Abstract
Curcuma longa possesses powerful antifungal activity, as demonstrated in many studies. In this study, the antifungal spectrum of Curcuma longa alcohol extract was determined, and the resulting EC50 values (mg/mL) of its extract on eleven fungi, including Fusarium graminearum, Fusarium chlamydosporum, Alternaria alternate, Fusarium tricinctum, Sclerotinia sclerotiorum, Botrytis cinerea, Fusarium culmorum, Rhizopus oryzae, Cladosporium cladosporioides, Fusarium oxysporum and Colletotrichum higginsianum, were 0.1088, 0.1742, 0.1888, 0.2547, 0.3135, 0.3825, 0.4229, 1.2086, 4.5176, 3.8833 and 5.0183, respectively. Among them, F. graminearum was selected to determine the inhibitory effects of the compounds (including curdione, isocurcumenol, curcumenol, curzerene, β-elemene, curcumin, germacrone and curcumol) derived from Curcuma longa. In addition, the antifungal activities of curdione, curcumenol, curzerene, curcumol and isocurcumenol and the synergies of the complexes of curdione and seven other chemicals were investigated. Differential proteomics of F. graminearum was also compared, and at least 2021 reproducible protein spots were identified. Among these spots, 46 were classified as differentially expressed proteins, and these proteins are involved in energy metabolism, tRNA synthesis and glucose metabolism. Furthermore, several fungal physiological differences were also analysed. The antifungal effect included fungal cell membrane disruption and inhibition of ergosterol synthesis, respiration, succinate dehydrogenase (SDH) and NADH oxidase.
Introduction
Plant disease is an important factor in agricultural production, and phytopathogenic fungi belonging to differen genera infect and cause diseases to numerous crops, thus causing economic losses in agriculture [1]. Fungal infestation and mycotoxin contamination are also the biggest global threat to the food and feed industries [2, 3]. The threats include Alternaria, Aspergillus, Botrytis, Colletotrichum, Fusarium, Penicillium, Mucor and Rhizopus [4]. Plant fungal diseases can cause plants to change their colour, wilt, deform, and even die, potentially leading to species extinction [5]. The rapid global re-emergence of Fusarium graminearum (F. graminearum), which causes head blight disease of wheat and barley, in the last decade along with contamination of grains with mycotoxins, has spurred basic research on the fungal causal agent. As a result, F. graminearum has quickly become one of the most intensively studied fungal phytopathogens [6]. The active ingredient in bio-pesticides or fungicides, a substance produced by plants themselves, can be easily decomposed and does not damage the ecological balance. Therefore, bio-pesticides or fungicides have been acknowledged as green pesticides [7]. With this knowledge, many researchers have focused on determining the antifungal components produced by plants over the past few years [8, 9], and significant progress has been made, including the discovery of carvone, azadirachtin and pyrethroids [10].
As a traditional Chinese herbal medicine, Curcuma longa (C. longa), a member of the Zingiberaceae family, has received much attention for producing many complex compounds that are useful in food, such as spices, flavouring and seasoning, and in cosmetic and pharmaceutical industries as pharmacological agents [11]. The therapeutic properties of C. longa include insecticidal [12, 13], antimicrobial [14], antifungal [15, 16, 17], antimalarial [18], antiviral [19] and antioxidant properties [15, 20]. C. longa has been reported to have toxic activities against fungi involved in the deterioration of agricultural products by interfering with the development of mycelia [21]. We previously reported that the ethanol and hexane extracts of C. longa have significant antifungal activities against the following ten pathogenic fungi: Botrytis cinerea, Chaetomium olivaceum, Fusarium graminearum, Mycogone perniciosa, Penicillium pallidum, Phoma wasabiae, Sclerotinia sclerotiorum, Verticillium dahlia, Plasmodiophora brassicae and Magnaporthe grisea [22– 25]. The following chemical components of C. longa have been reported: curcumenol, curdione, curcumin, isocurcumenol, curcumol, stigmasterol, zingiberene and curcumene [26–31]. Nevertheless, the antifungal mechanisms and active components of natural products derived from C. longa are still unknown [2, 15, 16]. Thus, it is necessary to systematically study the antifungal activity of the extract and its main active components and mechanism to evaluate the main targets for antifungal activity.
Materials and methods
Preparation of C. longa extract
Fresh rhizomes of C. longa were harvested in November 2015 from Chongzhou, Sichuan, P.R. China (located at 30°63′ latitude, 103°67′ longitude and an elevation of 520 m). The rhizome powder was macerated with 95% ethanol and sonicated by a bath sonicator at 40 Hz at room temperature (under 50°C). The extract was filtered and concentrated using a rotary evaporator [5], and it was stored at 4°C and protected from light prior to the next experiment.
Determination of antifungal activity of the extract
The following strains used in this study were respectively isolated from differentcrops that infected pathogenic fungi and were identified by their ITS sequences, including Fusarium graminearum (GenBank accession No: MF372579, from wheat), Fusarium tricinctum (MF372578, from kiwi fruit), Rhizopus oryzae (MF372577, from the fruit-body of Pleurotus ostreatus), Cladosporium cladosporioides (MF372580, from the fruit-body of Pleurotus ostreatus), Fusarium culmorum (MF372583, from morel’s ascocarp), Sclerotinia sclerotiorum (MF372581, from morel’s ascocarp), Alternaria alternate (MF373422, from strawberries infected by leaf spot disease), Fusarium chlamydosporum (MF383402, from root endophytes of Dendrobium crumenatum), Fusarium oxysporium (MF372600, from banana wilt) and Botrytis cinerea (MF510815, from Capsicum frutescens) and Colletotrichum higginsianum (MF510821, from wasabi japonica). These strains were all preserved at 4°C in the laboratory using PDA slant medium (200 g of potato, 20 g of glucose, 20 g of agar, and 1 L of dH2O; natural pH value).
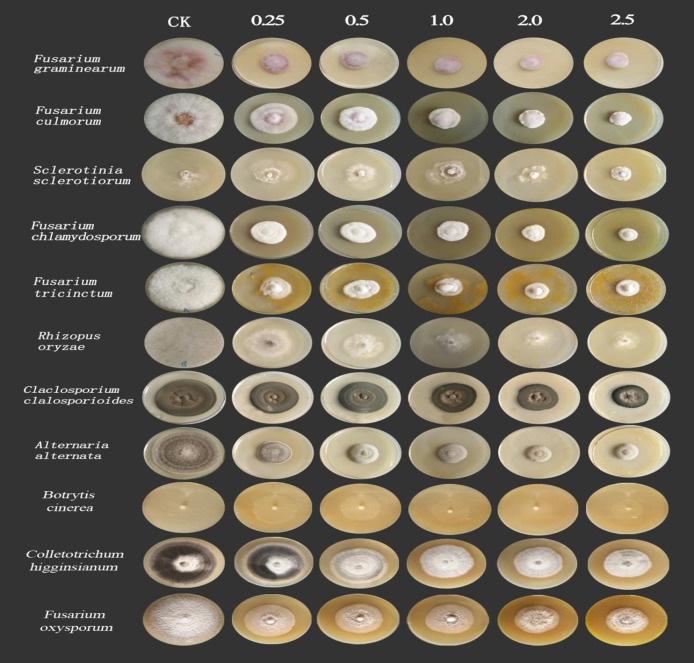
The antifungal activities of the extract against eleven pathogenic fungi were determined by the growth rate method [25] in this study. Briefly, the extract was mixed with dissolved PDA medium up to final concentrations (0, 0.25, 0.50, 1.00, 2.00 and 2.50 mg/mL, respectively, each concentrations were set up three parallel repetition), and the medium with no extract was used as the blank control group (CK). All the treatment and blank control groups were inoculated in triplicate with a mycelium plug (in diameter 4 mm) of each fungal phytopathogen strain in the centre of the plate. When the colonies of the blank control group completely covered the plate, the colony diameter of each plate was measured, and the inhibition rate (IR) was calculated by the following formula: IR (%) = 100%×(Dc-Dt)/Dc; where IR represents the inhibition rate, Dc represents the colony diameter of the blank control group, and Dt represents the colony diameter of the drug-treated group [32]. Finally, the regression equation, the half effect mass concentration and the correlation coefficient (R2) were calculated using DPS software (using the method of probability value analysis).
