Abstract
Objectives
Probe-based confocal laser endomicroscopy (pCLE) is a novel imaging modality that enables virtual optical biopsy in vivo. Loss of barrier function of the small bowel observed via pCLE as increased density of epithelial gaps (extrusion zones left in the intestinal lining after cells are shed) is predictive of relapse in adult patients with inflammatory bowel disease (IBD). This study aims to determine whether such observations on pCLE are similarly predictive of disease relapse in pediatric patients with IBD.
Methods
Pediatric patients with biopsy-proven IBD underwent pCLE during colonoscopy and subsequent clinical follow-up every 6 months. Relapse was defined as moderate to severe flare with endoscopic evidence of inflammation during the follow-up period. The relations between epithelial gap density, disease relapse, and imaging parameters were determined using Cox models.
Results
Twenty-four patients with IBD (13 with Crohn disease, 11 with ulcerative colitis) with a median age of 14 years (range 10–21) were studied for a median of 13 (4–33) months. The median duration of disease was 2.9 years (range 0–9). Increased epithelial gap density in the terminal ileum on pCLE of normal endoscopic appearing terminal ileum mucosa (N = 19) was predictive of disease relapse when 3 or more areas were imaged (N = 6, log-rank P = 0.02, C-statistic = 0.94).
Conclusions
In pediatric patients with IBD, barrier dysfunction observed on pCLE imaging of the small bowel was predictive of disease relapse.
Keywords: barrier function, confocal laser endomicroscopy, Crohn disease, endoscopy, inflammatory bowel disease, pediatric, ulcerative colitis
A major challenge in the management of patients with inflammatory bowel disease (IBD) is that the clinical course of disease can be highly variable (1). There are limited correlation between clinical activity, biological parameters, and endoscopic severity in patients with IBD (2,3). Despite intense search for predictors of relapse in IBD, limited biochemical and endoscopic factors have been found to be predictive of disease relapse. Higher serum C-reactive protein levels and fecal calprotectin levels are certainly found in patients who relapse, but the significant individual variability in the levels of these biomarkers has limited their usefulness in predicting relapses. Before the availability of biologic agents, mucosal healing was not found to prevent disease relapse but was associated with less endoscopic inflammation and less need for steroids (4). Mucosal healing, however, has since been shown to be predictive of clinical and endoscopic remission for patients on anti-tumor necrosis factor (TNR) therapy (5,6). Nonetheless, approximately 30% of the patients who achieve complete mucosal healing will still relapse within 2 years (5).
Studies during the past 2 decades have convincingly demonstrated that barrier disruption plays a significant and an important role in the pathogenesis of intestinal inflammation and severity of disease in IBD. Intestinal barrier dysfunction permits resident microbes to enter into the mucosa, which induce mucosal inflammation and secretion of proinflammatory cytokines such as TNF-α (7). Older assays for barrier function such as the lactulose/mannitol test (8) have not been useful clinically because the size of the sugar molecules (about 10−10 m) used in these test is not reflective of the size of the resident bacteria (about 10−6 m). The advent of confocal laser endomicroscopy (CLE) has enabled real-time in vivo assessment of mucosal barrier function (9,10). A surrogate marker for mucosal barrier function is the density of epithelial gaps (extrusion zones) in the intestinal surface as observed by CLE (11). The epithelial gaps or extrusion zones may be the potential entry sites for luminal microbes into the host (10). The epithelial gap density—a validated measure of epithelial cell extrusion (12)—is increased in most patients with IBD compared with healthy controls: 93% of the patients with Crohn disease and 75% of the patients with ulcerative colitis have elevated gap density (13). In adult patients with IBD, normal epithelial barrier function as determined by CLE examination was associated with only a 20% risk of disease relapse and a 5% risk of major events (hospitalization/surgery) occurring within 1 year (14,15). Conversely, barrier dysfunction corresponded to an 80% risk of relapse and a 45% risk of major events in follow-up. The availability of good endoscopic predictors of disease relapse obtained during the time of colonoscopy would enable significant improvements to the care of patients with IBD.
