Abstract
Importance
Cesarean birth has been associated with higher risk of offspring obesity, but previous studies have primarily focused on childhood obesity and have been hampered by limited control for confounders.
Objective
To investigate the relation between cesarean section and risk of offspring obesity.
Design
Prospective cohort study.
Participants
22,068 predominantly white individuals born to 15,271 women and followed from age 9–14 through age 20–28 years.
Main Outcomes and Measures
Risk of obesity based on IOTF or WHO cutoffs, depending on age. Secondary outcomes included risks of obesity associated with changes in mode of delivery, and differences in risk between birth-mode discordant siblings.
Results
4,921 individuals (22%) were born by cesarean section. The cumulative risk of obesity through the end of follow-up was 13% among all participants. The adjusted RR (95% CI) for obesity comparing cesarean to vaginal birth was 1.15 (1.06, 1.26). This association was stronger among women without known indications for Cesarean section (RR=1.30 (95% CI 1.09, 1.54)). Vaginal birth after cesarean was related to a 31% (95% CI: 17%, 47%) lower risk of offspring obesity compared to repeat cesarean section. In within-family analysis, individuals born by cesarean had a 64% (8%, 148%) higher odds of obesity than their siblings born vaginally.
Conclusions and Relevance
Cesarean birth was associated with offspring obesity after accounting for major confounding factors. While additional research is needed to clarify the mechanisms underlying this relation, clinicians and patients should weigh this risk when considering cesarean sections in the absence of a clear indication.
Keywords: cesarean section, vaginal delivery, offspring overweight, offspring obesity
INTRODUCTION
Nearly 1.3 million cesarean sections are performed yearly in the United States, making it the most common surgical procedure1 and accounting for one third of deliveries nationwide.2 When indicated, cesarean sections reduce morbidity risk to mother and fetus and, in many cases, are a life-saving intervention.3 Nevertheless, they have risks. Women undergoing a low-risk planned cesarean experience a 3-fold greater risk of major morbidity – including a 5-fold greater risk of cardiac arrest, a 3-fold greater risk of hysterectomy and puerperal infection and a 2-fold greater risk of thromboembolism – compared with women who have low-risk planned vaginal deliveries.4 Cesarean delivery is also related to an increased risk of maternal mortality.5 The most salient immediate risk to children is a higher frequency of respiratory complications.6,7 In addition, there is increasing evidence suggesting that children born by cesarean experience higher rates of adverse health outcomes later in life.8–10 With these concerns in mind, leading professional organizations have advocated for the prevention of primary cesarean delivery as a strategy to reduce the overall frequency of cesarean delivery.11
A growing literature suggests that cesarean section is related to a higher risk of offspring overweight and obesity. Two meta-analyses have reported pooled odds ratios (95% CI) of 1.22 (1.05, 1.42) for offspring obesity12 associated with C-section. However, inference from most of the existing studies has been hampered by limited sample size,13 suboptimal control for shared risk factors (e.g. pre-pregnancy BMI and common pregnancy complications)12,14,15 or both16. Therefore, it remains unclear whether the relation between mode of birth (Cesarean vs. vaginal) and offspring obesity is real or indicative of residual confounding. To overcome these limitations, we investigated the relation between cesarean section and offspring risk of obesity among participants of the Growing Up Today Study (GUTS), a large prospective cohort of individuals followed from childhood through early adulthood.
METHODS
GUTS is an ongoing prospective cohort study of young adults followed since 1996. A total of 16,882 children aged 9 to 14 years responded to the baseline questionnaire, and an additional 10,923 children aged 9 to 14 enrolled in 2004. Participants have been followed with yearly self-administered follow-up questionnaires between 1997 and 2001 and with biennial questionnaires thereafter17,18. Study details have been described previously.19,20 From the 23,655 GUTS participants for whom complete data on their mothers’ pregnancies were available, we excluded those for whom height and weight information was missing (n=221) and those who were not born of a singleton pregnancy (n=1,366). The final study included 22,068 individuals born to 15,271 women with follow up through 2011. The study was approved by the Human Subjects Committees of the Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital.
In each follow-up questionnaire, participants reported their height and weight, which are validly reported by preadolescents21, adolescents22–24 and adults25,26, although there is potential misclassification of obesity based on self-reported anthropometry. Body mass index (BMI) was calculated from these data as weight in kilograms divided by height in meters squared. For individuals under 18 years of age, we defined obesity according to age- and sex-specific cutoffs proposed by the International Obesity Task Force (IOTF, which provides continuity in BMI cutoffs used to define of overweight and obesity in children and in adults).27 For individuals older than 18 years, we defined obesity (BMI≥30 kg/m2) using World Health Organization cutoffs.28,29 Once an individual was classified as obese, they were considered obese for the reminder of follow-up.