Antifungal activities of eight chemical components derived from C. longa
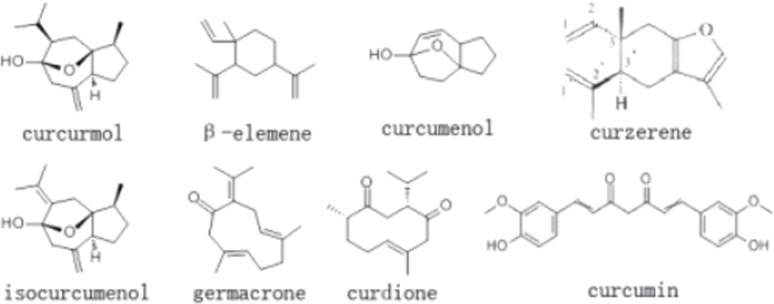
The antifungal activities of chemical components of C. longa against F. graminearum were also determined by the growth rate method. The chemical components used in this study, including curdione (CAS: 13657-68-6), isocurcumenol (CAS: 24063-71-6), curcumenol (CAS: 19431-84-6), curzerene (CAS: 17910-09-7), β-elemene (CAS: 515-13-9), curcumin (CAS: 458-37-7), germacrone (CAS: 6902-91-6) and curcumol (CAS: 4871-97-0), and their structural formulas are shown in Fig 1. A simultaneous high-performance liquid chromatography (HPLC) analysis was developed and validated for the determination of eight active components in C. longa. HPLC was performed on a Shimadzu SPD-10A (Kyoto, Japan) equipped with an LC-10AD pump, a DGU-10A degasser, a SPD-10AV ultraviolet visible (UV-vis) detector and an SIL-10AD auto-injector. The separation was conducted on a C18 reverse column (250mm×4.6mm, 5μm). The elution was performed on a gradient solvent system using acetonitrile and deionized water as mobile phases. The flow rate was 0.8 mL/min at 35°C. The UV-vis detector was monitored at 244 nm and the injection volume for all samples and standards was 40 μL. quantitative HPLC analysis of each compound was calculated according to its peakarea [33].
Fig 1. Structural formula of eight chemical components derived from C. longa.
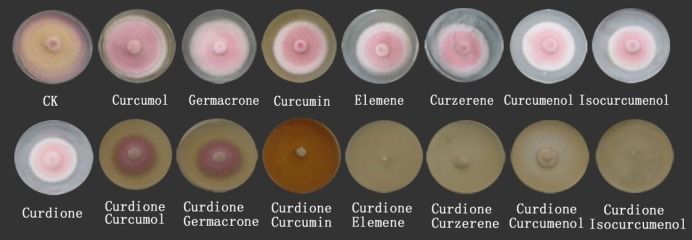
These chemical components were dissolved in PDA media at a final concentration of 0.5 mg/mL for each component, and the pure PDA medium was used as the control group (CK), the control group and the treatment group were set up three parallel repetitions. Then each group was inoculated with one mycelium plug using the above method. After approximately five days of incubation for the experimental plates at 25°C, the inhibitory effects of the drugs on eight groups were observed and calculated [32].
The component with the best antifungal effect (curdione) was mixed with seven other components in 1:1 ratio to form seven binary complexes, the seven complexes were dissolved in PDA media at a final concentration of 0.25 mg/mL for each component, and the antifungal effects of these complexes were investigated using the above method[25, 32].
Mechanism of C. longa extract against F. graminearum
Differential proteomic analysis
The F. graminearum mycelia (2 g for each treatment) group, which was treated with the extract (0.5 mg/mL) for 4 days at 25°C, was named the CLT group, and the group lacking the extract was named the control group (CK). Both samples were ground with 5 mL of Tris-saturated phenol (pH 8.0) and pre-chilled extraction buffer (containing 50 mM Tris-HCl, pH 8.5; 5 mM EDTA; 100 mM KCl; 2% v/v β-mercaptoethanol; and 30% sucrose). The mixture was transferred to centrifuge tubes, fully mixed at 4°C for 15 min and centrifuged at 6000 g for 3 min. The upper phenol layer was collected to EP tubes, and the precipitate was placed in an ice-cold bath with 4 volumes of 0.1 M ammonium acetate in methanol overnight. After the precipitate was washed 3–5 times and when its colour remained unchanged, it was washed with pre-chilled 80% acetone solution once again. Lysis buffer (50 mM Tris-HCl (pH 6.8), 100 mM DTT, 2% SDS, and glycerol) was added to the sample, and the sample was mashed using an ultrasonic instrument. The sample was then centrifuged, and the supernatant was retained. The concentration of protein was determined according to the literature [34], and the protein samples of the CLT and CK groups were adjusted to an equal concentration.
The protein sample was separated by loading it onto a 24 cm, linear pH gradient IPG strip (pH 4–7), and an oil layer was added to cover the surface to prevent water from evaporating overnight. The focus parameters were set (40 V hydration for 10 hrs; desalinization at 250 V for l h; 500 V for l h; 1000 V for l h; gradually stepped up to 8000 V, and 8000 V focus for 12 hrs). For the second electrophoretic dimension, the equilibrated immobilized pH gradient (IPG) strip was placed at the top of a 10% SDS-PAGE gel (34.6 mL of Acr/Bis (30%/0.8%); 1.5 mol/L Tris-HCl, pH 8.8; 32 μL oftetramethylethylenediamine (TEMED)and 800 μL of 10% ammonium sulphate (APS). SDS-PAGE was performed at 4 W per gel for approximately 40 min in a buffer containing 6 mol/L urea, 2% SDS, 0.05 mol/L Tris-HCl and 30% glycerol until the bromophenol blue flowed to the lower edge of the glass. According to the soft guide, the spots were detected, matched and normalized based on the density of the gel and the percent volume. Calculations for the spots were performed at different statistical levels by the Kolmogorov-Smirnov test using ImageMaster 7.0 [35–37].
Ten differentially expressed proteins were excised carefully from the 2-D gels, and the gel spots were dehydrated, rehydrated and digested to peptides according to the method described by literature [35]. The digested peptides were subjected to MALDI-TOF mass spectrometry using a 4700 plus MALDI-TOF Analyzer (Applied Biosystems, Foster City, CA). The peak list generation and peak picking for the peptide mass fingerprinting (PMF, http://www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH=PMF) and MS/MS data (http://www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH0MIS) were performed with an Autoflex TOF/TOF II (Bruker Daltonics, USA). The PMF and MS/MS data were used to derive the protein identity using the MAS-COT search engine (http://www.Matrixscience.com) applied to the NCBInr 20130413 release (24553352 sequences; 8469922479 residues). The significantly high MASCOT scores that resulted in a confidence interval (CI) greater than 95% for the PMF or TOF/TOF data of a spot were considered as a credibly identified protein. Such credible results were further confirmed through database searching using other programs, such as Profound [35].
Ergosterol content analysis
PDB (200 g of potato, 20 g of glucose and normal PH value) was added to the extract at final concentrations of 0, 0.125, 0.25 and 0.5 mg/mL, and each concentration were set up three parallel repetition, curdione and curcumenol were prepared into culture medium with the same concentration gradient, One mycelium punch strip of F. graminearum was inoculated into the PDB solution and cultured at 28°C under 180 rpm for 4 days. After filtering the mycelium, it was washed with sterile water and then dried at 60°C. Thereafter, it was ground with liquid nitrogen, and 50 mg of mycelium for each group was weighed and placed into a 50 mL centrifuge tube. Five millilitres of methanol/chloroform (75:25, v/v) was added to the dried mycelia and incubated overnight at room temperature. The next day, distilled water, chloroform and 5 mL of 0.5 mol/L phosphate buffer containing 2.0 mol/L KCl were added to the mycelia. After stratification, the chloroform phase was extracted, and the solution containing with 5 mL of methanol/ethanol (80:20, v/v) and1.4 mol/L KOH was then added. The mixture was saponified for 1 h at 60°C. Five millilitres of petroleum ether was then added (boiling range from 60°C to 90°C). The petroleum ether phases were evaporated to dryness with nitrogen gas. The precipitate was dissolved with ethanol to 1 mL. Weighed samples were prepared according to a previously described method [38]. Ergosterol was assayed by the HPLC method performed at 25°C using a mobile phase with methanol at a volumetric velocity of 1.0 mL/min. Ergosterol was detected with an UV detector at OD282 values [39]. The standard substance, ergosterol (HPLC purity ≥98%, Weikeqi Biological Technology Co., Ltd., Chengdu, China), was diluted in a concentration gradient with final concentrations of 2.5, 5, 10, 20, 40, 80 and 160 μg/mL. The standard curve was successfully established with the ergosterol concentration as the ordinate and the absorption peak area as the abscissa.