There are 2 CLE systems used for the study of patients with IBD: the endoscope-based CLE (eCLE) and the probe-based CLE (pCLE) system. The eCLE system is a confocal microscope (Optiscan, Notting Hill, Victoria, Australia) integrated into the distal tip of a conventional endoscope (Pentax, Tokyo, Japan). The eCLE system has not been available commercially since 2014. The pCLE system is a confocal probe that can be passed through the accessory channel of any endoscope system. The ColoFlex UHD probes used in this study generate dynamic (12 frames/second) images. The system has a field of view of 240 × 240 μm with a lateral resolution of 1 μm. The depth of imaging from the surface of the confocal lens is 55 to 65 μm. The pCLE system is used for all our previous studies of patients with IBD (11,15,16).
In this study, we hypothesized that epithelial barrier dysfunction also plays an important role in the development of mucosal inflammation in pediatric patients with IBD. Findings of pCLE of the small intestine during colonoscopy would be expected to have significant predictive value for disease relapse in pediatric patients with IBD. Our primary aim was to determine the predictive value of epithelial gap density measurements in the terminal ileum for disease relapse in pediatric patients with IBD. We also explored the relation of epithelial gap density with sex, disease duration, and endoscopically determined disease severity and location.
METHODS
We performed this prospective, blinded, observational cohort follow-up study of pediatric patients with IBD undergoing pCLE during the time of their standard of care colonoscopy at a tertiary care center (The Scientific Center of Children’s Health). IBD (Crohn disease or ulcerative colitis) were proved by previous biopsy, and patients who underwent pCLE from the period of October 2010 until May 2013 were included in this follow up study. The patients were studied from the time of their pCLE to October 2013. All patients or their designated family member signed appropriate informed consent per institutional policy to participate in the study. The study protocol was reviewed and approved by the Local Independent Ethics Committee and Academic Council of the Scientific Center of Children’s Health under Russian Academy of Medical Sciences. The study was registered at ClinicalTrials.Gov (NCT02003859).
The inclusion criteria for the study were biopsy-proven Crohn disease or ulcerative colitis and the ability to give informed consent. Exclusion criteria were as follows: previous allergies to fluorescein or shellfish, hepatic failure, reduced renal function (serum creatinine greater than 1.5 mg/dL), pregnancy, and nursing female patients. The clinical (demographics, history, physical examination findings), laboratory (C-reactive protein, fecal calprotectin, complete blood count etc.), and endoscopic information were recorded in a database. Details of the clinical disease activities and findings at the time of colonoscopies were classified as active disease (evaluation of symptoms) or inactive disease (asymptomatic patients undergoing cancer surveillance).
All patients underwent clinically indicated colonoscopy for the evaluation of disease status. All of the colonoscopies were performed under general anesthesia with sevoflurane according to the standard technique. At the time of the terminal ileal intubation, patients were given 10% fluorescein solution intravenously at a dose of 5 mg/kg body weight. Confocal images of the terminal ileum were obtained with the ultra-high-definition pCLE probe (UHD Coloflex, Mauna Kea Technologies, Paris, France) following a previously reported protocol (16). The technique is based on the principle of confocal fluorescence endomicroscopy. A laser of the wavelength of 488 nm is transmitted by a multifilament fiber-optic mini probe through the working channel of the endoscope to the mucosal surface. The mini-probe was introduced through the working channel of an endoscope with a diameter of 2.2 mm. To minimize artifacts as vascular leak and minimal tissue damage, the probe was placed in constant contact with the mucosal surface without excessive pressure or injury to the tissue. The most optimal position of the probe was deemed to be the one that was perpendicular to the surface of the mucosa. Maintaining a stable position of the probe is crucial in getting high-quality picture.
