Abstract
Background
Chronic helminth infections typically induce an immunoregulatory environment, with markedly reduced immune responses to both parasite-specific and unrelated bystander antigens. Here we tested whether these changes are also observed in human infections with Mansonella ozzardi, a neglected filarial nematode widely distributed across Latin America.
Methods
CD4+ T cell populations from microfilaremic (Fil+) and uninfected (Fil-) inhabitants in M. ozzardi-endemic riverine communities in Brazil were characterized by flow cytometry analysis. Plasma concentrations of a wide range of cytokines and chemokines were measured. We examined whether M. ozzardi infection is associated with suppressed in vitro lymphoproliferative and inflammatory cytokine responses upon stimulation with filarial antigen, unrelated antigens or mitogens.
Principal findings/Conclusions
Fil+ subjects had lower plasma levels of selected inflammatory cytokines, such as TNF-α, IL-8, and IL-6, than their Fil- counterparts. However, we found no evidence for attenuated T-cell responses to filarial antigens or co-endemic pathogens, such as malaria parasites and Toxoplasma gondii. CD4+ T cells expressing CD39, an ectonucleosidase involved in the generation of the anti-inflammatory molecule adenosine, were increased in frequency in Fil+ subjects, compared to uninfected controls. Significantly, such an expansion was directly proportional to microfilarial loads. Surprisingly, CD39 blocking with a neutralizing antibody suppressed antigen-driven lymphoproliferation in vitro, while decreasing inflammatory cytokine responses, in Fil+ and Fil- individuals. These findings suggest that circulating CD4+ CD39+ T cells comprise subsets with both regulatory and stimulatory roles that contribute to the immune homeostasis in chronic M. ozzardi infection.
Author summary
Helminth infections downregulate immunity and reduce host’s inflammatory responses, but the filarial nematode Mansonella ozzardi, which is widely distributed across Latin America, appears to represent an exception to this rule. We found similar lymphoproliferative responses to filarial and unrelated antigens and comparable regulatory cytokine responses in subjects harboring M. ozzardi microfilariae, compared to local uninfected controls. The proportion of CD4+ T cell subtypes expressing CD39 was significantly increased in infected subjects and correlated positively with their microfilarial density. However, antibody blocking of CD39, an ectonucleosidase involved in the synthesis of the immunosuppresive molecule adenosine, paradoxically reduced, rather than promoted, antigen-driven lymphoproliferation in vitro. We suggest that CD39+ CD4+ T cells circulating in microfilaremics comprise both regulatory and stimulatory cell subsets that are concomitantly expanded. The balance between these cell subsets with opposing regulatory functions may be crucial to maintain immune homeostasis during chronic M. ozzardi infections.
Introduction
Helminth parasites induce a wide range of mechanisms that downregulate immunity, limiting host’s inflammation-mediated tissue damage and favoring parasites’ persistence in typically asymptomatic carriers [1,2]. Nearly 40 years ago, T cell proliferation responses to parasite-specific antigens, but not to mitogens, were first shown to be severely dampened in chronic human infections with tissue-dwelling helminths, such as in schistosomiasis and lymphatic filariasis [3]. Likewise, reduced antigen-driven T-cell responses have also been documented in infections with lumen-dwelling intestinal nematodes [4]. The immunomodulatory environment in these long-lasting infections is characterized by cytokine responses skewed towards interleukin (IL)-10 and transforming growth factor (TGF)-β and the expansion of several regulatory T (Treg) and B cell subtypes, including the classical CD4+CD25hiCD127-FoxP3+ Treg cells. Immune responses to unrelated bystander antigens, such as co-occurring pathogens, allergens, and vaccines, are also often suppressed—a phenomenon termed spillover suppression [5]. Because major infectious diseases such as HIV infection, tuberculosis, and malaria are endemic to areas where several helminth infections are highly prevalent, spillover suppression has major public health implications [5]. However, it remains unclear to what extent similar regulatory networks operate in all chronic helminth infections of humans.
Three filarial nematodes of the genus Mansonella infect humans: M. streptocerca, which is endemic to Africa; M. perstans, which is commonly found in Africa but also occurs in South America [6]; and M. ozzardi, which is found exclusively in Latin America and the Caribbean islands [7]. M. ozzardi has a patchy geographic distribution from southern Mexico to northwestern Argentina, with overall prevalence ranging from <1% to 46% in affected communities [8,9]. Adult worms have been recovered from subcutaneous tissues of experimentally infected patas monkeys [10], but their habitat in human hosts remains uncertain. Microfilariae circulate in the peripheral blood all day long, with very little, if any, periodicity. Most human infections cause little or no disease and may remain undiagnosed and untreated for several years [8].
Infections with the filarial nematodes Wuchereria bancrofti, Brugia malayi, and M. perstans typically reduce immunity to malaria parasites and other pathogens, with an IL-10-dependent decrease in IL-12p70, interferon (IFN)-γ and IFN-γ-induced protein 10 (IP-10) responses upon T-cell stimulation [11–14]. Nevertheless, the immunomodulatory potential of M. ozzardi infections and their spillover effects remain largely unexplored—even in the Amazon Basin, where this filarial nematode and malaria parasites are endemic and co-infections with them are very likely.
Here we hypothesized that immune responses might be also downregulated in subjects chronically harboring M. ozzardi microfilariae. We first characterized peripheral blood mononuclear cell (PBMC) populations from people living in M. ozzardi-endemic communities of Brazil and found an expansion of CD4+ T cell subtypes expressing CD39 in infected subjects, compared to local uninfected controls. CD39 is an ectonucleosidase that catalyzes the phosphohydrolysis of extracellular ATP and ADP to AMP, which is in turn used by CD73 to synthesize the immunosuppresive molecule adenosine [15]. We next examined whether M. ozzardi infection is associated with suppressed T-cell responses driven by filarial and unrelated antigens and whether this is mediated through the induction of high levels of regulatory cytokines. Finally, we explored the effects of in vitro neutralization of CD39 expression on antigen-driven lymphoproliferative responses and cytokine production.
Methods
Ethics statement
Study protocols were approved by the Institutional Review Board of the Institute of Biomedical Sciences, University of São Paulo, Brazil (1133/CEP, 2013). Written informed consent was obtained from all patients or their parents or guardians, if participants were minors aged <18 years.
Study area
The field study was carried out in six villages—Monte Verde, Valparaíso, Boa Vista, Retiro, Nova Vida, and São Pedro—situated along the banks of Purus River, in northwestern Brazil (S1 Fig). These villages are located in the municipality of Boca do Acre (8°45’19"N, 67°23’50"W), southern Amazonas state, with a combined population of 1,300 inhabitants. They had previously been shown to be endemic for M. ozzardi, with an average prevalence of infection estimated at 27.3% [16]. Although M. ozzardi vectors have not been characterized in this area, simuliid black flies of the species Simulium amazonicum, S. argentiscutum, S. oyapockense s.l., and S. roraimense are believed to transmit this helminth across the Amazon Basin of Brazil [17]. Low-level malaria transmission is recorded year-round in these communities, with Plasmodium vivax accounting for over 95% of the infections in 2013. Other tissue-dwelling helminths such as Schistosoma mansoni and W. bancrofti are not endemic to this region.
During a pilot study in March 2013, we carried out house-to-house visits in the target communities and randomly screened 287 inhabitants for M. ozzardi microfilariae. By combining thick-smear microscopy and polymerase chain reaction (PCR) on finger-prick blood samples, we found 41 (14.3%) subjects harboring M. ozzardi microfilariae, with prevalences of infection ranging between 6.1% and 30.4% across communities. As described in similar endemic settings [18], prevalence of infection increased with age, from 4.4% in children below 10 years to 57.1% in adults over 50 years.
Study population
During the next visit to the target communities, in September 2013, we invited subjects previously found to harbor microfilariae, as well as a subsample of individuals found to be uninfected or not screened during the pilot survey, to contribute 40 mL of venous blood for microfilariae detection and PBMC and plasma separation. Because most infected subjects were adults, we prioritized adults as uninfected controls in order to have a similar age composition in the comparison groups. Blood was drawn between 9:00 am and 3:00 pm. Infections diagnosed during the pilot study and those newly diagnosed during this second survey were treated with a single dose of 0.2mg/kg of ivermectin [19] after blood sampling. No post-treatment samples were obtained.
Study subjects were given plastic containers containing 10% formalin and asked to provide a stool sample. Stool specimens were examined for eggs, cysts and larvae of intestinal parasites with a standard sedimentation method [20], which was preferred over the standard Kato-Katz method because we used formalin-diluted stool samples. No study participant had intestinal parasites detected by stool examination; furthermore, none of them had malaria diagnosed by using a quantitative real-time PCR targeting the 18S rRNA gene [21]. Plasma samples from all study participants were screened for IgG antibodies to Toxoplasma gondii by using the Serion ELISA classic IgG kit (Institut Viron/Serion, Würzburg, Germany); all of them were seropositive.
