. corticosteroid medication | 0.248 | 3.621 | 0.408–32.140 | |||
| Obstructive lung disease | 0.070 | 7.000 | 0.853–57.448 | 0.057 | 8.231 | 0.936–73.999 |
| Tumor characteristics | ||||||
| High CRS (3–5) [21] | 0.728 | 1.148 | 0.527–2.502 | |||
| Bilobar disease | 0.615 | 1.238 | 0.539–2.845 | |||
| Treatment | ||||||
| Neoadjuvant chemo | 0.405 | 0.704 | 0.308–1.608 | |||
| > 1 liver segment surgery | 0.146 | 1.075 | 0.445–2.598 | 0.079 | 2.422 | 0.902–6.502 |
| Major liver surgery | 0.872 | 1.808 | 0.814–4.018 | |||
| RFA performed | 0.979 | 1.011 | 0.463–2.206 | |||
| APR performed | 0.010 | 4.535 | 1.425–14.433 | 0.027 | 4.382 | 1.180–16.277 |
| Operation > 8 hours | 0.008 | 2.986 | 1.333–6.688 | 0.489 | 1.417 | 0.528–3.802 |
| Blood loss > 500ml | 0.167 | 1.768 | 0.788–3.968 | 0.315 | 1.671 | 0.613–4.554 |
A binary logistic regression analysis was performed with complication rate as the dependent variable (n = 106). Variables with a p-value < 0.17 were entered in the multivariable analysis.
RFA = radiofrequency ablation, APR = abdominoperineal resection, CRS = clinical risk score, BMI = body mass index, ASA-score = American Society of Anaesthesiologists physical status classification system.
Table 3. Regression analysis of the comprehensive complication index (CCI) of patients with complications.
| Univariable analysis | Multivariable analysis | ||
|---|---|---|---|
| Factors | P value | P value | Standardized Beta |
| Patient characteristics | |||
| Female sex | 0.618 | ||
| Age > 60 years | 0.762 | ||
| BMI > 30 | 0.239 | ||
| Current smoking | 0.383 | ||
| Comorbidity | |||
| ASA score ≥ 3 | 0.259 | ||
| Cardiovascular medication | 0.874 | ||
| Diabetic medication | 0.712 | ||
| Syst. corticosteroid medication | 0.138 | 0.290 | -0.134 |
| Obstructive lung disease | 0.323 | ||
| Tumor characteristics | |||
| High CRS (3–5)[21] | 0.233 | ||
| Bilobar disease | 0.498 | ||
| Treatment | |||
| Neoadjuvant chemo | 0.458 | ||
| > 1 liver segment surgery | 0.993 | ||
| Major liver surgery | 0.462 | ||
| RFA performed | 0.021 | 0.040 | -0.263 |
| APR performed | 0.306 | ||
| Operation > 8 hours | 0.377 | ||
| Blood loss > 500ml | 0.421 | ||
A linear regression was performed in all patients who developed complications (n = 63), with CCI score as the dependent variable. Variables with a p-value < 0.17 were entered in the multivariable analysis.
RFA = radiofrequency ablation, APR = abdominoperineal resection, CRS = clinical risk score, BMI = body mass index, ASA-score = American Society of Anaesthesiologists physical status classification system.
When comparing the CCI in the patients who actually suffered from complications, univariable analysis showed that RFA-treated patients had a lower complication severity (27.9 ± 13.0) compared to non-RFA-treated patients. (39.6 ± 23.3; p = 0.021). This difference in complication severity maintained significance in the multivariable analysis (p = 0.040; Table 3).
