Abstract
Background
Prior epidemiological evidences suggest that hepatitis B virus (HBV) infection is linked to cancers other than hepatocellular carcinoma. This prospective hospital registry-based case-control study aimed to investigate the sero-epidemiological association between chronic HBV infection and various types of cancer.
Methods
95,034 patients with first-diagnosed non-hepatocellular malignancy in a tertiary hospital between 2007 and 2014; and 118,891 non-cancer individuals as controls from a health promotion center were included. Cases and controls were compared for HBV surface antigen (HBsAg) positivity by conditional regression with adjustment for age, hypertension, diabetes, body mass index, alcohol consumption, smoking status and cholesterol level in both genders.
Results
An analysis of matched data indicated significant associations of HBV infection with lymphoma (adjusted odds ratio[AOR] 1.53 [95% CI 1.12–2.09] in men and 3.04 [1.92–4.82] in women) and biliary cancer (2.59[1.98–3.39] in men and 1.71[1.16–2.51] in women). Cervical (1.49[1.11–2.00]), uterine (1.69[1.09–2.61]), breast (1.16[1.02–1.32]), thyroid (1.49[1.28–1.74]), and lung cancers (1.79[1.32–2.44]) in women; and skin cancer (5.33[1.55–18.30]) in men were also significantly related to HBV infection.
Conclusions
Chronic HBV infection is associated with several malignant disorders including lymphoma, and biliary, cervical, uterine, breast, thyroid, lung, and skin cancers. Our findings may offer additional insights into the development of these neoplasms and may suggest the need to consider HBV screening in cancer patients and cancer surveillance in HBV-infected subjects.
Introduction
Infection with hepatitis B virus (HBV) is a major global public health challenge with a broad spectrum of clinical manifestations, and approximately 240 million individuals are infected with this virus.[1] HBV displays a substantial hepatic tissue tropism, and can induce chronic liver damage and subsequent hepatocarcinogenesis. [2, 3]
In addition to a potential to produce cholangiocarcinomas, prior epidemiological reports have suggested that chronic HBV infection also increases the risk of extra-hepatic malignancies such as non-Hodgkin lymphoma (NHL), pancreatic cancer, and gastric cancer. [4–7] Such associations could be explained by several biological effects of HBV: 1) As observed in autopsies, HBV may move systemically through the bloodstream and infect non-hepatic tissues including lymph nodes, kidney, pancreas, and gastrointestinal tract [8–10]; 2) given the existence of viral DNA and antigens in organ sites other than liver, HBV may replicate within such extra-hepatic reservoirs and play oncogenic roles [11–16]; 3) HBV may target organs adjacent to the liver, such as pancreas and stomach, which share common vascular and bile duct systems [17]; and 4) chronic immune stimulation of B-lymphocytes associated with persistent hepatic infection may be directly involved in lymphomagenesis.[18]
To date, most of studies of the linkage between HBV infection and extra-hepatic cancers have involved population-based cohorts and case-control series with only a limited number of types of malignancy.[4, 5, 7, 19, 20] Moreover, per-cancer observations derived from these studies were not consistent across publications, because of differences between the studied populations in terms of prevalence of HBV, screening practices, and monitoring duration, together with differences of ethnicity and exposure to carcinogens.[21, 22] The information obtained could point to a clinical need for periodic surveillance of HBV carriers for cancers at specific sites or of particular types, as already occurs globally in the case of hepatocellular carcinoma (HCC), and provide insight into the development of various malignancies.
Considering the potential benefit of the relevant findings, we aimed to investigate the sero-epidemiological connections between infection with HBV and various malignancies other than HCC, using data from an ongoing prospective registry of cancer patients, along with control data for a non-cancerous population in a health care service.
Patients and methods
Registry-based cancer and non-cancer populations
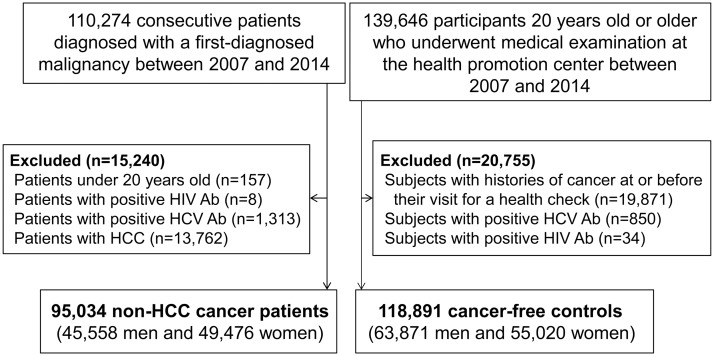
In this retrospective registry-based case-control study, we identified 110,274 consecutive patients diagnosed with a first malignancy in a prospectively-constructed hospital-based cancer registry of Asan Medical Center between January 2007 and December 2014, as shown in Fig 1. This cohort registry was part of the National Cancer Registration Program initiated by the Ministry of Health and Welfare, Republic of Korea in 1980, and has continued to collect information on patient demographics including sex, age, date of diagnosis, cancer site, morphology, and pathological tumor type. Details of the cancer registry in our center, the largest tertiary referral hospital in Seoul, Korea, have been given elsewhere.[23] Of the consecutive patients, 15,240 were excluded for the following reasons: those under 20 years old (n = 157); those with human immunodeficiency virus antibody (n = 8) or hepatitis C antibody (n = 1,313); and those diagnosed with HCC, a well-established consequence of chronic hepatitis B (n = 13,762).[2] Finally, 95,034 new cancer patients (45,558 men and 49,476 women) were included in the main analyses of this study. All cases of malignancy were diagnosed based on histological confirmation of tissue or bone marrow. The diagnostic and staging work-ups for each patient were determined according to the standard protocol for the specific tumor type mainly based on guidelines recommended by the National Comprehensive Cancer Network (www.nccn.org).