Mode of delivery (cesarean vs. vaginal) was reported by the participants’ mothers in 2009 using a questionnaire aimed at collecting lifetime pregnancy information.30 A validation study conducted among 154 women enrolled in NHS and the Collaborative Perinatal Project found perfect maternal recall of cesarean section at an average of 32 years after delivery.31 The same validation study also showed that long-term maternal recall of many pregnancy related events, including diagnosis of major pregnancy complications (hypertensive disorders of pregnancy, gestational diabetes, placental abruption and placenta previa), offspring birth weight, gestational age at delivery, and pregnancy multiplicity, were highly reproducible and specific.31
Information on covariates of interest in GUTS was prospectively collected from the GUTS participants’ mothers as part of their participation in NHS-II (maternal pre-pregnancy BMI, pre-pregnancy smoking, race/ethnicity, region of residence at delivery) and in the GUTS baseline and follow-up questionnaires (participant birth date, sex, breastfeeding duration). Maternal age at delivery was calculated as the difference, in years, between participants’ date of birth and the mothers’ date of birth.
Age-standardized pre-pregnancy and pregnancy characteristics were calculated for all participants and according to delivery mode. To evaluate the association between cesarean section and offspring obesity, we calculated relative risks (RR) and their 95% confidence intervals (CI) using log-binomial regression models with generalized estimating equations (GEE) to adjust for potential confounders while accounting for correlations in outcomes between siblings. We obtained crude and multivariable-adjusted estimates of this relation. The multivariable adjusted models included terms for maternal age at delivery (continuous), race (white, other), region (Northeast, Midwest, West, South), year of birth (<=1984, 1985–89, >1989), pre-pregnancy BMI (<18.5, 18.5–24.99, 25–29.99, 30+ kg/m2), maternal height (continuous), gestational diabetes (yes, no), pre-eclampsia (yes, no), pregnancy induced hypertension (yes, no), gestational age at delivery (<37, 37–39, 40–42, 43+ weeks), birth weight group (<5, 5–6.9, 7–8.4,8.5–9.9, 10+ lbs), pre-pregnancy smoking (never, past, current), previous Cesarean section (yes, no), offspring sex (boy, girl) and birth order (continuous). Missing categories were created for variables with missing value. We also fitted marginal structural models where the probability of undergoing a Cesarean section was predicted for each woman based on these same factors and subsequently used to weight each observation using stabilized weights.32,33 In addition, we fitted sex-stratified and age-stratified models and assessed the significance of heterogeneity by adding cross-product terms between mode of delivery and age at BMI report or sex to the main multivariable model. We also performed additional analyses treating BMI at each follow-up period as a continuous or binary outcome (obesity yes/no), to avoid problems related to change in classification over time while still capturing changes within individuals over time. While some lifestyle and behavioral factors during childhood are risk factors for obesity, we did not consider them as confounders because none precede both exposure and outcome and therefore cannot, by definition, confound the association between cesarean section and offspring obesity. In fact, it has been shown that inclusion of this type of covariates does not improve precision when the outcome is binary and may instead introduce bias. 34,35
To address the possibility of residual confounding, we conducted a series of alternate analyses. We first restricted the analysis to participants without known risk factors for Cesarean section (maternal pre-pregnancy BMI<25kg/m2, no gestational diabetes, no hypertensive disorders of pregnancy, never smoker, maternal age <30 years old, gestational age at delivery between 37 and 42 weeks, and birth weight between 5 and 9.9 lbs). We also evaluated adjusting for maternal BMI as a continuous variable allowing for non-linear relations. We then estimated the effect of change in delivery mode on offspring obesity using data from successive pregnancies of the same woman. Specifically, we estimated the effect on offspring obesity of vaginal birth among women with a previous cesarean delivery (vaginal birth after cesarean; VBAC) relative to women with repeated cesarean deliveries, as well as the effect of cesarean delivery among women with a previous vaginal delivery reltive to a repeated vaginal delivery. Lastly, to minimize the effect of post-natal environment and time-invariant maternal factors, we performed a within-family analysis comparing the risk of obesity for siblings discordant in mode of delivery.34–37 In this last analysis we used conditional logistic regression to estimate the odds ratio (OR) and 95% CI comparing participants born by cesarean to their vaginally born siblings. In addition, we evaluated the potential for residual confounding by weight gain during pregnancy in the subset of participants for whom this information was available (N=11,067). All analyses were conducted using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Of the 22,068 individuals in the study, 4,921 (22%) were born by Cesarean section. The cumulative risk of obesity through the end of follow-up was 13% among all participants. Age-standardized maternal and offspring characteristics, overall and by mode of delivery, are presented in Table 1. Women who delivered by Cesarean had a higher pre-pregnancy BMI, and were more likely to have experienced gestational diabetes, pre-eclampsia and pregnancy induced hypertension. They were also more likely to have had a previous Cesarean delivery. C-sections were also more frequent in pre-term and post-term births, and for offspring who were either low-birth weight or macrosomic. The Cesarean delivery rate was highest between 1985 and 1989 and decreased thereafter.
Table 1.