Frame-by-frame confocal video recordings of the terminal ileum (1–3 minutes) were collected from each patient and stored for analysis. Review and analysis of pCLE images were conducted in a post hoc manner by an expert confocal endomicroscopist (JJL) in a blinded fashion (16). Analysis of epithelial cells and gaps were performed manually using previously published criteria (11). In brief, adequately imaged villi, defined as villi with at least 75% surface area visualized on the pCLE image, with at least 3 different consecutive views of the villi seen were analyzed for total cell and epithelial gap counts. All confocal image videos were also scored for other pCLE-imaging parameters that were reported in previous studies: number of areas sampled, presence of diseased areas on pCLE images, clear visualization of epithelial cells on at least 70% of the video, proximity to ileocecal valve as evident by presence of colonic mucosa in the terminal ileum video.
For the derivation of epithelial gap density, villi with the highest frequency of epithelial gaps for any individual patient were used (range: 3–10 villi evaluated per patient). The gap density was derived by normalizing the total number of epithelial gaps per 1000 epithelial cells counted. Normal gap density was defined as under 10 gaps per 1000 epithelial cells counted; as reported in our previous study (13), increased epithelial gap density is defined as greater than 10 gaps per 1000 epithelial cells counted.
All patients were studied in the gastroenterology department of the Scientific Center of Children’s Health by their prospective gastroenterologists every 3 to 6 months. Patient outcomes in the follow-up period after colonoscopy with pCLE were tracked by review of electronic medical record system 1C (Enterprise 8, Moscow, Russia) for flares, hospitalizations, surgeries, and medication changes. Between hospitalizations, patients were instructed to contact their referring physician via phone or e-mail or were studied by pediatrician domiciliary.
We defined flares in follow-up using a combination of clinical symptoms and endoscopic evidence of mucosal inflammation. Because Harvey-Bradshaw index and Mayo scores are not used for routine clinical care of patients in Russia, clinical symptoms suggestive of disease flare included increased frequency of bowel motions, or reduced stool consistency, that is, diarrhea, presence of blood in the stool, and increased abdominal pain. For laboratory evaluations, increased C-reactive protein and/or fecal calprotectin, decreased hemoglobin were considered to be evidence of flare. All of the patients with flare symptoms underwent endoscopic evaluations, and those patients with evidence of mucosal inflammation on colonoscopy were classified as having flares. Both clinicians and patients were unaware of the findings of the ileal pCLE-imaging results. Therefore, all patient management decisions were made independently of pCLE results.
Statistical Analysis
Sample Size Calculation
The sample size calculation was performed based on the predictive value of epithelial gap density data of adult patients with IBD in our previous study (15). Assuming a difference in the relapse rate of 60% (10% normal vs 70% increased), a total of 20 patients (10 per group) would be required to achieve 80% power with type I error (α) of 0.05. Because nonparametric methods were anticipated to be used, we increased the patient enrollment by 20%, for a total of 24 patients.
The primary end-point of the study was a composite variable of IBD-related hospitalization or surgery in the follow-up period. Continuous variables that were normally distributed were expressed as mean ± standard deviation, while non-normally distributed continuous variables were expressed as median (range). Event-free survival probabilities were estimated by the Kaplan-Meier method, and the comparison between patients with normal and elevated gap densities was conducted via the log-rank test. The Cox proportional hazards model was used to assess gap density as a continuous predictor for the risk of major clinical events. Two-sided P values <0.05 were considered to be significant. For secondary analysis, we assessed the relation between epithelial gap density and other outcomes of interest using appropriate nonparametric tests. All analyses were conducted using the STATA data analysis and statistical software (StataCorp LP, College Station, TX).
RESULTS
The baseline study patient characteristics are shown in Table 1. There were 24 patients (13 CD, 11 UC) with a median follow-up of 13 months (range 4–33). There were 13 girls (54%) and 11 boys (46%) with a median age of 14 years (range 10–21). The median duration of disease at the time of pCLE was 2.9 years (range 0–9).
TABLE 1.