Fig 1. Flow diagram of the study.
To obtain controls as nearly as possible representative of the healthy population we used 139,646 subjects 20 years old or older who underwent medical examination at the health promotion center of our institution over the period of the study. Individuals with histories of cancer at or before their visit for a health check were excluded (n = 19,871) on the basis of assessment by self-questionnaire during the health checkup, hospital records, and data of the Korea National Cancer Registry and Korean National Health Insurance Service database, which have been validated as useful resources for research.[24] Subjects with human immunodeficiency virus antibody (n = 34) or hepatitis C antibody (n = 850) were also excluded. Altogether 118,891 individuals (63,871 men and 55,020 women) were selected.
The study was undertaken with the approval of the Institutional Review Board of Asan Medical Center (IRB No.2015-0513) and in accordance with the Declaration of Helsinki (1989) of the World Medical Association. The need for informed consent from the cancer patients was waived. According to the regulations of the National Health Insurance Scheme, which covers almost the entire population of Korea, it is mandatory for cancer patients to provide information to the cancer registry without specific agreement. In the case of the control participants, written informed consent for the use of anonymized personal data for research purposes was routinely obtained prior to the health screening. In this context, informed consent for this retrospective study was waived.
Collection of cancer status data
The cancer registry data of our center contains demographic and clinical information including age, sex, time of diagnosis, cancer classification, and histologic type of cancer for all cases of cancer [23]. Using the International Classification of Diseases codes (version 10), we classified all malignant neoplasms as one or other of the following: head and neck (00–14, 30–32), esophagus (15), stomach (16), colon (18–19), rectum (20), gallbladder (23), intra- and extra-hepatic bile duct (22, 24), pancreas (25), lung (34), bone and soft tissue (40–41, 49), skin (43–44), breast (50), cervix (53), uterus (54–55), ovary (56), prostate (61), kidney (64–65), bladder (67), brain (71), thyroid (73), lymphoma (81–89), and hematologic malignancies including leukemia and multiple myeloma (90–96).
The following data for each patient were obtained from complete inpatient and outpatient medical records using the clinical database system of our institution (Asan BiomedicaL Research Environment, ABLE): presence of hypertension and diabetes, history of alcohol consumption and smoking, body mass index (BMI), and total cholesterol. The controls completed a health questionnaire at baseline that included information about daily alcohol and smoking consumption. Routine measurement of BMI and total cholesterol were carried out as part of the health check-up.
Test for hepatitis B virus infection
Every cancer patient and individual attending for a health checkup in our hospital undergoes a blood test for HBV infection as part of the routine examination on the first visit. Because most HBV infections in Korea occur during the perinatal period or in early childhood, one measurement indicating hepatitis B surface antigen (HBsAg) positivity is regarded as reflecting chronic HBV infection.[25] Serum HBsAg was measured using the Architect assay (Abbott Laboratories, Chicago, IL, USA) or an immunoradiometric assay kit (DiaSorin, Vercelli, Italy).
Statistical analysis
To examine the sero-epidemiological connections between infection with HBV and various types of cancer, we employed individual case-control matching with well-known confounders for cancer development.[26] That is, each patient in the case group was randomly matched with an individual in the control group provided they had the same profile in terms of smoking, hypertension, diabetes, and alcohol drinking, and only small differences in age (<3 year), BMI (<3 kg/m2) and cholesterol (<10 mg/dL). To analyze this matched data, we used the conditional logistic regression model. In addition, we conducted individual analyses of tumor subtypes for cancers that were found to be significantly associated with HBV infection. In the matched cohorts, the control group was regarded as the reference, and the odds ratios (ORs) together with 95% confidence intervals (CIs) are presented for each cancer subtypes. Univariate and multivariate multinomial logit models were fitted for significant cancer subtypes.
All statistical analyses were conducted using R software version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/). In particular, the R packages for ‘survival’ [1] and ‘nnet’ [2] were used for the conditional logistic model and multinomial logit model fitting.[27, 28] All statistical inferences were two-sided, and a P-value less than 0.05 was considered statistically significant.
Results
Characteristics of the study and control populations
Table 1 shows the baseline characteristics of the cancer and control subjects. The median age of the cancer patients was 65 years (interquartile range [IQR] 56–73 years) for men and 56 years (IQR 47–66 years) for women. There were substantial differences between cases and controls in terms of age, smoking habits, alcohol consumption, BMI and total cholesterol level: men and women with neoplasms were more likely to be older, more hypertensive and diabetic than those without cancer; current smoking and alcohol use were more frequent in the control group than in the cancer patients; and the men with cancer had lower BMIs and lower levels of serum total cholesterol than the controls, while the women with cancer had higher BMIs. Women in both groups were less likely than men to be current or former smokers, and to consume any alcohol.