Age-standardized Maternal and Offspring Characteristics According to Delivery Mode of First-in-study Pregnancy. Data from Growing Up Today Study participants born to 15,271 women.
| Variables | Overalla (n=15,271) | Mode of delivery | |
|---|---|---|---|
|
| |||
| Vaginal (n=11,727) | Cesarean (n=3,544) | ||
| Maternal Characteristics | |||
| Age at delivery, mean(SD), yb | 30.2 (3.9) | 30.1 (3.9) | 30.7 (3.9) |
| White | 14462 (94.7) | 11117 (94.8) | 3342 (94.3) |
| Geographic Region | |||
| Northeast | 5421 (35.5) | 4187 (35.7) | 1230 (34.7) |
| Midwest | 5375 (35.2) | 4140 (35.3) | 1233 (34.8) |
| West | 2291 (15) | 1747 (14.9) | 542 (15.3) |
| South | 2153 (14.1) | 1618 (13.8) | 528 (14.9) |
| Missing | 46 (0.3) | 35 (0.3) | 14 (0.4) |
| Gestational diabetes | 580 (3.8) | 375 (3.2) | 198 (5.6) |
| Preeclampsia | 809 (5.3) | 481 (4.1) | 344 (9.7) |
| Pregnancy induced hypertension | 809 (5.3) | 493 (4.2) | 315 (8.9) |
| Previous C-section | 1206 (7.9) | 293 (2.5) | 890 (25.1) |
| Pre-pregnancy BMI | |||
| <18.5 kg/m2 | 1939 (12.7) | 1583 (13.5) | 365 (10.3) |
| 18.5–25 kg/m2 | 11804 (77.3) | 9112 (77.7) | 2690 (75.9) |
| 25–30 kg/m2 | 1084 (7.1) | 762 (6.5) | 330 (9.3) |
| 30+ kg/m2 | 321 (2.1) | 176 (1.5) | 135 (3.8) |
| Missing | 122 (0.8) | 94 (0.8) | 25 (0.7) |
| Pre-pregnancy smoking | |||
| Never smokers | 10751 (70.4) | 8303 (70.8) | 2456 (69.3) |
| Past smokers | 2764 (18.1) | 2123 (18.1) | 641 (18.1) |
| Current smokers | 458 (3.0) | 340 (2.9) | 120 (3.4) |
| Missing | 1283 (8.4) | 962 (8.2) | 326 (9.2) |
| Offspring Characteristics | |||
| Year of birth | |||
| <=1984 | 6063 (39.7) | 4761 (40.6) | 1301 (36.7) |
| 1985–1989 | 6093 (39.9) | 4609 (39.3) | 1492 (42.1) |
| >=1990 | 3115 (20.4) | 2357 (20.1) | 751 (21.2) |
| Sex,% | |||
| Female | 8827 (57.8) | 6825 (58.2) | 2002 (56.5) |
| Male | 6444 (42.2) | 4902 (41.8) | 1542 (43.5) |
| Gestational age at delivery | |||
| <37 weeks | 932 (6.1) | 622 (5.3) | 308 (8.7) |
| 37–39 weeks | 3405 (22.3) | 2556 (21.8) | 836 (23.6) |
| 40–42 weeks | 9819 (64.3) | 7763 (66.2) | 2066 (58.3) |
| 43+ weeks | 1069 (7.0) | 739 (6.3) | 333 (9.4) |
| Missing | 61 (0.4) | 47 (0.4) | 4 (0.1) |
| Birth weight group | |||
| <5 lbs | 260 (1.7) | 129 (1.1) | 131 (3.7) |
| 5–6.9 lbs | 3146 (20.6) | 2439 (20.8) | 698 (19.7) |
| 7–8.4 lbs | 7712 (50.5) | 6086 (51.9) | 1620 (45.7) |
| 8.5–9.9 lbs | 3711 (24.3) | 2768 (23.6) | 957 (27) |
| 10+ lbs | 351 (2.3) | 211 (1.8) | 131 (3.7) |
| Missing | 92 (0.6) | 82 (0.7) | 7 (0.2) |
| Breastfeeding duration | |||
| Never | 1497 (9.8) | 1067 (9.1) | 425 (12.0) |
| <=6 months | 5910 (38.7) | 4480 (38.2) | 1428 (40.3) |
| >6 months | 6841 (44.8) | 5406 (46.1) | 1435 (40.5) |
| Missing | 1023 (6.7) | 774 (6.6) | 252 (7.1) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Data are presented as number (percentage) of patients unless otherwise indicated and are standardized to the age distribution of the study population.
Value is not age adjusted.
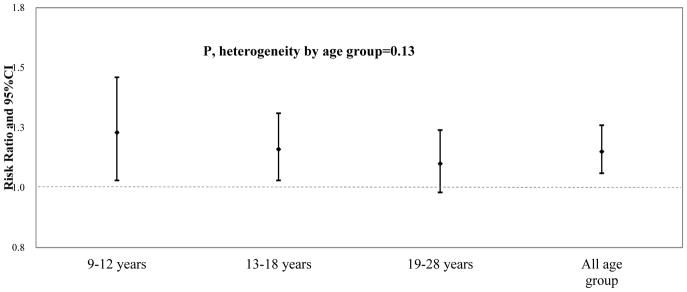
Cesarean birth was associated with higher risk of obesity in crude analyses (RR=1.30, 95% CI: 1.21–1.41). After adjustment for potential confounders, the association attenuated but remained statistically significant (Table 2). The multivariate adjusted RR for developing obesity comparing Cesarean to vaginal birth was 1.15 (95% CI: 1.06, 1.26; p=0.002). Most of the attenuation resulted from adjustment for maternal pre-pregnancy BMI. Of the 2,766 individuals who were classified as obese at some point during follow-up, 1,206 were classified at a later follow-up period as non-obese. When these individuals were excluded from the analyses, the corresponding adjusted RR (95% CI) was 1.16 (1.03, 1.30). When using repeated measures of BMI over time, the mean difference (95% CI) in BMI over the entire follow-up period between individuals born by Cesarean and vaginally-delivered individuals was 0.29 (0.18, 0.40) kg/m2 in the multivariate analyses. The association between cesarean section and offspring obesity was similar across strata of age. The multivariate adjusted RRs (95% CI) for obesity were 1.23 (1.03, 1.46) at ages 9–12, 1.16 (1.03, 1.31) at ages 13–18, and 1.10 (0.98, 1.24) at ages 19–28 (p, heterogeneity=0.13) (Figure 1 and eTable 1). Associations were also similar for females (1.12, 0.99–1.27) and males (1.18, 1.04–1.34) (p, heterogeneity=0.62).