Survival in matched analysis simultaneous vs. colorectal-first
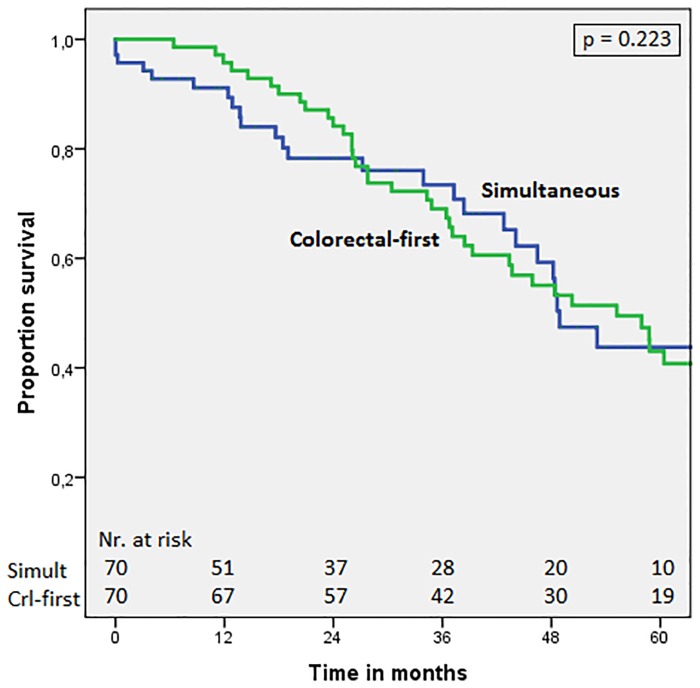
To confirm the findings of previous research of comparable survival in the simultaneous versus the colorectal-first approach, we performed a matched analysis. A matched pair analysis was performed to reduce bias due to confounding variables. Seventy patients who underwent colorectal-first surgery were successfully matched to 70 patients (70/106; 66.0%) who underwent simultaneous treatment. Table 4 shows the clinicopathological characteristics of matched patients with synchronous liver metastases undergoing simultaneous versus colorectal-first treatment. Fig 1 shows comparable survival curves in the simultaneous group and colorectal-first group, with 5-year overall survival of 43.8% and 43.0% and median overall survival of 48.9 months (95%CI = 42.8–55.0) and 55.2 months (95%CI = 41.7–68.7), respectively (p = 0.223).
Table 4. Clinicopathological characteristics of matched patients with synchronous liver metastases undergoing simultaneous or colorectal-first treatment.
| Simultaneous (n = 70) | Colorectal first (n = 70) | P-value | |
|---|---|---|---|
| Patient characteristics | |||
| Mean age ± SD | 62.2 ± 11.6 | 62.3 ± 9.0 | 0.947 |
| Male gender | 37 (52.9%) | 34 (48.6%) | 0.720 |
| Extent of liver surgery | 0.475 | ||
| ≥3 segments | 25 (35.7%) | 27 (38.6%) | |
| 1 or 2 segments | 13 (18.6%) | 14 (20.0%) | |
| RFA or wedge resection | 32 (45.7%) | 29 (41.4%) | |
| RFA | 0.424a | ||
| RFA as part of treatment | 30 (42.9%) | 25 (35.7%) | |
| • RFA + resection | 19 (29.9%) | 11 (15.7%) | |
| • Only RFA | 11 (15.7%) | 20.0%) | |
| Of which percutaneous | 0 | 10 (14.3%) | |
| Characteristics tumor | |||
| Low clinical risk score (0–2)[21] | 37 (52.9%) | 36 (51.4%) | 1.000 |
| Diam. CRLM in cm (median ± IQR) | 2.5 ± 2.5 | 3.0 ± 3.5 | 0.106 |
| Neoadjuvant chemotherapy | 35 (50.0%) | 32 (45.7%) | 0.690 |
| Primary tumor at rectal site | 36 (51.4%) | 34 (48.6%) | 0.832 |
| Bilobar liver disease | 23 (32.9%) | 32 (45.7%) | 0.160 |
Matching was performed based on the characteristics: location of primary tumor, major/minor liver surgery, type of liver procedure, clinical risk score, sex, age and neoadjuvant chemotherapy.
P values were calculated using the paired T test, McNemar test or Wilcoxon signed rank test.
a RFA vs. no RFA in simultaneous vs. colorectal-first treatment.
Fig 1. Overall survival in matched patients: Simultaneous treatment vs. colorectal-first.
Comparison of patients with synchronous CRLM undergoing the simultaneous treatment or the colorectal-first approach. P-value = 0.223 (stratified log-rank test).
