Abstract
The concept of tissue-restricted differentiation of postnatal stem cells has been challenged by recent evidence showing pluripotency for hematopoietic, mesenchymal, and neural stem cells. Furthermore, rare but well documented examples exist of already differentiated cells in developing mammals that change fate and trans-differentiate into another cell type. Here, we report that endothelial cells, either freshly isolated from embryonic vessels or established as homogenous cells in culture, differentiate into beating cardiomyocytes and express cardiac markers when cocultured with neonatal rat cardiomyocytes or when injected into postischemic adult mouse heart. Human umbilical vein endothelial cells also differentiate into cardiomyocytes under similar experimental conditions and transiently coexpress von Willebrand factor and sarcomeric myosin. In contrast, neural stem cells, which efficiently differentiate into skeletal muscle, differentiate into cardiomyocytes at a low rate. Fibroblast growth factor 2 and bone morphogenetic protein 4, which activate cardiac differentiation in embryonic cells, do not activate cardiogenesis in endothelial cells or stimulate trans-differentiation in coculture, suggesting that different signaling molecules are responsible for cardiac induction during embryogenesis and in successive periods of development. The fact that endothelial cells can generate cardiomyocytes sheds additional light on the plasticity of endothelial cells during development and opens perspectives for cell autologous replacement therapies.
Tissues that are not normally renewed, such as brain or striated muscle, were not thought to contain true stem cells and thus were believed to be poorly capable or incapable of regenerating after an injury.
In recent years, work from several laboratories has contributed to change this concept radically. Several investigators have succeeded in isolating and expanding neural stem cells that can generate neurons, oligodendrocytes, and astrocytes from fetal and adult brain (reviewed in refs. 1 and 2). Furthermore, neural stem cells, as well as hematopoietic and mesenchymal stem cells, can give rise to different tissues such as liver, brain, blood, or skeletal muscle, suggesting the presence of one or more types of truly pluripotent stem cells (refs. 3–13; see also ref. 14 for a recent review). The complete repertoire of the developmental options of a given stem cell is not yet known, although new examples are being accumulated at an impressive rate.
On the other hand, environmentally dictated changes of fate (trans-determination) are not restricted to stem cells and may involve progenitor cells at different steps of a given differentiation pathway (trans-differentiation). Examples of this latter process are known to occur in the mammalian esophagus and in the chick iris, where smooth muscle cells trans-differentiate to skeletal muscle (15, 16), or in vessels where endothelial cells trans-differentiate to pericytes/smooth muscle cells (17).
Although the understanding of the molecular control and of the developmental significance of these processes awaits further experimental work, the possibility of using stem/progenitor cells for tissue-specific cell therapy opens exciting perspectives for future clinical application. In this context, heart tissue is obviously a major target, considering that lesions of the myocardium are among the most common causes of death in the Western world. Although in the past there have been occasional and unsubstantiated reports of reserve cells in adult mammalian heart, it is generally assumed that the pool of cardiomyocytes is established shortly after birth, when proliferation ceases and, thereafter any loss of myocardial tissue cannot be repaired. Increasing vascularization to prevent further cell death is the leading strategy in this kind of research (18). Very recently, it has been reported that bone marrow hemangioblasts contribute new vessels to the postischemic myocardium (19) and, strikingly, that c-Kit-positive bone marrow hematopoietic stem cells differentiate into cardiomyocytes, endothelium, and smooth muscle when injected into a postischemic ventricular wall (20). Here, we report that both endothelial progenitors in the embryo and differentiated endothelial cells from the umbilical vein can differentiate into beating cardiomyocytes when cocultured with neonatal rat cardiomyocytes or when injected near the damaged area of the heart after occlusion of a coronary vessel. This kind of trans-differentiation, which seems to be independent from signaling molecules active in embryogenesis, widens the concept of myocardial regeneration and opens new perspectives for cell therapy in heart diseases.
Materials and Methods
Cell Culture.
Primary endothelial embryonic cells (EEC) were isolated from explants of mouse dorsal aorta from embryonic day 9 as described (21). For clonal expansion, cells were plated at a concentration of 104 per 60-mm dish on a feeder layer of mitomycin C (2 μg/ml)-treated STO cells. Clonal isolates were collected and tested by reverse transcription–PCR for the expression of VE-cadherin, CD34, Myf-5, and MyoD as described (21). More than 90% of the selected clones were positive for endothelial markers and negative for myogenic markers. Clones were used between the second and third passages.
The 44B endothelial cell line (22), derived from murine embryonic stem cells, H5V endothelial cells (23), derived from mouse heart, and 1G11 endothelial cells (24), isolated from adult mouse lung, were grown as described. Neural stem cells, isolated from the MLC1F/3F-nLacZ mouse strain, were grown as described (10). Human umbilical vein endothelial cells (HUVEC) were isolated and grown as described (25). STO and 3T3 mouse fibroblasts were grown in DMEM supplemented with 10% FCS.