Table 2.
Crude and Multivariable-Adjusted Risk Ratios (RR) and 95% Confidence Intervals (CI) for Offspring Obesity Associated With Cesarean vs. Vaginal Delivery. Data from the Growing Up Today Study, 1996–2011.
| Variable | Obese/participants (%) | RR (95%CI) ) for Offspring Obesity | |
|---|---|---|---|
| Main analyses | 2,766/22,068 (13%) | ||
| Vaginal Delivery | 2,012/17,147 (12%) | 1.00 (ref) | |
| Cesarean Delivery (Crude) | 754/4,921 (15%) | 1.30 (1.21, 1.41) | <0.001 |
| Cesarean Delivery (Model 1)1 | 754/4,921 (15%) | 1.15 (1.06, 1.26) | 0.002 |
| Sensitivity analyses | |||
| Propensity-score based estimate2 | 754/4,921 (15%) | 1.17 (1.08, 1.27) | <0.001 |
| Treating maternal BMI as continuous variable3 | 754/4,921 (15%) | 1.13 (1.03, 1.23) | <0.001 |
| Repeated measures estimate4 | 754/4,921 (15%) | 1.23 (1.11, 1.37) | <0.001 |
| Restricted to no known risk factors for cesarean5 (N=8566) | 200/1,503 (13%) | 1.30 (1.09, 1.54) | 0.004 |
| Restricted to siblings (N=12903) | 417/2748 (15%) | 1.24 (1.10, 1.41) | <0.001 |
Model 1 was adjusted for maternal age at delivery, race (white, other), maternal pre-pregnancy BMI group (<18.5, 18.5–24.99, 25–29.99, 30+ kg/m2), maternal height, gestational diabetes (yes, no), preeclampsia (yes, no), pregnancy induced hypertension(yes, no), child sex (male, female), year of birth (<=1984, 1985–1989, >1989), gestational age at delivery (<37, 37–39, 40–42, 43+ weeks), previous Cesarean section(yes, no), birth order, birth weight group (<5, 5–6.9, 7–8.4,8.5–9.9, 10+ lbs), pre-pregnancy smoking (never, past, current) and region of residence at birth (Northeast, Midwest, West, South).
Propensity-score based estimate using marginal structural model where the probability of undergoing a cesarean section was predicted for each woman based on these same factors and subsequently used to weight each observation using stabilized weights.
This model adjusted for the same covariates in model 1 but modeling pre-pregnancy BMI as a continuous variable with a linear term and a quadratic term instead of categorical.
GEE estimate using repeated obesity status during each follow up cycle.
Subgroup of participants without known risk factors for cesarean (maternal pre-pregnancy BMI<25kg/m2, no gestational diabetes, no hypertensive disorders of pregnancy, never smoker, maternal age<30 years old, gestational age at delivery between 37 and 42 weeks, and birth weight between 5 and 9.9 lbs).
Figure 1. Adjusted Risk Ratios (RR) and 95% Confidence Intervals (CI) for Cesarean section and Offspring Obesity.
Multivariate model was adjusted for maternal age at delivery, race (white, other), maternal pre-pregnancy BMI group (<18.5, 18.5–24.99, 25–29.99, 30+ kg/m2), previous cesarean (yes/no), maternal height, gestational diabetes (yes, no), preeclampsia (yes, no), pregnancy induced hypertension(yes, no), child sex (male, female), year of birth (<=1984, 1985–1989, >1989), gestational age at delivery (<37, 37–39, 40–42, 43+ weeks), birth order, birth weight group (<5, 5–6.9, 7–8.4,8.5–9.9, 10+ lbs), pre-pregnancy smoking (never, past, current) and region of residence at birth (Northeast, Midwest, West, South). Data from the Growing Up Today Study, 1996–2011
The association between cesarean delivery and offspring obesity remained in analyses aimed at addressing the possibility of residual confounding. Similar results were found when confounders were accounted for using propensity-score based methods, when maternal pre-pregnancy BMI was modeled as a continuous variable, when repeated obesity status at each follow-up period was considered, when analyses were restricted to individuals whose mothers had no known risk factor for cesarean section or to siblings (Table 2 and eTable 1). Further adjustment for breastfeeding duration and gestational weight gain did not change the conclusions (data not shown).