Table 1. Characteristics of the study populations.
| Pooled cohort | ||||
| Variable | Men | Women | ||
|
Cases (n = 45,558) |
Controls (n = 63,871) |
Cases (n = 49,476) |
Controls (n = 55,020) |
|
| Age, years* | 65 (56–73) | 53 (46–60) | 56 (47–66) | 52 (44–59) |
| Hypertension | 15,189 (33.3%) | 12,155 (19.0%) | 11,123 (22.3%) | 6,447 (11.7%) |
| Diabetes | 7,686 (16.9%) | 5,199 (8.1%) | 4,291 (8.7%) | 2,092 (3.8%) |
| Any alcohol use | 16,540 (36.3%) | 40,791 (63.9%) | 6,051 (12.2%) | 10,510 (19.1%) |
| Smoking status | ||||
| Never | 13,583 (29.8%) | 15,002 (23.5%) | 46,135 (93.2%) | 50,599 (92.0%) |
| Former | 22,070 (48.4%) | 24,761 (38.8%) | 2,171 (4.4%) | 2,201 (4.0%) |
| Current | 9,905 (21.7%) | 24,108 (37.7%) | 1,170 (2.4%) | 2,220 (4.0%) |
| Body mass index, kg/m2* | 23.8 (21.8–25.8) | 24.3 (22.6–26.1) | 23.2 (21.1–25.5) | 22.0 (20.2–24.1) |
| Serum Cholesterol, mg/dL* | 166 (139–193) | 190 (168–214) | 181 (156–207) | 188 (166–212) |
| Matched cohort | ||||
| Variable | Men | Women | ||
|
Matched cases (n = 39,414) |
Matched controls (n = 39,414) |
Matched cases (n = 46,330) |
Matched controls (n = 46,330) |
|
| Age, years* | 63 (55–71) | 63 (55–70) | 55 (46–64) | 55 (46–64) |
| Hypertension | 12,419 (31.5%) | 12,419 (31.5%) | 9595 (20.7%) | 9595 (20.7%) |
| Diabetes | 5779 (14.7%) | 5779 (14.7%) | 3293 (7.1%) | 3293 (7.1%) |
| Any alcohol use | 14,890 (37.8%) | 14,890 (37.8%) | 5555 (12.0%) | 5555 (12.0%) |
| Smoking status | ||||
| Never | 11,251 (28.5%) | 11,251 (28.5%) | 44333 (95.7%) | 44333 (95.7%) |
| Former | 19506 (49.5%) | 19506 (49.5%) | 1166 (2.5%) | 1166 (2.5%) |
| Current | 8657 (22.0%) | 8657 (22.0%) | 831 (1.8%) | 831 (1.8%) |
| Body mass index, kg/m2* | 23.9 (22.0–25.8) | 24.0 (22.3–25.6) | 23.1 (21.1–25.4) | 22.8 (21.0–25.0) |
| Serum Cholesterol, mg/dL* | 170 (147–195) | 171 (148–195) | 183 (159–208) | 183 (160–208) |
* Median (interquartile range)
Among the 45,558 men with cancer included in the study, stomach (n = 10,977), lung (n = 5,756), and colon (n = 4,101) cancers were the most common malignancies. Breast (n = 12,487), thyroid (n = 9,815) and stomach (n = 5,373) cancers were most frequent among the 49,476 women with malignancies. These results resemble the cancer incidence data in the national population-based cancer registry in Korea.[29]
In terms of matched set, baseline characteristics of the matched cohort, which included 85,744 subjects with malignancy and 85,744 healthy controls (1:1 ratio), are presented in Table 1. The distribution of common risk factors for the various cancers was comparable in the matched cohort.
Association between hepatitis B virus infection and site-specific cancers
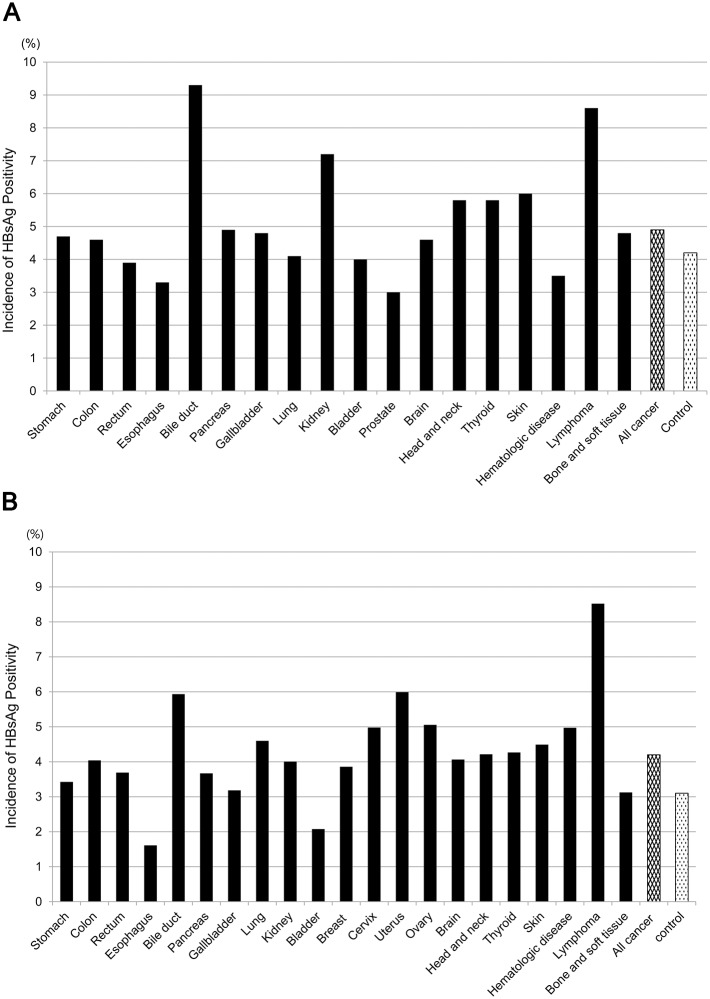
With regard to HBV-infected status, we identified 4,328 HBsAg-positive cancer patients (4.6%) of which 2,237 were men (4.9%) and 2,091 were women (4.2%). The sero-prevalence rate was significantly lower in the controls, with 4,401 HBsAg-positive subjects (3.7%) made up of 2,670 men (4.2%) and 1,731 women (3.1%) (Ps<0.001 for both gender). Fig 2A and 2B give the prevalence of HBsAg positivity in the cancer population according to specific tumor site. Positive HBsAg was relatively frequent in biliary cancer and lymphoma in both males (9.3% and 8.6%, respectively) and females (5.9%; and 8.5%, respectively), and the same was true for renal cancers in men (7.2%) and uterine cancers in women (6.0%).