Primary neonatal rat cardiomyocytes were isolated as described (26) and plated at 2 × 105 cells per ml, treated with mitomycin C (2 μg/ml to prevent overgrowth of contaminating fibroblasts), and used for the coculture experiments.
Endothelial cells (either genetically labeled or previously infected with lentiviral or adenoviral vectors; see below) were added to the cardiomyocytes at a 1:4 ratio in DMEM containing 10% FCS. After 2 days, the cultures were shifted to DMEM containing 5% horse serum and at various periods thereafter were processed for analysis.
When indicated, endothelial cells were treated with different concentrations of bone morphogenetic protein 4 (BMP4; either 1 or 10 nM) and fibroblast growth factor 2 (FGF2; either 1 or 10 nM) for different periods and thereafter were stained for the expression of myosin heavy chain (MyHC).
For the coupling experiment, rat cardiomyocytes were labeled with 10 nM 6′-carboxyfluorescein for 10 min at 37°C in serum-free medium. The cultures were washed extensively with serum-containing medium and then endothelial cells, labeled with an adenoviral vector containing the β-galactosidase gene, lacZ, were added. After 4 days, cultures were fixed and stained with an anti-β-galactosidase antibody.
Vectors.
An adenoviral vector carrying the lacZ reporter gene was used as previously described (27). The third-generation lentiviral vector pRRLsin.PPT-PGK.GFPpre expressing green fluorescent protein (GFP) was used as described (28).
In Vivo Analysis.
For in vivo assay, 2 × 105 GFP-labeled endothelial cells or 3T3 fibroblasts were injected into the myocardial layer of adult NOD-SCID mice in a total volume of 2 μl, using a 29-gauge needle adapted to a Hamilton syringe. After 2 weeks, the heart was removed, and cryostat sections were stained with MF20 antibody. Heterotopic cardiac transplantations in mice were performed as described by Corry et al. (29). The recipient NOD-SCID mice were anesthetized with ketamine (35 mg/kg) i.p. + xylazine (5 mg/kg) i.p. The aorta and vena cava were separated between the renal vessels and the bifurcation of the iliac vessels. The donor heart was harvested from a NOD-SCID mouse, and the left descending coronary artery was cauterized to induce ischemic damage. Immediately afterward, GFP-labeled, aorta-derived cells were injected into the heart parenchyma near the lesion site or into an uninjured heart (as a control) with a microsyringe fitted with a 29-gauge needle. The ascending aorta of the donor heart was then connected by an end-to-side anastomosis with the recipient aorta. A similar anastomosis was also created between the recipient vena cava and the superior vena cava of the donor heart. The total ischemic time averaged 15 min. Heterotopic cardiac survival was monitored by direct palpation of the heartbeat through the abdominal wall.
Cytochemistry.
β-Galactosidase activity was detected at the light microscopy level as described (30). For detection of β-galactosidase activity at the electron microscopy level, cells were fixed with glutaraldehyde (2.5%) and paraformaldehyde (1%) in 0.1 M PBS at pH 7.4 for 1.5 h. After extensive rinsing in PBS, the cells were stained in histochemical Bluo-Gal solution at 30°C overnight. The Bluo-Gal reaction mixture contains 1 mg/liter 5-bromo-4-chloro-3-indolyl β-galactopyranoside (X-Gal), 10 mM potassium ferricyanide, 10 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS. The cells then were postfixed in 1% OsO4 in 0.2 M collidine buffer (pH 7.4) for 1.5 h and stained with 2% uranyl acetate for 45 min. After rinsing and dehydration in alcohol and propylene oxide, the samples were embedded in epoxy resin. Ultrathin sections (80 nm) were examined and photographed with a Zeiss EM109 transmission electron microscope. For immunocytochemistry, the following antibodies were used: a monoclonal (MF20) and a polyclonal anti-MyHC (30), anti-cardiac troponin I monoclonal (31) donated by S. Schiaffino (Dept. of Pathology, Univ. of Padua), and anti-von Willebrand factor from PharMingen. Immunocytochemistry on tissue sections and cultured cells was carried out as described (30).
Reverse Transcription–PCR Analysis.
Reverse transcription–PCR was performed as described (32). Oligonucleotides used for amplification of the following genes were as follows: VE-cad (227 bp), 5′-GGATGCAGAGGCTCACAGAG-3′ and 3′-CTGGCGGTTCACGTTGGACT-5′; for Flk-1 (270 bp), 5′-TCTGTGGTTCTGCGTGGAGA-3′ and 3′-GTATCATTTCCAACCACCCT-5′; for CD34 (300 bp), 5′-TTGACTTCTGCAACCACGGA-3′ and 3′-TAGATGGCAGGCTGGACTTC-5′; for Myf-5 (132 bp), 5′-GAGCTGCTGAGGGAACAGGTGGAGA-3′ and 3′-GTTCTTTCGGGACCAGACAGGGCTG-5′; for MyoD (144 bp), 5′-CACTACAGTGGCGACTCAGACGCG-3′ and 3′-CCTGGACTCGCGCACCGCCTCACT-5′.