Mansonella ozzardi microfilaremia and filarial IgG4 antibodies
Laboratory diagnosis of M. ozzardi was based on microscopic examination of thick smears and quantitative PCR on all samples. Subjects positive by either method were defined as microfilaremic (Fil+) and those negative by both methods were defined as Fil-. Thick blood smears were stained with Giemsa; at least 100 fields were examined for microfilariae, under 1000 × magnification, before a slide was declared negative. We standardized an in-house, SYBR green-based real-time PCR approach to quantify the number of copies of amplicons, which was used as a proxy of microfilarial density. DNA templates for PCR amplification were isolated from 200 μL of whole venous blood on a QIAcube automated platform (Qiagen, Hilden, Germany), using QIAamp DNA blood kits (Qiagen), and eluted in 200 μL for water. Each 20-μL reaction mixture contained 2 μL of sample DNA (corresponding to 2 μL of whole blood), 10 μL of 2× Maxima SYBR Green qPCR master mixture (Fermentas, Burlington, Canada) and 0.5 μM of each primer (forward, 5’-CTT ATC ATC AGG TGA TAT TAA T-3’; reverse, 5’-TTA GTT TCT TTT CCT CCG CT-3’). These primers allow the amplification of a M. ozzardi-specific 295-base pair (bp) fragment of the internal transcribed spacer (ITS)-2 of the ribosomal DNA (rDNA) gene [22]. Standard curves were prepared with serial tenfold dilutions of the target sequence, cloned into pGEM-T Easy vectors (Promega, Madison, WI, USA), to allow for calculating the number of ITS-2 amplicons/μL of blood. We used a Mastercycler gradient real-time PCR cycler (Eppendorf, Hamburg, Germany) for PCR amplification with a template denaturation step at 95°C for 10 min, followed by 40 cycles of 15 sec at 95°C and 1 minute at 55°C, with fluorescence acquisition at the end of each extension step. Amplification was immediately followed by a melting program consisting of 15 sec at 95°C, 15 sec at 55°C, and a stepwise temperature increase of 0.2°C/sec until 95°C, with fluorescence acquisition at each temperature transition. No-template controls (containing all reagents for amplification except for the DNA template) were run for every qPCR microplate.
Filaria-specific IgG4 antibodies were determined by ELISA as described [23], using a B. malayi adult worm extract (BmA) as a solid-phase capture antigen.
Plasma cytokines
We compared plasma levels of the following cytokines in Fil+ and Fil- subjects: epidermal growth factor (EGF), eotaxin, fibroblast growth factor (FGF)-basic, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), hepatocyte growth factor (HGF), IFN-α, IFN-γ, IL-1ra, IL-1β, IL-2, IL-2r, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p40/p70), IL-13, IL-15, IL-17, IP-10, monocyte chemoattractant protein (MCP)-1, monokine induced by IFN-γ (MIG, also known as CXCL9), macrophage inflammatory protein (MIP)-1α, MIP-1β, CCL5 (also known as RANTES, Regulated on Activation, Normal T Cell Expressed and Secreted), TNF-α, and vascular endothelial growth factor (VEGF). Samples were tested using the Human Cytokine Magnetic 30-Plex Panel (Invitrogen), and data were acquired with a Bio-Plex 200 System (Bio-Rad) using Luminex 100 xMAP technology (Luminex, Austin, USA).
Peripheral blood mononuclear cell separation and phenotyping
PBMC were separated by gradient centrifugation with Ficoll-Paque Plus (GE Healthcare, Little Chalfont, United Kingdom), up to 6 hours after blood collection, cryopreserved in liquid nitrogen, and thawed as described [24]. PBMC were evaluated for viability and only samples with >90% viability were used in subsequent analyses.
CD4+ T cell subtypes circulating in the peripheral blood of Fil+ and Fil-subjects were defined according to the (co-)expression of regulatory and activation markers. Regulatory molecules included: (a) the transcription factor FoxP3, (b) the TNF receptor family costimulatory receptor OX-40 (CD134), (c) the glucocorticoid-inducible TNF receptor family-related protein (GITR/CD357), (d) the TNF receptor II (TNFRII/CD120b), (e) the ectonucleosidase CD39 (also known as NTPDase1), (f) the programmed cell death protein 1 (PD-1/ CD279), (g) the lymphocyte-activation gene 3 product (LAG-3/CD223), (h) the membrane-bound C-terminal pro-region of TGF-β known as latency-associated peptide (LAP-TGF-β), and (i) the primary inhibitory receptor CTLA-4 (CD152), which competes with CD28 for CD80 and CD86 binding on antigen-presenting cells. The activation markers were: (a) HLA-DR, which identifies a Treg cell subset involved in contact-dependent immune suppression, and (b) CD69, an early activation marker with inhibitory properties. Treg cells were phenotypically defined as CD4+CD25hi cells that express FoxP3 but not the α chain of the IL-7 receptor (CD127).
After thawing, 106 viable cells/mL were transferred to V-bottomed 96-well microplates with 200 μL of staining buffer (PBS with 2% fetal bovine serum and 2 mm EDTA) per well and centrifuged at 600 × g for 5 min. For surface staining, the cell pellet was incubated with directly conjugated antibodies at room temperature, in the dark, for 30 min and washed twice with 200 μL of staining buffer. To analyze the intracellular expression of FoxP3 and Ki67, a nuclear protein that regulates cell division, cells were fixed and permeabilized using the Transcription Factor Fixation/Permeabilization kit of eBiosciences (San Diego, CA, USA) and then incubated with specific, labeled antibodies. In this context, we used intracellular Ki67 expression as an indicator of T-cell proliferation [25]. Monoclonal antibody panels are listed in S1, S2 and S3 Tables. Samples were acquired on an LSR Fortessa flow cytometer (BD Biosciences, San Jose, CA, USA) using the FACSDiva software (BD Biosciences). Anti-mouse IgG-coated beads (BD Biosciences) stained with each fluorochrome separately were used for software-based compensation. We used the Live/Dead Fixable Blue Dead Cell Stain for UV excitation (Invitrogen, Carlsbad, CA, USA) for dead cell exclusion and collected ≥ 300,000 events (panels 1 and 2, S1 and S2 Tables) or ≥ 600,000 events (panel 3, S3 Table) in each live gate. Only samples with >80% viability were analyzed. Fluorescence minus one (FMO) controls were used to control for spectral overlap. Boolean analysis was applied to evaluate co-expression of different molecules by the same cells. Data analysis was carried out using FlowJo software version 8.8.6 (Tree Star, Ashland, OR, USA).
Antigens and PBMC proliferation assays
We used BmA, prepared as described [26], to elicit filarial-specific lymphocyte proliferation in vivo. As bystander antigens, we used: (a) commercially available Staphylococcus aureus enterotoxin B (SEB; Sigma-Aldrich), (b) a soluble tachizoite extract prepared with the RH strain of T. gondii (TgT) [27], and (c) red blood cells (RBC) infected with P. vivax schizonts (PvS). To prepare PvS, patient-derived infected blood was filtered through Fresenius Kabi BioR01 Plus filters (Bad Homburg, Germany) to remove WBC and cultured for 44–46 h in McCoy’s 5A medium (Invitrogen) supplemented with 20% human AB serum until intracellular parasites reached the mature schizont stage (≥ 4 nuclei) [28]. RBC infected with mature schizonts were enriched by using MACS separation columns (Miltenyi, Auburn, CA, USA) as described [29].
To examine whether M. ozzardi is associated with T-cell hyporesponsiveness, we stimulated PBMC from Fil+ and Fil- subjects with the antigens described above. To this end, 106 PBMC/well were labelled with CellTrace Violet (Invitrogen) and incubated in 96-well microplates with either BmA (13 μg/mL), SEB (10 μg/mL), TgT (12 μg/mL), or 105 P. vivax-infected RBC/well, to a final volume of 200 μL/well. BmA and SEB were used with 10 μg/mL of anti-CD28/CD49d co-stimulus (BD FastImmune, San Jose, CA, USA). Cells were incubated for 72 h at 37°C in RPMI-1640 medium supplemented with 10% inactivated fetal bovine serum, 10 mM HEPES, 2 mM L-glutamine, 1 mM sodium pyruvate, 55 μM 2-mercaptoethanol, and a 1% (vol/vol) solution containing 100 U/mL of penicillin, 10 μg/mL of streptomycin, and 25 μL/mL of amphotericin B in a humidified chamber with 5% CO2. Medium alone and 10 μg/mL of phytohemagglutinin (PHA-P, Sigma-Aldrich, St. Louis, MO, USA) were used as negative and positive controls, respectively. After acquisition of ≥ 300,000 events in each live gate on an LRS Fortessa or FACSCanto II flow cytometer (BD Biosciences), the proportion of PBMC that had divided at least once during the culture period was calculated using the FlowJo software. Here, we subtracted the proportion of divided PBMC in medium-only wells (“unstimulated”) from that of antigen-containing wells to obtain net proportions of divided cells. Only samples with >80% viability were analyzed.
To test the effect of in vitro CD39 blocking on lymphoproliferation, we compared the proportion of dividing PBMC after stimulation with SEB (10 μg/mL) and anti-CD28/CD49d (10 μg/mL), as described above, in the absence (medium alone) or the presence of 2 μg/mL of anti-CD39 antibody (Biolegend, San Diego, CA, USA) [30]. At this concentration, this antibody was previously shown, by flow cytometry, to abolish surface CD39 recognition by the FITC-labeled anti-CD39 monoclonal antibody used in PBMC phenotyping. As a positive control, we used 2 μg/mL of a neutralizing anti-IL-10 antibody (Biolegend). Separate lymphoproliferation assays were carried out in parallel with the addition of 2 mM ATP or adenosine (both from Sigma-Aldrich) to cells that had been pre-incubated with the blocking anti-CD39 antibody.
Cytokine production after PBMC stimulation in vitro
We used standard intracellular cytokine staining to evaluate the production and accumulation, after antigen stimulation, of Th1- and Th2-type cytokines within the endoplasmic reticulum of CD4+ cells from Fil+ and Fil- subjects. To this end, 0.5 × 106 PBMC/well were cultured in U-bottomed 96-well microplates for 4 hours at 37°C in the absence (medium alone) or the presence of BmA (13 μg/mL) or SEB (10 μg/mL) combined with anti-CD28/CD49d co-stimulus (10 μg/mL). We next added 10 μg/mL of brefeldin A, to retain the cytokines within the PBMC, followed by further 19 hours at 37°C in a CO2 incubator. To evaluate how CD39 blocking affected cytokine production by CD4+ T cells, 2 μg/mL of purified anti-CD39 antibody (Biolegend) were added to selected wells shortly after antigen stimulation. Next, PBMC were transferred to V-bottomed 96-well microplates, for cell surface staining with anti-CD3 and anti-CD4 monoclonal antibodies for 30 min, followed by incubation with the permeabilization buffer supplied by eBioscience, for 1 hour at room temperature, and staining with monoclonal antibodies. The monoclonal antibody panel used for intracellular staining of IL-2, TNF-α, IFN-γ, IL-4, IL-5, and IL-13 is listed in S4 Table. We used a FACSCanto II flow cytometer (BD Biosciences) to acquire ≥ 300,000 events in each live gate, in samples with >80% viability, and FlowJo software for data analysis.