Fig 2. Prevalence of HBV infection by gender and primary tumor site among cancer patients and non-cancer subjects.
HBsAg positivity was more frequent in cancer cases than in controls for both genders (4.9% vs. 4.2% for men; and 4.2% vs. 3.1% for women, both Ps<0.001) (A) Male patients with bile duct cancer (9.3%), lymphoma (8.6%), and kidney cancer (7.2%) were more frequently infected with HBV than controls. (B) In women with malignances, positive HBsAg was more frequently seen in patients with lymphoma (8.5%), uterine cancer (6.0%), and biliary cancer (5.9%).
In men, bile duct cancer (adjusted odds ratio [AOR] 2.59, 95% CI 1.98–3.39, P<0.001), lymphoma (AOR 1.53, 95% CI 1.12–2.09, P = 0.007), and dermatologic cancer (AOR 5.33, 95% CI 1.55–18.30, P = 0.008) were significantly correlated with HBV status, as shown in Table 2. In women, both biliary cancer (AOR 1.71, 95% CI 1.16–2.51, P = 0.007) and lymphoma (AOR 3.04, 95% CI 1.92–4.82, P<0.001) were significantly linked to HBV infection in the exact matching model with adjustment of cancer risk factors, which was consistent with the male data (Table 3). In terms of female-specific cancers, additional matching analyses yielded significant associations for breast (AOR 1.16, 95% CI 1.02–1.32, P = 0.033), cervical (AOR 1.49, 95% CI 1.11–2.00, P = 0.007), and uterine cancers (AOR 1.69, 95% CI 1.09–2.61, P = 0.019). HBsAg status was significantly correlated with lung (AOR 1.79, 95% CI 1.32–2.44, P<0.001) and thyroid cancers (AOR 1.49, 95% CI 1.28–1.74, P<0.001) in the matched women’s cohort, but not in the matched men’s cohort (AOR 0.91, 95% CI 0.75–1.11, P = 0.370 for lung cancer; and AOR 1.24, 95% CI 0.96–1.60, P = 0.104 for thyroid cancer).
Table 2. Relationship between hepatitis B virus infection and site-specific cancers in men.
| Cancer Site | Number of patients in the pooled cohort | Matched analysis* | |||
|---|---|---|---|---|---|
| Total | HBsAg+ | OR | 95% CI | P value | |
| Stomach | 10,977 | 519 | 1.03 | 0.90–1.17 | 0.709 |
| Colon | 4,101 | 189 | 1.13 | 0.90–1.42 | 0.275 |
| Rectum | 3,293 | 127 | 0.82 | 0.63–1.05 | 0.120 |
| Esophagus | 1,089 | 36 | 1.00 | 0.62–1.62 | 1.000 |
| Bile duct | 2,622 | 245 | 2.59 | 1.98–3.39 | <0.001 |
| Pancreas | 2,123 | 105 | 1.17 | 0.84–1.62 | 0.358 |
| Gallbladder | 545 | 26 | 0.94 | 0.48–1.86 | 0.862 |
| Lung | 5,756 | 236 | 0.91 | 0.75–1.11 | 0.370 |
| Kidney | 1,595 | 115 | 1.16 | 0.85–1.59 | 0.343 |
| Bladder | 1,273 | 51 | 0.96 | 0.63–1.45 | 0.833 |
| Prostate | 3,920 | 116 | 0.62 | 0.47–0.81 | <0.001 |
| Brain | 1,586 | 73 | 0.90 | 0.65–1.26 | 0.553 |
| Head and neck | 1,323 | 77 | 1.33 | 0.94–1.88 | 0.113 |
| Thyroid | 2,510 | 146 | 1.24 | 0.96–1.60 | 0.104 |
| Skin | 282 | 17 | 5.33 | 1.55–18.30 | 0.008 |
| Hematologic disease | 1,009 | 35 | 0.46 | 0.29–0.74 | 0.002 |
| Lymphoma | 1,303 | 112 | 1.53 | 1.12–2.09 | 0.007 |
| Bone and soft tissue | 251 | 12 | 1.38 | 0.55–3.42 | 0.493 |
CI = confidence interval; HBsAg = hepatitis B virus surface antigen; OR = odds ratio.
* Controls were matched to cases by age, hypertension, diabetes, alcohol consumption, smoking status, and serum cholesterol level.
Table 3. Relationship between hepatitis B virus infection and site-specific cancers in women.