Results
Endothelial Cells Isolated from the Embryonic Dorsal Aorta Can Differentiate into Cardiomyocytes.
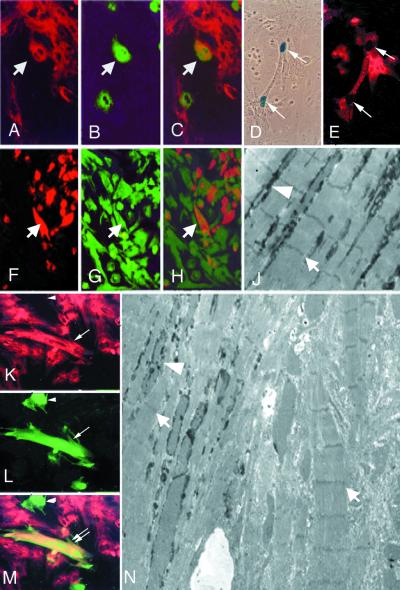
Clonal isolates from the embryonic aorta (21) were expanded on a feeder layer of mitomycin C-treated STO fibroblasts. Under these experimental conditions, the EEC express endothelial markers such as Flk-1, VE-cadherin, and CD34 but not myogenic markers such as Myf5 or MyoD (data not shown). During their growth, the cells were infected with the lentiviral vector pRRLsin.PPT.PGK.GFPpre (28) expressing GFP. More than 90% of the population expressed GFP after a single round of infection. Fluorescent cells were then mixed with a 4-fold excess of neonatal rat cardiomyocytes and cultured for 5 days. After this period, cultures were fixed and stained with MF20 antibody that recognizes sarcomeric MyHCs. Fig. 1 A–C shows that, although the majority of GFP-expressing cells did not express sarcomeric myosin, several cells (≈10% of the fluorescent population) were double labeled. Because the lentiviral vector is replication incompetent, the double-labeled cells represent aorta-derived, endothelial progenitors that have differentiated into striated muscle. Almost invariably, these double-labeled cells were adjacent to or intermixed with cardiomyocytes, suggesting that cell–cell contact is needed for the activation of myogenesis in these progenitors; indeed, medium conditioned from cardiomyocyte cultures did not induce myogenesis in aorta-derived clonal isolates (not shown). For ultrastructural studies, EEC were infected with an adenoviral vector expressing the lacZ reporter gene (27) and then mixed with unlabeled neonatal rat cardiomyocytes. Electron microscopy analysis of cocultures revealed well developed sarcomeres and junctions in many clustered cells, several of which were labeled with Bluo-Gal. Fig. 1N shows an example of two adjacent cardiomyocytes, only one of which shows electron-dense precipitates in the cytoplasm. A magnification of the same field (Fig. 1J) reveals the presence of Bluo-Gal precipitates among the well developed sarcomeres.
Figure 1.
In vitro cardiac differentiation of endothelial cells. (A–C) Double fluorescence of a coculture of neonatal rat cardiomyocytes, stained with anti-MyHC MF20 (red in A) with endothelial progenitors labeled with a GFP lentiviral vector (green in B). Arrows indicate a double-labeled cell (orange in the merged figure, C). (D and E) Coculture of endothelial progenitors, freshly isolated from MLC1/3F-nLacZ day 9 embryos with neonatal rat cardiomyocytes, stained with X-Gal (D) and with anti-cardiac troponin I (E). Arrows indicate a binucleated cell expressing β-galactosidase in the nucleus and cardiac troponin I in the cytoplasm. (F–H) Double fluorescence of a coculture of neonatal rat cardiomyocytes, prelabeled with 6′-carboxyfluorescein (green in G), with endothelial progenitors labeled with an adenoviral vector expressing lacZ and stained with an anti-β-galactosidase antibody (red in F). Arrows indicate one of several endothelial cells where fluorescein has entered through open junctions (orange in the merged figure, H). (K–M) Double fluorescence of a coculture of neonatal rat cardiomyocytes, stained with anti-MyHC MF20 (red in K) with HUVEC labeled with a GFP lentiviral vector (green in L). The double arrow indicates one double-labeled cell (orange in the merged figure, M), whereas the arrowhead indicates a HUVEC that has not trans-differentiated. (N) Electron micrograph showing two close cells, both containing sarcomeres (arrows), one of which is also labeled with Bluo-Gal (arrowhead), whereas the other is not (×3,000). (J) Higher magnification (×5,000) of an area of N of a cell showing Bluo-Gal labeling (arrowhead) among sarcomeres (arrow).