Baseline patient characteristics
| Crohn disease (N = 13) | Ulcerative colitis (N = 11) | |
|---|---|---|
| Age, y; mean ± SD | 14.9 ± 3.8 | 14.3 ± 3.3 |
| % Male | 38 | 55 |
| Disease duration, y; median (range) | 4 (1.5–10.5) | 4 (1–10) |
| Endoscopic disease location, % | ||
| Ileal | 1 (77) | |
| Ileo-colonic | 10 (8) | |
| Colonic | 2 (15) | |
| Pancolitis | 7 (64) | |
| Left-side/proctitis | 4 (36) | |
| Endoscopic disease severity, % | ||
| Normal | 4 (31) | 2 (18) |
| Mild | 2 (15) | 7 (64) |
| Moderate | 2 (18) | |
| Severe | 7 (54) | |
| Medications, % | ||
| Aminosalicylate | 9 (69) | 7 (64) |
| Steroids | 4 (31) | 1 (9) |
| Immuno-modulator | 8 (61) | 1 (9) |
| Biologics | 5 (38) | 2 (18) |
SD = standard deviation.
The disease distributions for CD were as follows: ileo-colitis in 9 patients (70%), ileitis in 2 patients (15%), and colitis in 2 patients (15%). For UC, the disease distributions were as follows: pancolitis in 7 patients (64%), distal colitis in 3 (27%), and proximal colitis in 1 (9%). For IBD therapy, 6 patients (25%) were on anti-TNF agents, 8 (33%) were on 5-ASA, 2 (8%) were on steroids, 1 (4%) was on no therapy, the remaining 7 patients (29%) were on different combination regimens. During the follow-up period, 9 patients (38%) had clinical relapse that met the predefined criteria (clinical symptoms of flare with biochemical and endoscopic evidence of mucosal inflammation). We did not find any clinical or endoscopic factors to be predictive of disease relapse in the follow-up period.
Correlation of Epithelial Gap Density and Disease Relapse
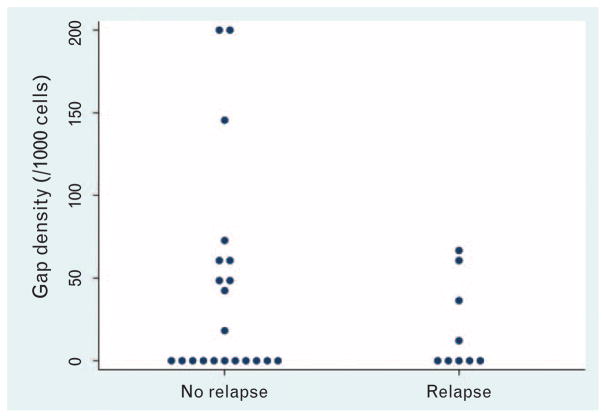
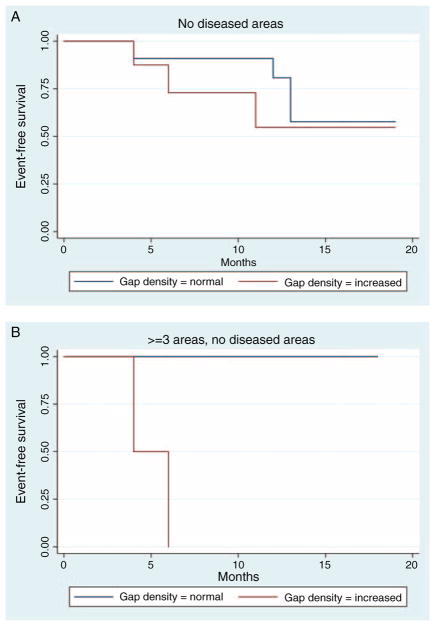
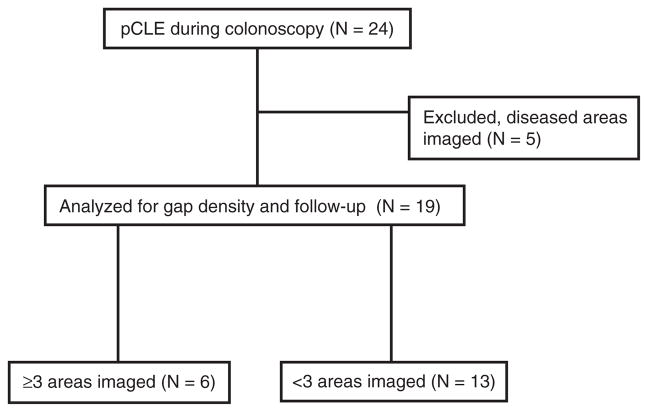
Representative pCLE images of the terminal ileum from study patients are shown in Fig. 1. The gap density distribution of all study patients (N = 24) are shown in Fig. 2. We excluded confocal videos with disease areas imaged from further analysis for disease relapse, because architectural changes in the villous in patients with disease areas imaged have microerosions near inflamed mucosa, which will increase the epithelial gaps