We also evaluated cytokine levels in culture supernatants harvested after 72-h incubation of PBMC with BmA (13 μg/mL) or SEB (10 μg/mL) combined with anti-CD28/CD49d (10 μg/mL), or medium alone. Again, 2 μg/mL of purified anti-CD39 antibody (Biolegend) were added to selected wells after antigen stimulation. We used the PeliKine compact ELISA kit (Sanquin, Amsterdam, Netherlands) to measure IL-6, IL-13, IL-10, IL-4, and IFN-γ levels.
Statistical analysis
Because most continuous variables had an overdispersed distribution, results were summarized as medians and interquartile ranges (IQR). Comparisons between samples from different subjects were done with nonparametric Mann-Whitney U tests (for continuous variables) or χ2 tests (for proportions). Paired data were compared with Wilcoxon signed rank tests for continuous variables. Nonparametric correlation coefficients (rs) were estimated using Spearman rank correlation tests. All analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Significance was defined at the 5% level. We used the Benjamini-Hochberg procedure [31] to control for the false discovery rate (q) when many comparisons were conducted in the same sample set and one or more of these tests resulted in a significant difference. We ranked all individual P values from the smallest to the largest and compared each of them to its Benjamini-Hochberg critical value, given by (i/m)q, where i is the rank and m is the total number of tests. We set q at 0.10, meaning that we accept up to 10% of the associations with significant results being false positives. The largest P value that has P<(i/m)Q was considered significant, as well as all of the P values smaller than it. Calculations of the Benjamini-Hochberg critical value were done using the Excel spreadsheet available at: http://www.biostathandbook.com/multiplecomparisons.html.
Results
Characteristics of study participants
Fifty microfilaremic (Fil+) subjects aged between 10 and 98 years and 34 uninfected (Fil-) controls from the same communities aged between 7 and 84 years contributed plasma and PBMC samples. The number of ITS-2 amplicons ranged between 1,300 and 2,973,350 copies/μL in microfilaremics. Fil+ and Fil- subjects did not differ significantly according to their age and sex distribution, hemoglobin levels, total IgE levels and counts of white blood cells (WBC), lymphocytes, T lymphocytes, and T CD4+ lymphocytes (Table 1). Moreover, clinical signs and symptoms were reported in comparable frequencies by Fil+ and Fil- individuals (S5 Table).
Table 1. Demographic, hematologic, and clinical characteristics of microfilaremic subjects (Fil+) and uninfected controls (Fil-).
| Characteristic | Value for group | P value | |
|---|---|---|---|
| Fil- | Fil+ | ||
| No. of subjects | 34 | 50 | |
| Age in years (range) | 37.1 (7–84) | 43.8 (10–98) | 0.207 |
| Gender (% male) | 52.9 | 56.0 | 0.722 |
| Village | |||
| Boa Vista | 4 | 4 | 0.013 |
| Monte Verde | 11 | 5 | |
| Nova Vida | 11 | 11 | |
| Retiro | 3 | 14 | |
| São Pedro | 0 | 4 | |
| Valparaíso | 5 | 12 | |
| Hemoglobin levels (g/100 mL) | 14.5 (11.8–21.2) | 14.2 (11.7–18.40) | 0.665 |
| Anemia (%) | 2.9 (n = 1) | 6.0 (n = 3) | 0.410 |
| No. of WBCs (109/L) | 7.8 (4.7–11.9) | 8.2 (3.9–17) | 0.658 |
| No. of lymphocytes (109/L) No. of granulocytes (109/L) |
2.5 (1.3–3.5) 4.6 (2.2–9.10) |
2.3 (1.2–4.1) 5.0 (1.9–43) |
0.193 0.266 |
| No. of T lymphocytes (109/L) | 1.74 (0.94–2.64) | 1.54 (0.66–3.58) | 0.271 |
| No. of CD4+ T lymphocytes (109/L) | 0.98 (0.43–1.71) | 0.96 (0.43–2.13) | 0.381 |
Data are presented as median (interquartile range) except if otherwise indicated and were compared with the Mann-Whitney test (continuous variables) or with the χ2 test (proportions).
IgG4 antibodies to BmA were measured by ELISA in available plasma samples from 82 study participants. These were significantly higher in microfilaremics than in uninfected controls (median, 3594 pg/mL vs. 1628 pg/mL, Mann-Whitney U test, P = 0.004). Twenty-six subjects, hereafter termed IgG4H, had BmA antibody levels above the overall median; 25 of them were microfilaremic. The IgG4L group comprised of 56 subjects (25 of them infected) with BmA antibody levels below the overall median. Therefore, half of the 50 Fil+ subjects but only one of the 32 Fil- subjects tested for antibodies were in the IgG4H group. Interestingly, the IgG4H and IgG4L groups had similar age and sex distribution, hemoglobin levels, and WBC counts, but the IgG4H population had significantly lower counts of lymphocytes, T lymphocytes, and T CD4+ lymphocytes (S6 Table). Specific IgG4 antibody concentrations in microfilaremics correlated positively with ITS-2 amplicon copy numbers (rs = 0.315, P = 0.004).
Inflammatory and regulatory cytokines in microfilaremics
Microfilaremics had significantly lower plasma concentrations of TNF-α (median, 9.7 vs. 18.4 pg/mL, P = 0.012), IL-4 (median, 0.0 vs. 9.2 pg/mL, P = 0.008), IL-8 (median, 4.7 vs. 28.5 pg/mL, P = 0.005), G-CSF (median, 52.4 vs. 101.9 pg/mL, P = 0.016), IL-6 (median, 3.8 vs. 8.0 pg/mL, P = 0.021), and MIP-1α (median, 16.1 vs. 28.9 pg/mL, P = 0.016), and significantly higher levels of the Th2-type mediator eotaxin (median, 47.7 vs. 37.0 pg/mL, P = 0.006), compared with uninfected controls (S7 Table). However, the slight difference between Fil+ and Fil- subjects in IL-10 concentrations (median, 17.4 vs. 13.7 pg/ml) did not reach statistical significance (P = 0.052). Levels of eotaxin, but not those of other cytokines or chemokines, were significantly correlated to the proportion of circulating CD4+ cells that were CD39+ (rS = 0.356, P = 0.002) and to the proportion of circulating Treg cells that that were CD39+ (rS = 0.274, P = 0.022).
Lymphoproliferative and cytokine responses following antigen stimulation in Fil+ and Fil- subjects
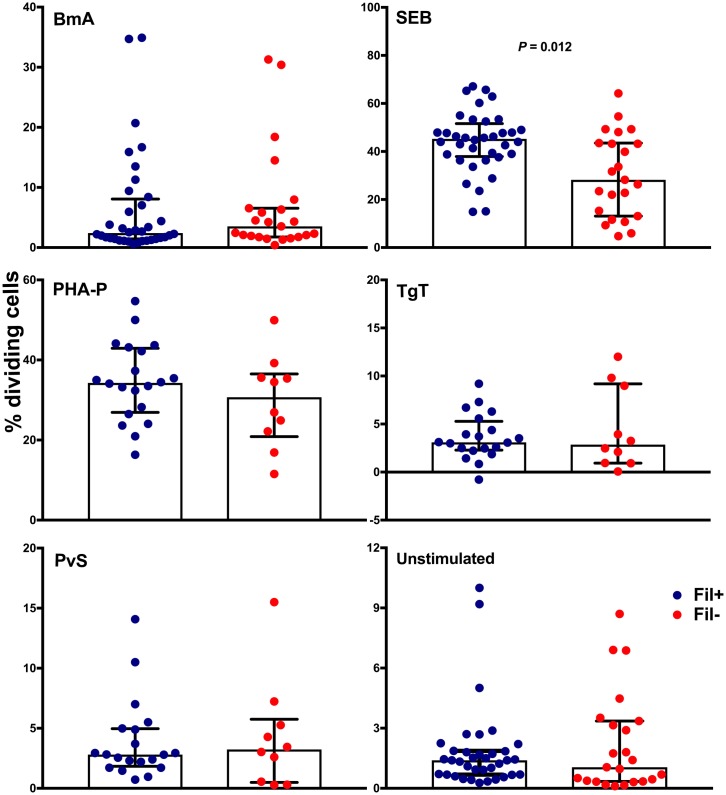
We found no evidence for lymphocyte hyporesponsiveness in M. ozzardi infection. Fig 1 shows comparable proliferation patterns of PBMC from microfilaremic and uninfected subjects that were stimulated with filarial (BmA) and two bystander antigens (TgT and PvS), as well as with mitogen (PHA-P). However, SEB-driven PBMC proliferation was significantly increased, rather than decreased, in microfilaremics, with median proportion of dividing cells of 45.2% vs. 31.7% (P = 0.012) in Fil+ and Fil- subjects, respectively. Moreover, antigen-driven cytokine responses did not differ significantly between infected and uninfected subjects. We found comparable proportions of CD4+ T cells from Fil+ and Fil- individuals producing IFN-γ, IL-2, IL-10, TNF-α, and Th2-type cytokines (IL-4, IL-5, and IL-13) in vitro following stimulation with BmA or SEB (S8 Table). Finally, we found similar concentrations of secreted IL-6, IL-13, IL-10, IL-4, and IFN-γ in PBMC culture supernatants from Fil+ and Fil- subjects stimulated with BmA and SEB (S9 Table).
Fig 1. Antigen-driven lymphocyte proliferation is not suppressed in Mansonella ozzardi infection.