Determination of respiratory inhibition rate
Fresh PDB medium was added to the extract at final concentrations (0, 0.125, 0.25, 0.5 and 1.0 mg/mL, each concentration were set up three parallel repetition), curdione and curcumenol were prepared into culture medium with the same concentration gradient, One mycelium plug of F. graminearum was inoculated into the PDB solution and cultured at 28°C and 180 rpm for 24 hrs. The amounts of dissolved oxygen were measured in vitro at 0, 0.5, 1 and 2 hrs after treatment using a JPB-607A Dissolved Oxygen Meter (Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China) according to method provided by the manufacturers. Based on the change in the dissolved oxygen content in the fungus suspension, the respiration rate of the fungus can be determined. The rate of respiratory inhibition of F. graminearum caused by the extract was calculated by the following formula: IR (%) = 100% × (R0-RI)/R0; where IR is the rate of respiratory inhibition (%) of F. graminearum caused by the extract; and R0 and RI represent the respiration rate of F. graminearum before and after addition of the extract (mg O2/L∙min), respectively [40].
Analyses of succinate dehydrogenase (SDH) and NADH oxidase activities
PDB solution was added to the extract at final concentrations of 0. 0.0625, 0.125, 0.25 and 0.5 mg/mL, each concentration was set up three parallel repetitions. One mycelium plug was inoculated into PDB solution and was cultured at 28°C and 180 rpm for 4 days. To filter the mycelium, it was washed with sterile water and then dried at 35°C. Thereafter, it was ground with liquid nitrogen, and 0.1 g of mycelium from each group was weighed accurately and placed into a centrifuge tube. The SDH and NADH activity was then measured for each group.
According to the SDH kit instructions (purchased from Suzhou Comin Biotechnology Co., Ltd; Suzhou, China), the activity of SDH was measured. First, distilled water was used to adjust the spectrophotometer to zero, and reagents were then added to a 1 mL glass cuvette to record the initial absorbance (A1) for 20 s at a 600 nm wavelength. Thereafter, the cuvette was quickly placed with the reaction liquid together into a 25°C water bath, and a consistent, accurate response for one minute was obtained. Te absorbance (A2) at 1 min 20 s at a 600 nm wavelength was recorded, and the SDH activity was then calculated using the following formula: SDH activity (U/g) = 1508×(A1-A2)/W. According to the kit instructions (the NOX kit from the Shanghai Institute of Biological Technology), the activity of NADH was measured. The spectrophotometer was adjusted to zero using distilled water, and the reagents were added to a 1 mL glass cuvette. The initial absorbance values (B1) at 20 s and at 1 min 20 s (B2) on 600 nm wavelength were respectively recorded, and then the NADH oxidase activity was calculated using the following formula: NADH oxidase activity (U/g) = 505×(B1-B2)/W.
Statistical analyses
Data values were expressed as the means±standard deviations of three independent experiments. The data from each group and for each extract concentration were compared using one-way analysis of variance, followed by Dunnett’s test formultiple comparisons, and the tests were performed using SPSS 17.0 system, P-value less than or equal to 0.05 was considered to be statistically significant.
Results
Determination of antifungal activity
In this study, eleven fungi were used as the test pathogens to determine the antifungal spectrum for C. longa extracts. The results showed that when the concentration of the extract reached 1.0 mg/mL, it had a strong inhibitory effect on various pathogenic fungi (Table 1, Fig 2). The EC50 values of the extract on Sclerotinia sclerotiorum, Fusarium chlamydosporum, Fusarium tricinctum, Rhizopus oryzae, Fusarium graminearum, Fusarium culmorum, Cladosporium cladosporioides, Alternaria alternate, Botrytis cinerea, Fusarium oxysporum and Colletotrichum higginsianum were 0.3825, 0.1742, 0.2547, 1.2086, 0.1088, 0.4229, 4.5176, 0.1888, 0.1749, 0.3758 and 0.6594 mg/mL, respectively. These fungal belong to sordariomycetes, dothideomycetes, leotiomycetes, hyphomycetes, zygomycetes and eurotiomycetes (Table 1).
Table 1. Regression equation of inhibition rates for the C. longa extract antagonizing sixteen phytopathogenic fungi.
| No | Pathogen | Classification1 | IR(%)2 | TRE(Y =)3 | R2 | EC504 | References |
|---|---|---|---|---|---|---|---|
| 1 | Phoma wasabiae | Dothideomycetes | 67.08±2.64 | 1.2923x+5.3148 | 0.9468 | 0.5707 | Liu et al., 2008 |
| 2 | Alternaria alternata | Dothideomycetes | 61.17±2.14 | 0.3714x+5.2689 | 0.9665 | 0.1888 | This study |
| 3 | Chaetomium olivaceum | Sordariomycetes | 54.78±2.74 | 1.3975x+5.0791 | 0.9796 | 0.8778 | Long et al., 2007 |
| 4 | Penicillium pallidum | Sordariomycetes | 74.85±3.44 | 0.5344x+5.7772 | 0.9572 | 0.0351 | Li et al., 2011 |
| 5 | Mycogone perniciosa | Sordariomycetes | 71.72±2.80 | 0.9128+5.3180 | 0.9538 | 0.4484 | Li et al., 2011 |
| 6 | Fusarium chlamydosporum | Sordariomycetes | 67.97±2.72 | 0.5526x+5.4194 | 0.9763 | 0.1742 | This study |
| 7 | Fusarium culmorum | Sordariomycetes | 63.50±2.58 | 0.7917x+5.2959 | 0.9881 | 0.4229 | This study |
| 8 | Fusarium graminearum | Sordariomycetes | 63.80±2.30 | 0.3616x+5.3484 | 0.9825 | 0.1088 | This study |
| 9 | Fusarium oxysporium | Sordariomycetes | 41.20±2.06 | 0.3584x+4.7888 | 0.8821 | 3.8833 | This study |
| 10 | Fusarium tricinctum | Sordariomycetes | 65.40±2.94 | 0.5397x+5.3205 | 0.9658 | 0.2547 | This study |
| 11 | Colletotrichum higginsianum | Sordariomycetes | 34.50±1.73 | 0.6589x+4.5384 | 0.9896 | 5.0183 | This study |
| 12 | Verticillium dahlia | Sordariomycetes | 78.19±3.52 | 0.7244x+5.6466 | 0.9557 | 0.1281 | Li et al., 2011 |
| 13 | Botrytis cinerea | Leotiomycetes | 53.50±2.56 | 0.3484x+5.1755 | 0.9387 | 0.3135 | This study |
| 14 | Sclerotinia sclerotiorum | Leotiomycetes | 63.80±2.55 | 0.7771x+5.3243 | 0.9749 | 0.3825 | This study |
| 15 | Rhizopus oryzae | Zygomycetes | 46.27±2.31 | 0.9423x+4.9225 | 0.9803 | 1.2086 | This study |
| 16 | Claclosporiumclaclosporioides | Hyphomycetes | 29.90±1.50 | 0.7384x+4.5164 | 0.9395 | 4.5176 | This study |
Note
1) The pathogen belongs to the classified classes.
2) IR represents inhibition rate, which was determined when the used extract concentration was 0.5 mg/ml.
3) TRE indicates the toxicity regression equation in which “x” represents the logarithm of the mass concentration of the extract, Y represents the inhibition rate and R2 represents the correlation coefficient.
4) The unit for EC50 was mg/mL.
Fig 2. Effects of eight components of C. longa on eleven pathogenic fungi.
The extract concentration unit ranged from 0.25 to 2.5 mg/mL.