Survival in matched analysis in RFA with or without liver resection vs. liver resection alone
Thirty-five patients (35/47; 74.5%) who underwent a treatment including RFA, either as a sole treatment or in combination with liver resection (RFA ± liver resection treatment), were successfully matched to 35 patients in whom no RFA was applied and only underwent liver resection. Table 5 shows the clinicopathological characteristics of all matched patients. Fig 2 shows comparable survival curves in the RFA group and the liver resection group, with 5 year overall survival of 49.2% and 56.3% and median overall survival of 48.4 months (95%CI = 18.3–78.4) and 70.2 months (95%CI = 31.1–109.3), respectively (p = 0.782). Likewise, Fig 3 shows comparable survival curves in the RFA group and the liver resection group concerning disease-free survival (DFS), with a 5 year DFS of 39.1% and 30.1% and a median DFS of 44.1 months (95%CI = 29.2–59.0) and 38.4 months (95%CI = 11.7–65.1), respectively (p = 0.683).
Table 5. Clinicopathological characteristics of matched patients undergoing RFA as a part of treatment vs. liver resection only.
| Liver resection (n = 35) | RFA ± resection (n = 35) | P-value | |
|---|---|---|---|
| Patient characteristics | |||
| Mean age ± SD | 59.6 ± 12.2 | 64.2 ± 10.6 | 0.037 |
| Male sex | 20 (57.1%) | 20 (57.1%) | 1.000 |
| Extent of liver intervention | 0.549 a | ||
| ≥3 segments | 6 (17.1%) | 5 (14.3%) | |
| 1 or 2 segments | 6 (17.1%) | 6 (17.1%) | |
| Wedge resection | 23 (65.7%) | 15 (42.8%) | |
| Only RFA | - | 9 (25.7%) | |
| Type of colorectal surgery | 0.228 | ||
| Abdominoperineal resection | 8 (22.9%) | 8 (22.9%) | |
| Low anterior resection | 14 (40.0%) | 16 (45.7%) | |
| Colon | 13 (37.1%) | 11 (31.4%) | |
| Characteristics tumor | |||
| Low clinical risk score (0–2) [21] | 20 (57.1%) | 22 (62.9%) | 0.774 |
| Diam. CRLM in cm (median ± IQR) | 2.2 ± 2.0 | 1.9 ± 1.8 | 0.268 |
| Neoadjuvant chemotherapy | 24 (68.6%) | 25 (71.4%) | 1.000 |
| Primary tumor at rectal site | 23 (65.7%) | 24 (68.6%) | 1.000 |
| Bilobar disease | 6 (37.1%) | 23 (65.7%) | <0.001 |
Matching was performed based on the characteristics: type of colorectal surgery, major/minor liver surgery, clinical risk score, age and neoadjuvant chemotherapy.
a McNemar test for a 3x3 comparison of ‘>3 segments’ vs. ‘1 or 2 segments’ vs. ‘wedge/RFA’.
P values were calculated using the paired T test, McNemar test or Wilcoxon signed rank test.
Fig 2. Overall survival in matched patients: RFA ± resection vs. only resection.
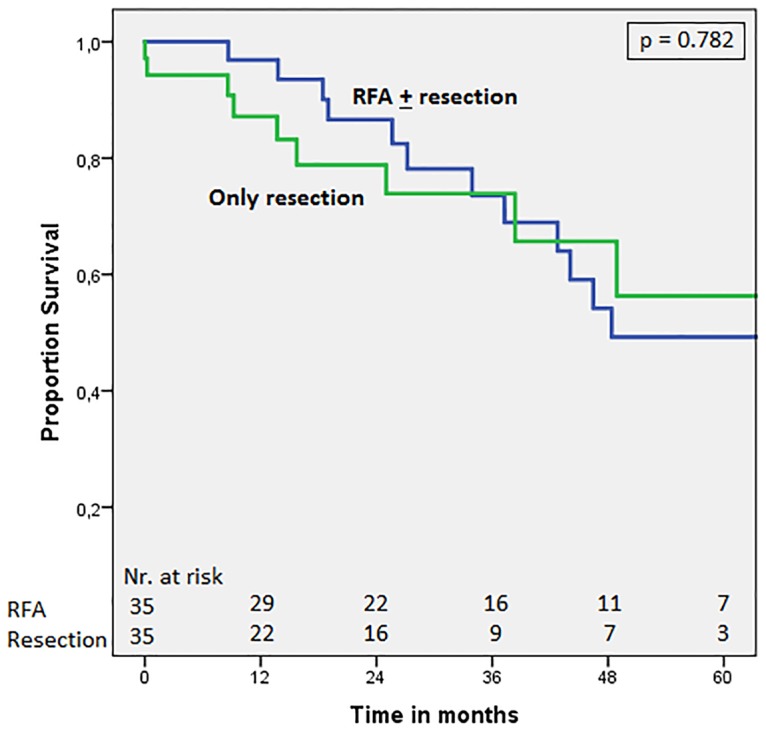
Comparison of patients with synchronous CRLM undergoing surgical treatment including RFA (RFA ± resection) or only liver resection. P-value = 0.782 (stratified log-rank test).
