Abstract
The unfolded protein response (UPR) is a highly regulated signaling pathway that is largely conserved across eukaryotes. It is essential for cell homeostasis under environmental and physiological conditions that perturb the protein folding in the endoplasmic reticulum (ER). Arabidopsis is one of the outstanding multicellular model systems in which to investigate the UPR. Here, we described a protocol to induce the UPR in plants, specifically arabidopsis, and to estimate their ability to cope with ER stress through the quantification of physiological parameters.
Keywords: Unfolded protein response, Endoplasmic reticulum stress, Temporary ER stress, Prolonged ER stress, Tunicamycin, Arabidopsis
1 Introduction
Adverse environmental changes and physiological conditions of growth can interfere with crucial biosynthetic processes and disturb proper protein folding in the endoplasmic reticulum (ER), leading to a potentially lethal condition generally termed “ER stress” [1, 2]. To overcome ER stress, the unfolded protein response (UPR), a highly regulated signaling pathway largely conserved across eukaryotes, is actuated [3]. The UPR activates a network of pathways, which generally promote cell adaptation and restore ER homeostasis; however, under unmitigated stress conditions they also can ignite programmed cell death (PCD) both in animals [1, 4] and plants [5, 6]. Despite the relevance of the UPR in cell growth and stress adaptation and the abundant information on the function of the canonical stress sensors and transducers in the UPR [5, 7, 8], we lack a complete understanding of the molecular players involved in the temporally distinct UPR-related events (i.e., UPR initiation, recovery from temporary ER stress, survival from mild and prolonged ER stress, and ignition of PCD under unmitigated ER stress), especially in plants [9].
Depending on the duration of ER stress, three main experimental approaches have been implemented to investigate the contribution of the UPR players in ER stress resolution. The downstream transcriptional initiation of the adaptive UPR signaling response can be monitored at a molecular level by assaying the expression levels of the UPR marker genes in time-course treatment with ER stress-inducing agents (for detailed methods, see [3, 10]). The plant ability to recover after temporary ER stress or to cope with prolonged and mild ER stress is evaluated at phenotypic level on seedlings transferred or germinated and grown on ER stress inducer-containing media [5, 6]. Finally, ER stress-induced cell death can be evoked by growing seedlings under prolonged and severe ER stress conditions and quantified by standard methods to test for PCD (e.g., ion leakage and TUNEL staining) [6, 11].
Common chemical agents used to experimentally induce ER stress and activate the UPR in plants are tunicamycin (Tm) or dithiothreitol (DTT) [5, 12, 13]. Tm inhibits the N-acetylglucosamine phosphotransferases; this prevents N-linked glycosylation of nascent polypeptides in the ER lumen. DTT is a potent reducing agent that prevents disulfide bond formation during folding of polypeptides in the ER (see Note 1). Here, we describe phenotypic analyses most commonly adopted to evaluate ER stress sensitivity of arabidopsis seedlings after a short ER stress-inducing treatment or during prolonged ER stress.
2 Materials
Basic equipment for plant sterile tissue culture.
Plant growth chamber conditions: continuous white light at 21 °C, 100 μEinstein/m2 s, 65% humidity.
Plant growth medium: half-strength (1/2) Linsmaier and Skoog (LS) medium, 1% sucrose, pH 5.7.
Tunicamycin (Tm) from Streptomyces sp. (see Note 2): prepare 10 mg/mL of Tm stock solution by dissolving 0.005 g Tm in 0.5 mL of DMSO yielding a clear solution (see Notes 3 and 4).
Dimethyl sulfoxide (DMSO).
70% ethanol.
5% commercial bleach freshly prepared.
Acetone: 80% solution in water.
Petri dishes, square with grids, 100 mm × 100 mm, sterile, polystyrene.
Micropore gas-permeable surgical tape.
Sterilized toothpicks.
Razor blades.
Analytical balance.
Microplate, 96 well, flat bottom, clear.