Antifungal activity of the chemical component of C. longa
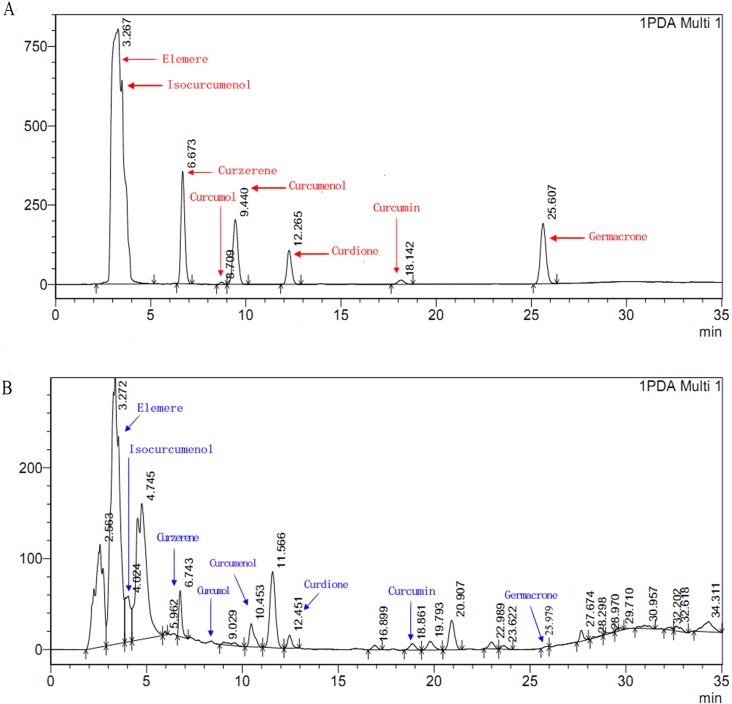
The HPLC chromatogram of the eight components and the extract of C. longa were compared and analyzed. It was found that the percentages of curdione, isocurcumenol, curcumenol, curzerene, β-elemene, curcumin, germacrone, curcumol in the extract of C. longa were 0.87%, 4.18%, 1.86%, 0.17%, 2.11%, 37.19%, 0.60%, 0.07%, respectively (Fig 3).
Fig 3.
Analytical HPLC profiles of the eight components (A) and the extract of C. longa(B).
Eight chemical constituents of C. longa all had inhibitory effects on the mycelia growth of F. graminearum (Table 2, Fig 4). Among the eight chemicals, curdione had the best inhibitory effect on the growth of F. graminearum with an inhibitory rate of 52.9%. We next investigated the antifungal effect of curdione combined with seven other chemicals. The results showed that the inhibitory effect was greatly enhanced (Table 2, Fig 4). The antifungal rate of curdione combined with isocurcumenol and β-elemene reached 100%, while the inhibitory rates of curdione combined with curcumin curzerene, curcumenol, curcumol and germacrone, were 93.6%, 88.9%, 82.7%, 63.6%, and 56.4%, respectively.
Table 2. Inhibition rates of eight main components from C. longa and binary complexes of curdione and other components on F. graminearum.
| Component | Cas No. | Molecular formula | Single component | Binary complex |
|---|---|---|---|---|
| Curdione | 13657-68-6 | C15H24O2 | 52.9±2.38 | / |
| Isocurcumenol | 24063-71-6 | C15H22O2 | 48.8±2.05 | 100 |
| Curcumenol | 19431-84-6 | C15H22O2 | 47.6±2.38 | 82.7±3.80 |
| Curzerene | 17910-09-7 | C15H20O | 42.9±2.06 | 88.9±3.89 |
| Beta elemene | 515-13-9 | C22H20O12 | 36.9±1.88 | 100 |
| Curcumin | 458-37-7 | C21H20O6 | 34.5±1.62 | 93.6±2.80 |
| Germacrone | 6902-91-6 | C15H22O | 17.9±0.95 | 56.4±1.97 |
| Curcumol | 4871-97-0 | C15H24O2 | 10.7±0.59 | 63.6±2.61 |
Fig 4. Inhibitory efficacy of eight components and seven binary complexes derived from C. longa against F. graminearum.
Differential proteomic analysis
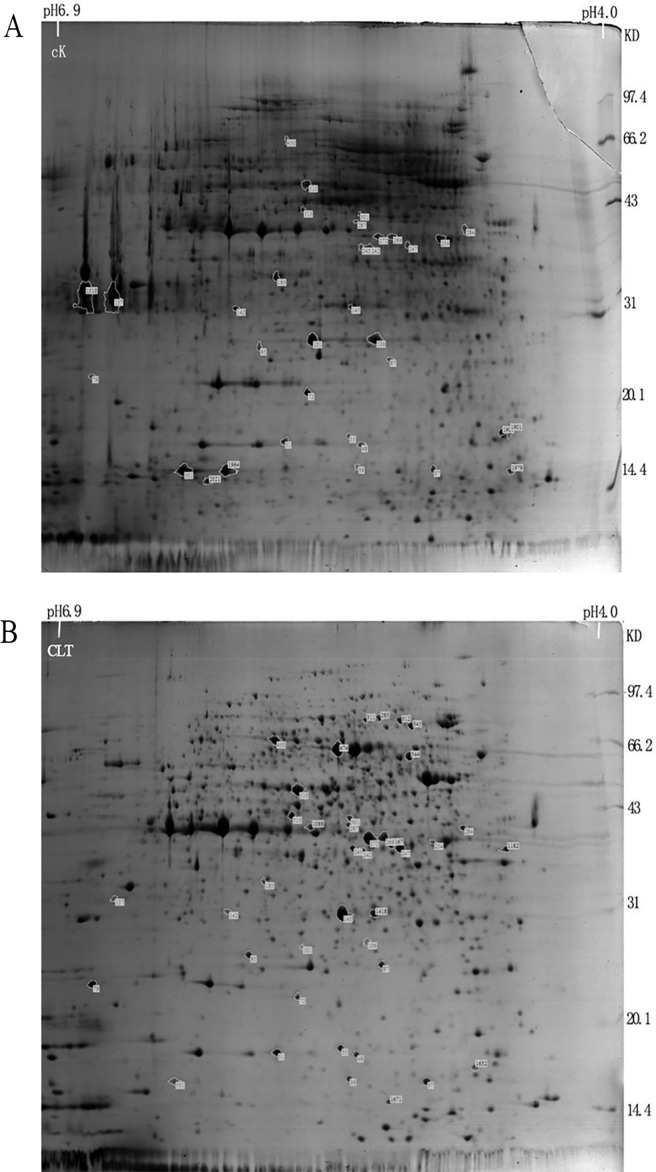
To further elucidate the antifungal mechanism, two-dimensional gel electrophoresis (2-DGE) was utilized to compare the proteins of the bacterial strains that had not been treated (CK) with C. longa with those that had been treated (CLT). The fungal 2-DGE map consisted of at least 2021 reproducible protein spots, 46 of which were classified as differentially expressed proteins (shown in Fig 5),46 of which were classified as differentially expressed proteins (Fig 5).
Fig 5.
Separation of total soluble proteins extracted from extract-treated (B) and untreated (A) F. graminearum cells on silver-stained gels over a pH range of 4–6.9.
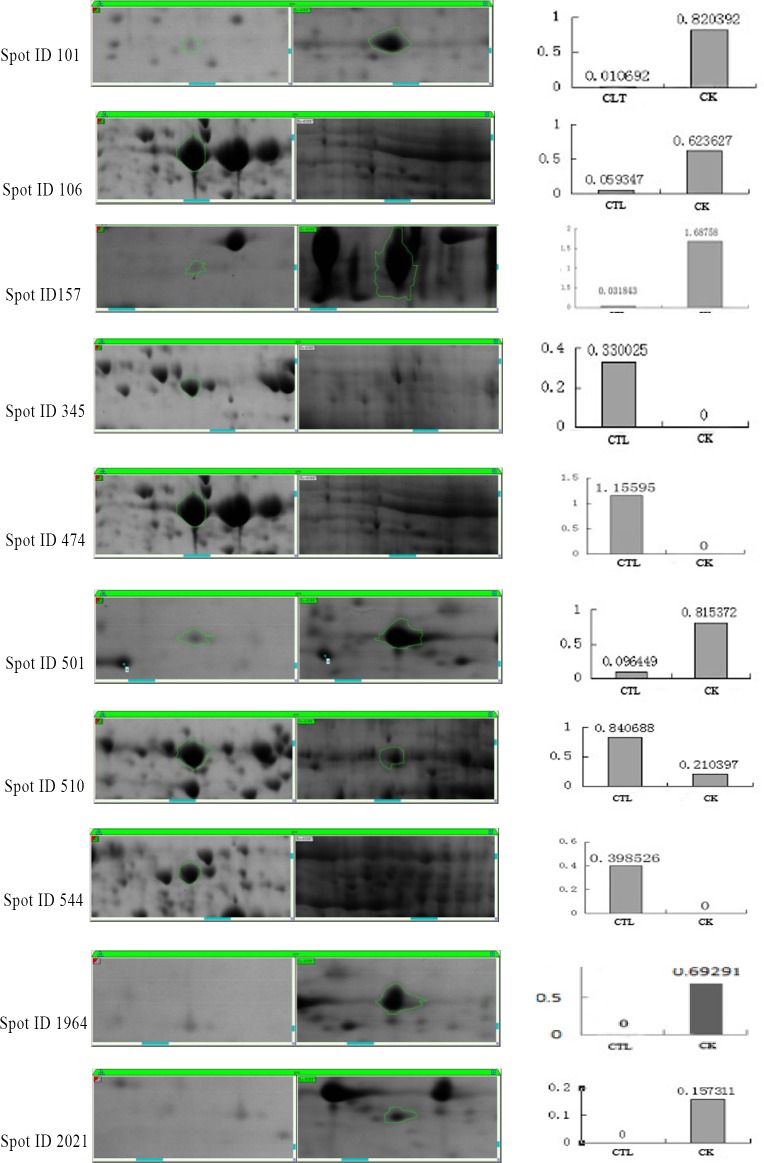
According to the peptide fingerprint data, using the MASCOT search engine NCBInr20130413 (24553352 sequences; 8469922479 residues), the differentially expressed protein species were preliminary identified. Ten representative proteins were identified as follows: three glyceraldehyde 3-phosphate dehydrogenase proteins (GAPDH) (spot 101, 106 and 501), the core domain-containing protein of the tRNA synthetase family II (spot 157), a possible forkhead box (spot 345), a possible FG09834 protein spot 474), a phosphoglycerate kinase (spot 510), a possible FPSE protein (spot 544), a possible FG03122 protein (spot 1694) and the zinc binuclear structural domain-containing fungal protein (spot 2021). These proteins are involved in energy metabolism, tRNA synthesis and glucose metabolism (shown in Table 3 and Fig 6.).