PBMC from microfilaremic (Fil+) and uninfected (Fil-) subjects were labeled with CellTrace Violet and incubated with filarial (B. malayi adult worm extract [BmA]) and unrelated antigens (Staphylococcus aureus enterotoxin B [SEB], Toxoplasma gondii taquizoite extract [TgT] and red blood cells infected with Plasmodium vivax schizonts [PvS]), mitogen (phytohemagglutinin [PHA-P]), or medium alone (“unstimulated”). The net % of divided cells (stimulated minus unstimulated) was estimated by flow cytometry and shown in all panels, except for the lower right panel, which shows % of unstimulated divided cells. Data are presented as medians and interquartile ranges for 20 Fil+ and 10 Fil- subjects (TgT, PvS, and PHA-P) or 36 Fil+ subjects and 23 Fil- subjects (BmA, SEB, and medium alone) and were compared using the Mann-Whitney U test. The only significant P value after controlling for a false discovery rate (q) set at 0.10 is shown (number of comparisons m = 6).
Higher frequency of CD39-expressing CD4+ T cells and Treg cells in Fil+ subjects
We compared the expression of regulatory and activation markers in circulating CD4+ T cells from Fil+ and Fil- subjects, using the gating strategy shown in S2 and S3 Figs. Fil+ and Fil- subjects had similar frequencies of CD4+ T cells expressing most regulatory and activation markers (Table 2). However, microfilaremics had a significantly higher frequency of CD4+ T cells expressing CD39 than uninfected controls (median, 7.2% vs. 5.5% of all circulating CD4+ T cells). A similar difference in the frequency of CD4+ T cells expressing CD39 was observed when comparing IgG4H and IgG4L subjects (S10 Table).
Table 2. Frequency of CD4+ T cells expressing regulation and activation markers in microfilaremic subjects (Fil+) and uninfected controls (Fil-).
| Marker | Value for group (% of CD4+ T cells) | P value | |
|---|---|---|---|
| Fil- | Fil+ | ||
| No. of subjects | 33 | 48 | |
| CD39 | 5.52 (3.89–7.34) | 7.23 (5.58–9.23) | 0.005* |
| CTLA-4 | 0.56 (0.22–3.32) | 0.52 (0.18–5.55) | 0.338 |
| Intracellular CTLA-4 | 21.60 (10.90–31.40) | 20.00 (10.30–41.20) | 0.839 |
| HLA-DR | 4.30 (2.34–6.91) | 4.89 (2.50–8.18) | 0.131 |
| PD-1 | 9.12 (5.55–12.60) | 9.34 (6.10–14.50) | 0.112 |
| TNFRII | 5.66 (3.63–14.80) | 6.86 (4.06–13.90) | 0.147 |
| GITR | 0.36 (0.12–1.39) | 0.51 (0.07–1.89) | 0.082 |
| LAG-3 | 0.09 (0.03–0.18) | 0.09 (0.03–1.46) | 0.826 |
| LAP-TGF-β | 3.96 (1.96–14.80) | 4.38 (2.35–15.8) | 0.713 |
| OX-40 | 9.22 (5.35–14.60) | 7.92 (5.38–14.10) | 0.809 |
| CD69 | 4.21 (0.97–23.15) | 5.62 (1.34–28.60) | 0.606 |
Data are presented as medians (interquartile ranges) and were compared with the Mann-Whitney U test.
* indicates a significant difference after controlling for a false discovery rate (q) set at 0.10 (number of comparisons m = 11).
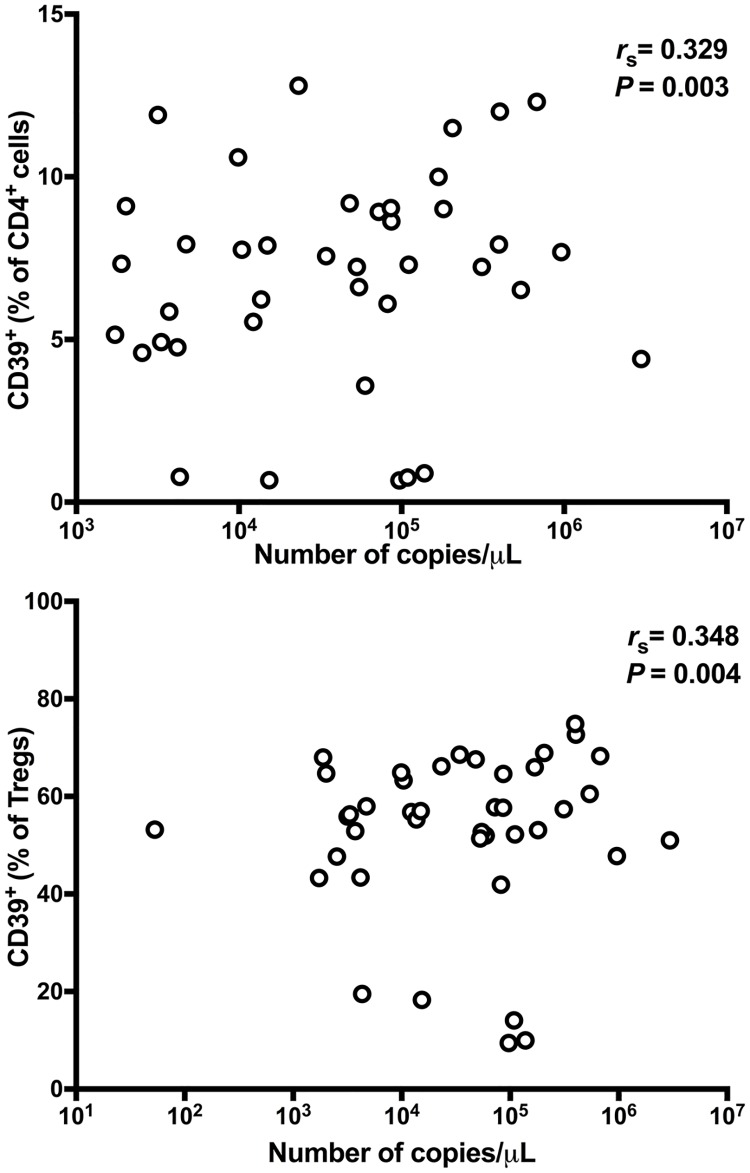
We next examined the classical Treg cell compartment, phenotypically defined as CD4+CD25hiCD127-FoxP3+ cells, using the gating strategies shown in S4 and S5 Figs. Treg cells comprised identical proportions (median, 1.5%) of CD4+ T cells circulating in Fil+ and Fil- subjects, indicating that M. ozzardi infection did not induce an expansion of the Treg cell compartment. However, the analysis of surface marker expression on Treg cells revealed a significantly greater proportion of CD39+ Tregs in microfilaremics compared to uninfected controls (median, 69.7% vs. 60.9%). CD39+ Treg cells accounted for a median of 1.1% and 0.9% of all CD4+ T cells circulating in infected and uninfected individuals, respectively. Therefore, ~15–16% of CD39+ cells within the CD4+ compartment were classical Treg cells. No significant difference was found in the frequencies of Treg cells expressing all other molecules listed in Table 3, except for the CD39-PD-1+ subset (increased in Fil+ subjects). Overall, similar results were obtained when comparing IgG4H and IgG4L subjects (S11 Table). Interestingly, CD39+ Treg cells from microfilaremics and uninfected controls more often co-express a wide range of other regulatory molecules (CTLA-4, LAP-TGF-β, LAG-3, TNFRII, GITR, and OX-40) and activation markers (HLA-DR and CD69) than do CD39- Treg cells (S6 Fig). These findings are consistent with the notion that CD39 expression characterizes a functionally more “suppressive” Treg subtype [32]. Because the frequencies of CD39+ CD4+ T cells and CD39+ classical Treg cells correlated positively with the number of M. ozzardi-specific ITS-2 amplicon copies in microfilaremics (Fig 2), we conclude that the M. ozzardi-associated expansion of CD39+ T-cell populations is directly proportional to the microfilarial load.
Table 3. Frequency of CD4+CD25hiCD127-FoxP3+ Treg cells expressing regulation and activation markers in microfilaremic subjects (Fil+) and uninfected controls (Fil-).
