Abstract
Background
Some human poxvirus infections can be acquired through zoonotic transmission. We report a previously unknown poxvirus infection in 2 patients, 1 of whom was immunocompromised; both patients had known equine contact.
Methods
The patients were interviewed and clinical information was abstracted from the patients’ medical files. Biopsies of the skin lesions were collected from both patients for histopathology, immunohistochemistry, and transmission electron microscopy analysis. Oral and skin swabs were collected from animals with frequent contact with the patients, and environmental sampling including rodent trapping was performed on the farm where the immunosuppressed patient was employed. “Pan-pox and high Guanine-cytosine” polymerase chain reaction assays were performed on patient, animal, and environmental isolates. Amplicon sequences of the viral DNA were used for agent identification and phylogenetic analysis.
Results
Specimens from both human cases revealed a novel poxvirus. The agent shares 88% similarity to viruses in the Parapoxvirus genus and 78% to those in the Molluscipoxvirus genus but is sufficiently divergent to resist classification as either. All animal and environmental specimens were negative for poxvirus and both patients had complete resolution of lesions.
Conclusions
This report serves as a reminder that poxviruses should be considered in cutaneous human infections, especially in individuals with known barnyard exposures. The clinical course of the patients was similar to that of parapoxvirus infections, and the source of this virus is currently unknown but is presumed to be zoonotic. This report also demonstrates the importance of a comprehensive approach to diagnosis of human infections caused by previously unknown pathogens.
Keywords: poxvirus, skin infection, parapoxvirus, immunocompromised, imiquimod
Four genera of poxviruses contain species that infect humans: Orthopoxvirus, Parapoxvirus, Yatapoxvirus, and Molluscipoxvirus [1, 2]. Variola virus (the etiologic agent of smallpox) and molluscum contagiosum virus are primarily human pathogens, whereas the other poxviruses are zoonoses and humans are incidental hosts. The skin is the primary portal of entry for most poxviruses. These viruses are epitheliotropic and produce lesions that progress through well-described stages over several weeks [3]. Lesions may be restricted to specific body sites as in the case of parapoxvirus infections or generalized as seen in some orthopoxvirus infections such as smallpox and monkeypox.
Human infection with zoonotic poxviruses occurs as a result of direct or indirect contact (via fomites) with infected animals. Such infections are typically self-limiting but may be protracted and may involve varying treatment modalities in immunocompromised individuals, as demonstrated in this case report.
Case Reports
Patient 1
On 16 November 2012, a 17-year-old woman from eastern Tennessee developed an erythematous macule on her right cheek. She had an orthotopic heart transplant in 2007 and was maintained on tacrolimus and mycophenolate-mofetil. On 18 November the lesion on her cheek grew in size and a new macule emerged on her right temple. Over the next 1–2 weeks, the lesions progressed from macules to papules to 1 to 1.5 cm brown nodules. The lesions were pruritic and painful. The patient had right cervical lymphadenopathy but no fever. On 20 November, the patient sought medical attention at a local children’s hospital, where she was referred to a dermatologist. The dermatologist excised the right temple lesion and submitted it for dermatopathologic evaluation. Histopathology revealed ballooning keratinocytes with eosinophilic cytoplasmic inclusions suggestive of a poxvirus infection. The dermatologist prescribed topical and oral acyclovir and oral minocycline.
On 29 November, the patient returned to her dermatologist for recurrence of the right temple lesion and development of 4 additional facial lesions, which followed the same progression as the prior lesions. The dermatologist then referred her to a pediatric infectious disease specialist. The following day, at the consultation, 6 facial lesions at varying stages of progression were noted. The physician prescribed 5% imiquimod cream for thrice-weekly application and 2% mupirocin ointment for twice-daily application. The physician requested assistance from the Centers for Disease Control and Prevention (CDC) for laboratory confirmation of a suspected poxvirus infection. On 4 December, examination of the patient revealed progression of the lesion on the glabella and 3 new lesions on the chin. The crust overlying the oldest nodule on the cheek was separating from the underlying skin (Figure 1A).