Fig 3. Disease-free survival in matched patients: RFA ± resection vs. only resection.
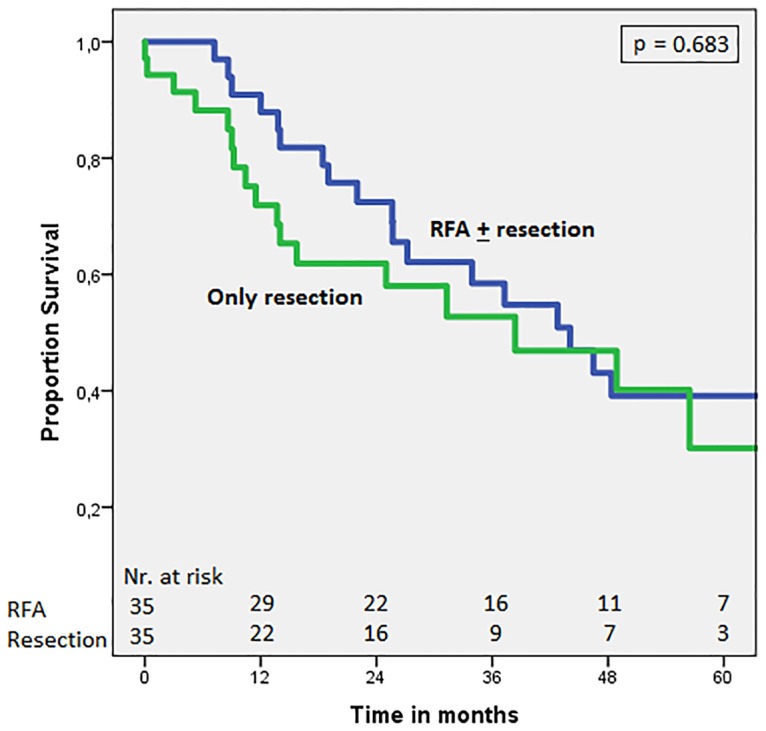
Comparison of patients with synchronous CRLM undergoing surgical treatment including RFA (RFA ± resection) or only liver resection. P-value = 0.683 (stratified log-rank test).
Discussion
The main finding of this study in patients with synchronous colorectal liver metastases who underwent simultaneous surgery of the primary tumor and its liver metastases, is that the CCI, as a comprehensive score of all occurring complications including their weighted severity, showed that RFA had a lower complication severity compared to liver resection only. Additionally, RFA treatment of the liver metastases—either alone or as an adjunct to liver resection- has a comparable overall survival and disease-free survival as liver resection alone. Of note, the surgical procedure performed for treatment of the primary tumor (APR) and a patient characteristic (age > 60) were associated with a higher complication rate. To corroborate findings as published by most other medical centers, we could also demonstrate in a matched analysis that patients undergoing simultaneous surgery had a similar overall survival compared to patients undergoing colorectal-first surgery. Of note, two out of nine centers in the review by Siriwardena et al. presented a worse survival for the patients undergoing simultaneous surgery compared to staged resection [24].
There is considerable variation in survival rates among studies comparing RFA with liver resection, both for the overall and the disease free survival [13,15,25–27]. In our study, almost half of patients undergoing simultaneous treatment were treated with RFA. Twenty-eight out of the 47 RFA-treated patients underwent RFA because of bilobar liver metastases. The other treatment option for bilobar CRLM is a two-stage hepatectomy, in which around 30% of the patients planned for a 2-stage hepatectomy actually never undergo the second procedure [28–30]. In a recent study with matched patients -matching based on oncological prognostic markers-, survival rate in a two-stage hepatectomy was compared to a one-stage hepatectomy [31]. In the latter patient group, parenchyma-sparing, ultrasound guided liver surgery was performed with complete tumor clearance in one procedure. The authors found that drop out (38.1%) in the two-stage group was not caused by selection of patients with oncological more aggressive tumors, but by the inability to obtain complete liver tumor clearance in one procedure. In parallel, we suggest that the use of RFA to obtain complete tumor clearance in one procedure in these patients will have the best chance on intentional curative treatment.