| Marker | Value for group (% of Treg cells) | P value | |
|---|---|---|---|
| Fil- | Fil+ | ||
| No. of subjects | 33 | 48 | |
| CD69 | 3.51 (1.68–9.60) | 3.32 (1.69–8.23) | 0.592 |
| CTLA-4 | 0.49 (0.23–6.24) | 0.69 (0.42–7.21) | 0.417 |
| Intracellular CTLA-4 | 78.60 (62.20–83.05) | 77.00 (68.90–78.60) | 0.673 |
| HLA-DR | 26.75 (8.31–45.67) | 28.95 (14.30–55.20) | 0.494 |
| PD-1 | 3.37 (1.76–9.50) | 4.50 (1.98–10.30) | 0.132 |
| TNFRII | 45.40 (28,98–57.90) | 42.25 (27.00–51.30) | 0.972 |
| GITR | 0.33 (0.12–1.16) | 0.43 (0.18–1.45) | 0.161 |
| LAG-3 | 0.13 (0.05–0.98) | 0.17 (0.05–1.07) | 0.173 |
| LAP-TGF-β | 7.08 (3.84–16.98) | 6.21(3.92–14.80) | 0.912 |
| OX-40 | 3.62 (1.59–12.56) | 3.68 (1.61–13.60) | 0.784 |
| CD39 | 60.90 (48.05–62.40) | 69.70 (62.3–70.12) | 0.009* |
| CD39+CD69+ | 3.31 (0.98–8.90) | 2.84 (0.73–12.50) | 0.286 |
| CD39+CTLA-4+ | 0.55 (0.10–14.70) | 0.91 (0.28–25.40) | 0.967 |
| CD39+intracellular CTLA-4+ | 84.80 (34.56–89.60) | 83.00 (68.40–91.30) | 0.351 |
| CD39+HLA-DR+ | 33.80 (12.39–66.70) | 33.05 (16.40–64.30) | 0.855 |
| CD39+PD-1+ | 3.55 (1.39–15.20) | 4.19 (1.61–14.30) | 0.323 |
| CD39+TNFRII+ | 48.85 (24.62–74.88) | 44.75 (36.10–69.72) | 0.516 |
| CD39+GITR+ | 0.35 (0.06–1.30) | 0.44 (0.07–2.10) | 0.416 |
| CD39+LAG-3+ | 0.00 (0.00–3.94) | 0.19 (0.00–1.18) | 0.077 |
| CD39+LAP-TGF-β + | 7.71 (3.52–9.40) | 6.18 (2.71–17.10) | 0.681 |
| CD39+OX-40+ | 4.53 (2.32–20.15) | 4.50 (1.71–18.30) | 0.891 |
| CD39-CD69+ | 3.34 (0.68–12.98) | 2.47 (0.30–13.71) | 0.210 |
| CD39-CTLA-4+ | 0.83 (0.08–4.50) | 0.98 (0.04–6.30) | 0.897 |
| CD39-intracellular CTLA-4+ | 61.4 (38.12–67.60) | 65.50 (37.60–72.70) | 0.176 |
| CD39-HLADR+ | 12.35 (6.32–38.40) | 13.40 (8.16–37.20) | 0.435 |
| CD39-PD-1+ | 4.43 (0.87–12.70) | 7.79 (2.98–16.75) | 0.001* |
| CD39-TNFRII+ | 31.3 (8.45–46.72) | 33.05 (15.40–47.50) | 0.414 |
| CD39-GITR+ | 0.14 (0.02–1.70) | 0.11 (0.01–1.58) | 0.520 |
| CD39-LAG-3+ | 0.00 (0.00–1.20) | 0.00 (0.00–1.78) | 0.041 |
| CD39-LAP-TGF-β+ | 4.90 (1.05–12.20) | 2.65 (0.62–11.73) | 0.895 |
| CD39-OX-40+ | 2.36 (0.08–6.50) | 2.93 (0.07–7.12) | 0.645 |
Data are presented as medians (interquartile ranges) and were compared with the Mann-Whitney U test.
* indicates significant differences after controlling for a false discovery rate (q) set at 0.10 (number of comparisons m = 31).
Fig 2. The expansion of CD4+CD39+ T-cell populations in microfilaremics is directly proportional to their microfilarial load.
The frequencies of CD4+CD39+ T cells (% of all CD4+ T cells that express CD39; upper panel) and CD39+ Treg cells (% of all CD4+CD25hiCD127-FoxP3+ T cells that express CD39; lower panel) were plotted against the number of Mansonella ozzardi-specific ITS-2 amplicon copies obtained by quantitative real-time PCR, a proxy of microfilarial load. Data analyzed for 48 subjects using the Spearman rank correlation test.
CD39 blocking in vitro inhibits lymphocyte proliferation and alters cytokine responses after antigen stimulation
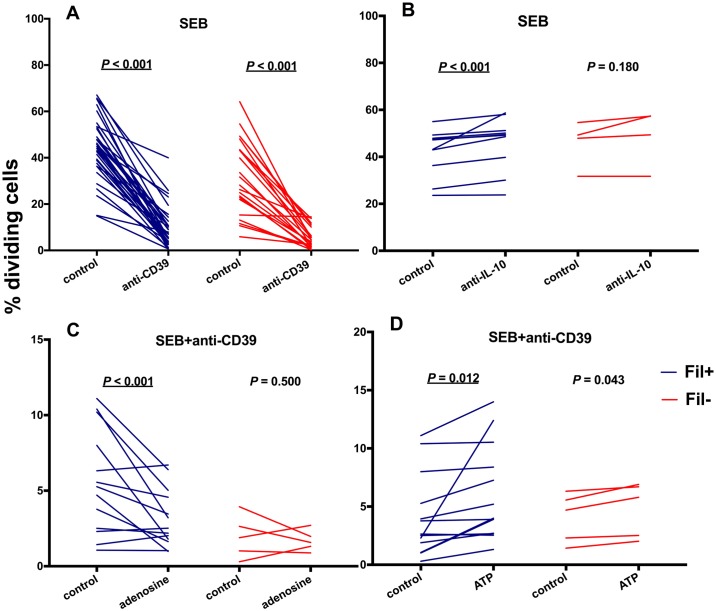
To explore the immunomodulatory consequences of surface expression of CD39 in circulating CD4+ T cells, we used an anti-CD39 antibody to neutralize this molecule [30]. We observed that SEB-driven lymphocyte proliferation decreased significantly in the presence of CD39-blocking antibody (Fig 3A), although proliferation increased, as expected [2], in the presence of neutralizing anti-IL-10 antibody (Fig 3B). SEB-induced lymphocyte proliferation in the presence of anti-CD39 antibody was further inhibited by adding 2mM adenosine to the culture medium (Fig 3C), being partially restored by adding 2mM ATP (Fig 3D). Interestingly, comparable inhibitory effects of CD39 blocking on cell proliferation in vitro were observed in Fil+ and Fil- subjects.
Fig 3. CD39 blocking markedly inhibits antigen-driven lymphocyte proliferation.
PBMC from microfilaremic (Fil+) and uninfected (Fil-) subjects were labeled with CellTrace Violet and incubated with Staphylococcus aureus enterotoxin B (SEB). The net % of divided cells was compared in the presence or absence of anti-CD39 antibody (“anti-CD39” and “control”, respectively; panel A), in the presence or absence of anti-IL-10 antibody (“anti-IL-10” and “control”, respectively; panel B), in the presence of anti-CD39 antibody with or without 2 mM adenosine added to the culture medium (“adenosine” and “control”, respectively; panel C), and in the presence of anti-CD39 antibody with or without 2 mM ATP added to the culture medium (“ATP” and “control”, respectively; panel D). Individual data are presented for 36 Fil+ and 21 Fil- subjects (panel A), 10 Fil+ and 4 Fil- subjects (panel B) or 13 Fil+ and 5 Fil- subjects (panels C and D); they were compared using the Wilcoxon signed rank test. Significant P values after controlling for a false discovery rate (q) set at 0.10 are underlined (number of comparisons m = 4 for each group, Fil+ and Fil-).
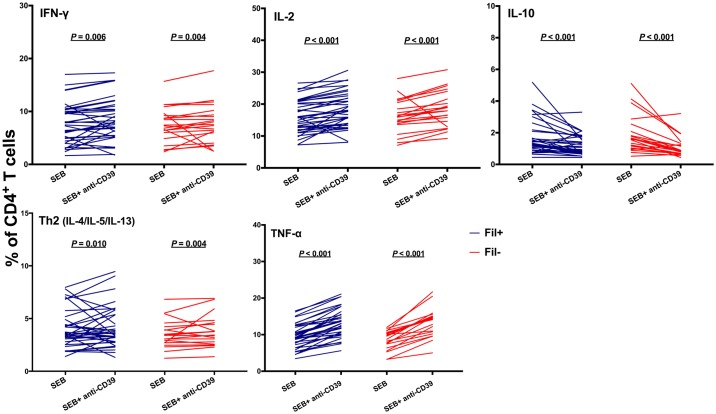
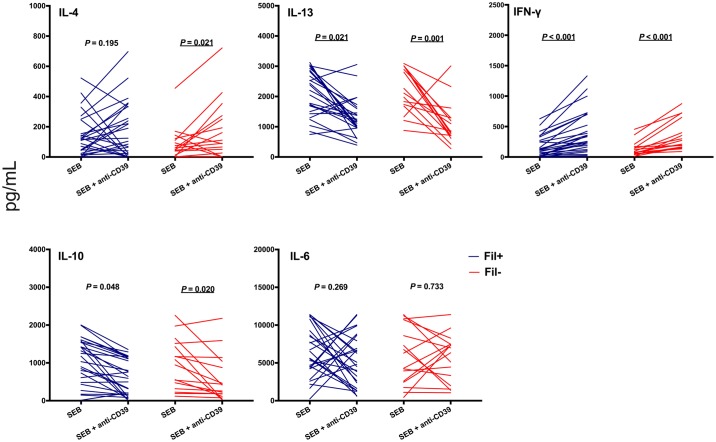
Antibody-mediated CD39 neutralization also exerted profound effects on SEB-driven cytokine production. We observed increased proportions of CD4+ T cells producing IFN-γ, IL-2, TNF-α, and Th2-type cytokines, but a decreased proportion of CD4+ T cells producing IL-10, in the presence of anti-CD39 antibody (Fig 4). These changes were reversed in the presence of 2mM adenosine (S7 Fig). Accordingly, levels of IL-4, and IFN-γ in culture supernatants increased, while those of IL-13, and IL-10 significantly decreased, following CD39 blocking in vitro (Fig 5). CD39 blocking caused similar changes in SEB-driven cytokine responses in Fil+ and Fil- subjects (Figs 4 and 5), although not all comparisons reached statistical significance due to the relatively small sample sizes.
Fig 4. CD39 blocking alters antigen-driven cytokine production by CD4+ T cells.
PBMC from microfilaremic (Fil+) and uninfected (Fil-) subjects were stimulated with Staphylococcus aureus enterotoxin B (SEB) in the presence or absence of anti-CD39 antibody and stained for intracellular cytokines. The % of CD4+ T cells producing each cytokine was estimated by flow cytometry. Individual data are presented for 40 Fil+ and 28 Fil- subjects and were compared using the Wilcoxon signed rank test. Significant P values after controlling for a false discovery rate (q) set at 0.10 are underlined (number of comparisons m = 5 for each group, Fil+ and Fil-).
Fig 5. CD39 blocking alters antigen-driven cytokine secretion in PBMC culture supernatants.