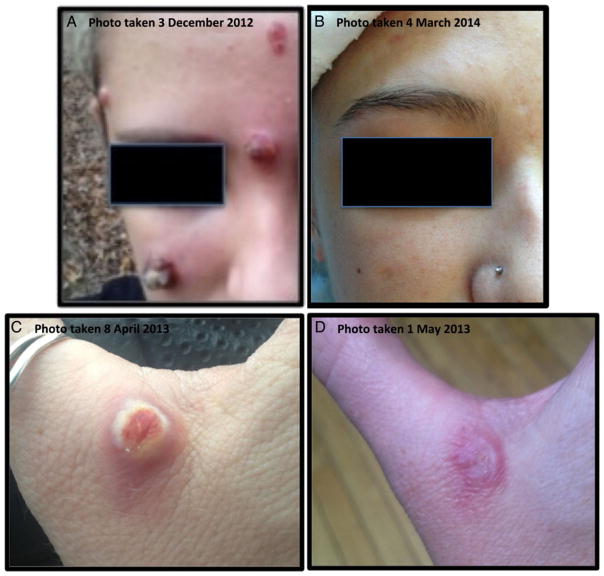
Figure 1.
A, Large 1 to 1.5 cm facial nodules on patient 1 approximately 3 weeks after lesions developed. B, Patient 1’s lesions 14 months after excision and cryotherapy. C, Marble-sized lesion in the web space between the right index finger and the thumb of patient 2 about 3 weeks after the lesion emerged. D, Patient 2’s lesion 3 weeks after excision and cauterization.
Despite the application of imiquimod for 2 weeks, the existing lesions progressed. On 13 December, the patient returned to the dermatologist, who excised the larger nodules and removed the smaller lesions with cryotherapy. The dermatologist discontinued imiquimod to lessen the risk of scarring. Four months after excision, the patient had atrophic scars and underwent laser therapy to mitigate scarring. Fourteen months later, only minimal scarring was evident (Figure 1B).
The patient owned and cared for a horse, which was kept at a stable with other horses, a hinny, dogs, cats, and chickens. The patient had been working at this stable daily for 4 years and regularly handled hay, animal manure, and animal feed and collected chicken eggs. None of these animals had mucus membrane or skin lesions. This history of long-term exposure to a stable environment coupled with the initial pathology result of a poxvirus infection led to the suspicion that the patient was infected with a parapoxvirus. The patient reported no contact with small ruminants, cattle, rodents, or other wild animals. She had no recent travel or exposure to individuals with proliferative or vesicular skin lesions.
Patient 2
On 8 April 2013, an immunocompetent 28-year-old woman from western Missouri presented to her physician with a marble-sized nodule in the web space between the right index finger and thumb. She had right axillary lymphadenopathy but was afebrile. On 10–17 March, the patient had traveled to northern Tanzania on a philanthropic mission providing care for donkeys and dogs. On 12 March, the patient sustained a rope burn on her right hand at the site of the lesion while restraining a donkey. On the following day, the patient rescued another donkey from an animal watering hole during which her hands were submerged in murky water. On 18 March, the patient returned to the United States and noticed a small papule at the site of the abrasion. By 30 March, the papule had developed into a nodule (Figure 1C). Given her travel history, she was referred to an infectious disease specialist and dermatologist. The dermatologist excised the lesion for dermatopathologic evaluation and the area was cauterized. Topical Neosporin cream as needed and 10 days of oral doxycycline and levofloxacin were prescribed for the patient. The day after the excision, the patient experienced painful swelling of her right arm consistent with cellulitis; all symptoms resolved after 4–5 days. As of May 1, three weeks after the excision, the patient reported resolution of the skin lesion (Figure 1D). Histopathology showed eosinophilic inclusions suggestive of poxvirus infection and surface bacterial colonization. The dermatologist requested CDC assistance for laboratory confirmation.
While in Tanzania, the patient was primarily in contact with donkeys. The patient recalled seeing wounds on some of the donkeys’ withers and legs. The patient was part of a group of 8 volunteers, and none of her colleagues developed lesions. In the United States, the patient owned dogs, cats, and a horse. The horse was kept in a nearby stable and the patient saw her horse 1 month prior to the trip. None of these animals had visible lesions.