Because MF20 antibody recognizes sarcomeric myosin in both skeletal and cardiac muscle, we analyzed the cocultures for the expression of cardiac-specific markers. Furthermore, because expansion in culture may alter the potency of freshly isolated cells, we repeated the experiments by isolating and pooling individual clones (each clone containing ≈100–200 cells under these conditions) and cocultured about 1,000 EEC with 4,000 cardiomyocytes in microspot cultures. Clones had been derived from MLC1F/3F-nLacZ embryos, expressing the n-LacZ reporter gene only in the nuclei of differentiated muscle (33). The cocultures were stained with X-Gal and reacted with a monoclonal antibody directed against cardiac-specific troponin I, one of the few sarcomeric proteins expressed in cardiac but not in skeletal muscle (31). Fig. 1 D and E shows a binucleated cell expressing nuclear β-galactosidase and cardiac troponin I in the cytoplasm. Because expression of the reporter gene is cell autonomous, the cell indicated must derive from the embryonic aorta and, because it coexpresses a cardiac marker, it has differentiated into a cardiomyocyte.
Functional evidence of cardiac differentiation is provided by electrical coupling of cardiac cells, through the formation of active gap junctions. To test this possibility, neonatal rat cardiomyocytes were prelabeled with 6′-carboxyfluorescein, a membrane-permeant dye that is esterified inside cells and cannot therefore recross the plasma membrane (34). Fluorescein-labeled cardiomyocytes were then cocultured with EEC that had been previously labeled with an adenoviral vector expressing the lacZ reporter gene (27). Fig. 1 F–H shows that in these cultures several cells express both fluorescein and β-galactosidase, indicating that β-galactosidase-positive endothelial progenitors have been electrically coupled to fluorescein-containing cardiomyocytes. The specificity of this coupling was verified by exposing sister dishes to the phorbol ester phorbol 12-myristate 13-acetate (10−8 M), which is known to block gap junctions; in this case, no double-labeled cells were detected (data not shown).
Endothelial Cells Contribute to Myocardium Formation in a PostIschemic Heart.
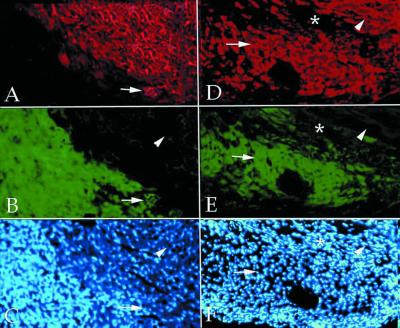
To test whether endothelial embryonic cells are also capable of differentiating into cardiomyocytes in an in vivo environment, 2 × 105 GFP-labeled endothelial progenitors were injected into the left ventricle of adult NOD-SCID mice. After 2 weeks, the mice were killed and the heart was sectioned in a cryostat. Each section was stained with MF20. Fig. 2 shows that injected fluorescent cells were detected in a relatively coherent mass along the track of the needle, usually located under the epicardium and sometimes between different myocardial layers, closely adhering to cardiomyocytes (Fig. 2 A and B). The arrow shows a rare example of two double-labeled cells that had differentiated into myosin-positive cardiomyocytes in vivo. To test whether cardiac differentiation of endothelial cells would result in significant tissue repair of postischemic myocardium, 2 × 105 GFP-labeled endothelial progenitors were injected into the left ventricle of adult NOD-SCID mice where the left anterior descending coronary had been cauterized. After injection of the cells, the heart was transplanted into the abdomen of a recipient NOD-SCID mouse, connecting the ascending aorta of the donor heart with the recipient aorta, and the superior vena cava with the recipient vena cava. After 2 weeks, the transplanted heart was removed, and cryostat sections were stained with anti-MyHC. As shown in Fig. 2 D and E, in the area of injection the majority of GFP-labeled cells had differentiated into myosin-positive cells, with the typical shape of cardiomyocytes, indicating that new myocardium had been produced by injected endothelial cells. In transplanted control hearts (that had not been infarcted), very few of the injected cells expressed MyHCs, as in control hearts in situ (data not shown).
Figure 2.
In vivo cardiac differentiation of endothelial cells. Double fluorescence of a section of normal mouse heart (A–C) and of an infarcted mouse heart (D–F), 2 weeks after injection of endothelial progenitors labeled with a GFP lentiviral vector (green in B and E). The sections have been stained with anti-MyHC MF20 (red in A and D). Nuclear staining (Hoechst) is shown in C and F. In the uninjured heart, very few double-labeled cells could be detected (arrows in A–C). In contrast, a large number of double-labeled cells were present in the infarcted heart (arrows in D–F) in the area of injection; arrowheads indicate normal myocardium, and asterisks indicate an infarcted area devoid of injected cells.