density, thus preventing accurate assessment of barrier function in normal-appearing mucosa. In our previous studies, only endoscopically normal-appearing mucosa were imaged and analyzed for epithelial gap density. Among videos with no diseased areas imaged (N = 19), the relation between presence of epithelial gaps and disease relapse was found to significantly depend on the number of areas imaged (P = 0.01) (Fig. 3A). Specifically, increased epithelial gap density observed on pCLE was predictive of disease relapse when 3 or more areas were imaged (logrank P = 0.02; C-statistic = 0.94) (Fig. 3B). In these patients (N = 6), 100% of the patients with increased gap density developed clinical relapse, whereas none of the patients with normal gap density relapsed (Fig. 3A). For patients who had only 1 or 2 areas were imaged (N = 13), the epithelial gap density was not predictive of disease relapse (logrank P = 0.32, C-statistic = 0.60) (Fig. 3A). The flow chart for all study patients is shown in Table 2.
FIGURE 1.
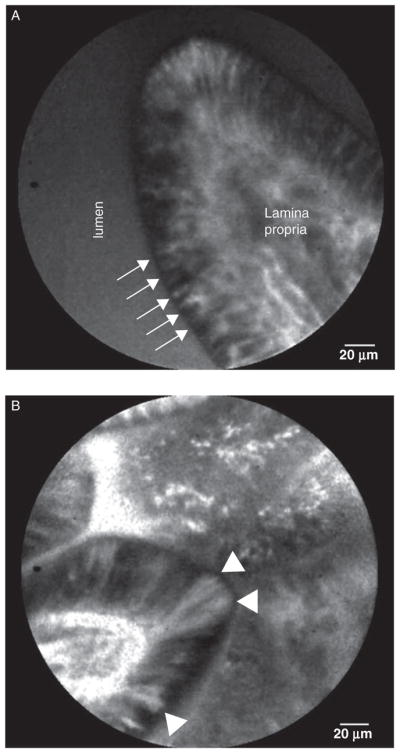
Representative images of villi used for epithelial gap density derivation. A, normal villous with no epithelial gaps seen. Arrows indicate individual intestinal epithelial cells. B, abnormal villous with several epithelial gaps present, arrow heads indicate epithelial gaps.
FIGURE 2.
Epithelial gap densities of the terminal ileum based onp-CLE in all study patients. Epithelial gap density is expressed as the number of epithelial gap per 1000 cells counted. pCLE = probe-based confocal laser endomicroscopy.
FIGURE 3.
Event-free survival in study patients. A, Kaplan-Meier plot of event-free survival in all study patients with no disease areas imaged, by normal and elevated gap densities based on pCLE of the terminal ileum. B, Kaplan-Meier plot of event-free survival in patients with IBD with normal and elevated gap densities based on pCLE imaging who had ≥3 areas imaged and met other criteria defined in previous studies. pCLE = probe-based confocal laser endomicroscopy; IBD = inflammatory bowel disease.
TABLE 2.
Study flow chart
pCLE = probe-based confocal laser endomicroscopy.