Microplate reader equipped with 663 and 645 nm wavelengths.
3 Methods
3.1 Recovery After Temporary ER Stress Treatment
Carry out all procedures at room temperature and if aseptic conditions are required, work in a laminar flow hood.
Surface-sterilize the arabidopsis seeds (see Note 5): in a micro-centrifuge tube, soak the seeds in 1 mL 70% ethanol for 1 min, suspend them by turning the tube or vortex, pour out the liquid, rinse two times in distilled sterile water, suspend the seeds in 1 mL 5% commercial bleach for 1 min, then rinse three times in sterile distilled water (see Note 6). Store the seeds at 4 °C for 2 days to synchronize them.
Autoclave for 25 min the plant growth medium with 12 g/L agar (see Note 7) and pour 40 mL into each square Petri dish.
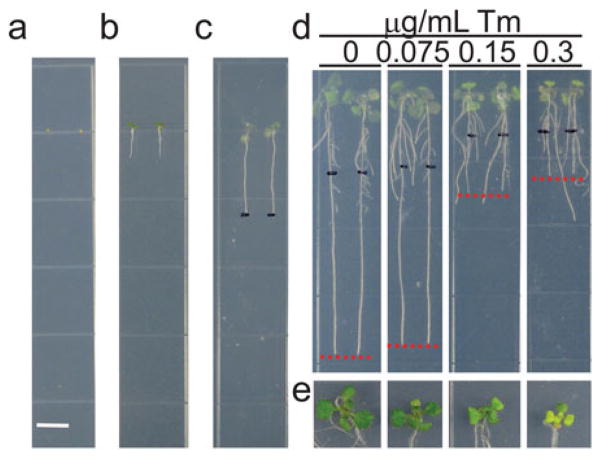
Use a sterile toothpick to sow the arabidopsis seeds on the solid plant growth medium placing each seed at an equally spaced distance (Fig. 1a).
Seal the plates with one layer of micropore gas-permeable surgical tape to allow gaseous exchange.
Germinate and grow the seeds in vertical position in a plant growth chamber for 5 days.
Autoclave for 25 min the plant growth medium and store it at room temperature.
Freshly prepare 0.075, 0.15, and 0.30 mg/mL Tm stock solutions by 133, 66, and 33× dilution, respectively, of the 10 mg/mL Tm stock solution using 1/2 LS liquid medium.
Autoclave for 25 min the plant growth medium with 12 g/L agar (see Note 5). Cool down the medium until 50 °C (see Note 8) and keep it homogeneously mixed with a magnetic stir bar.
Add 0.075, 0.15, and 0.30 mg/mL Tm stock solutions, respectively, to the cooled down ½ LS medium by 1000 dilution to make plant growth medium containing 0.075, 0.15, and 0.30 μg/mL Tm (see Notes 9 and 10).
As mock control, the same preparation procedure is carried out replacing the Tm in the ½ LS medium with 0.0005% DMSO.
Transfer the 5-day-old seedlings using a sterile toothpick to plant growth medium plates containing DMSO, 0.075, 0.15, or 0.30 μg/mL Tm (Fig. 1b). Seal the plates with one layer of micropore gas-permeable surgical tapes to permit gaseous exchange and place them on vertical position into the plant growth chamber.
After 3 days, use a sterile toothpick to transfer the seedlings to plant growth medium plates without Tm (or DMSO). Mark the root tip on the back of the plate using an extra-fine point permanent marker and place the plates on vertical position into the plant growth chamber (Fig. 1c).
After 3 days, observe the growth phenotype (Fig. 1d and e) and quantify the plant growth parameters as described below.
If using Col-0 ecotype wild-type arabidopsis, the plants show growth defects already at 0.15 μg/mL Tm (Fig. 1d and e).
Fig. 1.