We then estimated the effect of change in delivery mode on offspring obesity. Among women with a previous C-section (N=2,815), offspring’s obesity risk was 31% (17%, 47%) (RR (95%CI)=0.69 (0.53, 0.83)) lower after a VBAC compared to a repeat Cesarean delivery. Among women with a previous vaginal delivery (N=12,815), the estimated increased risk in offspring obesity (1.13, 0.98–1.30) (Table 3) was comparable to the equivalent estimate in the entire population (1.15, 1.06–1.26), although it failed to reach statistical significance.
Table 3.
Risk Ratios (RR) and 95% Confidence Intervals (CI) for Offspring Obesity Stratified by the Type of Delivery in the Previous Pregnancy. Data from the Growing Up Today Study, 1996–2011.
| Delivery Mode | Obesity | ||
|---|---|---|---|
|
| |||
| Obese/participants (%) | RR (95% CI) | p-value | |
| Previous cesarean section (n=2,815) | |||
| Repeat Cesarean | 313/2,032 (15%) | 1.0 (ref) | |
| Vaginal Birth After Caesarean (Model 1)1 | 66/783 (8%) | 0.69 (0.53, 0.83) | 0.005 |
| Vaginal Birth After Caesarean (Model 2)2 | 66/783 (8%) | 0.71 (0.55, 0.91) | 0.008 |
| Vaginal Birth After Caesarean (Model 3)3 | 66/783 (8%) | 0.73 (0.58, 0.91) | 0.005 |
| Previous Vaginal Delivery (n=12,815) | |||
| Successive Vaginal Delivery | 1,322/11,537 (11%) | 1.0 (ref) | |
| Cesarean Delivery (Model 1)1 | 184/1,278 (14%) | 1.13 (0.98, 1.30) | 0.090 |
| Cesarean Delivery (Model 2)2 | 184/1,278 (14%) | 1.12 (0.98, 1.28) | 0.100 |
| Cesarean Delivery (Model 3)3 | 184/1,278 (14%) | 1.17 (1.01, 1.35) | 0.038 |
Adjusted for maternal age at delivery, race (white, other), maternal pre-pregnancy BMI group (<18.5, 18.5–24.99, 25–29.99, 30+ kg/m2), maternal height, gestational diabetes (yes, no), preeclampsia (yes, no), pregnancy induced hypertension(yes, no), child sex (male, female), year of birth (<=1984, 1985–1989, >1989), gestational age at delivery (<37, 37–39, 40–42, 43+ weeks), birth order, birth weight group (<5, 5–6.9, 7–8.4,8.5–9.9, 10+ lbs), pre-pregnancy smoking (never, past, current) and region of residence at birth (Northeast, Midwest, West, South).
Adjusted for the same covariates in model 1 but modeling pre-pregnancy BMI as a continuous variable with a linear term and a quadratic term instead of categorical.
Propensity-score based estimate using a marginal structural model where the probability of undergoing a cesarean section was predicted for each woman based on these same factors and subsequently used to weight each observation using stabilized weights.
Lastly, we used data from successive pregnancies and siblings to perform a within-family analysis of discordant mode of delivery among the 12,903 individuals with one or more siblings in GUTS in order to minimize potential confounding by shared post-natal environment and time-invariant maternal factors. In this analysis, the odds of obesity were 64% (8%, 149%) higher among individuals born by Cesarean than their vaginally born siblings. The association was similar for ages 9–18 years and for ages 19–28 years (Table 4).
Table 4.
Within-family Odds Ratios (OR) and 95% Confidence Intervals (CI) for Offspring Obesity Associated With Cesarean vs. Vaginal Delivery. Data from the Growing Up Today Study, 1996–2011
| Variable | Within-family analysis | ||
|---|---|---|---|
|
| |||
| Obese/participants (%) | OR (95%CI) | ||
| Overall | |||
| Vaginal Delivery | 1,091/10,155 (11%) | 1.00 (ref) | |
| Cesarean Delivery (Model 1)* | 417/2,748 (15%) | 1.64 (1.08, 2.48) | 0.02 |
| 9–18 years old | |||
| Vaginal Delivery | 719/10,113 (7%) | 1.00 (ref) | |
| Cesarean Delivery (Model 1)* | 301/2,739 (11%) | 1.67 (1.01, 2.76) | 0.044 |
| 19–28 years old | |||
| Vaginal Delivery | 677/6,714 (10%) | 1.00 (ref) | |
| Cesarean Delivery (Model 1)* | 233/1,772 (13%) | 1.72 (0.89, 3.32) | 0.107 |
Conditional logistic regression model adjusted for maternal age at delivery, race (white, other), maternal pre-pregnancy BMI group (<18.5, 18.5–24.99, 25–29.99, 30+ kg/m2), gestational diabetes (yes, no), preeclampsia (yes, no), pregnancy induced hypertension(yes, no), child sex (male, female), year of birth (<=1984, 1985–1989, >1989), gestational age at delivery (<37, 37–39, 40–42, 43+ weeks), previous Cesarean section(yes, no), birth order, birth weight group (<5, 5–6.9, 7–8.4,8.5–9.9, 10+ lbs), pre-pregnancy smoking (never, past, current) and region of residence at birth (Northeast, Midwest, West, South).