Table 3. Mass spectrometry results of 10 representative differentially expressed proteins.
| Match | GI | Annotation | Species | Function | Mw | Matching |
|---|---|---|---|---|---|---|
| 101 | gi|46123759 | Glyceraldehyde 3-phosphate dehydrogenase | Gibberellazeae | Energy metabolism | 36328 | 128 |
| 106 | gi|46123759 | Glyceraldehyde 3-phosphate dehydrogenase | Gibberellazeae | Energy metabolism | 36328 | 139 |
| 157 | gi|310790817 | Core domain-containing protein of the tRNAsynthetase family II | He Sheng anthrax | tRNA synthesis | 57978 | 68 |
| 345 | gi|342878255 | Possible fork head box | Fusarium knife | growth | 67072 | 130 |
| 474 | gi|46136637 | Possible FG09834 protein | Gibberellazeae | 63541 | 244 | |
| 501 | gi|46123759 | Glyceraldehyde 3-phosphate dehydrogenase | Gibberellazeae | Energy metabolism | 36328 | 52 |
| 510 | gi|46116300 | Phosphoglycerate kinase | Gibberellazeae | Glucose metabolism | 44882 | 145 |
| 544 | gi|408396407 | Possible FPSE protein | Fusarium pseudograminearum | 57551 | 93 | |
| 1694 | gi|46114560 | Possible FG03122 protein | Gibberellazeae | 43523 | 66 | |
| 2021 | gi|429850883 | Zinc binuclear structural domain-containing fungal protein | Colletotrichumgloeosporioides | 73952 | 81 |
Fig 6. Comparative results of ten differentially expressed protein spots.
The sample treated with the extract from C. longa was labelled CTL, and the sample lacking extract was labelled as CK. “0” represents no production.
As seen from Fig 6, GAPDH proteins appeared at a lower abundance in the CLT group compared to the CK group, the expression of spot 101 dropped from 0.82 to 0.01, spot 106 dropped from 0.62 to 0.07, spot 501 dropped from 0.82 to 0.10. In addition, in the CLT group, the core domain-containing protein of the tRNA synthetase family II (the expression dropped from 1.69 to 0.03), possible FG03122 protein (the expression dropped from 0.69 to 0) and the zinc binuclear structural domain-containing fungal protein (the expression dropped from 0.16 to 0) had very low expression (Fig 6). And there were four proteins with a higher abundance in the CLT group compared to the CK group, including a possible forkhead box (the expression increased from 0 to 0.33), a possible FG09834 protein (the expression increased from 0 to 1.16), a phosphoglycerate kinase (the expression increased from 0.21 to 0.84), and a possible FPSE protein,(the expression increased from 0 to 0.40), which showed in Table 3 and Fig 6.
Ergosterol content
In this experiment, the results showed that when the concentration of the extract of C. longa, curdione and curcumenol increased, the ergosterol content was decreased. The ergosterol content of the various treatment groups are listed in Table 4. When the concentrations of the extract were 0.125, 0.25 and 0.5 mg/mL, the ergosterol content decreased significantly, ranging from 38.33 to 16.04 μg/g. When the concentrations of curdione were 0.125, 0.25 and 0.5 mg/mL, the ergosterol content decreased significantly (ranging from 38.33 to 17.51 μg/g). Curcumenol also had effect on F. graminearum ergosterol contents, but not as well as the extract and curdione (shown in Table 4).
Table 4. Ergosterol contents of F. graminearum treated with C. longa extract, curdione and curcumenol.
| Groups | Concentration (mg/mL) | Ergosterol content (μg/g) | ||
|---|---|---|---|---|
| Extract of C. longa | Curdione | Curcumenol | ||
| ck | 0 | 38.33±1.92 a | 38.33±1.92 a | 38.33±1.92 a |
| 1 | 0.125 | 28.36±1.28 b | 27.88±1.12 b | 30.22±1.40 b |
| 2 | 0.250 | 22.33±1.03 c | 21.47±0.77 c | 26.32±0.89 c |
| 3 | 0.500 | 16.04±0.80d | 17.51±0.88 d | 20.09±0.90 d |
Note: Values with different superscript letters (a~d) indicated that there were significant differences within the columns (p<0.05).
The effect of the extract on the respiratory chain of F. graminearum
In this experiment, as the concentration of the extract derived from C. Longa, curdione and curcumenol increased, the rate of respiratory inhibition of the extract on F. graminearum gradually increased (Table 5).The extract and curdione had strong effect on F. graminearum respiration. When the concentration of the extract and curdione reached 0.5 mg/mL, the rate of respiratory inhibition reached 70.8% and 66.5%, respectively. When the concentration of the extract and curdione reached 1.0 mg/mL, the rate of respiratory inhibition reached as high as 75.0% and 74.9%, respectively. Curcumenol also had effect on F. graminearum respiration, but not as well as the extract and curdione (Table 5).
Table 5. Respiratory inhibition of F. graminearum treated with C. longa extract, curdione and curcumenol.
| Groups | Concentration(mg/mL) | Respiratory inhibition rate (%) | ||
|---|---|---|---|---|
| Extract of C. longa | Curdione | Curcumenol | ||
| 1 | 0.125 | 16.7±0.8 a | 23.8±0.7 a | 15.4±0.6 a |
| 2 | 0.250 | 54.2±2.3 b | 34.7±1.2 b | 34.4±1.4 b |
| 3 | 0.500 | 70.8±3.5 c | 66.5±1.9 c | 40.7±1.5 c |
| 4 | 1.000 | 75.0±3.5 d | 74.9±2.1 d | 41.8±1.6 c |
Note: Values with different superscript letters (a~d) indicated that there were significant differences within the columns (p<0.05).