METHODS
Clinical Specimens
One formalin-fixed, paraffin-embedded (FFPE) skin specimen from each patient was examined by histopathology, immunohistochemistry (IHC), transmission electron microscopy (TEM), and molecular assays.
Histopathology, Immunohistochemistry, and TEM
Sections, 3 micrometer in thickness, were cut from FFPE skin biopsy specimens and stained with hematoxylin and eosin. IHC for parapoxvirus was performed utilizing an immunoalkaline phosphatase technique. The antiparapoxvirus antibodies used were a polyclonal sheep anti–orf virus antibody and a polyclonal antibovine popular stomatitis virus antibody known to cross-react with orf. The FFPE sections from both patients were deparaffinized in xylene and embedded for thin-section TEM as previously described [4].
Polymerase Chain Reaction Assays
Extracted DNA was evaluated with the use of a pan-pox polymerase chain reaction (PCR) for general detection of poxvirus. The pan-pox PCR is a combination of 2 assays based on Guanine-cytosine (GC) content: a low-GC PCR for the detection of orthopoxvirus DNA, and a high-GC PCR for the specific detection of molluscipoxvirus and parapoxvirus DNA [5]. The amplicon sequences from the high-GC PCR assay were used for virus identification and phylogenetic analysis.
Animal and Environmental Sampling
On 26 December 2012, oral swabs were collected from 20 animals in contact with patient 1. The animals were inspected and no visible lesions consistent with poxvirus infections were present. In light of the second case, and because the both patients had contact with equines, a second extensive investigation was conducted in May 2013 at the farm where patient 1 worked. The following occurred during the field investigation: (1) interviews of the farm owner and veterinarian were conducted to determine the origin, movements, and health history of animals on the property; (2) a thorough physical examination was conducted of all domestic animals present in the barn environment, and oral swabs and swabs of skin irregularities (scabs, scar, and wounds) were obtained from 12 animals; (3) swab specimens of fomites (animal stall walls, surfaces, bridles) with likely contact with the oral mucosa of the equines were obtained; and (4) 180 traps were set for rodents over 2 successive nights. For patient 2, oral swabs were collected in May 2013 from the patient’s 9 animals. Table 1 lists the animal and environmental specimens collected. DNA was extracted from all swabs using the BioRobot EZ1 system DNA tissue kit (Qiagen) according to the modified pretreatment protocol described previously, and specimens were tested using the pan-pox PCR assay [5].
Table 1.
Sampling of Animals That Had Frequent Contact With Patients and List of Surfaces/Fomites Sampled on the Farm and the Horse Stable
| Patient | Sampling Location/Site | Animal/Fomite (No.) | Type of Specimen | No. of Specimens | Comments |