DISCUSSION
In this large cohort of U.S. individuals followed from childhood, through adolescence and young adulthood, Cesarean section was associated with a 15% increase in the risk of offspring obesity after adjusting for major confounding factors. The relation was similar across strata of age and remained consistent in a large number of sensitivity analyses. This association was stronger (30% increased risk) among individuals without known risk factors for C-section. Analyses of change in mode of delivery across multiple pregnancies revealed that individuals born through VBAC were 31% less likely to become obese than those born through a repeat Cesarean. Moreover, within-family analysis showed that individuals born through Cesarean section were 64% more likely to be obese than their siblings born through vaginal birth. The consistency of these findings across multiple strategies to account for potential confounding factors, in particular the analyses restricted to individuals without known risk factors for cesarean section and those conducted within families, strongly suggest that this relation may not be due to confounding factors but may instead represent a true biological effect.
While evidence is still building, the observed higher risk of offspring obesity associated with Cesarean section may be a consequence of differences in gastrointestinal microbiota established at birth.38,39 Vaginally delivered infants have greater exposure to their mother’s vaginal and gastrointestinal microbiota compared to infants delivered by Cesarean, who are mainly exposed to their mother’s skin microbiota and to external environmental bacterial communities at birth.40–42 This early-life difference in mode of delivery leads to altered gut microbiota pattern in offspring.39 Compared with infants born vaginally, newborns delivered by Cesarean section harbor more staphylococcus, less bifidobacteria, and less diverse bacteria species in microbiota colonization, a pattern that has been linked to increased capacity for energy harvest and risk of overweight and obesity at later life.39,43,44 Studies documenting differences in microbiota according to mode of delivery have mainly been limited to the first year of life. Whether differences in offspring microbiota are sustained long-term remains to be evaluated.
Our findings extend and refine evidence in this area. Despite inconsistent findings from individual studies,44–51 two recent meta-analyses reported a 22% increased odds of adult obesity12 associated with Cesarean delivery. However, many of the studies included in these meta-analyses—particularly in the meta-analyses for adult obesity—failed to account for important potential confounders, most importantly for maternal pre-pregnancy BMI.12,15 Several additional studies have reported on the relation with childhood obesity since the publication of these meta-analyses. A study of 2,988 Canadian children found a non-statistically significant higher risk of obesity among children born through Cesarean section after adjusting for maternal pre-pregnancy BMI (multivariate OR=1.20, 95% CI: 0.87–1.65).14 Similarly, a study of German children found that Cesarean section was related to a higher risk of offspring obesity at 2 years of age (n=1734, OR, 1.68, 95%CI, 1.10–2.58), but not at 6 (n=1244, OR, 1.49, 95% CI, 0.55–4.05) or 10 years of age (n=1170, OR, 1.16, 95% CI, 0.59–2.29) after adjusting for maternal pre-pregnancy BMI.52 Despite the lack of statistical significance of the findings of these two studies, which could be explained by their limited sample size, the magnitude of the associations reported is similar to our estimates.
The most important limitation of our study is that we lack data on intrapartum indications for cesarean delivery. However, the most common intrapartum indications of Cesarean delivery, namely fetal intolerance of labor and arrest of labor 53, are not known risk factors for childhood obesity and are therefore unlikely to be important confounders of the relation between Cesarean section and offspring obesity. Similarly, we do not have detailed data on other potentially important information about labor and delivery such as whether or not women underwent labor, whether or not membranes were ruptured nor detailed information on antibiotic use during pregnancy or labor and delivery. An additional limitation is the under-representation of minorities in our cohort. However, there are no a priori reasons to believe this relation would differ across race or ethnicity. In addition, all mothers in our studies were nurses participating in a long-term health study. While this facilitated their long-term follow-up, that of their offspring and the prospective collection of high-quality detailed data, it may hamper the generalizability of the findings to the general population. For example, pre-pregnancy BMI was lower than that of women of reproductive age in the US around the same time.54 An additional limitation is that estimates of the prevalence of obesity using self-reported information may be lower than estimates based on direct anthropometry. Nevertheless, it is unlikely that misclassification of obesity status was related to mode of birth. Hence, the most likely effect of this error is to attenuate the association towards the null. Finally, we lacked information on offspring microbiota or other potential biological mediators to further explore the underlying mechanisms.
Nevertheless, the current study has multiple strengths and was able to address the most salient limitations of previous studies. The prospective study design, large sample size, and long-term follow-up allowed us to examine the relation of cesarean section and offspring obesity risk from childhood through early adulthood and to provide precise estimates of the association. The availability of key pre-pregnancy and pregnancy information allowed for multiple sensitivity analyses aimed at addressing residual confounding. In addition, information on multiple pregnancies from the same woman and extensive family data enabled us to estimate, for the first time in this literature, the effects of changes in mode of delivery and to minimize the effect of confounding due to environmental factors and time-invariant maternal characteristics by conducting within-family analyses.