PBMC from microfilaremic (Fil+) and uninfected (Fil-) subjects were stimulated with Staphylococcus aureus enterotoxin B (SEB) in the presence or absence of anti-CD39 antibody. After 72 h of culture, supernatants were harvested and assessed for cytokines. Data are presented as individual cytokine concentrations (pg/mL) for 27 Fil+ and 15 Fil- subjects and were compared using the Wilcoxon signed rank test. Significant P values after controlling for a false discovery rate (q) set at 0.10 are underlined (number of comparisons m = 5 for each group, Fil+ and Fil-).
Increased Ki67 expression in circulating CD39+ CD4+ T cells and Treg cells is reversed by CD39 blocking
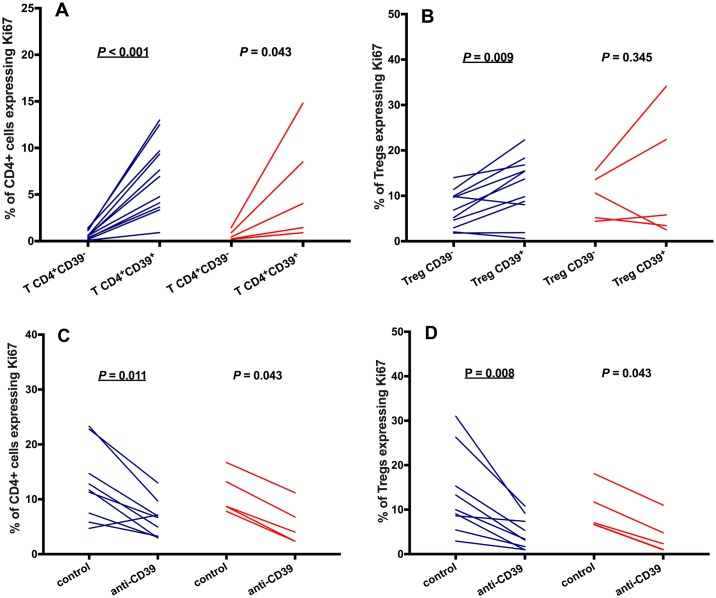
Intracellular Ki67 expression was measured to explore the effects of CD39 on T-cell proliferation. We first observed an increased expression of Ki67 by CD39+ CD4+ T cells, compared to their CD39- counterparts. This change was statistically significant in microfilaremics but not in uninfected controls, although the trends were similar (Fig 6A). We also found an increased expression of Ki67 in the CD39+ subset of Treg cells, compared to CD39- Treg cells, in microfilaremics (Fig 6B). These and previous findings [32] indicate that CD39 expression delineates CD4+ T cell subsets with enhanced proliferative ability. Indeed, CD39 blocking significantly reduced the proportion of Ki67+ CD39+ CD4+ T cells (Fig 6C) and Ki67+ CD39+ Treg cells (Fig 6D). The trends were similar regardless of the infection status, but only reached statistical significance among microfilaremics. This further confirms the suppressive effect of CD39 neutralization on T-cell proliferation.
Fig 6. Reduced Ki67 expression in CD4+CD39+ T cells following CD39 blocking.
PBMC from microfilaremic (Fil+) and uninfected (Fil-) subjects were stained for intracellular Ki67, an indicator of T-cell proliferation. We first compared the proportion of Ki67+ cells among CD4+CD39+ and CD4+CD39- T cells (panel A) and CD39+ and CD39- Treg (CD4+CD25hiCD127-FoxP3+) cells (panel B). Next, we compared the proportion of Ki67+ cells among CD4+ T cells (panel C) and Treg cells (panel D) in the presence or absence of anti-CD39 antibody (“anti-CD39” and “control”, respectively). Individual data are shown for 11 Fil+ and 5 Fil- subjects (panels A and B) or 9 Fil+ and 5 Fil- subjects (panels C and D) and were compared using the Wilcoxon signed rank test. Significant P values after controlling for a false discovery rate (q) set at 0.10 are underlined (number of comparisons m = 5 for each group, Fil+ and Fil-).
Discussion
The filarial nematode M. ozzardi challenges the common view that chronic helminth infections will necessarily elicit immunomodulatory responses such as those thoroughly described in schistosomiasis, lymphatic filariasis, and intestinal nematode infections [1,3,4]. We observed decreased plasma levels of some inflammatory cytokines in microfilaremics, compared to uninfected controls living in the same communities, but Fil+ and Fil- groups had similar proportions of CD4+ T cells producing IFN-γ, IL-2, IL-10, TNF-α, and Th2-type cytokines, as well as similar levels of secreted IL-6, IL-13, IL-10, IL-4, and IFN-γ in culture supernatants, following antigen stimulation. A concomitant increase in plasma concentrations of both inflammatory (IL-6) and regulatory (IL-10) cytokines was recently described in M. ozzardi infections in Brazil [33], but further comparisons with our data are limited by the lack of concurrent analyses of antigen-driven cytokine production in vitro [33].
Moreover, T-cell responses to filarial and unrelated antigens are not attenuated in subjects harboring M. ozzardi microfilariae. Our data suggest that M. ozzardi infection, which is highly prevalent in riverine communities across the Amazon Basin [8], is unlikely to suppress T-cell responses to co-occurring pathogens, such as malaria parasites, in endemic populations.
How Treg cells exert their suppressive effects remains incompletely understood, but CD39 is thought to play a crucial immunoregulatory role in these cells [15]. Indeed, surface expression of CD39 appears to confer enhanced proliferative and suppressive ability to induced Treg cells [32,34]. Moreover, CD39 boosts the differentiation of type 1 regulatory (Tr1) cells, characterized by the production of IL-10 and lack of FoxP3 expression, which are able to limit inflammation and favor immune tolerance [34]. CD39 hydrolyses extracellular ATP (eATP) and ADP into AMP, while another ectonucleotidase, CD73, dephosphorylates AMP to adenosine. eATP activates P2 purignergic and pyrimidinergic receptors expressed by T cells, B cells, dendritic cells, macrophages, and neutrophils, triggering a range of proinflammatory responses. Adenosine, in contrast, is a labile molecule that binds to the adenosine receptor 2A expressed on effector T cells and dampens cell proliferation and inflammatory cytokine secretion [15]. Adenosine concentrations are further regulated by adenosine deaminase (ADA), which catalyzes the deamination of adenosine generating inosine and ammonia. Human ADA1 binds CD26 on T-cells, favoring adenosine turn-over [35]. Therefore, the interplay of CD39 with CD73 and CD26 regulates the levels of eATP, ADP, AMP, and adenosine, with major consequences for the purinergic control of inflammation and adaptative immune responses [34].
Increased expression of CD39 by circulating Treg cells has been associated with disease progression in tuberculosis [36] and HIV/AIDS [37–39]. Moreover, CD39 is overexpressed by several types of cancer cells, in addition to tumor-infiltrating T cells, suggesting that CD39 and purinergic signaling can directly modulate tumor growth, metastasis and angiogenesis, in addition to inducing immune tolerance, further favoring cancer progression [40]. Indeed, blocking CD39 in vivo with specific antagonists or monoclonal antibodies currently in preclinical development has been suggested as an immunomodulatory intervention to enhance effector T-cell responses in HIV infection [37,38] and in cancer [40].
However, the pool of circulating CD4+ T cells expressing CD39 comprises not only Treg cells with enhanced co-expression of regulatory markers and greater suppressive ability. In fact, 5–7% of all circulating CD4+ T cells expressed CD39 in our study subjects, and CD39+ Treg cells represented only 15–16% of the pool of circulating CD39+CD4+ T cells in microfilaremic and uninfected study subjects. Moreover, CD39 is constitutively expressed by the vast majority (>90%) of B cells and monocytes and by small proportions (5% or less) of CD8+ T cells [40]. A particularly interesting subset of CD39+CD4+ T cells comprises the partially characterized CD25-FoxP3- “inducer” T (Tind) cells that promote and potentiate, rather than suppress, T-cell proliferation and inflammatory cytokine production [41,42]. Tind cells appear to be functionally similar to newly described activated effector CD4+ T cells that co-express CD39 and CD26, have no suppressive effect, and are prone to apoptosis [43]. Co-culture with Tind cells enhances the proliferation of CD4+CD25-CD39- “responder” T cells (Tres) from healthy donors, an effect that can be partially reversed by anti-CD39 monoclonal antibodies [41]. Moreover, co-culture of Tres with Tind cells enhances the production of IFN-γ, TNF-α, GM-CSF, IL-6, and IL-10 [41]. These findings imply that increased CD39 expression and regulatory ability are not necessarily coupled in all circulating CD4+ T cell subsets. The dramatic decrease in antigen-driven cell proliferation observed in both microfilaremic and uninfected individuals in the presence of CD39-blocking antibody (Fig 3A) is consistent with a predominance of Tind cells in the CD39+CD4+ pool of circulating T cells from our study population. Therefore, the relative proportions of CD39+ Treg and Tind cells may determine the patterns of immune homeostasis observed in human populations exposed to different environmental antigens and pathogens. In addition, this balance may determine the outcome of immunomodulatory interventions based on CD39 blocking.
The main limitation of this study is its cross-sectional design. Therefore, we were able to identify statistically significant associations between current infection and the proportion of certain CD4+ T cell populations circulating in the peripheral blood, but our study design does not allow us to infer causal relationships. Moreover, phenotypic characterization of CD4+ T cells is restricted to the circulating compartment, which may not be representative of the T cell population in infected subjects. Analyses of post-treatment samples could theoretically help to delineate infection-related changes in T-cell responses that can be reversed in the absence of helminth-derived antigenic stimulation. However, given that ivermectin, the first-line treatment for M. ozzardi infection, is highly efficacious against microfilariae but does not appear to kill adult worms [8,19], PBMC samples collected after treatment may still be stimulated by circulating excreted-secreted soluble antigens that are chronically released by adult worms.