|---|---|---|---|---|---|
| Patient 1 | Farm | Horses (3) | Oral swabs, skin scabs, lesion swabs, and blood/serum | 26 | Clinical specimens were collected from all animals on the farm in December 2013 and May 2014. |
| Hinny (1) | Oral and lesion swabs | 3 | |||
| Dogs (2) | Oral and lesion swabs | 5 | |||
| Indoor cats (2) | Oral swabs | 2 | |||
| Barn cat (1) | Oral and lesion swabs | 3 | The cat had frequent contact with rodents and would bring dead rodents into the animal barn. | ||
| Chickens (3) | Oral swabs | 3 | The chickens were brought to the farm 1 month prior to the patient’s symptom onset. | ||
| Patient’s home | Dogs (2) | Oral swabs | 2 | ||
| Cats (2) | Oral swabs | 2 | |||
| Friend’s home | Dogs (2) | Oral swabs | 2 | ||
| Cats (2) | Oral swabs | 2 | |||
| Animal stall | Walls (3) Windows (4) Feeding and water troughs (9) |
Swabs | 20 | Scratches were observed on the wall, which indicates frequent animal contact. | |
| Tack room | Bridles and harness (9) | Swabs | 10 | ||
| Patient 2 | Stable | Horse (1) | Oral swabs | 2 | The horse was housed in a stable with 20 other horses. |
| Patient’s home | Dogs (3) | Oral swabs | 6 | ||
| Cats (5) | Oral swabs | 10 |
RESULTS
Histopathology and Immunohistochemistry
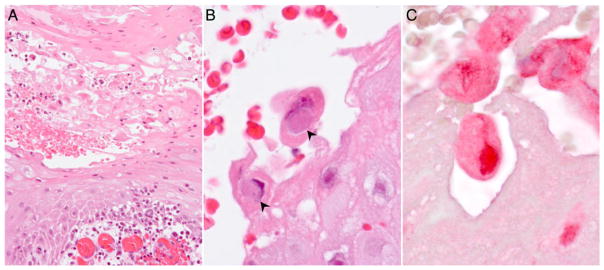
The biopsies from both patients showed lesions typical of cutaneous poxvirus infection. The epidermis was acanthotic with a serocellular crust (Figure 2A). Keratinocyte ballooning degeneration and necrosis were prominent, and scattered, large, eosinophilic to amphophilic cytoplasmic inclusions were seen within the degenerating keratinocytes (Figure 2B). The dermis showed edema, capillary proliferation, and diffuse lymphocytic to mixed inflammatory infiltrates. Superficial colonization by bacteria was also noted. IHC demonstrated positive staining of keratinocyte cytoplasm using polyclonal sheep antisera generated against orf virus (Figure 2C). No staining was observed with the antisera generated against bovine popular stomatitis virus.
Figure 2.
A, Photomicrograph of the lesion from patient 2 shows acantholysis, keratinocyte ballooning degeneration, and dermal capillary proliferation. B, Cytoplasmic inclusions are seen within degenerating keratinocytes (arrowheads). C, Immunohistochemistry staining shows parapoxvirus antigens in red within the keratinocytes.
Transmission Electron Microscopy
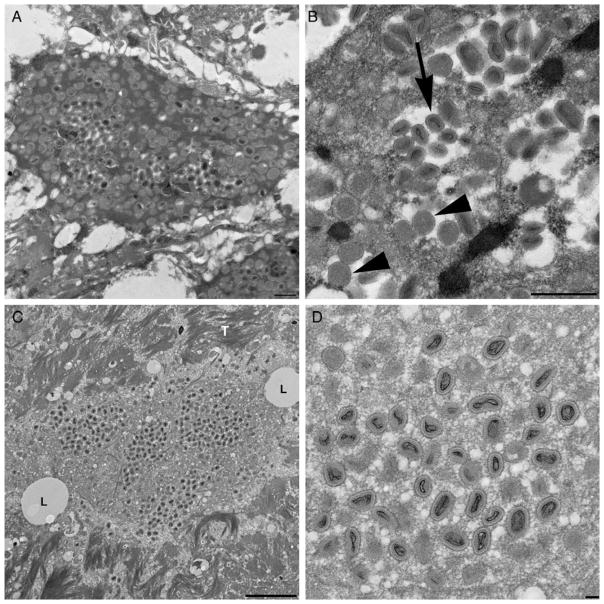
The FFPE sections from both patients prepared for TEM revealed ovoid virions morphologically consistent with a poxvirus. Viral particles measured 200–300 nm by 100–150 nm (Figure 3).
Figure 3.
A, Tissue from patient 1 with intracellular poxvirus particles. B, Mature (arrow) and immature (arrowhead) viral particles at higher magnification. Similar findings were seen in micrographs taken of tissue from patient 2 (C and D). L denotes lipids; T denotes tonofilaments.