In conclusion, we observed an association between Cesarean delivery and increased risk of offspring obesity that persisted through early adult life. We also report for the first time a protective effect of VBAC on offspring obesity and a significant difference in risk of obesity between siblings discordant for mode of birth. The relation between Cesarean section and offspring obesity was stronger in analyses restricted to individuals without known risk factors for Cesarean section and in within-family analyses. These findings suggest that this relation may be a true adverse outcome of Cesarean delivery that clinicians and patients should weigh when considering cesarean sections in the absence of a clear medical or obstetric indication. Since large randomized trials of Cesarean vs. vaginal birth may not be ethically feasible, additional research from large, prospective studies with high quality data on pre-pregnancy, pregnancy and delivery information is needed to address whether these findings are generalizable to minorities and to investigate whether increased obesity rates translate to increased risk of adverse cardio-metabolic outcomes among individuals born by Cesarean section.
Supplementary Material
Acknowledgments
Funding/Support: Supported by National Institutes of Health grants UM1-CA176726, P30-DK046200, U54-CA155626, T32-DK007703-16, HD066963, HL096905, DK084001 and MH087786. Dr. Zhang is supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health.
Footnotes
Author Contributions: Drs. Chavarro and Yuan had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chavarro, Blaine
Acquisition of data: Chavarro, Missmer
Analysis and interpretation of data: Yuan, Chavarro, Field, Gaskins
Drafting of the manuscript: Yuan
Critical revision of the manuscript for important intellectual content: Chavarro, Gaskins, Blaine, Zhang, Gillman, Missmer, Field
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank the thousands of participants in the Growing Up Today Study and their mothers.
Role of the Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Pfuntner AWL, Stocks C. HCUP Statistical Brief #165. Agency for Healthcare Research and Quality; Rockville, MD: Oct, 2013. Most Frequent Procedures Performed in U.S. Hospitals, 2011. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb165.pdf. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: Final Data for 2013. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015;64(1):1–68. [PubMed] [Google Scholar]
- 3.Gregory KD, Jackson S, Korst L, Fridman M. Cesarean versus vaginal delivery: whose risks? Whose benefits? Am J Perinatol. 2012;29(1):7–18. doi: 10.1055/s-0031-1285829. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Liston RM, Joseph KS, et al. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ : Canadian Medical Association Journal. 2007;176(4):455–460. doi: 10.1503/cmaj.060870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilakivi-Clarke L, Cho E, deAssis S, et al. Maternal and prepubertal diet, mammary development and breast cancer risk. J Nutr. 2001;131:154S–157S. doi: 10.1093/jn/131.1.154S. [DOI] [PubMed] [Google Scholar]
- 6.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. 2008;336 doi: 10.1136/bmj.39405.539282.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loebel G, Zelop CM, Egan JF, Wax J. Maternal and neonatal morbidity after elective repeat Cesarean delivery versus a trial of labor after previous Cesarean delivery in a community teaching hospital. J Matern Fetal Neonatal Med. 2004;15(4):243–246. doi: 10.1080/14767050410001668653. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen NM, Bager P, Stenager E, et al. Cesarean section and offspring’s risk of multiple sclerosis: a Danish nationwide cohort study. Mult Scler. 2013;19(11):1473–1477. doi: 10.1177/1352458513480010. [DOI] [PubMed] [Google Scholar]
- 9.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208(4):249–254. doi: 10.1016/j.ajog.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Blustein J, Liu J. Time to consider the risks of caesarean delivery for long term child health. BMJ. 2015:350. doi: 10.1136/bmj.h2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711. doi: 10.1097/01.AOG.0000444441.04111.1d. [DOI] [PubMed] [Google Scholar]
- 12.Darmasseelane K, Hyde MJ, Santhakumaran S, Gale C, Modi N. Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta-analysis. PLoS ONE. 2014;9(2):e87896. doi: 10.1371/journal.pone.0087896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li HT, Zhou YB, Liu JM. Cesarean section might moderately increase offspring obesity risk. Am J Clin Nutr. 2012;96(1):215–216. doi: 10.3945/ajcn.112.038760. author reply 216. [DOI] [PubMed] [Google Scholar]
- 14.Flemming K, Woolcott CG, Allen AC, Veugelers PJ, Kuhle S. The association between caesarean section and childhood obesity revisited: a cohort study. Arch Dis Child. 2013;98(7):526–532. doi: 10.1136/archdischild-2012-303459. [DOI] [PubMed] [Google Scholar]
- 15.Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes (Lond) 2013;37(7):893–899. doi: 10.1038/ijo.2012.195. [DOI] [PubMed] [Google Scholar]
- 16.Li H-T, Zhou Y-B, Liu J-M. The Impact of Cesarean Section on Offspring Overweight and Obesity: A Systematic Review and Meta-Analysis. Obstetrical & Gynecological Survey. 2014;69(1):9–11. doi: 10.1097/1001.ogx.0000442815.0000427237.0000442812a. [DOI] [Google Scholar]