Progression of the CRLM has been observed after removal of the primary tumor, which suggests that simultaneous resection of both the colorectal primary and the liver tumor might yield better oncological outcome [32–34]. Two large multicentre studies [1,6] and our results show that the overall survival comparing colorectal-first and simultaneous strategies is similar. A recent study analyzed the effect of resection of the primary tumor on synchronous liver metastases with a multivariate model to predict progressive disease, in which the only adverse prognostic variable was undergoing an upfront primary colorectal resection [35].
In our study, the extent of liver surgery in simultaneous procedures did not substantially contribute to the rate and severity of complications. This favorable complication pattern may be due to selection of patients, since the combination of APR with major liver resection was scarce. Our results of a lower complication rate in RFA patients with simultaneous treatment is in concordance with a consensus statement on RFA treatment in general as compared to liver resection [12]. Concerning treatment of the primary colorectal cancer, we and others show both higher complication rates in patients undergoing APR, compared to both LAR and colon surgery [36–38].
A limitation of this study is its retrospective design. This study, however has its merits because it describes an observation of clinical decision-making. We realize that this study has limited power due to the number of patients, however, this is the largest study to date analyzing RFA in simultaneous resections.
In conclusion, patients who underwent RFA of liver metastases show similar oncological outcome and lower complication severity compared to liver resection in simultaneous treatment of both the primary colorectal carcinoma and the liver metastases. Hence, RFA is a useful treatment option in simultaneous resections.
Supporting information
All complications registered, stratified by both treatment (liver resection, resection + RFA and RFA) and by (anatomical) reason of complications (bowel-related, liver-related, general).
(DOCX)
Data Availability
This study was approved by the local Medical Ethical committee (METc2015/343), and was judged not to be within the scope of the Medical Research in Human Subjects Act (WMO). In the authors’ retrospective study concerning oncological disease, it is impossible to obtain written consent of all patients, since about 50% of patients had died of the disease at the time of writing the manuscript. The local ethics committee approved the authors’ method of including patients in their observational, retrospective study, provided that they obey the Dutch law regulations. As they cannot obtain patient consent due to the aforementioned reasons, to ensure patient confidentiality they cannot make the data publicly available. The data can be provided upon request to qualified researchers who conform to Dutch legislations regarding patient confidentiality. The principal investigator of this type of studies (retrospective, observational, and judged not to be within the scope of the Medical Research in Human Subjects Act (WMO)) is the primary contact for sending data requests. Contact information for requests for data: J. Bottema, research coordinator of the department of surgery at the University Medical Center Groningen (e-mail: j.t.bottema@umcg.nl).
Funding Statement
The authors received no funding for this work.
References
- 1.Mayo SC, Pulitano C, Marques H, Lamelas J, Wolfgang CL, de Saussure W, et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg. 2013;216(4):707–716. doi: 10.1016/j.jamcollsurg.2012.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mentha G, Roth AD, Terraz S, Giostra E, Gervaz P, Andres A, et al. “Liver first” approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg 2008;25(6):430–435. doi: 10.1159/000184734 [DOI] [PubMed] [Google Scholar]
- 3.Jegatheeswaran S, Mason JM, Hancock HC, Siriwardena AK. The liver-first approach to the management of colorectal cancer with synchronous hepatic metastases: a systematic review. JAMA Surg 2013;148(4):385–391. doi: 10.1001/jamasurg.2013.1216 [DOI] [PubMed] [Google Scholar]
- 4.Muangkaew P, Cho JY, Han H-S, Yoon Y-S, Choi Y, Jang JY, et al. Outcomes of simultaneous major liver resection and colorectal surgery for colorectal liver metastases. J Gastrointest Surg 2016;20(3):554–563. doi: 10.1007/s11605-015-2979-9 [DOI] [PubMed] [Google Scholar]