Arabidopsis Col-0 growth phenotype after recovery from short-term Tm treatment. (a) Arabidopsis seeds sown at an equally spaced distance on ½ LS solid medium for vertical growth. Scale bar: 0.65 mm. (b) 5-day-old seedlings were transferred to ½ LS solid medium plates containing Tm or DMSO for short-term ER stress treatment. (c) 8-day-old seedlings were transferred from Tm-plates to ½ LS solid plates and the root tips were marked on the back of the plate using a black extra-fine point marker. (d) Root growth inhibition of Tm-treated seedlings compared to untreated seedlings after 3 days of recovery from short-term Tm treatment. Red lines show the primary root tip. (e) Close-up view of the shoot of seedlings shown in (d)
3.2 Prolonged ER Stress Treatment
Carry out all procedures at room temperature and if aseptic conditions are required, work in a laminar flow hood.
Autoclave for 25 min the plant growth medium and store it at room temperature.
Autoclave for 25 min the plant growth medium with 12 g/L agar (see Note 5).
Freshly prepare 0.04, 0.06, and 0.08 mg/mL Tm stock solutions by 250, 166, and 125× dilution of 10 mg/mL Tm stock solution, respectively, using 1/2 LS liquid medium.
Add 0.04, 0.06, and 0.08 mg/mL Tm stock solutions, respectively, to the cooled down ½ LS medium by 1000 dilution to make plant growth medium containing 40, 60, and 80 ng/mL Tm (see Notes 9 and 11). As mock control, the same preparation procedure is carried out replacing the Tm in the ½ LS medium with 0.0005% DMSO.
Surface-sterilize the arabidopsis seeds (see Note 3) and store them at 4 °C for 2 days.
Use a sterile toothpick to sow the surface-sterilized seeds on the ½ LS medium containing DMSO, 40, 60, and 80 ng/mL Tm. Place each seed at an equally spaced distance (Fig. 1a)
Seal the plates with one layer of micropore gas-permeable surgical tape to allow gaseous exchange.
Germinate and grow the seeds in vertical position in a plant growth chamber.
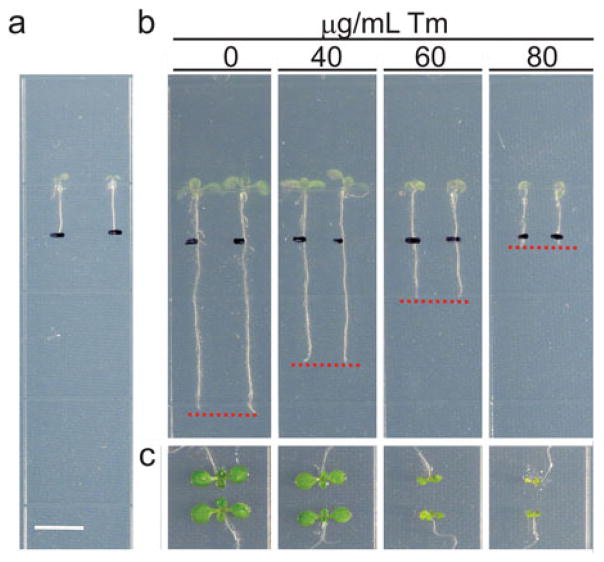
After 5 days, mark the root tip on the back of the plate using an extra-fine point marker (Fig. 2a) and place the plates back on vertical position into the plant growth chamber.
Observe the growth phenotype 7–14 days after germination (Fig. 2b and c), but check the plant growth each day during the assay to precisely monitor the ER stress sensitivity of the seedlings. Quantify the plant growth parameters as described below.
If using Col-0 ecotype wild-type arabidopsis, the plants show growth defects starting from 40 ng/mL Tm (Fig. 2b and c).
Fig. 2.