| Cancer Site | Number of patients in the pooled cohort | Matched analysis* | |||
|---|---|---|---|---|---|
| Total | HBsAg+ | OR | 95% CI | P value | |
| Stomach | 5,373 | 184 | 0.98 | 0.79–1.21 | 0.825 |
| Colon | 2,921 | 118 | 1.15 | 0.87–1.52 | 0.320 |
| Rectum | 1,842 | 68 | 1.29 | 0.89–1.88 | 0.183 |
| Bile duct | 1,584 | 94 | 1.71 | 1.16–2.51 | 0.007 |
| Pancreas | 1,499 | 55 | 0.93 | 0.60–1.44 | 0.739 |
| Esophagus | 62 | 1 | 0.50 | 0.05–5.51 | 0.571 |
| Gallbladder | 691 | 22 | 1.06 | 0.55–2.05 | 0.866 |
| Lung | 2,653 | 122 | 1.79 | 1.32–2.44 | <0.001 |
| Kidney | 674 | 27 | 1.16 | 0.63–2.14 | 0.640 |
| Bladder | 289 | 6 | 1.20 | 0.37–3.93 | 0.763 |
| Breast | 12,487 | 482 | 1.16 | 1.02–1.32 | 0.033 |
| Cervix | 2,370 | 118 | 1.49 | 1.11–2.00 | 0.007 |
| Uterus | 1,102 | 66 | 1.69 | 1.09–2.61 | 0.019 |
| Ovary | 1,048 | 53 | 0.67 | 0.34–1.31 | 0.240 |
| Brain | 2,484 | 101 | 1.09 | 0.82–1.47 | 0.549 |
| Head and neck | 427 | 18 | 1.33 | 0.63–2.82 | 0.451 |
| Thyroid | 9,815 | 419 | 1.49 | 1.28–1.74 | <0.001 |
| Skin | 245 | 11 | 1.83 | 0.68–4.96 | 0.232 |
| Hematologic disease | 744 | 37 | 1.26 | 0.73–2.18 | 0.406 |
| Lymphoma | 974 | 83 | 3.04 | 1.92–4.82 | <0.001 |
| Bone and soft tissue | 192 | 6 | 2.00 | 0.50–8.00 | 0.327 |
CI = confidence interval; HBsAg = hepatitis B virus surface antigen; OR = odds ratio.
*Controls were matched to cases by age, hypertension, diabetes, alcohol consumption, smoking status, and serum cholesterol level.
Subtype analyses of HBV-related cancers
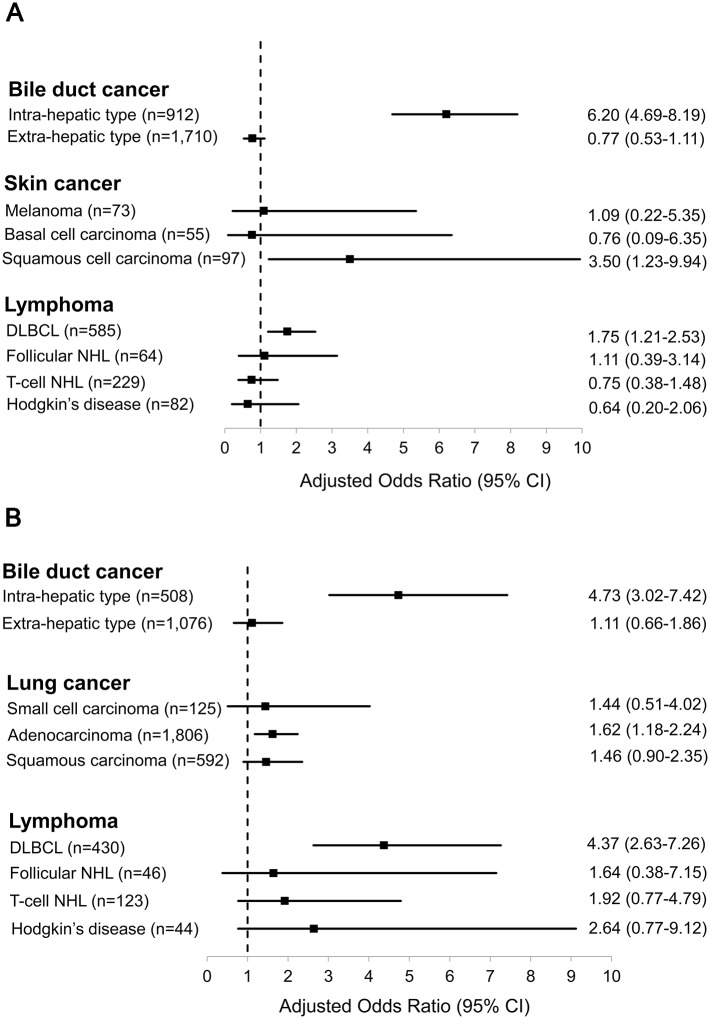
We next performed subgroup matched analyses for the individual cancer types identified as having significant associations with HBV infection by the conditional logistic regression method (Fig 3). For bile duct cancer, intra-hepatic cholangiocarcinoma (IHCCC), not extra-hepatic cholangiocarcinoma, was significant correlated with hepatitis B surface antigenemia in both genders (AOR 6.20, 95% CI 4.69–8.19, P<0.001 for men; and AOR 4.73, 95% CI 3.02–7.42, P<0.001 for women). In analyses based on the prevalent histological sub-types of the other HBV-related malignancies, there were significant positive links between HBV infection and diffuse large B cell lymphoma (DLBCL) (AOR 1.75, 95% CI 1.21–2.53, P = 0.003 for men; and AOR 4.37, 95% CI 2.63–7.26, P<0.001 for women); squamous cell carcinoma for skin malignancies in men (AOR 3.50, 95% CI 1.23–9.94, P = 0.019); and adenocarcinoma for lung cancer in women (AOR 1.62, 95% CI 1.18–2.24, P = 0.003). No significant correlation was observed for the remaining subtypes of the relevant cancers.
Fig 3. Subtype analyses of individual HBV-related cancers identified by the exact matching method in our series.
(A) In men, the intra-hepatic type of bile duct cancer, DLBCL, and skin squamous cell carcinoma were significantly association with HBV infection (all Ps<0.05). (B) In women, lung adenocarcinoma as well as DLBCL and intra-hepatic cholangiocarcinoma were correlated with positive HBsAg (all Ps<0.05). No significant relationships were noted for other major sub-classification of the relevant cancers.