In conclusion, it is tempting to speculate that the balance between suppressive CD4+CD39+ Treg cells and immunostimulatory CD4+CD25-FoxP3-CD39+ Tind cells is a key factor contributing to the unexpected patterns of immune regulation found in M. ozzardi infection. We suggest that an increased frequency of Tind cells in our microfilaremics might have prevented lymphocyte hyporesponsiveness despite the concomitant expansion of the CD4+CD39+ Treg subset. However, the CD39+ cell pool remains uncharacterized in other chronic helminth infections that induce typical immunomodulatory responses. Therefore, further studies on CD39+ cells may help to unveil some of the biochemical and molecular pathways whereby different helminths manipulate their hosts’ immunity.
Supporting information
MV = Monte Verde, VP = Valparaíso, BV = Boa Vista, RT = Retiro, NV = Nova Vida, and SP = Sao Pedro. The location of the town of Boca do Acre is also shown.
(DOCX)
A, Time; B, Singlets; C, Lymphocytes were selected for their size and complexity; D, Selection of viable cells; E, Selection of CD3+ cells; F, Selection of CD4+ T cells. Expression of TNFRII (G) PD-1 (H), CD69 (I), CTLA-4 (J), and HLA-DR (L) was measured as shown.
(DOCX)
A, Time; B, Singlets; C, Lymphocytes were selected for their size and complexity; D, Selection of viable cells; E, Selection of CD3+ cells; F, Selection of CD4+ T cells. Expression of intracelular CD39 (G), CTLA-4 (H), OX-40 (I), LAP-TGF-β (J), GITR (L), and LAG-3 (M) was evaluated as shown.
(DOCX)
A, Time; B, Singlets; C, Lymphocytes were selected for their size and complexity; D, Selection of viable cells; E, Selection of CD3+ cells; F, Dual labelling for CD4 and CD25 to define CD4+CD25+ cells; G, Selection of CD4+CD25+ cells that do not express CD127; H, Selection, from the CD25+CD4+CD127- population, of lymphocytes co-expressing FOXP3 and CD39; H1, CD4+CD25+CD127-CD39+FOXP3- T cells; H2, CD4+CD25-CD127+CD39+FOXP3+ T cells; H3, CD4+CD25+CD127-CD39-FOXP3+ T cells. Expression of TNFRII (I) PD-1 (J), CD69 (L), CTLA-4 (M), and HLA-DR (N) was evaluated as shown.
(DOCX)
A, Time; B, Singlets; C, Lymphocytes were selected for their size and complexity; D, Selection of viable cells; E, Selection of CD3+ cells; F, Dual labelling for CD4 and CD25 to define CD4+CD25+ cells; G, Selection of CD4+CD25+ cells that do not express CD127; H, Selection, from the CD25+CD4+CD127- population, of lymphocytes co-expressing FOXP3 and CD39; H1, CD4+CD25+CD127-CD39+FOXP3- T cells; H2, CD4+CD25-CD127+CD39+FOXP3+ T cells; H3, CD4+CD25+CD127-CD39-FOXP3+ T cells. Expression of intracellular CTLA-4 (I), OX-40 (J), LAP-TGF-β (L), GITR (M), and LAG-3 (N) was evaluated as shown.
(DOCX)
We compared the frequencies of CD39+ and CD39- Treg cells (defined as CD4+CD25hiCD127-FoxP3+ T cells) that expressed a range of regulatory and activation markers. Data are shown for 48 Fil+ and 33 Fil- subjects and were compared using the Wilcoxon signed rank test. Only significant P values after controlling for a false discovery rate (q) set at 0.10 (m = 9) are shown.
(DOCX)
PBMC from microfilaremic (Fil+) and uninfected (Fil-) subjects were stimulated with Staphylococcus aureus enterotoxin B (SEB) in the presence or absence of anti-CD39 antibody, stained for intracellular cytokines, and then incubated with 2mM adenosine. The % of CD4+ T cells producing each cytokine was estimated by flow cytometry. Data are presented for 11 Fil+ and 5 Fil- subjects and were compared using the Wilcoxon signed rank test. Only significant P values after controlling for a false discovery rate (q) set at 0.10 (m = 5 for each group [Fil+ and Fil-] and each pair of experimental conditions [SEB vs. SEB+anti-CD39, SEB vs. SEB+anti-CD39+adenosine; SEB+anti-CD39 vs. SEB+anti-CD39+adenosine]) are shown.
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Subjects with BmA antibody levels above the overall median were defined as IgG4H, while those with BmA antibody levels below the overall median were defined as IgG4L.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank all blood sample donors for their participation in this study; Dr. Lígia A. Gonçalves and Dr. Pablo S. Fontoura for help in field work; Dr. Mauro R. Tucci for clinical care of study participants; Dr. Heitor Franco de Andrade Jr. and Andrea Costa (Institute of Tropical Medicine, University of São Paulo) for the generous gift of TgT antigen; and Maria José Menezes for expert laboratory support.
Data Availability
Because original data files contain information that may potentially lead to the identification of study participants, requests for access to original data shoud be submited to the Institutional Review Board of the Institute of Biomedical Sciences, University of São Paulo, Brazil, at cep@icb.usp.br. The study identification number is 1133/CEP, 2013.
Funding Statement
Research was supported by a research grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; http://www.fapesp.br/en/), Brazil (2013/12723-7 to MUF). NFL (2013/26928-0 and 2016/06970-0) and RMG-L (2010/51938-0) were supported by scholarships from FAPESP; MUF receives a senior researcher scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; http://www.cnpq.br/), Brazil. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Maizels RM, Yazdanbakhsh M (2003) Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3: 733–44. doi: 10.1038/nri1183 [DOI] [PubMed] [Google Scholar]
- 2.Metenou S, Nutman TB (2013) Regulatory T cell subsets in filarial infection and their function. Front Immunol 4: 305 doi: 10.3389/fimmu.2013.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yazdanbakhsh M (1999) Common features of T cell reactivity in persistent helminth infections: lymphatic filariasis and schistosomiasis. Immunol Lett 65: 109–15. [DOI] [PubMed] [Google Scholar]
- 4.Maizels RM, McSorley HJ (2016) Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 138: 666–75. doi: 10.1016/j.jaci.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Riet E, Hartgers FC, Yazdanbakhsh M (2007) Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212: 475–90. doi: 10.1016/j.imbio.2007.03.009 [DOI] [PubMed] [Google Scholar]
- 6.Tavares da Silva LB, Crainey JL, Ribeiro da Silva TR, Suwa UF, Vicente AC, et al. (2017) Molecular verification of New World Mansonella perstans parasitemias. Emerg Infect Dis 23: 545–7. doi: 10.3201/eid2303.161159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medeiros JF, Crainey JL, Pessoa FA, Luz SL (2017) Mansonelliasis In: Marcondes CB, editor. Arthropod borne diseases. Cham (Switzerland): Springer International Publishing, p. 562. [Google Scholar]
- 8.Lima NF, Veggiani Aybar CA, Dantur Juri MJ, Ferreira MU (2016) Mansonella ozzardi: a neglected New World filarial nematode. Pathog Glob Health 110: 97–107. doi: 10.1080/20477724.2016.1190544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raccurt CP (2018) Mansonella ozzardi and its vectors in the New World: an update with emphasis on the current situation in Haiti. J Helminthol, in press doi: 10.1017/S0022149X17000955 [DOI] [PubMed] [Google Scholar]
- 10.Orihel TC, Lowrie RC Jr, Eberhard ML, Raccurt C, Kozek WJ, et al. (1981) Susceptibility of laboratory primates to infection with Mansonella ozzardi from man. Am J Trop Med Hyg 30: 790–4. [DOI] [PubMed] [Google Scholar]
- 11.Metenou S, Dembélé B, Konate S, Dolo H, Coulibaly SY, et al. (2009) Patent filarial infection modulates malaria-specific type 1 cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population. J Immunol 183: 916–24. doi: 10.4049/jimmunol.0900257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metenou S, Babu S, Nutman TB (2012) Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS 7: 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolo H, Coulibaly YI, Dembele B, Konate S, Coulibaly SY, et al. (2012) Filariasis attenuates anemia and proinflammatory responses associated with clinical malaria: a matched prospective study in children and young adults. PLoS Negl Trop Dis 6: e1890 doi: 10.1371/journal.pntd.0001890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Layland LE, Specht S (2014) Helpful or a hindrance: co-infections with helminths during malaria. Adv Exp Med Biol 828: 99–129. doi: 10.1007/978-1-4939-1489-0_5 [DOI] [PubMed] [Google Scholar]
- 15.Takenaka MC, Robson S, Quintana FJ (2016) Regulation of the T cell response by CD39. Trends Immunol 37: 427–39. doi: 10.1016/j.it.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medeiros JF, Py-Daniel V, Barbosa UC, Ogawa GM (2009). Occurrence of Mansonella ozzardi (Nematoda, Onchocercidae) in riverine communities of the Purus River, Boca do Acre municipality, Amazonas State, Brazil. Cad Saude Publica 25: 1421–6. [DOI] [PubMed] [Google Scholar]
- 17.Shelley AJ, Coscarón S 2001. Simuliid blackflies (Diptera: Simuliidae) and ceratopogonid midges (Diptera: Ceratopogonidae) as vectors of Mansonella ozzardi (Nematoda: Onchocercidae) in northern Argentina. Mem Inst Oswaldo Cruz 96: 451–8. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros JF, Py-Daniel V, Barbosa UC, Izzo TJ. 2009. Mansonella ozzardi in Brazil: prevalence of infection in riverine communities in the Purus region, in the state of Amazonas. Mem Inst Oswaldo Cruz 104: 74–80. [DOI] [PubMed] [Google Scholar]