Arabidopsis Col-0 growth phenotype after prolonged Tm treatment. (a) Root tips of 5-day-old seedlings were marked on the back of the plate using a black extra-fine point marker. Scale bar: 0.65 mm. (b) Root growth inhibition of Tm-treated seedlings compared to untreated seedlings grown for 10 days vertically. Red lines show the primary root tip. (c) Close-up view of the shoot of seedlings shown in (b)
3.3 Plant Growth Parameters
The effect of the prolonged ER stress on arabidopsis seeds can be quantified through the quantification of emergence of the true leaves (Fig. 2c). Count the number of seedlings having true leaves on Tm-containing plates and normalize this value with respect to the total number of all sown seeds. Similarly, count the number of seedlings having true leaves on DMSO-containing plates and normalize this value on the total number of all sown seeds [12].
Fresh shoot weight is an easy readout of plant sensitivity of both short-term ER stress and prolonged ER stress. Using a razor blade, cut the plant shoots at a consistent cut-position on the hypocotyl. Gently dry the shoots on a paper towel and measure the weight with an analytical balance. To quantify the relative growth values, the shoot weight of the seedlings grown on Tm is divided by the shoot weight of the seedlings grown on DMSO-plates. For each measurement, use a pool of 6–10 shoots for each of the at least six technical replicates for each of the three biological replicates.
Chlorophyll (Chl) content of the leaves can be used as an indicator of overall plant health after short-term or prolonged Tm treatment. After measuring fresh shoot weight, place the pool of leaves into a microcentrifuge tube and extract chlorophyll using 1 mL cold 80% acetone at 4 °C for 24 h (see Note 12) in the dark (see Note 13). Quickly transfer 0.3 mL of the supernatant to a flat-bottom 96-well microplate and use 0.3 mL 80% acetone blank as reference. Measure the absorbance (A) of the chlorophyll content using a microplate reader at 663 and 645 nm wavelengths. The chlorophyll concentration is calculated as follows: Chl (mg/g) = [8.02 × A663 + 20.20 × A645] × V/1000 × W, where V = volume of the extract (mL) and W = weight of fresh leaves (g) [13, 14].
The primary root elongation declines as the concentration of the ER stress inducer increases into the growth media in a dose-dependent manner (Figs. 1d and 2b) [15]. For this reason, ER stress sensitivity can be quantified measuring the primary root length of seedlings grown on Tm-plates. Take pictures of the plants treated as described in Subheading 3.1, steps 1–14 or 3.2, steps 1–10 (see Note 14). Measure the root length from the root tip to the mark made in the Subheading 3.1, step 13 or 3.2, step 9. To quantify the relative root elongation value, the root length of the seedlings grown on Tm is divided by the root length of the seedlings grown on DMSO-plates.
Acknowledgments
This study was primarily supported by National Institutes of Health (GM101038) with contributing support from the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (award number DE-FG02-91ER20021), and AgBioResearch.
Footnotes
Even if DDT is commonly used to cause protein misfolding in the ER, it is not a specific ER stress inducer, since it blocks disulfide bond formation both in the ER and in the cytosol, and causes oxidative stress and the expression of defense genes [12, 15].
The hazard category for Tm is acute toxicity, oral (category 2) H300 (fatal if swallowed). Tm is light sensitive.
To avoid formation of aerosols, directly inject the DMSO solvent using a 1-mL syringe with a needle through the lid of the tube containing the solution and mix.
To avoid freezing and thawing, aliquot the 10 mg/mL stock solution into relatively small amounts (0.01 mL aliquots) and store them at −20 °C freezer in the dark.
ER stress sensitivity is highly affected by the seeds quality. Use seeds with high germination quality and freshly collected from healthy plants.
Do not leave seeds in ethanol or bleach for too long because the treatment may be toxic to the seeds.
Mix homogeneously the solution with a magnetic stir bar before and after to autoclave, to avoid settling of unmelted agar at the bottom of the bottle.
Tm is sensitive to high temperature.
Prepare the Tm-containing medium plates right before using them.