The relation between epithelial gap density with disease duration, sex, endoscopic disease severity, and location was explored in a secondary analysis. We did not find any significant correlation of gap density to the clinical factors and endoscopic findings examined.
DISCUSSION
Limited reliable endoscopic predictors of disease relapse for patients with IBD have been identified. In our present study, we found that mucosal barrier dysfunction as measured by increased epithelial gap density in the pediatric patients was predictive of moderate-to-severe disease relapse with a median follow-up period of 13 months. The predictive value required that at least 3 endoscopically normal areas were imaged at the time of colonoscopy with pCLE.
The frequency of relapse observed in our study is comparable with previous studies in patients with IBD. In adult patients with IBD, barrier function assessment using pCLE was shown to be predictive of disease relapse in 2 previous studies. When the pCLE-imaging protocols were studied, epithelial gap density in the terminal ileum of pediatric patients with IBD appears to be a potent predictor of moderate-to-severe relapse: 100% of the patients with increased gap density relapsed during the follow-up period. Conversely, normal barrier function (normal gap density) was associated with no clinical relapse.
The findings of CLE for epithelial gap density were validated in a 3-way study of CLE against conventional confocal microscopy and light microcopy (12). The interobserver and intraobserver Spearman’s correlation coefficient of epithelial cell counts were 0.86 and 0.99, respectively (12). As expected, we found that random pCLE imaging of the terminal ileum that included disease areas and less than 3 normal mucosal areas imaged could not accurately predict disease relapse (Fig. 3A). Our study highlights the importance of sampling adequate number of sites in the terminal ileum for the purpose of defining barrier dysfunction. In our previous studies, we sampled 5 areas in the terminal ileum for a total of 10 minutes for the derivation epithelial gap density as an assessment of barrier function. As shown in this study, 3 areas is the minimum number of sampling needed to determine the barrier function status for the prediction of disease relapse (Fig. 3B). This is consistent with other reports that statistically, a minimum of 3 samples should be taken to define an average of an observed event. Thus, only pCLE videos that met previously defined criteria to assess barrier function could be used to predict clinical flares. In particular, at least 3 endoscopically normal areas in the terminal ileum should be imaged with pCLE to provide a quantitative assessment of epithelial integrity for accurate prediction of disease course.
Although pCLE imaging of the terminal ileum and colon were performed in all patients, only videos of the terminal ileum were analyzed. This was due to the fact that all previous studies of CLE imaging of the intestinal barrier function and epithelial extrusion zones have been performed in the terminal ileum. The epithelial gap density as a semi-quantitative measure of epithelial cell extrusion was also validated in studies of the terminal ileum. The epithelial gaps or extrusion zones in the colon are more difficult to ascertain on pCLE images and in patients with IBD, particularly those with pancolitis, there would be no disease-free areas for confocal evaluation.
Our study results indicate that examination of mucosal barrier function with pCLE may have a role in the management of pediatric patients with IBD by identifying those at high risk of relapse. Our study findings will need to be validated in a multi-centered, prospective controlled clinical trial. A careful assessment of mucosal barrier function may facilitate the development of a risk stratification scheme to more effectively predict disease relapse. This risk stratification scheme may form the basis of a more personalized management algorithm for pediatric patients with IBD, thereby allowing early interventions and improved outcomes. An endoscopic indicator for impending relapse may help clinicians to tail the management of patients based on the risk of relapse and complications. Increased epithelial gap density appears be a surrogate marker for enhanced epithelial cell shedding induced by enhanced TNF-α and other proinflammatory cytokine activities.
There are several limitations to our existing study: this is a single-centered study of relatively small number of pediatric patients with IBD; therefore, generalizability of our results may be limited. Second, the center has significant pCLE experience, with each of the operators having performed more than 50 procedures. Another limitation of the study is the small number of patients in the study (N = 24) did not reveal predictive value of clinical or endoscopic findings, although previous larger studies have shown that the presence of colitis and certain disease type (fistula) was predictive of relapse. The results were analyzed by expert confocal microscopists who performed the initial adult pCLE studies as well as the validation studies in the rodent models. The effect of less-experienced pCLE operators and confocal microscopists on the collection and interpretations of the results was not examined. Lastly, we have included Crohns disease and ulcerative colitis patients in this study and not restricted entry criteria for patients. The follow-up period was also variable for the study cohort, ranging from 4 to 33 months.