Different Types of Endothelial Cells Exhibit Different Potential for Conversion into Cardiomyocytes.
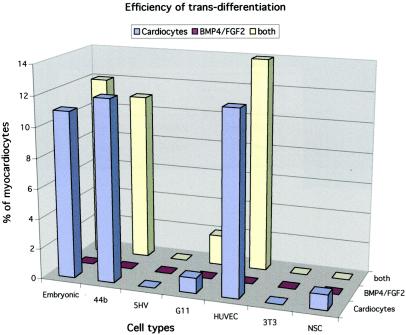
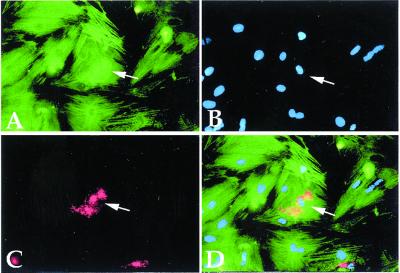
To investigate how widespread this potential is among other endothelial cell types as well as in other types of stem cells, we infected with GFP-expressing lentiviral vector the 44B endothelial cell line (22) derived from murine embryonic stem cells, H5V endothelial cells (23) derived from mouse heart, and 1G11 endothelial cells (24) isolated from adult mouse lung. We also infected neural stem cells isolated from the same MLC1F/3F-nLacZ mouse strain that had been previously shown to differentiate efficiently into skeletal muscle (10). As a negative control, we infected 3T3 mouse fibroblasts. Finally, to test whether this capacity was also present in human cells, we similarly infected early-passage HUVEC. After infection, all cell lines were cocultured with neonatal rat cardiomyocytes, and the number of cardiomyocytes expressing GFP was counted in 30 randomly selected microscopic fields and expressed as a percentage of the test (GFP+) cell population. Fig. 3 shows that the potential to generate cardiomyocytes was present also in the 44B cells, derived from embryonic stem cells and in the human HUVEC (Fig. 1 K–M). It was much reduced in 1G11 and in neural stem cells, and it was practically absent in H5V and in 3T3 fibroblast cells. The observation that HUVEC can also differentiate into cardiomyocytes is intriguing as these are relatively well differentiated endothelial cells, expressing markers characteristic of mature endothelium. Trans-differentiation is normally verified by the transient coexpression of markers of two different cell types in the same cytoplasm. We thus double-stained HUVEC at successive days of coculture with rat cardiomyocytes with an antibody that recognizes von Willebrand factor and another antibody that recognizes sarcomeric myosin. Whereas for the first 2 days of coculture we could not detect double-labeled cells, starting on day 3, we observed numerous large, flat cells that reacted strongly with the anti-von Willebrand factor antibody and that contained well aligned sarcomeres in the same cytoplasm (Fig. 4). At later days of coculture, double-labeled cells became rare, and only occasionally did we detect fully differentiated cardiomyocytes, with a strong reactivity to von Willebrand factor antibody, localized in a single perinuclear area rather than dispersed throughout the cytoplasm (data not shown). This observation suggests that the gene encoding von Willebrand factor had been down-regulated in the trans-differentiated cardiomyocytes, and the residual protein was in the process of being degraded.
Figure 3.
In vitro cardiac differentiation of different types of endothelial and nonendothelial cells. Endothelial progenitors from embryonic aorta (EEC) and from embryonic stem cells (44b), endothelial cells from adult heart (5HV) and lung (1G11), and from human umbilical vein (HUVEC), mouse fibroblasts (3T3), and neural stem cells (NSC) were infected with the GFP-expressing vector and cocultured with neonatal rat cardiomyocytes as described in Materials and Methods. Cardiac differentiation in cocultures was scored by counting double-labeled cells (GFP+/MyHC+) in 30 randomly selected fields and expressing the number obtained as a percentage of total GFP+ cells. Data are the average of at least three separate experiments with SE ranging within 10% of the mean. In a separate set of experiments, the same cells were exposed to BMP4 and FGF2 as detailed in Materials and Methods, and after 5 days they were stained for the expression of MyHC. No positive cells were scored in two separate experiments. Finally, in one experiment, the same molecules were added to cocultures of the same cell lines with cardiomyocytes.
Figure 4.
In vitro cardiac trans-differentiation of endothelial cells. Double fluorescence of a coculture of neonatal rat cardiomyocytes with HUVEC, stained with anti-MyHC polyclonal antibody (green in A), with anti-von Willebrand factor monoclonal antibody (red in C). Nuclear staining (Hoechst) is shown in B. The arrow indicates von Willebrand factor-containing granules in the cytoplasm of a differentiated cardiomyocyte (orange in the merged figure, D).
Cardiac Differentiation of Endothelial Cells Is Regulated by Different Signals Than Those Activating Embryonic Cardiogenesis.