The optimal range of concentration of Tm has to be experimentally determined. As initial screen, use 0.075 and 0.15 μg/mL Tm and then decrease or increase the Tm concentration depending on the degree of Tm sensitivity of the analyzed arabidopsis ecotype.
The optimal range of concentration of Tm has to be experimentally determined. As initial screen, use 20 and 40 ng/mL Tm and then decrease or increase the Tm concentration depending on the degree of Tm sensitivity of the analyzed arabidopsis ecotype and on the purpose of your assay. To evaluate Tm sensitivity under mild ER stress conditions, use low Tm concentration that can be increased to get unmitigated ER stress conditions and consequent cell death.
Complete chlorophyll extraction is fulfilled when the shoots are completely white. If the leaves are still partially green, keep the tissues at 4 °C in the darkness for longer time.
During chlorophyll extraction and analyses, keep the samples in the dark, because chlorophylls are light-sensitive pigments.
To better distinguish the roots, take pictures of the back of the plates.
References
- 1.Chen Y, Brandizzi F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013;23:547–555. doi: 10.1016/j.tcb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J-X, Howell SH. Managing the protein folding demands in the endoplasmic reticulum of plants. New Phytol. 2016 doi: 10.1111/nph.13915. [DOI] [PubMed] [Google Scholar]
- 3.Ruberti C, Brandizzi F. Conserved and plant-unique strategies for overcoming endoplasmic reticulum stress. Front Plant Sci. 2014;5:69. doi: 10.3389/fpls.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Brandizzi F. AtIRE1A/AtIRE1B and AGB1 independently control two essential unfolded protein response pathways in Arabidopsis. Plant J Cell Mol Biol. 2012;69:266–277. doi: 10.1111/j.1365-313X.2011.04788.x. [DOI] [PubMed] [Google Scholar]
- 6.Mishiba K, et al. Defects in IRE1 enhance cell death and fail to degrade mRNAs encoding secretory pathway proteins in the Arabidopsis unfolded protein response. Proc Natl Acad Sci U S A. 2013;110:5713–5718. doi: 10.1073/pnas.1219047110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J-X, Srivastava R, Che P, Howell SH. An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Plant Cell. 2007;19:4111–4119. doi: 10.1105/tpc.106.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata Y, Fedoroff NV, Koizumi N. Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell. 2008;20:3107–3121. doi: 10.1105/tpc.108.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruberti C, Kim S-J, Stefano G, Brandizzi F. Unfolded protein response in plants: one master, many questions. Curr Opin Plant Biol. 2015;27:59–66. doi: 10.1016/j.pbi.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Brandizzi F. Analysis of unfolded protein response in Arabidopsis. Methods Mol Biol. 2013;1043:73–80. doi: 10.1007/978-1-62703-532-3_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z-T, et al. The membrane-associated transcription factor NAC089 controls ER-stress-induced programmed cell death in plants. PLoS Genet. 2014;10(3):e1004243. doi: 10.1371/journal.pgen.1004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L, et al. The lumen-facing domain is important for the biological function and organelle-to-organelle movement of bZIP28 during ER stress in Arabidopsis. Mol Plant. 2013;6:1605–1615. doi: 10.1093/mp/sst059. [DOI] [PubMed] [Google Scholar]
- 13.Ritchie RJ. Consistent sets of spectro-photometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res. 2006;89:27–41. doi: 10.1007/s11120-006-9065-9. [DOI] [PubMed] [Google Scholar]
- 14.Ni Z, Kim E-D, Chen ZJ. Chlorophyll and starch assays. 2009 Available at: http://dx.doi.org/10.1038/nprot.2009.12.
- 15.Deng Y, Srivastava R, Howell SH. Protein kinase and ribonuclease domains of IRE1 confer stress tolerance, vegetative growth, and reproductive development in Arabidopsis. Proc Natl Acad Sci U S A. 2013;110:19633–19638. doi: 10.1073/pnas.1314749110. [DOI] [PMC free article] [PubMed] [Google Scholar]