Discussion
Although HBV is mainly thought to be hepatotrophic and hepatocarcinogenic,[30] the current analysis of matched data for a broad spectrum of cancers in a prospective cancer registry demonstrates that chronically-infected HBV status is closely associated with a variety of non-HCC malignancies such as lymphoma and biliary cancer, regardless of patient’s gender; cervical, uterine, breast, thyroid and lung cancers in females; and skin cancers in males.
Although one case-control study conducted in the U.S. with low HBV prevalence showed no associations,[31] several previous studies have suggested that HBV may play a causal role in the development of lymphoma, especially DLBCL, which is the most common and aggressive type of NHL.[5, 32] Our analysis also revealed that, of the various lymphoma subclasses, DLBCL had the strongest linkage with positive HBsAg (AOR 1.75 for men and 4.37 for women). Although little is known of how HBV may generate tumors, possible routes may be inferred from the known mechanisms by which HCV produces lymphomas, such as chronic B-cell proliferation in response to antigenic stimulation, high-affinity interaction between viral envelope and B-cell receptors and direct viral infection of B cells.[18, 33]
In our series, hepatitis B surface antigenemia increased the risk of IHCCC ---but not of extra-hepatic biliary neoplasms ----by 6.2- and 4.7- fold for men and women, respectively. Similar findings have often been reported in other population-based and case-control studies in different regions.[6, 32, 34] Active HBV replication and viral integration into the human genome within IHCCC tumors may provide the basis of the effect, since molecular genetic studies have demonstrated the presence of HBV DNA in IHCCC tissue specimens.[35, 36] Recent experiments also suggest that because progenitor cells can differentiate into human hepatocytes and cholangiocytes, HBV may induce cholangiocytic carcinogenesis by the same mechanism as it does for hepatocyte carcinogenesis.[34, 37] In addition, bile duct damage and fibrosis mediated by HBV-inducing chronic liver disease may be a potential process leading to IHCCC.[34, 35]
In contrast with our observations in males and females, several cancerous conditions, including pancreatic and gastric cancers that are of widespread health interest, have been shown to be significantly associated with HBV positivity.[4, 16, 20, 38, 39] A study by Hassan and colleagues from an HBV non-endemic country reported a relationship of pancreatic cancer with prior hepatitis B infection and positive anti-hepatitis B virus core (HBc) antibody, rather than current infection and positive HBsAg.[4] Also, Taiwanese and Swedish population cohort studies and Chinese case-control studies found only marginal associations between pancreatic cancer and the presence of HBsAg.[16, 20, 39] On the other hand, in contrast to our cohort from Korea where gastric cancer ranked 2nd in incidence, Chinese samples with stomach cancer were more frequently related to current HBV infection, compared to individuals with benign gastric diseases or other hospitalized subjects.[7, 16] These discrepant features of the association between hepatitis B viremia and foregut malignancies may be due to differences in study design, populations investigated, the incidences of cancer or hepatitis, and periods when the observations were made.
Our investigation appears to be the first to find evidence that chronic HBV infection is correlated with the presence of gynecologic, endocrinologic, pulmonary, and dermatologic cancers, which were little studied in prior investigations of this kind. Although the underlying mechanisms have not been clearly identified, some biological phenomena may be relevant: 1) integration of both HBV and human papilloma virus (HPV) into the same sites in the human genome, as well as inappropriate responses of human leukocyte antigen and lymphocytes to the viruses;[40, 41] 2) association of prolonged viral hepatitis with chronic inflammatory impairment of other organs leading to chronic obstructive pulmonary disease, autoimmune thyroiditis, and lichen planus of the skin with malignant potential;[42–45] and 3) detection of HBV DNA and HBV surface antigens in skin tissue and breast milk.[10, 46, 47] In relation to HBV-associated lung cancer, the bloodstream may be a possible route for HBV transmission, given the blood-borne transmission of HPV suggested by the development of adenocarcinoma of the lung.[47] Although correlations were revealed in the present study, further basic and clinical investigations will be needed to establish whether chronic HBV infection plays a substantial oncogenic role on the development of the various malignancies.
Our findings suggest important clinical consequences. The key point raised by this study is the need for a risk-adapted strategy for serologic testing of hepatitis B in patients with potentially associated cancers in order to prevent accidental HBV reactivation and hopefully to delay tumor progression, although this effect has not yet been proven other than in HBV-related HCC. However, the current U.S. Preventive Services Task Force recommendations do not advocate screening for hepatitis B in any cancer patients.[48] Conversely, it may be desirable that possible target cancers, no doubt not restricted to HCC, be regularly included in surveillance programs of individuals with chronic hepatitis B according to their individual associations with HBV. The cost-effectiveness of the proposed policies must be established prior to actual clinical application.
Some limitations of our cross-sectional study should be considered. Time-dependent longitudinal observation studies, together with experimental evidence, are needed to clarify the causal relationship between viral infection and the subsequent development of specific cancers. Another consideration is the relationship between resolved HBV infection without presence of the surface antigen and malignant conditions, which was not examined in the present study. Indeed, over 65% of the Korean general population are sero-positive for anti-HBc,[25] and hence anti-HBc status is not routinely checked in general clinical practice. In addition, we did not fully take into account all specific cancer-related causal factors, such as Helicobacter pylori for gastric cancer and HPV for cervical cancer.[26] Future studies should be investigated to investigate the additional effect of hepatitis B virus on the specific pathogen-induced carcinogenesis. However, in our analyses we adjusted common risk factors of the various cancers as confounders (i.e., age, BMI, alcohol consumption, cigarette smoking, diabetes, hypertension, and serum cholesterol), more comprehensively than other relevant studies.[4, 5, 7, 16, 19–22] Lastly, since control subjects had voluntarily visited the hospital for a general health check-up, there is a potential for selection bias. The matched analysis using conditional logistic regression used in this study may reduce the possibility of the bias.