Molecular Assays and DNA Sequence Analysis
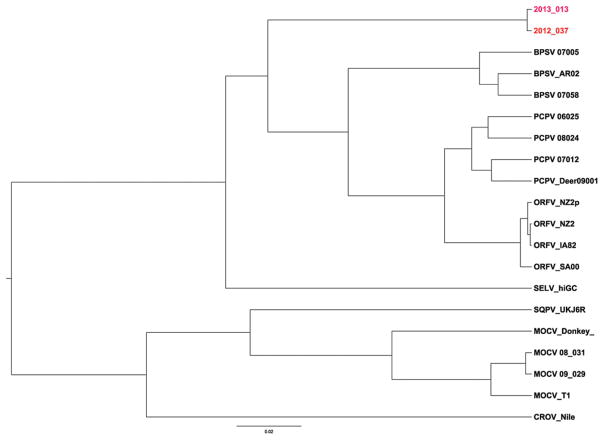
The low-GC PCR assay revealed no amplification, whereas results of the high-GC PCR assay confirmed a poxvirus infection in both patients; however, the patients’ isolates were negative for molluscipox and parapoxviruses using real-time PCR assays specific for these viruses. Phylogenetic analysis of the amplified sequences from both patients’ isolates indicated a novel, high-GC poxvirus (Figure 4). Results of a National Center for Bio-technology Information Basic Local Alignment Search Tool search indicated the amplicon sequence had an 88% similarity to viruses in the Parapoxvirus genus and 78% to molluscum contagiosum virus within the Molluscipoxvirus genus. Notably, known zoonotic parapoxviruses (orf, pseudocowpox virus, and bovine popular stomatitis virus) share 92% similarity to each other along the same amplicon sequenced from both patients’ isolates. All 98 specimens (from 29 animals and 25 environmental sites) were negative for poxvirus using the pan-pox PCR assay, and no rodents were trapped.
Figure 4.
Phylogenetic tree showing the relationship of viruses isolated from patient 1 (2012_037) and patient 2 (2013_013) to other poxviruses. There was a single-nucleotide change between the patients’ isolates. The phylogenetic tree was constructed from 620 nucleotide sequences of a high-GC polymerase chain reaction amplicon targeting the highly conserved viral RNA polymerase gene. GenBank accession numbers of individual amplicons are listed: patient 1 (KM491712), patient 2 (KM491713), 3 strains of bovine popular stomatitis virus (BPSV 07005, GQ902054.1; BPSV_AR02, AY386265.1; BPSV 07058, GQ902053.1), 4 strains of orf virus (ORFV_NZ2p, AX754989; ORFV_NZ2, DQ184476.1; ORFV_IA82, AY386263.1;ORFV_SA00, AY386264), a red squirrel pox from the United Kingdom (SQRV_UKHE601899), a molluscum contagiosum–like virus from a donkey (MOCV_Donkey JQ269324), 3 pseudocowpox virus strains (PCPV 06025, GQ902049.1; PCPV 08024, GQ902050.1; PCPV 07012, GQ902051.1), a pseudocowpox-like virus from a US deer hunter (PCPV_Deer09001) [6], a sealpox virus (SELV_hiGC,), 3 strains of molluscum contagiosum virus (MOCV_T1, U60315.1; MOCV 08_031, GQ902057; MOCV 09_029), and Nile crocodilepox virus (CROV_Nile, DQ356948.1). The DNA sequences were aligned with the use of the BioEdit and Clustal alignment programs. Phylogenetic analyses were performed with the use of the Bayesian analysis software packages BEAST and BEAUti, version 1.7.5. The analyses ran a Markov chain Monte Carlo chain length of 5 000 000, with a Hasegawa–Kishino–Yano nucleotide substitution model, strict molecular clock, and sampling of every 1000 states. To root the dendrogram, a myxoma virus, MYXV_wel (JX565582) not shown, is used as the outgroup. The 0.02 scale bar denotes the genetic distance in substitutions per site.