- 17.Field AE, Sonneville KR, Falbe J, et al. Association of sports drinks with weight gain among adolescents and young adults. Obesity (Silver Spring, Md) 2014;22(10):2238–2243. doi: 10.1002/oby.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111(3):e221–226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 19.Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care. 2006;29(10):2231–2237. doi: 10.2337/dc06-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112(5):999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokmakidis SP, Christodoulos AD, Mantzouranis NI. Validity of self-reported anthropometric values used to assess body mass index and estimate obesity in Greek school children. J Adolesc Health. 2007;40(4):305–310. doi: 10.1016/j.jadohealth.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Elgar FJ, Roberts C, Tudor-Smith C, Moore L. Validity of self-reported height and weight and predictors of bias in adolescents. J Adolesc Health. 2005;37(5):371–375. doi: 10.1016/j.jadohealth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Strauss RS. Comparison of measured and self-reported weight and height in a cross-sectional sample of young adolescents. Int J Obes Relat Metab Disord. 1999;23(8):904–908. doi: 10.1038/sj.ijo.0800971. [DOI] [PubMed] [Google Scholar]
- 24.Goodman E, Hinden BR, Khandelwal S. Accuracy of Teen and Parental Reports of Obesity and Body Mass Index. Pediatrics. 2000;106(1):52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- 25.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled height and past weight among younger women. Int J Obesity. 1995;19:570–572. [PubMed] [Google Scholar]
- 26.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. New England Journal of Medicine. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization technical report series. 1995;854:1–452. [PubMed] [Google Scholar]
- 29.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 30.Michels KB, Willett WC, Graubard BI, et al. A longitudinal study of infant feeding and obesity throughout life course. Int J Obes (Lond) 2007;31(7):1078–1085. doi: 10.1038/sj.ijo.0803622. [DOI] [PubMed] [Google Scholar]
- 31.Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10(6):774–777. [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA. Constructing Inverse Probability Weights for Marginal Structural Models. American Journal of Epidemiology. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braback L, Ekeus C, Lowe AJ, Hjern A. Confounding with familial determinants affects the association between mode of delivery and childhood asthma medication - a national cohort study. Allergy Asthma Clin Immunol. 2013;9(1):1710–1492. doi: 10.1186/1710-1492-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- 36.Almqvist C, Cnattingius S, Lichtenstein P, Lundholm C. The impact of birth mode of delivery on childhood asthma and allergic diseases--a sibling study. Clin Exp Allergy. 2012;42(9):1369–1376. doi: 10.1111/j.1365-2222.2012.04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khashan AS, Kenny LC, Lundholm C, Kearney PM, Gong T, Almqvist C. Mode of obstetrical delivery and type 1 diabetes: a sibling design study. Pediatrics. 2014;134(3):2014–0819. doi: 10.1542/peds.2014-0819. [DOI] [PubMed] [Google Scholar]
- 38.Neu J, Rushing J. Cesarean Versus Vaginal Delivery: Long-term Infant Outcomes and the Hygiene Hypothesis. Clinics in perinatology. 2011;38(2):321–331. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musso G, Gambino R, Cassader M. Obesity, Diabetes, and Gut Microbiota: The hygiene hypothesis expanded? Diabetes Care. 2010;33(10):2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huurre A, Kalliomäki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of Delivery – Effects on Gut Microbiota and Humoral Immunity. Neonatology. 2008;93(4):236–240. doi: 10.1159/000111102. [DOI] [PubMed] [Google Scholar]
- 42.Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138(9):1796S–1800S. doi: 10.1093/jn/138.9.1796S. [DOI] [PubMed] [Google Scholar]
- 43.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 44.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 2011;35(4):522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 45.Barros FC, Matijasevich A, Hallal PC, et al. Cesarean section and risk of obesity in childhood, adolescence, and early adulthood: evidence from 3 Brazilian birth cohorts. Am J Clin Nutr. 2012;95(2):465–470. doi: 10.3945/ajcn.111.026401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldani MZ, Barbieri MA, da Silva AA, Gutierrez MR, Bettiol H, Goldani HA. Cesarean section and increased body mass index in school children: two cohort studies from distinct socioeconomic background areas in Brazil. Nutr J. 2013;12(1):104. doi: 10.1186/1475-2891-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huh SY, Rifas-Shiman SL, Zera CA, et al. Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch Dis Child. 2012;97(7):610–616. doi: 10.1136/archdischild-2011-301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H. A national epidemiological survey on obesity of children under 7 years of age in nine cities of China, 2006. Zhonghua Er Ke Za Zhi. 2008;46(3):174–178. [PubMed] [Google Scholar]
- 49.Rooney BL, Mathiason MA, Schauberger CW. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern Child Health J. 2011;15(8):1166–1175. doi: 10.1007/s10995-010-0689-1. [DOI] [PubMed] [Google Scholar]
- 50.Steur M, Smit HA, Schipper CM, et al. Predicting the risk of newborn children to become overweight later in childhood: the PIAMA birth cohort study. Int J Pediatr Obes. 2011;6(2–2):1. doi: 10.3109/17477166.2010.519389. [DOI] [PubMed] [Google Scholar]
- 51.Zhou L, He G, Zhang J, Xie R, Walker M, Wen SW. Risk factors of obesity in preschool children in an urban area in China. Eur J Pediatr. 2011;170(11):1401–1406. doi: 10.1007/s00431-011-1416-7. [DOI] [PubMed] [Google Scholar]
- 52.Pei Z, Heinrich J, Fuertes E, et al. Cesarean delivery and risk of childhood obesity. J Pediatr. 2014;164(5):1068–1073. doi: 10.1016/j.jpeds.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 53.Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29–38. doi: 10.1097/AOG.0b013e31821e5f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272(3):205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.