In conclusion, chronic and current infection with HBV may be positively associated with the presence of several non-hepatocellular malignancies, including cervical and uterine cancers and even breast, thyroid, lung and skin cancers that have not previously been studied in this context. These findings should provide helpful practical information relevant to both HBV screening in cancer patients and cancer surveillance in hepatitis B patients.
Supporting information
This file contains raw data about clinical variables of each patient in this study.
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Locarnini S, Hatzakis A, Chen DS, Lok A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J Hepatol. 2015; 62(1 Suppl): S76–86. doi: 10.1016/j.jhep.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 2.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002; 36: 1206–1213. doi: 10.1053/jhep.2002.36780 [DOI] [PubMed] [Google Scholar]
- 3.Yu MC, Tong MJ, Coursaget P, Ross RK, Govindarajan S, Henderson BE. Prevalence of hepatitis B and C viral markers in black and white patients with hepatocellular carcinoma in the United States. J Natl Cancer Inst. 1990; 82: 1038–1041. [DOI] [PubMed] [Google Scholar]
- 4.Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, et al. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008; 26: 4557–4562. doi: 10.1200/JCO.2008.17.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. 2010; 11: 827–834. doi: 10.1016/S1470-2045(10)70167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008; 103: 1716–1720. doi: 10.1111/j.1572-0241.2008.01796.x [DOI] [PubMed] [Google Scholar]
- 7.Wei XL, Qiu MZ, Jin Y, Huang YX, Wang RY, Chen WW, et al. Hepatitis B virus infection is associated with gastric cancer in China: an endemic area of both diseases. Br J Cancer. 2015; 112: 1283–1290. doi: 10.1038/bjc.2014.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowoslawski A, Krawczynski K, Brzosko WJ, Madalinski K. Tissue localization of Australia antigen immune complexes in acute and chronic hepatitis and liver cirrhosis. Am J Pathol. 1972; 68: 31–56. [PMC free article] [PubMed] [Google Scholar]
- 9.Trepo CG, Zucherman AJ, Bird RC, Prince AM. The role of circulating hepatitis B antigen/antibody immune complexes in the pathogenesis of vascular and hepatic manifestations in polyarteritis nodosa. J Clin Pathol. 1974; 27: 863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol. 1984; 65 Pt 3): 651–655. doi: 10.1099/0022-1317-65-3-651 [DOI] [PubMed] [Google Scholar]
- 11.Hoefs JC, Renner IG, Askhcavai M, Redeker AG. Hepatitis B surface antigen in pancreatic and biliary secretions. Gastroenterology. 1980; 79: 191–194. [PubMed] [Google Scholar]
- 12.Yoshimura M, Sakurai I, Shimoda T, Abe K, Okano T, Shikata T. Detection of HBsAg in the pancreas. Acta Pathol Jpn. 1981; 31: 711–717. [DOI] [PubMed] [Google Scholar]
- 13.Olivera-Martinez MA, Gallegos-Orozco JF. Recurrent viral liver disease (hepatitis B and C) after liver transplantation. Arch Med Res. 2007; 38: 691–701. doi: 10.1016/j.arcmed.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 14.Jilbert AR, Freiman JS, Gowans EJ, Holmes M, Cossart YE, Burrell CJ. Duck hepatitis B virus DNA in liver, spleen, and pancreas: analysis by in situ and Southern blot hybridization. Virology. 1987; 158: 330–338. [DOI] [PubMed] [Google Scholar]
- 15.Ogston CW, Schechter EM, Humes CA, Pranikoff MB. Extrahepatic replication of woodchuck hepatitis virus in chronic infection. Virology. 1989; 169: 9–14. [DOI] [PubMed] [Google Scholar]
- 16.Wei XL, Luo HY, Li CF, Jin Y, Zeng ZL, Ju HQ, et al. Hepatitis B virus infection is associated with younger median age at diagnosis and death in cancers. Int J Cancer. 2017. doi: 10.1002/ijc.30719 [DOI] [PubMed] [Google Scholar]
- 17.Blum HE, Stowring L, Figus A, Montgomery CK, Haase AT, Vyas GN. Detection of hepatitis B virus DNA in hepatocytes, bile duct epithelium, and vascular elements by in situ hybridization. Proc Natl Acad Sci U S A. 1983; 80: 6685–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011; 117: 1792–1798. doi: 10.1182/blood-2010-06-275818 [DOI] [PubMed] [Google Scholar]
- 19.Ulcickas Yood M, Quesenberry CP Jr., Guo D, Caldwell C, Wells K, Shan J, et al. Incidence of non-Hodgkin’s lymphoma among individuals with chronic hepatitis B virus infection. Hepatology. 2007; 46: 107–112. doi: 10.1002/hep.21642 [DOI] [PubMed] [Google Scholar]
- 20.Iloeje UH, Yang HI, Jen CL, Su J, Wang LY, You SL, et al. Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL-HBV cohort study. Liver Int. 2010; 30: 423–429. doi: 10.1111/j.1478-3231.2009.02147.x [DOI] [PubMed] [Google Scholar]
- 21.Amin J, Dore GJ, O’Connell DL, Bartlett M, Tracey E, Kaldor JM, et al. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol. 2006; 45: 197–203. doi: 10.1016/j.jhep.2006.02.014 [DOI] [PubMed] [Google Scholar]
- 22.Andersen ES, Omland LH, Jepsen P, Krarup H, Christensen PB, Obel N, et al. Risk of all-type cancer, hepatocellular carcinoma, non-Hodgkin lymphoma and pancreatic cancer in patients infected with hepatitis B virus. J Viral Hepat. 2015; 22: 828–834. doi: 10.1111/jvh.12391 [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Cho JH, Lyu Y, Lee SH, Hwang KH, Lee MS. Construction and validation of hospital-based cancer registry using various health records to detect patients with newly diagnosed cancer: experience at Asan Medical Center. J Prev Med Public Health. 2010; 43: 257–264. doi: 10.3961/jpmph.2010.43.3.257 [DOI] [PubMed] [Google Scholar]
- 24.Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev. 2012; 13: 6163–6168. [DOI] [PubMed] [Google Scholar]
- 25.Ahn YO. Strategy for vaccination against hepatitis B in areas with high endemicity: focus on Korea. Gut. 1996; 38 Suppl 2: S63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet. 2014; 383: 549–557. doi: 10.1016/S0140-6736(13)62224-2 [DOI] [PubMed] [Google Scholar]
- 27.Therneau T. A package for survival analysis in S version 2.38. 2015 [cited Jan 12 2017]. https://CRAN.R-project.org/package=survival.