DISCUSSION
We present 2 cases of a cutaneous poxvirus infection involving a previously unknown, currently unclassified virus. The DNA sequence from the highly conserved viral RNA polymerase gene (J6R) suggests that this virus is most closely related to the Parapoxvirus genus but belongs to a unique clade that is distinct and divergent. IHC data demonstrate antigenic similarities between the new virus and the orf virus but not bovine popular stomatitis virus. Studies such as that of Housawi et al suggest a link between cross-reactivity and genetic relatedness among parapoxviruses [7]. In that study, the authors detected variations in the cross-reactivity of 27 monoclonal antibodies (mAbs) produced against orf virus for different parapoxvirus species and strains [7]. Notably, the number of cross-reactive mAbs decreased when strains were more distantly related. Only 2 mAbs reacted with the squirrel poxvirus (SQPV_UKJ6R in Figure 4), whereas 6 mAbs reacted with the seal parapoxvirus (SELV_hiGC in Figure 4). Thus, our IHC result may suggest a closer relationship between the novel poxvirus and the orf virus than bovine popular stomatitis virus, but due to the paucity of studies on the cross-reactivity of parapoxviruses, no further deductions can be made about the similarities of this new virus to other known parapoxviruses.
Based on clinical presentation, it is reasonable to compare this novel virus with parapoxvirus infections. Parapoxviruses are widespread and infect a wide range of mammals including ungulates and seals, and human infection occurs as a result of direct or indirect (via fomites) contact with infected animals [8–10]. The viruses are hardy and highly resistant to environmental degradation under ambient conditions [11]. Patients with parapoxvirus infections typically have a well-defined history of animal contact such as slaughtering, meat processing, or bottle feeding [6, 10, 12]. We were unable to identify the origin of this new virus for either patient. Despite this, we cannot rule out a zoonotic source, as poxviruses have a wide host range and both patients had habitual exposure to the species-rich environment of a horse stable. The high degree of genetic similarity between the 2 viruses could suggest a common geographic origin; however, patient 2 also had contact with donkeys in Tanzania, and the timing of lesion origination suggests that she acquired the virus in Tanzania. Having failed to identify a potential source of fomite contamination originating in the United States (ie, equipment transported from Missouri to Tanzania), we are unable to resolve this conundrum.
The appearance and progression of our patients’ lesions was similar to those of the parapoxviruses. Persons infected with parapoxviruses generally present with solitary or regionally restricted lesions, usually on hands or arms rather than a disseminated rash, as is seen with some orthopoxvirus infections (smallpox, monkeypox). Furthermore, parapoxviruses replicate in regenerating epidermal keratinocytes; these cells are rich in nucleotide pools required for viral replication [13]. Both patients in this series had a compromised epidermal surface; patient 1 had mild acne and patient 2 sustained a rope burn at the site of the lesion 1 week prior to symptom onset.
These patients also highlight the differences in presentation between immunocompromised and immunocompetent persons. In immunocompetent patients, parapoxvirus lesions are generally self-limited, as was seen in patient 2. In contrast, immunocompromised patients often develop large, rapidly growing, exophytic lesions in atypical sites such as the face [14–17], as observed in patient 1.
Treatment strategies for cutaneous poxvirus infections depend on the patient’s immune status and clinical course. In immunocompromised patients with orf virus lesions, application of imiquimod, an immunomodulatory agent, has been shown to result in the clearance of lesions within days to weeks [14–16], although in most of these cases, imiquimod was used in conjunction with other treatment modalities. Patient 1’s lesions appeared to be refractory to imiquimod therapy, as the lesions continued to grow despite its application. Thus, the therapeutic effect of imiquimod in poxvirus infection remains unclear. Surgical excision and cryotherapy are also treatment options, which proved successful in both patients [17, 18]. Patient 2 experienced symptoms consistent with cellulitis, which is not uncommon in cutaneous infections.
Although we were unable to identify the source(s) of this novel poxvirus, we recommend the use of nonporous (rubber or latex) gloves for persons in contact with stable or barn environments, or involved in animal handling, particularly those individuals who are immunosuppressed or have open wounds on the hands. Also, all open wounds should be covered when handling animals, and skin should be immediately washed after contact with animals as poxviruses are known to infect damaged skin.
In summary, this is a report of a novel poxvirus infection in 2 patients with a common exposure to domestic animals, including equids. This report highlights the importance of a comprehensive approach to diagnosis. Collaboration between multiple specialists aided in dictating appropriate treatment modalities. In this instance, the identification of a novel poxvirus related to Parapoxvirus helped guide treatment options and possible outcomes.