In the embryo, signals emanating from the underlying endoderm activate master regulatory genes such as GATA4 and Nkx-2.5 in the cranial splanchnic mesoderm, resulting in cellular commitment to cardiogenesis. These signals can be replaced by BMP2 or BMP4 and FGF2 or FGF4 but not by other members of these families of signaling molecules (35). We thus treated endothelial cells with various concentrations of BMP4 and FGF2 but in no case observed any cell that had differentiated into a cardiomyocyte. We also added these molecules to cocultures of endothelial progenitors and rat cardiomyocytes and did not observe any increase in the number of endothelial cells recruited to a cardiac fate (Fig. 3). Together, these data indicate that the signals that induce myocardial differentiation in endothelial cells are different from those that activate cardiogenesis in the early embryo.
Discussion
Endothelial Cells May Differentiate into Cardiomyocytes in Vitro and in Vivo.
Data reported in this article demonstrate that clonal isolates of endothelial cells derived from embryonic vessels are capable of forming functional cardiomyocytes when in the presence of differentiated cardiomyocytes. This is demonstrated by activation of cardiac-specific genes, development of well differentiated sarcomeres, electrical coupling with cardiomyocytes, and in vivo differentiation into myocardium when injected into a postischemic ventricular wall. When different types of endothelial cells were used in this assay, cells derived from embryonic or neonatal stages appeared capable of generating cardiomyocytes, whereas endothelial cells isolated from adult animals were not. This suggests that this kind of plasticity may be age-dependent and progressively lost during postnatal life. Still, the fact that well differentiated endothelial cells such as HUVEC can still give rise to cardiomyocytes indicates that these cells can trans-differentiate into mature myocardium. Indeed, we showed transient coexpression of von Willebrand factor, a typical endothelial marker, with sarcomeric MyHCs, a phenomenon demonstrating transition from one differentiated state (endothelium) to another (cardiac muscle). This is an example of trans-differentiation into cardiac muscle.
Cardiac Trans-Differentiation in Endothelial Cells Does Not Appear To Be Mediated by the Same Molecules That Induce Cardiogenesis in the Embryo.
Cardiac differentiation of endothelial cells occurs both in progenitors and in differentiated cells. In both cases, contact with differentiated cardiomyocytes is required, as conditioned medium from the same cells does not activate cardiogenesis. The need for cell–cell contact is consistent with the model we proposed to explain the phenomenon of stem/progenitor cell plasticity. In this model, vessel-associated progenitors penetrate a developing tissue during angiogenesis and are recruited by surrounding tissues to the local cell fate. Some cells may remain undifferentiated and, because of their peculiar origin, pluripotent (36). Indeed, there is now convincing evidence that endothelial progenitor cells may generate some pericytes (17, 37), in addition to those that are recruited from surrounding mesenchyme (38).
In the early embryo, signals emanating from the underlying endoderm activate cardiac genes in competent progenitors from the cranial splanchnic mesoderm. It has been recently reported that members of the BMP (2 and 4) and of the FGF (2 and 4) family of signaling molecules can replace the activity of the endoderm and activate cardiogenesis (35). However BMPs and FGFs did not induce cardiac trans-differentiation in endothelial cells, nor did they increase trans-differentiation rate in cocultures, strongly suggesting that the mechanism that recruits endothelial cells to a cardiogenic fate is probably different from the one operating in the embryo. Information on the signaling molecules and the responding molecular mechanisms that regulate recruitment of endothelial cells to a myocardial lineage is unavailable, as in all of the other cases of trans-determination or trans-differentiation described so far. In the case of the smooth to skeletal muscle trans-differentiation of the esophagus, recent work has established that the Myf5 gene is activated upon the switch, but the nature of the activating signals is unknown (39). The real challenge in the near future will be represented by the unraveling of these processes, a key step to design future controlled cell replacement therapies.
How Many Cell Types Can Regenerate Functional Myocardium?