- 28.Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth ed New York: Springer; 2002. [Google Scholar]
- 29.Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer. 2015; 137: 2060–2071. doi: 10.1002/ijc.29670 [DOI] [PubMed] [Google Scholar]
- 30.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006; 295: 65–73. doi: 10.1001/jama.295.1.65 [DOI] [PubMed] [Google Scholar]
- 31.Anderson LA, Pfeiffer R, Warren JL, Landgren O, Gadalla S, Berndt SI, et al. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev. 2008; 17: 3069–3075. doi: 10.1158/1055-9965.EPI-08-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fwu CW, Chien YC, You SL, Nelson KE, Kirk GD, Kuo HS, et al. Hepatitis B virus infection and risk of intrahepatic cholangiocarcinoma and non-Hodgkin lymphoma: a cohort study of parous women in Taiwan. Hepatology. 2011; 53: 1217–1225. doi: 10.1002/hep.24150 [DOI] [PubMed] [Google Scholar]
- 33.De Re V, De Vita S, Marzotto A, Gloghini A, Pivetta B, Gasparotto D, et al. Pre-malignant and malignant lymphoproliferations in an HCV-infected type II mixed cryoglobulinemic patient are sequential phases of an antigen-driven pathological process. Int J Cancer. 2000; 87: 211–216. [DOI] [PubMed] [Google Scholar]
- 34.Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009; 100: 1765–1770. doi: 10.1038/sj.bjc.6605063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006; 37: 1211–1216. doi: 10.1016/j.humpath.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015; 47: 1003–1010. doi: 10.1038/ng.3375 [DOI] [PubMed] [Google Scholar]
- 37.Tanaka M, Tanaka H, Tsukuma H, Ioka A, Oshima A, Nakahara T. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepat. 2010; 17: 742–748. doi: 10.1111/j.1365-2893.2009.01243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berrington de Gonzalez A, Yun JE, Lee SY, Klein AP, Jee SH. Pancreatic cancer and factors associated with the insulin resistance syndrome in the Korean cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2008; 17: 359–364. doi: 10.1158/1055-9965.EPI-07-0507 [DOI] [PubMed] [Google Scholar]
- 39.Huang J, Magnusson M, Torner A, Ye W, Duberg AS. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: a nationwide study in Sweden. Br J Cancer. 2013; 109: 2917–2923. doi: 10.1038/bjc.2013.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siu SS, Cheung TH, Chan PK, Lin CK, Lo KW. Patients with malignant or pre-malignant cervical lesion have increased risk of becoming hepatitis B carrier. J Exp Clin Cancer Res. 2007; 26: 77–81. [PubMed] [Google Scholar]
- 41.Ferber MJ, Montoya DP, Yu C, Aderca I, McGee A, Thorland EC, et al. Integrations of the hepatitis B virus (HBV) and human papillomavirus (HPV) into the human telomerase reverse transcriptase (hTERT) gene in liver and cervical cancers. Oncogene. 2003; 22: 3813–3820. doi: 10.1038/sj.onc.1206528 [DOI] [PubMed] [Google Scholar]
- 42.Kanazawa H, Hirata K, Yoshikawa J. Accelerated decline of lung function in COPD patients with chronic hepatitis C virus infection: a preliminary study based on small numbers of patients. Chest. 2003; 123: 596–599. [DOI] [PubMed] [Google Scholar]
- 43.Fallahi P, Ferrari SM, Politti U, Giuggioli D, Ferri C, Antonelli A. Autoimmune and neoplastic thyroid diseases associated with hepatitis C chronic infection. Int J Endocrinol. 2014; 2014: 935131 doi: 10.1155/2014/935131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995; 123: 615–620. [DOI] [PubMed] [Google Scholar]
- 45.Gawkrodger DJ, Stephenson TJ, Thomas SE. Squamous cell carcinoma complicating lichen planus: a clinico-pathological study of three cases. Dermatology. 1994; 188: 36–39. doi: 10.1159/000247083 [DOI] [PubMed] [Google Scholar]
- 46.Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology. 1993; 18: 781–789. [DOI] [PubMed] [Google Scholar]
- 47.de Oliveira PR, Yamamoto AY, de Souza CB, de Araujo NM, de Andrade Gomes S, Heck AR, et al. Hepatitis B viral markers in banked human milk before and after Holder pasteurization. J Clin Virol. 2009; 45: 281–284. doi: 10.1016/j.jcv.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 48.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311: 507–520. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains raw data about clinical variables of each patient in this study.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.