Acknowledgments
We thank Dr Brett Peterson from the Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention (CDC); Dr Charles Hatcher from the Tennessee Department of Agriculture; Glenis Moore from the Tennessee Department of Health; Dr Edward J. Primka III of Dermatology Associates of Knoxville; and Mr Randy Schillers and Ms Cindy Butler of the Missouri Department of Health for their participation.
Footnotes
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fenner F, Burnet FM. A short description of the poxvirus group (vaccinia and related viruses) Virology. 1957;4:305–14. doi: 10.1016/0042-6822(57)90065-x. [DOI] [PubMed] [Google Scholar]
- 2.Dhar AD, Werchniak AE, Li Y, et al. Tanapox infection in a college student. N Engl J Med. 2004;350:361–6. doi: 10.1056/NEJMoa031467. [DOI] [PubMed] [Google Scholar]
- 3.Tack DM, Reynolds M. Zoontic poxviruses associated with companion animals. Animals. 2011;1:19. doi: 10.3390/ani1040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayat M. Principles and techniques of electron microscopy: biological applications. 3. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- 5.Li Y, Meyer H, Zhao H, Damon IK. GC content-based pan-pox universal PCR assays for poxvirus detection. J Clin Microbiol. 2010;48:268–76. doi: 10.1128/JCM.01697-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roess AA, Galan A, Kitces E, et al. Novel deer-associated parapoxvirus infection in deer hunters. N Engl J Med. 2010;363:2621–7. doi: 10.1056/NEJMoa1007407. [DOI] [PubMed] [Google Scholar]
- 7.Housawi FMT, Roberts GM, Gilray JA, et al. The reactivity of monoclonal antibodies against orf virus with other parapoxviruses and the identification of a 39 kDa immunodominant protein. Arch Virol. 1998;143:2289–303. doi: 10.1007/s007050050461. [DOI] [PubMed] [Google Scholar]
- 8.Nollens HH, Gulland FM, Jacobson ER, et al. Parapoxviruses of seals and sea lions make up a distinct subclade within the genus Parapoxvirus. Virology. 2006;349:316–24. doi: 10.1016/j.virol.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Robinson AJ, Mercer AA. Parapoxvirus of red deer: evidence for its inclusion as a new member in the genus parapoxvirus. Virology. 1995;208:812–5. doi: 10.1006/viro.1995.1217. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Human orf virus infection from household exposures—United States, 2009–2011. MMWR Morb Mortal Wkly Rep. 2012;61:4. [PubMed] [Google Scholar]
- 11.Rheinbaben F, Wolff MH. Handbook of antiviral disinfectants. Berlin: Springer-Verlag; 2002. [Google Scholar]
- 12.MacNeil A, Lederman E, Reynolds MG, et al. Diagnosis of bovine-associated parapoxvirus infections in humans: molecular and epidemiological evidence. Zoonoses Public Health. 2010;57:e161–4. doi: 10.1111/j.1863-2378.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- 13.Moss B. Poxiviridae: the viruses and their replication. 4. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 14.Lederman ER, Green GM, DeGroot HE, et al. Progressive ORF virus infection in a patient with lymphoma: successful treatment using imiquimod. Clin Infect Dis. 2007;44:e100–3. doi: 10.1086/517509. [DOI] [PubMed] [Google Scholar]
- 15.Zaharia D, Kanitakis J, Pouteil-Noble C, Euvrard S. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:e62–4. doi: 10.1111/j.1432-2277.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- 16.Ara M, Zaballos P, Sanchez M, et al. Giant and recurrent orf virus infection in a renal transplant recipient treated with imiquimod. J Am Acad Dermatol. 2008;58:S39–40. doi: 10.1016/j.jaad.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Ballanger F, Barbarot S, Mollat C, et al. Two giant orf lesions in a heart/lung transplant patient. Eur J Dermatol. 2006;16:284–6. [PubMed] [Google Scholar]
- 18.Tan ST, Blake GB, Chambers S. Recurrent orf in an immunocompromised host. Br J Plast Surg. 1991;44:465–7. doi: 10.1016/0007-1226(91)90209-3. [DOI] [PubMed] [Google Scholar]