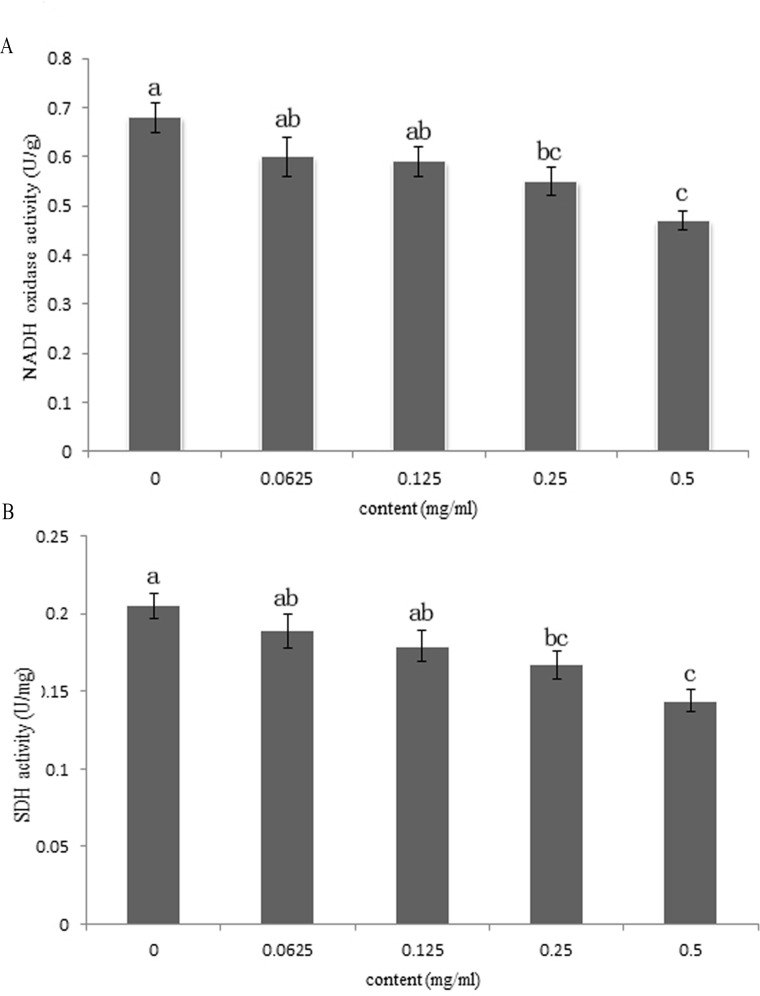
The experimental results showed that the NADH oxidase and SDH activities decreased with an increase in the concentration of the extract, and the gradient was obvious (shown in Fig 7). When the concentration of the extract reached 0.5 mg/mL, there were significant differences in NADH oxidase activity between the experimental group and the blank control group. When the concentrations of the extract were 0.125 and 0.25 mg/mL, there was a specific difference between the blank control group and the experimental group regarding NADH oxidase and SDH activity.
Fig 7.
Changes of NADH oxidase (A) and SDH (B) activities. Values with different superscript letters (a-~c) showed significant differences among the pillars (p<0.05).
Discussion
C. longa possesses powerful antifungal activity, as demonstrated in some studies [21, 41]. Based on previous studies and this study, the extract of C. longa has inhibitory effects on over twenty pathogenic fungi, including Aspergillus flavus [16], Fusarium verticillioides [15], Curvularia pallescens, Colletotrichum falcatum, Aspergillus niger, Aspergillus terreus, Fusarium oxysporum,Fusarium moniliforme [17], Fusarium graminearum [2], Phoma wasabiae, Alternaria alternate, Botrytis cinerea, Chaetomium olivaceum, Penicillium pallidum, Mycogone perniciosa, and Verticillium dahlia [22–24], these pathogenic fungi belong to sordariomycetes, dothideomycetes, leotiomycetes, hyphomycetes, zygomycetes and eurotiomycetes, etc. These data indicated that C. longa showed a broad-spectrum antifungal effect.
In this study, eight chemical constituents of C. longa, includingcurdione, isocurcumenol, curcumenol, curzerene, β-elemene, curcumin, germacrone and curcumol, were allverified showing inhibitory effects on the mycelia growth of F. graminearum while theircontents in the rhizomeextract of C. longa were 0.87%, 4.18%, 1.86%, 0.17%, 2.11%, 37.19%, 0.60%, 0.07%, respectively. Not only that, we lately reportedthat curdione, curcumenol, β-elemene, germacrone were existed in the leaves’extract of C. longa with the contents of 5.91%, 3.96%, 7.92%, 2.62%, respectively [42]. It could be seen that the contents ofthese compounds with antifungal activities were relatively high. However, their antifungal effectswerenot shown consistently, which suggested that different chemical components differ indifferent fungal targets. Hu et al reported that the inhibition behaviour of C. longa on fungal growth (Aspergillus flavus) is involved in its ability to disrupt the integrity of plasma membrane and mitochondrial dysfunction, inducing metabolic stagnation [16]. These results suggested that those compounds showed better mutual synergetic effects. Curcumin and β-elemene have been reported to exert significant antifungal activity [43, 44]. In this study, we found that curdione had the best antifungal activity, but the binary complexes of curdione with seven other components had better antifungal activity. The antifungal activitiesof curdione, curcumenol, curzerene, curcumol and isocurcumenol and their synergistic interactions were demonstrated for the first time.Persistent efforts and attempts have been made to dissect the action mode of traditional Chinese medicine in recent years, which has provided certain evidence for inter-herbal interactions. However, the interactions among different components in a single herb have been largely neglected. In Qi’s present study provides evidence that there are complicated interactions among the three components of Curcuma wenyujin Y.H. Chen et C Ling in which germacrone, curdioneand furanodiene were the three main components in the herb with an approximate molar ratio of 1:3:3 [45,46]. In this paper, the study showed that there are complicated interactions among curdione and other seven compounds, but the mechanism of the interaction between them needs to be further studied.The antifungal mechanism of C. longa has not been systematically elucidated although its powerful antifungal activity has been demonstrated by many reports. Currently, the antifungal drugs can be categorized into three types according to their functional mechanisms as follows: acting on the synthesis of the cell wall, e.g., caspofungin, which inhibits the synthesis of the main components of the fungal cell wall β-(1,3)-D-glucans; acting on cholesterol synthesis in fungal cell membranes, e.g., ketoconazole and amphotericin; and acting on the synthesis of fungal nucleic acids (Flucytosine) [47]. In this study, we analysed and identified the differential proteomic expression of F. graminearum using two-dimensional gel electrophoresis (2-DE). GAPDH (spot 101, 106 and 501) not only plays an important role in glycolysis but also in non-metabolic processes, including transcription activation and apoptosis. GAPDH is found in various organismal cells and is primarily involved in glycolysis, gluconeogenesis, the Calvin cycle and other energetic metabolic pathways. GAPDH is also one of the most basic enzymes required to sustain life and is always described as exhibiting higher order multifunctionality in the context of maintaining cellular iron homeostasis [48]. GAPDH was downregulated in the treatment group in addition to the core domain-containing protein of the tRNA synthetase family II (spot 157) and Zinc binuclear structural domain-containing fungal protein (spot 2021) (Table 3, Fig 5). Most of these proteins involved in translation and suggested that inhibition of translation might be the common pathway in the antifungal effect. Similar results had been obtained in a previous study [37], which reported that Pseudomonas aeruginosa treated with AgNPs–GE induces the expression of energy metabolism proteins. These results indicate that C. longa can potentially disrupt the synthesis of critical proteins and enzymes that may ultimately inhibit the growth of fungi. However, other proteins involving in protein synthesis, suchas the forkhead box protein (spot 345), were upregulated by C. longa. The forkhead box protein family are transcription factors that have a helical structure in their DNA-binding region. The forkhead protein is not only used as a typical transcription factor to regulate gene transcription but is also directly associated with condensed chromatin and participates in its reconstruction, coordinating with other transcription factors to participate in transcriptional regulation. The forkhead protein was upregulated in the treatment group. Phosphoglycerate kinase (spot 510), which catalyses the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP, was upregulated [49], as was FG09834 protein (spot474) and FPSE protein (spot 544). Thus, these proteins may participate in the synthesis of stress response-related factors that are expressed in response to C. longa treatment.
At present, many studies on the antifungal mechanism have been primarily focused on the following two actions: the formation of transmembrane pores or ion channels on the cellular membrane, leading to the leakage of essential metabolites, and the disruption of the cell wall structure, interfering with cell wall synthesis [50]. Ergosterol is an important component of membrane lipids, similar to vertebrate cholesterol, and it modulates the fluidity, permeability and thickness of the membrane. These sterols preferentially associate with sphingolipids in microdomains that have been postulated to have important roles in membrane organization and function [51, 52]. Ergosterol can be combined with phospholipids to stabilize the membrane structure, which can regulate the mobility of the fungal cell membrane and plays an important role in ensuring the integrity of the membrane structure, membrane-binding enzyme activity, cell viability and cell transport. Once ergosterol absent, abnormal function of the fungal cell membrane or even cell rupture will occur [53, 54]. In this study found that C. longa can inhibit the synthesis of ergosterol, and there were also studies examined that some antifungal analogue inhibited the growth of fungi by inhibiting the synthesis of ergosterol [55]. Hence, the membrane was also an important antifungal target of C. longa.