In conclusion, our data suggest that barrier dysfunction, as detected using pCLE imaging of the terminal ileum, is predictive of moderate-to-severe relapse in pediatric patients with IBD. The addition of pCLE to routine colonoscopy, although it is associated with significant cost, may significantly aide clinicians to risk stratify patients and ultimately lead to better management of pediatric patients with IBD.
What Is Known
Epithelial gap density on probe-based confocal laser endomicroscopy of the terminal ileum is a marker for barrier function in patients with inflammatory bowel disease.
In adult patients with inflammatory bowel disease, increased gap density is predictive of disease relapse.
What Is New
In pediatric patients with inflammatory bowel disease, probe-based confocal laser endomicroscopy imaging can be used to determine barrier dysfunction in the terminal ileum.
Increased epithelial gap density is predictive of moderate to severe disease relapse if 3 or more areas are imaged with probe-based confocal laser endomicroscopy.
Acknowledgments
This study was supported in part by the Scientific Center of Children’s Health to A.S. (purchase of research confocal equipment). J.J.L. is a recipient of Canadian Institutes of Health Research New Investigator Salary award and received pilot grant from the Center for Microbial Pathogenesis and Host Inflammatory Responses (NIH P20 GM103625) at the University of Arkansas for Medical Sciences.
The authors would like to thank Directorate of the Scientific Center of Children’s Health.
Footnotes
www.clinicaltrials.gov registration number: NCT02003859.
The authors report no conflicts of interest.
References
- 1.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–8. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–5. doi: 10.1136/gut.35.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones J, Loftus EV, Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–24. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–22. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 5.Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–8. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 6.Af Bjorkesten CG, Nieminen U, Sipponen T, et al. Mucosal healing at 3 months predicts long-term endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2013;48:543–51. doi: 10.3109/00365521.2013.772230. [DOI] [PubMed] [Google Scholar]
- 7.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104:1627–32. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- 9.Kiesslich R, Goetz M, Angus EM, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. 2007;133:1769–78. doi: 10.1053/j.gastro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Liu JJ, Davis EM, Wine E, et al. Epithelial cell extrusion leads to breaches in the intestinal epithelium. Inflamm Bowel Dis. 2013;19:912–21. doi: 10.1097/MIB.0b013e3182807600. [DOI] [PubMed] [Google Scholar]
- 11.Liu JJ, Madsen KL, Boulanger P, et al. Mind the gaps: confocal endomicroscopy showed increased density of small bowel epithelial gaps in inflammatory bowel disease. J Clin Gastroenterol. 2011;45:240–5. doi: 10.1097/MCG.0b013e3181fbdb8a. [DOI] [PubMed] [Google Scholar]
- 12.Liu JJ, Rudzinski JK, Mah SJ, et al. Epithelial gaps in a rodent model of inflammatory bowel disease: a quantitative validation study. Clin Transl Gastroenterol. 2011;2:e3. doi: 10.1038/ctg.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turcotte JF, Kao D, Mah SJ, et al. Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos) Gastrointest Endosc. 2013;77:624–30. doi: 10.1016/j.gie.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–53. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turcotte JF, Wong K, Mah SJ, et al. Increased epithelial gaps in the small intestine are predictive of hospitalization and surgery in patients with inflammatory bowel disease. Clin Transl Gastroenterol. 2012;3:e19. doi: 10.1038/ctg.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JJ, Wong K, Thiesen AL, et al. Increased epithelial gaps in the small intestines of patients with inflammatory bowel disease: density matters. Gastrointest Endosc. 2011;73:1174–80. doi: 10.1016/j.gie.2011.01.018. [DOI] [PubMed] [Google Scholar]