Recent evidence has shown that it is possible to decrease the loss of viable myocardium and improve cardiac function with bone marrow-derived progenitor cells (19, 20). In particular, in one report cytokine-mobilized CD34+ hematopoietic progenitor cells were used. A subfraction of this population, CD34+/CD117Dim, included cells expressing vascular endothelial growth factor receptor 2 (VEGFR-2, flk-1), which is considered a marker of the angioblast lineage. When injected in a peripheral vein of nude rats that had undergone myocardial infarction, these cells induced vasculogenesis and neoangiogenesis with limitation of the infarct area and improved cardiac function (19). In another recent report, injection of Kit+/Lin− progenitor cells from bone marrow directly in the border of the infarcted myocardium was shown to determine conversion of these cells into cardiomyocytes, accompanied by a recovery of cardiac function (20). Whether this Kit+/Lin− population includes hemangioblasts is not known, although the conversion of these cells into endothelial cells was demonstrated. An interesting possibility, which might reconcile results from all of these reports, is that cardiomyocytes can be generated from hemangioblasts only under particular conditions. In fact, our data show that cardiomyocyte conversion of angioblastic lineages can be obtained by direct contact of cells with cardiomyocytes. It is therefore possible that the “default” differentiation pathway of angioblast progenitor cells is the endothelium, whereas cardiomyocyte differentiation can be induced only with cell contact between the angioblast and a differentiated cardiomyocyte. This may happen physiologically, during fetal and perinatal development (tissue growth) and, in pathological conditions, every time there is a minor injury to the myocardium, resulting in activation of surviving cardiomyocytes (tissue repair). The mechanism of recruitment may differ between growth and repair; if cell–cell contact is required, then the presence of differentiated cells inside the mass of the injected population needs to be explained. It is possible that injected GFP+ cells, once differentiated by contact with resident cardiocytes, induce trans-differentiation of cells in the interior part and/or that newly formed cardiocytes may proliferate (as happens up to birth) and increase their total number. Both of these events may be stimulated by the inflammatory microenvironment. Even if these mechanisms of amplification occur in vivo, more severe lesions, where the necrotic area is large, may not be repaired, particularly late in life when this trans-differentiation potential is probably lost or drastically reduced (as suggested by its absence in endothelial cells from adult animals). This is probably why the heart is considered to be composed of irreplaceable cells and to be unable to regenerate.
Based on these observations, it may be argued how widespread this cardiogenic potential is among different progenitor and stem cells. Indeed, the cells used in this study, although derived from a different stage and anatomical site from those used in the work by Orlic et al. (20), share with them some of the features of hemangioblasts, including the expression of c-Kit that is present also in mature endothelial cells (40). It should be noted, however, that both hematopoietic stem cells and circulating endothelial progenitors are relatively difficult cell types to expand in vitro, whereas HUVEC are easily available in large numbers and therefore more suitable for molecular studies on this process. On the other hand, neural stem cells, which can efficiently generate blood (4) and skeletal muscle (10), appear poorly capable of generating cardiomyocytes, at least under the experimental conditions used in this study. The experimental conditions used are extremely important, given our ignorance of the underlying molecular mechanism, and should make any current conclusion tentative, as, for example, it has been recently reported that in the context of the developing chicken embryo, neural stem cells do actually contribute to the myocardium (9).
In conclusion, our observation that human endothelial cells, such as those derived from the umbilical vein, also share the capacity of generating cardiomyocytes opens perspectives for cell replacement therapy. It may be possible to create an archive of patients' umbilical cord cells to be used later in life. Furthermore, the isolation and in vitro expansion of circulating endothelial progenitors is a procedure that has already been used successfully in myocardial ischemia (18). Together with recruitment from hematopoietic stem cells and increased angiogenesis, these data should set the stage for molecular studies of stem/progenitor plasticity and hence for future clinical applications.
Acknowledgments
This paper is dedicated to Franco Tato, who died on July 7, 2001. We thank S. Schiaffino for the gift of the anti-troponin I antibody. This work was supported by grants from Telethon/Ermenegildo Zegna donation, the European Community, Fondazione Cenci Bolognetti, Agenzia Spaziale Italiana, American Heart Association, Italian Ministry of Health (1%), and Italian Ministry of University (Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica).
Abbreviations
- EEC
endothelial embryonic cells
- HUVEC
human umbilical vein endothelial cells
- BMP
bone morphogenetic protein
- FGF
fibroblast growth factor
- MyHC
myosin heavy chain
- GFP
green fluorescent protein
- X-Gal
5-bromo-4-chloro-3-indolyl β-galactopyranoside
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Temple S, Alavarez-Buylla A. Curr Opin Neurobiol. 1999;9:135–141. doi: 10.1016/s0959-4388(99)80017-8. [DOI] [PubMed] [Google Scholar]