The fungal respiratory system was sensitive to many well-known inhibitors such as antimycin A, and the primary site of action of antifungal antibiotic is to block electron transfer between the flavoprotein of the NADH-dehydrogenase and cytochrome B segment of the respiratory chain of fungi [56]. NADH oxidase and SDH play important roles in the respiratory chain. Thus, we further investigated the effect of C. longa on NADH oxidase and SDH activities of F. graminearum. NADH oxidase is a redox enzyme that can directly oxidize NADH to NAD+ in the presence of oxygen, and it plays an important role in regulating the metabolism of microorganisms. The initial study of NADH oxidase showed that it plays an important role in oxidative stress and can be used as a scavenger of intracellular oxygen [57, 58]. SDH is a binding enzyme of the mitochondrial inner membrane, which belongs to the membrane-bound enzyme, and it is one of the hubs that connects electron transfer and oxidative phosphorylation, which provides an electron to the respiratory chain in mitochondria and contributes to a variety of prokaryotic cell oxygen demands and capacity. SDH is a marker enzyme for the mitochondria. SDH is also an important enzyme in the energy metabolism of microbial cells, and its activity is a sensitive marker of cell energy metabolism [59]. Pyrrolnitrin has been reported to inhibit Bacillus megaterium primarily by forming complexes with phospholipids and to block electron transfer of Saccharomyces cerevisiae between succinate or reduced nicotinamide adenine dinucleotide (NADH) and coenzyme Q [57]. The finding revealed that C. longa could suppress the activity of NADH oxidase and SDH, which might interfere with the TCA cycle and inhibit the ATP synthesis in the mitochondria of F. graminearum, suggesting that there are inhibitory sites on the respiratory chain of C. longaon F. graminearum.
This study showed that the ethanol extract of C. longa can disrupt the synthesis of critical proteins and enzymes, which may ultimately inhibit the growth of fungi. The antifungal effects were found to be related to the disruption of fungal cell membrane systems, specifically the inhibition of ergosterol synthesis and the respiratory chain. Future studies are needed to develop the target of each chemical component of C. longa and to determine the cost-benefit balance and to develop the components into series of environmentally sustainable biofungicides.
Acknowledgments
This work was supported by the project of the National Key Research and Development Program of China (2017YFD020201105), the Sichuan Province Scientific Support Program (2016KZ0006, 2012ZZ0001) and the Chengdu Scientific Research Plan (2015-NY02-00100-NC).
Data Availability
All relevant data are within the paper. All deep-sequencing dataset are available from the NCBI GeneBank database (accession number(s) chenciqiong, 57563241).
Funding Statement
This work was supported by National Key Research and Development Program of China (2017YFD020201105), the Sichuan Province Scientific Support Program (2016KZ0006, 2012ZZ0001) and the Chengdu Scientific Research Plan (2015-NY02-00100-NC). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Aqueveque P, Céspedes CL, Becerra J, Aranda M, Sterner O. Antifungal activities of secondary metabolites isolated from liquid fermentations of Stereum hirsutum (Sh134-11) against Botrytis cinerea (grey mould agent). Food and Chemical Toxicology. 2017; 109 (2):1048–1054. [DOI] [PubMed] [Google Scholar]
- 2.Kumar KN, Venkataramana M, Allen JA, Chandranayaka S, Murali HS, Batra HV. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT- Food Science and Technology. 2016; 69:522–528. [Google Scholar]
- 3.Pitt JI, & Hocking AD. Fungi and food spoilage. Dordrecht, Heidelberg, London, New York: Springer; 2009; (3rd ed, p. 519). [Google Scholar]
- 4.Bautista-Baños S, Sivakumar D, Bello-Pérez A, Villanueva-Arce R, Hernández-López M. A review of the management alternatives for controlling fungi on papaya fruit during the postharvest supply chain. Crop Protection. 2013; 49 (7):8–20. [Google Scholar]
- 5.Madden LV, Hughes G, Bosch FVD. The Study of plant disease epidemics. Aps Press American Phytopathological Society. 2007. [Google Scholar]
- 6.Goswami RS, Kistler HC. Heading for disaster: Fusarium graminearum on cereal crops. Molecular Plant Pathology. 2004; 5(6): 515–25. doi: 10.1111/j.1364-3703.2004.00252.x [DOI] [PubMed] [Google Scholar]
- 7.Céspedes CL, Salazar JR, Ariza-Castolo A, Yamaguchi L, Avila JG., Aqueveque P, et al. Biopesticides from plants: Calceolaria integrifolia s.l. Environmental Research. 2014; 132: 391–406. doi: 10.1016/j.envres.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues AM., Theodoro PN, Eparvier V, Basset C, Silva MR, Beauchêne J, et al. Search for antifungal compounds from the wood of durable tropical trees. Journal of Natural Products. 2010; 73(10):1706–1707. doi: 10.1021/np1001412 [DOI] [PubMed] [Google Scholar]
- 9.Radwan MM, Tabanca N, Wedge DE, Tarawneh AH, Cutler SJ. Antifungal compounds from turmeric and nutmeg with activity against plant pathogens. Fitoterapia. 2014; 99:341–346. doi: 10.1016/j.fitote.2014.08.021 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen VN, Seo DJ, Park RD, Jung WJ. Antimycotic activities of Cinnamon-derived compounds against Rhizoctonia solani in vitro. Biocontrol. 2009; 54(5):697–707. [Google Scholar]
- 11.Chen IN, Chang CC, Ng CC, Wang CY, Shyu YT, Chang TL. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Foods Hum Nutr. 2008; 63(1):15–20. doi: 10.1007/s11130-007-0063-7 [DOI] [PubMed] [Google Scholar]
- 12.Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J Tradit Complement Med. 2017; 7(2):205–233. doi: 10.1016/j.jtcme.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Changtam C, Koning HP, Ibrahim H, Sajid MS, Gould MK, Suksamrarn A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur J Med Chem. 2010; 45(3): 941–956. doi: 10.1016/j.ejmech.2009.11.035 [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Mahajan S, Sharma R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnology Reports. 2015; 6: 51–55. doi: 10.1016/j.btre.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avanço GB, Ferreira FD, Bonfim NS, Peralta RM, Brugnari T, Mallmann CA, et al. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control. 2017; 73(B): 806–813. [Google Scholar]
- 16.Hu Y, Zhang J, Kong W, Zhao G, Yang M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chemistry. 2017; 220:1–8. doi: 10.1016/j.foodchem.2016.09.179 [DOI] [PubMed] [Google Scholar]
- 17.Singh G, Singh OP, Maurya S. Chemical and biocidal investigations on essential oils of some Indian Curcuma species. Progress in Crystal Growth and Characterization of Materials. 2002; 45(1–2):75–81. [Google Scholar]
- 18.Martinez-Correa HA, Paula JT, Kayano ACAV, Queiroga CL, Magalhães PM, Costa FTM, et al. Composition and antimalarial activity of extracts of Curcuma longa L. obtained by a combination of extraction processes using supercritical CO2, ethanol and water as solvents. The Journal of Supercritical Fliuds. 2017; 119: 122–129. [Google Scholar]
- 19.Dao TT, Nguyen PH, Won HK, Kim EH, Park J, Won BY, et al. Curcuminoids from Curcuma longa and their inhibitory activities on influenza A neuraminidases. Food Chem. 2012; 134(1): 21–28. [Google Scholar]
- 20.Priya R, Prathapan A, Raghu KG, Menon AN. Chemical composition and in vitro antioxidative potential of essential oil isolated from Curcuma longa L.leaves. Asian Pac.J.Trop.Biomed. 2012; 2, S695–S699. [Google Scholar]
- 21.DiasFerreira F, Mossini SA, DiasFerreira FM, Arrotéia CC, Costa CL, Nakamura CV, et al. The inhibitory effects of Curcuma longa L. essentialoil and curcumin on Aspergillus flavus Link growth and morphology. The Scientific World Journal. 2013; Article ID 343804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long ZF, Wei Y, Cui J, Liu SG, Wang LH. Active constituent of Curcuma longa for inhibiting and killing plant pathogenic fungi and its preparation and application. 2007; CN200710049042.4. (in Chinese) [Google Scholar]