- 5.Fukami Y, Kaneoka Y, Maeda A, Takayama Y, Onoe S, Isogai M. Simultaneous resection for colorectal cancer and synchronous liver metastases. Surg Today 2016;46(2):176–182. doi: 10.1007/s00595-015-1188-1 [DOI] [PubMed] [Google Scholar]
- 6.Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol 2007;14(12):3481–3491. doi: 10.1245/s10434-007-9522-5 [DOI] [PubMed] [Google Scholar]
- 7.Feng Q, Wei Y, Zhu D, Ye L, Lin Q, Li W, et al. Timing of hepatectomy for resectable synchronous colorectal liver metastases: for whom simultaneous resection is more suitable—a meta-analysis. PLoS One. 2014. August 5;9(8):e104348 doi: 10.1371/journal.pone.0104348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanji S, Mackillop WJ, Wei X, Booth CM. Simultaneous resection of primary colorectal cancer and synchronous liver metastases: a population-based study. Can J Surg 2017;60(2):122–128. doi: 10.1503/cjs.008516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzeng CW, Cooper AB, Vauthey JN, Curley SA A. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB 2014;16(5):459–468. doi: 10.1111/hpb.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato M, Tateishi R, Yasunaga H, Horiguchi H, Yoshida H, Matsuda S, et al. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: a national survey of 54,145 patients. J Gastroenterol 2012;47(10):1125–1133. doi: 10.1007/s00535-012-0569-0 [DOI] [PubMed] [Google Scholar]
- 12.Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol 2015. December;25(12):3438–3454. doi: 10.1007/s00330-015-3779-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karanicolas PJ, Jarnagin WR, Gonen M, Tuorto S, Allen PJ, DeMatteo RP, et al. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surg 2013;148(7):597–601. doi: 10.1001/jamasurg.2013.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hof J, Wertenbroek MWJLAE, Peeters PMJG, Widder J, Sieders E, de Jong KP. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg 2016;103(8):1055–1062. doi: 10.1002/bjs.10162 [DOI] [PubMed] [Google Scholar]
- 15.Eltawil KM, Boame N, Mimeault R, Shabana W, Balaa FK, Jonker DJ, et al. Patterns of recurrence following selective intraoperative radiofrequency ablation as an adjunct to hepatic resection for colorectal liver metastases. J Surg Oncol 2014;110(6):734–738. doi: 10.1002/jso.23689 [DOI] [PubMed] [Google Scholar]
- 16.Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev 2015;41(9):729–741. doi: 10.1016/j.ctrv.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 17.van Dijk TH, Tamas K, Beukema JC, Beets GL, Gelderblom AJ, de Jong KP, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol 2013;24(7):1762–1769. doi: 10.1093/annonc/mdt124 [DOI] [PubMed] [Google Scholar]
- 18.Bisschop C, van Dijk TH, Beukema JC, Jansen RLH, Gelderblom H, de Jong KP, et al. Short-Course Radiotherapy Followed by Neoadjuvant Bevacizumab, Capecitabine, and Oxaliplatin and Subsequent Radical Treatment in Primary Stage IV Rectal Cancer: Long-Term Results of a Phase II Study. Ann Surg Oncol 2017. Forthcoming. doi: 10.1245/s10434-017-5897-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kele PG, Van der Jagt EJ, Krabbe PFM, de Jong KP. Lack of anatomical concordance between preablation and postablation CT images: a risk factor related to ablation site recurrence. Int J Hepatol 2012;2012:870306 doi: 10.1155/2012/870306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien P-A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258(1):1–7. doi: 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 21.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- 23.Ho Daniel E., Imai Kosuke, King Gary S EA. MatchIt: Nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. [Google Scholar]
- 24.Siriwardena AK, Mason JM, Mullamitha S, Hancock HC, Jegatheeswaran S. Management of colorectal cancer presenting with synchronous liver metastases. Nat Rev Clin Oncol 2014;11(8):446–459. doi: 10.1038/nrclinonc.2014.90 [DOI] [PubMed] [Google Scholar]
- 25.Minami Y, Kudo M. Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver 2013;7(1):1–6. doi: 10.5009/gnl.2013.7.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265(3):958–968. doi: 10.1148/radiol.12111851 [DOI] [PubMed] [Google Scholar]