- 19.Basano SA, Fontes G, Medeiros JF, Aranha-Camargo JS, Souza-Vera LJ, et al. (2014) Sustained clearance of Mansonella ozzardi infection after treatment with ivermectin in the Brazilian Amazon. Am J Trop Med Hyg 90: 1170–5. doi: 10.4269/ajtmh.13-0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman WA, Pons JA, Janer JL (1934) The sedimentation concentration method in schistosomiasis mansoni. Puerto Rico J Public Health Trop Med 9: 283–91. [Google Scholar]
- 21.Barbosa S, Gozze AB, Lima NF, Batista CL, Bastos MS, et al. (2014) Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis 8: e3109 doi: 10.1371/journal.pntd.0003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales-Hojas R, Post RJ, Shelley AJ, Maia-Herzog M, Coscarón S, et al. (2001) Characterization of nuclear ribosomal DNA sequences from Onchocerca volvulus and Mansonella ozzardi (Nematoda: Filarioidea) and development of a PCR-based method for their detection in skin biopsies. Int J Parasitol 31: 169–77. [DOI] [PubMed] [Google Scholar]
- 23.Terhell AJ, Price R, Koot JW, Abadi K, Yazdanbakhsh M (2000) The development of specific IgG4 and IgE in a paediatric population is influenced by filarial endemicity and gender. Parasitology 121: 535–43. [DOI] [PubMed] [Google Scholar]
- 24.Gonçalves RM, Salmazi KC, Santos BA, Bastos MS, Rocha SC, et al. (2010) CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses? Infect Immun 78: 4763–72. doi: 10.1128/IAI.00578-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares A, Govender L, Hughes J, Mavakla W, de Kock M, et al. (2010) Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods 362: 43–50. doi: 10.1016/j.jim.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouser EE, Pollakis G, Yazdanbakhsh M, Harnett W, de Jong EC, et al. (2016) Brugia malayi antigen (BmA) inhibits HIV-1 trans-infection but neither BmA nor ES-62 alter HIV-1 infectivity of DC induced CD4+ Th-Cells. PLoS One 11: e0146527 doi: 10.1371/journal.pone.0146527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camargo ME, Ferreira AW, Mineo JR, Takiguti CK, Nakahara OS (1978) Immunoglobulin G and immunoglobulin M enzyme-linked immunosorbent assays and defined toxoplasmosis serological patterns. Infect Immun 21: 55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira TC, Rodrigues PT, Menezes MJ, Gonçalves-Lopes RM, Bastos MS et al. (2017) Genome-wide diversity and differentiation in New World populations of the human malaria parasite Plasmodium vivax. PLoS Negl Trop Dis 11: e0005824 doi: 10.1371/journal.pntd.0005824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mata-Cantero L, Lafuente MJ, Sanz L, Rodriguez MS (2014) Magnetic isolation of Plasmodium falciparum schizonts iRBCs to generate a high parasitaemia and synchronized in vitro culture. Malar J 13: 112 doi: 10.1186/1475-2875-13-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, et al. (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110: 1225–32. doi: 10.1182/blood-2006-12-064527 [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300. [Google Scholar]
- 32.Lu Y, Wang X, Gu J, Lu H, Zhang F, Li X, et al. (2015) iTreg induced from CD39+ naive T cells demonstrate enhanced proliferate and suppressive ability. Int Immunopharmacol 28: 925–30. doi: 10.1016/j.intimp.2015.03.039 [DOI] [PubMed] [Google Scholar]
- 33.Costa AG, Sadahiro A, Tarragô AM, Pessoa FAC, Loiola BP, et al. (2018) Immune response in Mansonella ozzardi infection modulated by IL-6/IL-10 axis in Amazon region of Brazil. Cytokine, in press doi: 10.1016/j.cyto.2017.09.033 [DOI] [PubMed] [Google Scholar]
- 34.Takenaka MC, Robson S, Quintana FJ. (2016) Regulation of the T-cell response by CD39. Trends Immunol 37: 427–39. doi: 10.1016/j.it.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong RP, Tachibana K, Hegen M, Munakata Y, Cho D, et al. (1997) Determination of adenosine deaminase binding domain on CD26 and its immunoregulatory effect on T cell activation. J Immunol 159: 6070–6. [PubMed] [Google Scholar]
- 36.Chiacchio T, Casetti R, Butera O, Vanini V, Carrara S, Girardi E, et al. (2009) Characterization of regulatory T cells identified as CD4+CD25highCD39+ in patients with active tuberculosis. Clin Exp Immunol 156: 463–70. doi: 10.1111/j.1365-2249.2009.03908.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulze Zur Wiesch J, Thomssen A, Hartjen P, Tóth I, Lehmann C, et al. (2011) Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol 85: 1287–97. doi: 10.1128/JVI.01758-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolova M, Carriere M, Jenabian MA, Limou S, Younas M, et al. (2011) CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog 7: e1002110 doi: 10.1371/journal.ppat.1002110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Abente J, Correa-Rocha R, Pion M (2016) Functional mechanisms of Treg in the context of HIV infection and the Janus face of immune suppression. Front Immunol 7: 192 doi: 10.3389/fimmu.2016.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allard B, Longhi MS, Robson SC, Stagg J. (2017) The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev 276: 121–44. doi: 10.1111/imr.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ndhlovu LC, Leal FE, Eccles-James IG, Jha AR, Lanteri M, et al. (2010) A novel human CD4+ T-cell inducer subset with potent immunostimulatory properties. Eur J Immunol 40: 134–41. doi: 10.1002/eji.200939258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moncrieffe H, Nistala K, Kamhieh Y, Evans J, Eddaoudi A, et al. (2010) High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol 185: 134–43. doi: 10.4049/jimmunol.0803474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang F, Yu M, Cavanagh MM, Hutter Saunders J, Qi Q, et al. (2016) Expression of CD39 on activated T cells impairs their survival in older individuals. Cell Rep 14: 1218–31. doi: 10.1016/j.celrep.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MV = Monte Verde, VP = Valparaíso, BV = Boa Vista, RT = Retiro, NV = Nova Vida, and SP = Sao Pedro. The location of the town of Boca do Acre is also shown.
(DOCX)
A, Time; B, Singlets; C, Lymphocytes were selected for their size and complexity; D, Selection of viable cells; E, Selection of CD3+ cells; F, Selection of CD4+ T cells. Expression of TNFRII (G) PD-1 (H), CD69 (I), CTLA-4 (J), and HLA-DR (L) was measured as shown.
(DOCX)
A, Time; B, Singlets; C, Lymphocytes were selected for their size and complexity; D, Selection of viable cells; E, Selection of CD3+ cells; F, Selection of CD4+ T cells. Expression of intracelular CD39 (G), CTLA-4 (H), OX-40 (I), LAP-TGF-β (J), GITR (L), and LAG-3 (M) was evaluated as shown.
(DOCX)
A, Time; B, Singlets; C, Lymphocytes were selected for their size and complexity; D, Selection of viable cells; E, Selection of CD3+ cells; F, Dual labelling for CD4 and CD25 to define CD4+CD25+ cells; G, Selection of CD4+CD25+ cells that do not express CD127; H, Selection, from the CD25+CD4+CD127- population, of lymphocytes co-expressing FOXP3 and CD39; H1, CD4+CD25+CD127-CD39+FOXP3- T cells; H2, CD4+CD25-CD127+CD39+FOXP3+ T cells; H3, CD4+CD25+CD127-CD39-FOXP3+ T cells. Expression of TNFRII (I) PD-1 (J), CD69 (L), CTLA-4 (M), and HLA-DR (N) was evaluated as shown.
(DOCX)
A, Time; B, Singlets; C, Lymphocytes were selected for their size and complexity; D, Selection of viable cells; E, Selection of CD3+ cells; F, Dual labelling for CD4 and CD25 to define CD4+CD25+ cells; G, Selection of CD4+CD25+ cells that do not express CD127; H, Selection, from the CD25+CD4+CD127- population, of lymphocytes co-expressing FOXP3 and CD39; H1, CD4+CD25+CD127-CD39+FOXP3- T cells; H2, CD4+CD25-CD127+CD39+FOXP3+ T cells; H3, CD4+CD25+CD127-CD39-FOXP3+ T cells. Expression of intracellular CTLA-4 (I), OX-40 (J), LAP-TGF-β (L), GITR (M), and LAG-3 (N) was evaluated as shown.
(DOCX)
We compared the frequencies of CD39+ and CD39- Treg cells (defined as CD4+CD25hiCD127-FoxP3+ T cells) that expressed a range of regulatory and activation markers. Data are shown for 48 Fil+ and 33 Fil- subjects and were compared using the Wilcoxon signed rank test. Only significant P values after controlling for a false discovery rate (q) set at 0.10 (m = 9) are shown.
(DOCX)
PBMC from microfilaremic (Fil+) and uninfected (Fil-) subjects were stimulated with Staphylococcus aureus enterotoxin B (SEB) in the presence or absence of anti-CD39 antibody, stained for intracellular cytokines, and then incubated with 2mM adenosine. The % of CD4+ T cells producing each cytokine was estimated by flow cytometry. Data are presented for 11 Fil+ and 5 Fil- subjects and were compared using the Wilcoxon signed rank test. Only significant P values after controlling for a false discovery rate (q) set at 0.10 (m = 5 for each group [Fil+ and Fil-] and each pair of experimental conditions [SEB vs. SEB+anti-CD39, SEB vs. SEB+anti-CD39+adenosine; SEB+anti-CD39 vs. SEB+anti-CD39+adenosine]) are shown.
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Subjects with BmA antibody levels above the overall median were defined as IgG4H, while those with BmA antibody levels below the overall median were defined as IgG4L.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
Because original data files contain information that may potentially lead to the identification of study participants, requests for access to original data shoud be submited to the Institutional Review Board of the Institute of Biomedical Sciences, University of São Paulo, Brazil, at cep@icb.usp.br. The study identification number is 1133/CEP, 2013.