- 23.Liu HH, Wei Y, Cui J, Huang YY, Wang LH, Liu SG, et al. Antifungal activities of the extracts from Curcuma phaeocaulis against Phoma wasabiae. Journal of Sichuan University (Natural Science Edition). 2008; 45 (5):1235–1238. (in Chinese) [Google Scholar]
- 24.Li HF, Wei Y, Long ZF, Huang YY, Cui J. Activity and chemical analyses of the hexane extract from Curcuma phaeocaulis against pathogenic fungi. Journal of Sichuan University (Natural Science Edition). 2011; 48 (1):191–195. (in Chinese) [Google Scholar]
- 25.Chen ZZ, Wei Y, Li X, Peng CC, Long ZF. Antifungal activity and mechanism of major compound isolated from hexane extract of Curcuma longa. Asian J. Chem. 2013; 25(12): 6597–6600. [Google Scholar]
- 26.Hamdi OAA, Awang K, Hadi AHA, Syamsir DR, Ng SW. Curcumenol from Curcuma zedoaria: a second monoclinic modification. Acta Crystallographica. 2010; 66(11): o2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou XL, Hayashi-Nakamura E, Takatani-Nakase T, Tanaka K, Takahashi K. Curdione plays an important role in the inhibitory effect of Curcuma aromatica on CYP3A4 in Caco-2 cells. Evidence-based Complementary and Alternative Medicine. 2011; 913898 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itokawa H, Shi Q, Akiyam T, Morris-Natschke SL, Lee KH. Recent advances in the investigation of curcuminoids. Chinese Medicine. 2008; 3(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakshmi S, Padmaj G, Remani P. Antitumour effects of isocurcumenol isolated from Curcuma zedoaria rhizomes on human and murine cancer cells. International Journal of Medicinal Chemistry. 2011; 2011: 253962 doi: 10.1155/2011/253962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y, Hang TJ, Zhang ZX, An DK. Comparison of curcumol contents in essential oil from four species of rhizoma Curcumae L. Chinese Traditional & Herbal Drugs. 2001; 32(7):600–602. (in Chinese) [Google Scholar]
- 31.Li SY, Yuan W, Deng GR, Wang P, Yang PY.Aggarwal BB. Chemical composition and product quality control of turmeric (Curcuma longa L.).Pharmaceutical Crops. 2011; 2(2):28–54. [Google Scholar]
- 32.Su X, Ma LJ, Chen AL, Zhang LQ. Antifungal and antibacterial activity of extracts from the husk of Carya cathayensis. Journal of Zhejiang Forestry College. 2008; 25(3):405–410. (in Chinese) [Google Scholar]
- 33.Nugroho A, Rohman A, Lukitaningsih E, Rakhmawati N, Sudjadi. Analysis of curcumin in ethanolic extract of Curcuma longa Linn. and Curcuma xanthorriza Roxb.,using high performance liquid chromatography with UV-detection. 2015; 9 (4): 188–194. [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976; 72(1~2):248–54. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Ge Q, Zhang J, Zhou J, Xu J. Proteomic analysis of Cd-responsive proteins in Solanum torvum. Plant Molecular Biology Reporter. 2013; 31(2):485–491. [Google Scholar]
- 36.Rodriguez-Celma J, Rellan-Álvarez R, Abadia A, Abadia J, Lopez-Millan AF. Changes induced by two levels of cadmium toxicity in the 2-DE protein profile of tomato roots. Journal of Proteomics.2010; 73(9):1694–1706. doi: 10.1016/j.jprot.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Wang Z, Wu R, Jiang D, Bai B, Tan D,et al. Proteomic analysis of the antibacterial mechanism of action of Juglone against Staphylococcus aureus. Natural Product Communications. 2016; 11(6):825–827. [PubMed] [Google Scholar]
- 38.Niemenmaa O, Galkin S, Hatakka A. Ergosterol contents of some wood-rotting basidiomycete fungi grown in liquid and solid culture conditions. International Biodeterioration & Biodegradation. 2008; 62 (2):125–134. [Google Scholar]
- 39.Dohnal V, Kaderová I, Ježková A, Skládanka J. Ergosterol content in selected grasses on the end of vegetation period. Acta Univ Agric Silvic Mendel Brun. 2007; 55(4): 9–14. [Google Scholar]
- 40.Chen YF, Huang KL, Gao JH, Ning ZX. Inhibition of the respiration of microorganism by α-bromo cinnamaldehyde and alkenoic acid estes. Food and Fermentation Industries. 1994; (3):26–29.(in Chinese) [Google Scholar]
- 41.Sanit S. Antifungal activity of crude extracts of some medicinal plants against Fusarium sp., the pathogen of dirty panicle disease in rice. Journal of medicinal plants research. 2016; 10 (19):248–255. [Google Scholar]
- 42.Chen Q, Liu QY, Chen CQ, Liu HM, Long ZF. Antifungal activity and GC/MS analysis of extracts from Curcuma phaeocaulis stems and leaves against plant pathogenic fungi. Journal of Sichuan University (Natural Science Edition). 2017; 54(1): 209–214 (in Chinese). [Google Scholar]
- 43.Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Research International. 2014; 2014: 186864 doi: 10.1155/2014/186864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniguchi S, Miyoshi S, Tamaoki D, Yamada S, Tanaka K, Uji Y,et al. Isolation of jasmonate-induced sesquiterpene synthase of rice: Product of which has an antifungal activity against Magnaporthe oryzae. Journal of Plant Physiology. 2014; 171 (8):625 doi: 10.1016/j.jplph.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 45.Kong Q, Sun F, Chen XP. Impact of fixed-dose combination of germacrone, curdione, and furanodiene on breast cancer cell proliferation. Cell Journal. 2013; 15 (2):160–165. [PMC free article] [PubMed] [Google Scholar]
- 46.Yang FQ, Wang YT, Li SP. Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and high-performance liquid chromatography. J Chromatogr A. 2006; 1134(1–2): 226–231. doi: 10.1016/j.chroma.2006.09.048 [DOI] [PubMed] [Google Scholar]
- 47.Meng S. Studies on antifungal activity and mechanism of bio-active components from Allium chinense. Hunan Normal University; 2006. (in Chinese). [Google Scholar]
- 48.Boradia VM, Raje M, Raje CI. Protein moonlighting in iron metabolism: glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Biochemical Society Transactions. 2014; 42 (6): 1796–1801. doi: 10.1042/BST20140220 [DOI] [PubMed] [Google Scholar]
- 49.Yon JM, Desmadril M, Betton JM, Minard P, Ballery N, Missiakas D, et al. Flexibility and folding of phosphoglycerate kinase. Biochimie. 1990; 72(6–7): 417–429. [DOI] [PubMed] [Google Scholar]
- 50.Hu LB, Zhou W, Zhang T, Yang ZM, Xu JH, Shi ZQ. Mechanism of inhibition to Fusarium moniliforme by antimicrobial peptide Fengycins. Microbiology China. 2010; 37(2):251–255. [Google Scholar]
- 51.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005; 438 (7068): 612~621. doi: 10.1038/nature04399 [DOI] [PubMed] [Google Scholar]
- 52.Munro S. Lipid rafts: elusive or illusive? Cell. 2003; 115(4): 377–388. [DOI] [PubMed] [Google Scholar]
- 53.Georgopapadakov NH, Walsh TJ. Human mycoses: Drugs and targets for emerging pathogens. Science.1994; 264 (15):371. [DOI] [PubMed] [Google Scholar]
- 54.Bentz BJ, Six DL. Ergosterol content of fungi associated with Dendroctonus ponderosae and Dendroctonus rufipennis. Annals of the Entomological Society of America. 2009; 99(2):189–194. [Google Scholar]
- 55.Ghannoum MA, Rice LB. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clinical Microbiology Reviews, 1999, 12 (4):501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong DT, Horng JS, Gordee RS. Respiratory Chain of a Pathogenic Fungus, Microsporum gypseum: Effect of the Antifungal Agent Pyrrolnitrin. Journal of Bacteriology. 1971; 106 (1):168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higuchi M, Yamamoto Y, Kamio Y. Molecular biology of oxygen tolerance in lactic acid bacteria: Function of NADH oxidase and Dpr oxidative stress. Bioscience and Bioengineering. 2000; 90 (5): 484–493. [PubMed] [Google Scholar]
- 58.Felipe FL, Hugenholtz J. Purification and characterization of the water forming NADH-oxidase from lactococcus. International Dairy Journal. 2001; 11(1):37–44. [Google Scholar]
- 59.Piasecka M, Wenda-Rózewicka L, Ogonski T. Computerized analysis of cytochemical reactions for dehydrogenases and oxygraphic studies as methods to evaluate the function of the mitochondrial sheath in rat spermatozoa. Andrologia. 2001; 33(1):1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper. All deep-sequencing dataset are available from the NCBI GeneBank database (accession number(s) chenciqiong, 57563241).