- 27.Weng M, Zhang Y, Zhou D, Yang Y, Tang Z, Zhao M, et al. Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta-analysis. PLoS One 2012;7(9):e45493 doi: 10.1371/journal.pone.0045493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam VWT, Laurence JM, Johnston E, Hollands MJ, Pleass HCC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford) 2013;15(7):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faitot F, Faron M, Adam R, Elias D, Cimino M, Cherqui D, et al. Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases: a case-matched analysis of surgical and oncological outcomes. Ann Surg 2014;260(5):822–828. doi: 10.1097/SLA.0000000000000976 [DOI] [PubMed] [Google Scholar]
- 30.Abbott DE, Sohn VY, Hanseman D, Curley SA. Cost-effectiveness of simultaneous resection and RFA versus 2-stage hepatectomy for bilobar colorectal liver metastases. J Surg Oncol 2014;109(6):516–520. doi: 10.1002/jso.23539 [DOI] [PubMed] [Google Scholar]
- 31.Viganò L, Torzilli G, Cimino M, Imai K, Vibert E, Donadon M, et al. Drop-out between the two liver resections of two-stage hepatectomy. Patient selection or loss of chance? Eur J Surg Onco 2016;42(9):1385–1393. [DOI] [PubMed] [Google Scholar]
- 32.Peeters CFJM, de Waal RMW, Wobbes T, Westphal JR, Ruers TJM. Outgrowth of human liver metastases after resection of the primary colorectal tumor: a shift in the balance between apoptosis and proliferation. Int J cancer 2006;119(6):1249–1253. doi: 10.1002/ijc.21928 [DOI] [PubMed] [Google Scholar]
- 33.Scheer MGW, Stollman TH, Vogel W V, Boerman OC, Oyen WJG, Ruers TJM. Increased metabolic activity of indolent liver metastases after resection of a primary colorectal tumor. J Nucl Med 2008;49(6):887–891. doi: 10.2967/jnumed.107.048371 [DOI] [PubMed] [Google Scholar]
- 34.van der Wal GE, Gouw ASH, Kamps JAAM, Moorlag HE, Bulthuis MLC, Molema G, et al. Angiogenesis in synchronous and metachronous colorectal liver metastases: the liver as a permissive soil. Ann Surg. 2012;255(1):86–94. doi: 10.1097/SLA.0b013e318238346a [DOI] [PubMed] [Google Scholar]
- 35.Slesser AA, Khan F, Chau I, Khan AZ, Mudan S, Tekkis PP, et al. The effect of a primary tumour resection on the progression of synchronous colorectal liver metastases: an exploratory study. Eur J Surg Oncol. 2015;41(4):484–492. doi: 10.1016/j.ejso.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 36.Ciga Lozano MÁ, Codina Cazador A, Ortiz Hurtado H. Oncological results according to type of resection for rectal cancer. Cir Esp. 2015;93(4):229–235. doi: 10.1016/j.ciresp.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 37.Wang X-T, Li D-G, Li L, Kong F-B, Pang L-M, Mai W. Meta-analysis of oncological outcome after abdominoperineal resection or low anterior resection for lower rectal cancer. Pathol Oncol Res 2015;21(1):19–27. doi: 10.1007/s12253-014-9863-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shubert CR, Habermann EB, Bergquist JR, Thiels CA, Thomsen KM, Kremers WK, et al. A NSQIP review of major morbidity and mortality of synchronous liver resection for colorectal metastasis stratified by extent of liver resection and type of colorectal resection. J Gastrointest Surg 2015;19(11):1982–1994. doi: 10.1007/s11605-015-2895-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All complications registered, stratified by both treatment (liver resection, resection + RFA and RFA) and by (anatomical) reason of complications (bowel-related, liver-related, general).
(DOCX)
Data Availability Statement
This study was approved by the local Medical Ethical committee (METc2015/343), and was judged not to be within the scope of the Medical Research in Human Subjects Act (WMO). In the authors’ retrospective study concerning oncological disease, it is impossible to obtain written consent of all patients, since about 50% of patients had died of the disease at the time of writing the manuscript. The local ethics committee approved the authors’ method of including patients in their observational, retrospective study, provided that they obey the Dutch law regulations. As they cannot obtain patient consent due to the aforementioned reasons, to ensure patient confidentiality they cannot make the data publicly available. The data can be provided upon request to qualified researchers who conform to Dutch legislations regarding patient confidentiality. The principal investigator of this type of studies (retrospective, observational, and judged not to be within the scope of the Medical Research in Human Subjects Act (WMO)) is the primary contact for sending data requests. Contact information for requests for data: J. Bottema, research coordinator of the department of surgery at the University Medical Center Groningen (e-mail: j.t.bottema@umcg.nl).