- 2.Gage F H. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari G, Cusella De Angelis M G, Coletta M, Stornaioulo A, Paolucci E, Cossu G, Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 4.Bjorson C R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger M F, MacKay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J D, Moorman M A, Simonetti D W, Craig S, Marshak D R. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature (London) 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 7.Paterson B E, Bowen W C, Patrene K D, Mars W M, Sullivan A K, Murase N, Boggs S S, Greenberger J S, Goff J P. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 8.Jackson K A, Mi T, Goodell M A. Proc Natl Acad Sci USA. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke D L, Johansson C B, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 10.Galli R, Borello U, Gritti A, Minasi M G, Bjornson C, Coletta M, Mora M, De Angelis M G, Fiocco R, Cossu G, Vescovi A L. Nat Neurosci. 2000;3:986–991. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- 11.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman I L, Grompe M. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 12.Brazelton T, R, Rossi F M V, Keshet G I, Blau H M. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 13.Mezey E, Chandross K J, Harta G, Maki R A, Scott R, McKercher S R. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 14.Anderson D J, Gage F H, Weissman I L. Nat Med. 2001;7:393–395. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- 15.Patapoutian A, Wold B J, Wagner R A. Science. 1995;270:1818–1821. doi: 10.1126/science.270.5243.1818. [DOI] [PubMed] [Google Scholar]
- 16.Mochii M, Mazaki Y, Mizuno N, Hayashi H, Eguchi G. Dev Biol. 1998;199:226–234. doi: 10.1006/dbio.1997.8800. [DOI] [PubMed] [Google Scholar]
- 17.DeRuiter M C, Poelmann R E, VanMunsteren J C, Mironov V, Markwald R R, Gittenberger-de Groot A C. Circ Res. 1997;80:444–451. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto A, Gwon H, Iwaguro H, Yamaguchi J, Uchida S, Masuda S, Silver M, Ma S, Kearney M, Isner J M, Asahara T. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 19.Kocher A A, Schster M D, Szabolcs M J, Takuma S, Burkhoff D, Wang J, Homma S, Edwards N M, Iliescu S. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 20.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson S M, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine D, et al. Nature (London) 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 21.De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis M G, Ponzetto C, Cossu G. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balconi G, Spagnuolo R, Dejana E. Arterioscler Thromb Vasc Biol. 2000;20:1443–1451. doi: 10.1161/01.atv.20.6.1443. [DOI] [PubMed] [Google Scholar]
- 23.Dong Q G, Bernasconi S, Lostaglio S, De Calmanovici R W, Martin-Padura I, Breviario F, Garlanda C, Ramponi S, Mantovani A, Vecchi A. Arterioscler Thromb Vasc Biol. 1997;17:1599–1604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- 24.Garlanda C, Parravicini C, Sironi M, De Rossi M, Wainstok de Calmanovici R, Carozzi F, Bussolino F, Colotta F, Mantovani A, Vecchi A. Proc Natl Acad Sci USA. 1994;91:7291–7295. doi: 10.1073/pnas.91.15.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Introna M, Breviario F, d'Aniello E M, Golay J, Dejana E, Mantovani A. Eur Heart J. 1993;14, Suppl. K:78–81. [PubMed] [Google Scholar]
- 26.De Luca A, Sargiacomo M, Puca A, Sgaramella G, De Paolis P, Frati G, Morisco C, Trimarco B, Volpe M, Condorelli G. J Cell Biochem. 2000;77:529–539. doi: 10.1002/(sici)1097-4644(20000615)77:4<529::aid-jcb2>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Lattanzi L, Salvatori G, Coletta M, Sonnino C, Cusella-De Angelis M G, Gioglio L, Murry C E, Kelly R, Ferrari G, Molinaro M, et al. J Clin Invest. 1998;101:2119–2128. doi: 10.1172/JCI1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Follenzi A, Ailles L E, Bakovic S, Geuna M, Naldini L. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 29.Corry R J, Winn H J, Russell P S. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Tajbakhsh S, Vivarelli E, Cusella-De Angelis G, Rocancourt D, Buckingham M, Cossu G. Neuron. 1994;13:813–821. doi: 10.1016/0896-6273(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 31.Ausoni S, Campione M, Picard A, Moretti P, Vitadello M, De Nardi C, Schiaffino S. J Biol Chem. 1994;269:339–346. [PubMed] [Google Scholar]
- 32.Ferrari S, Molinari S, Melchionna R, Cusella-De Angelis M G, Battini R, Kelly R, Cossu G. Cell Growth Differ. 1997;8:23–34. [PubMed] [Google Scholar]
- 33.Kelly R, Alonso G, Tajbakhsh S, Cossu G, Buckingham M. J Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donahue J K, Kikkawa K, Johns D C, Marban E, Lawrence J H. Proc Natl Acad Sci USA. 1997;94:4664–4668. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lough J, Barron M, Brogley M, Sugi Y, Bolender D L, Zhu X. Dev Biol. 1996;178:198–202. doi: 10.1006/dbio.1996.0211. [DOI] [PubMed] [Google Scholar]
- 36.Bianco P, Cossu G. Exp Cell Res. 1999;251:257–263. doi: 10.1006/excr.1999.4592. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Nature (London) 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 38.Hellstrom M, Kaln M, Lindahl P, Abramsson A, Betsholtz C. Development (Cambridge, UK) 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 39.Kablar B, Tajbakhsh S, Rudnicki M A. Development (Cambridge, UK) 2000;127:1627–1639. doi: 10.1242/dev.127.8.1627. [DOI] [PubMed] [Google Scholar]
- 40.Buzby J S, Knoppel E M, Cairo M S. Exp Hematol (Charlottesville, VA) 1994;22:122–129. [PubMed] [Google Scholar]