Abstract
Objective
To investigate the structural brain characteristics of adolescent patients with d-transposition of the great arteries (d-TGA), repaired with the arterial switch operation in early infancy, using quantitative volumetric magnetic resonance imaging (MRI).
Study design
Ninety-two patients with d-TGA from the Boston Circulatory Arrest Study (76% male; median age at scan 16.1 years) and 49 control subjects (41% male; median age at scan 15.7 years ) were scanned using a 1.5-Tesla magnetic resonance imaging (MRI) system. Subcortical and cortical gyral volumes and cortical gyral thicknesses were measured using surface-based morphometry. Group differences were assessed with linear regression.
Results
Compared with controls, patients with d-TGA demonstrated significantly reduced subcortical volumes bilaterally in the striatum and pallidum. Cortical regions that showed significant volume and thickness differences between groups were distributed throughout parietal, medial frontoparietal, cingulate, and temporal gyri. Among adolescents with d-TGA, volumes and thicknesses correlated with several perioperative variables, including age at surgery, cooling duration, total support time, and days in the cardiac intensive care unit.
Conclusion
Adolescents with d-TGA repaired early in life exhibit widespread differences from control adolescents in gray matter volumes and thicknesses, particularly in parietal, midline, and subcortical brain regions, corresponding to white matter regions already known to demonstrate altered microstructure. These findings complement observations made in white matter in this group and suggest that the adolescent d-TGA cognitive profile derives from altered brain development involving both white and gray matter.
Keywords: brain, MRI, congenital heart disease, cortical thickness
Advances in prenatal diagnosis, surgical treatment, and postoperative management of children born with congenital heart disease (CHD) have dramatically improved their survival. Currently, the number of adults with CHD in the United States has been estimated to be as high as 2.9 million.1 However, survivors often demonstrate neurodevelopmental morbidity for which the neuroanatomic correlates remain unclear.2
Ample evidence supports a relationship between CHD and adverse sequelae in the developing brain. Neuropathological evaluation in neonates who died shortly after cardiac surgery has demonstrated both white and gray matter injury, including cerebral cortex, subcortical gray matter, hippocampus, and cerebellum.3 Interestingly, the pattern of gray matter injury included both infarction and neuronal dropout, suggesting that in surviving children with CHD, not all gray matter injury will be evident as chronic infarction.
Magnetic resonance imaging (MRI) of infants before and after corrective surgery for CHD has revealed evidence of cerebral injury. These studies demonstrate a relationship between injury or diminished gray matter volume, particularly in frontal and parietal lobes, and predisposing risk factors including low preoperative cerebral blood flow, severity of perioperative hypoxia, lower mean systemic blood pressure in the first postoperative day, and type of CHD.4-7 Preoperatively, neonates with single ventricle defects and d-transposition of the great arteries (d-TGA) have imaging features of delayed cerebral maturation of both white matter and gray matter.8, 9 We have previously demonstrated alteration of deep white matter microstructure not apparent on conventional clinical MRI in adolescents with d-TGA surgically corrected in early infancy.10 As has been found in the premature infant, white matter injury can adversely affect the development of cortical gray matter, reflected in altered thickness.11, 12 Evidence for compromised gray matter beyond the perioperative period has been reported. Of note, morphometric study of a group of adolescents with surgically corrected CHD revealed reduction of total cortical gray matter and subcortical gray matter volumes.13, 14
Our group has previously reported the cognitive and behavioral deficits of children and adolescents with repaired d-TGA.15 We have now employed quantitative volumetric analysis of brain MRI data to compare parcellated gray matter volumes and cortical thicknesses in a group of adolescents with d-TGA repaired in early infancy with those of healthy control adolescents. Further, we explored associations between medical covariates and gray matter measures within the group with d-TGA.
Methods
In the Boston Circulatory Arrest Study (BCAS), infants < age 3 months with d-TGA undergoing the arterial switch operation were randomly assigned to two methods of vital organ support during hypothermic cardiopulmonary bypass (predominant deep hypothermic circulatory arrest versus predominant low-flow cardiopulmonary bypass) between April 1988 and February 1992. We have previously published trial methods and neurodevelopmental findings in the perioperative period and at ages 1, 4, 8, and 16 years.15-19
Adolescents recruited to the control group met criteria adapted from the NIH MRI study of normal brain development.20, 21 Children with known risk factors for brain disorders (e.g., intrauterine exposure to toxicants; histories of closed head injury with loss of consciousness, language disorder, or Axis 1 psychiatric disorder; first-degree relative with a lifetime history of an Axis 1 psychiatric disorder; or abnormality on neurological examination) were excluded. We also excluded control subjects for whom MRI was contraindicated (eg, pacemaker, metal implants), those with trisomy 21, adolescents with other forms of CHD requiring surgical correction, and subjects whose primary language was not English. This study was approved by the Boston Children’s Hospital Institutional Review Board and adhered to institutional guidelines. Parents provided informed consent, and adolescents provided assent.
MRI Acquisition
Subjects were scanned on identical GE Twin 1.5T systems (General Electric, Milwaukee, WI) at either Boston Children’s Hospital or Beth Israel Deaconess Medical Center. Volumetric series for each subject were acquired using a Spoiled Proton Gradient Recalled (SPGR) 3D sequence, with imaging parameters: acquisition matrix = 256 × 256, FOV = 220mm, slice thickness = 1.5mm, TR/TE = 35ms/6ms, flip angle = 45 degrees, resulting in a voxel size of 0.8594 × 0.8594 × 1.5mm3. Slices were obtained axially, aligned parallel to the anterior commissure-posterior commissure plane.
Structural brain MRIs were blindly interpreted by a neuroradiologist for quality of MRI data and the presence of structural abnormalities. Abnormalities were classified with respect to origin (acquired or developmental), type (infarction, mineralization, iron deposition, myelination delay, ventriculomegaly, abnormal signal characteristics), extent (focal or diffuse), and anatomic location in the brain. These data are a subset of those reported on in a previous manuscript.15
MRI Analysis and Anatomic Classification
Images were processed using the fully-automated tools in Freesurfer v5.0 (A.A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital). The technical details are described elsewhere.22-27 Briefly, this procedure involves partitioning volumetric MRI images into white matter, gray matter, and cerebrospinal fluid. The deep gray matter in each hemisphere is segmented into seven discrete subcortical structures with corresponding calculated volumes: thalamus, caudate, putamen, globus pallidus, hippocampus, amygdala, and nucleus accumbens. Next, the outer pial surface of the brain is delineated, as is the surface comprising the white matter/gray matter junction. Cortical thickness is obtained by taking the distance between these two surfaces. Finally, the cortical surface is parcellated into distinct units based on gyral and sulcal structure. Mean cortical thickness and volume is obtained for 30 gyri in each hemisphere. All images were inspected for image processing errors and manually corrected when necessary.
We classified parcellated regions according to position along their respective arterial trees using a standard atlas of cerebral vasculature.28, 29 Each region was assigned to one of three groups (proximal, middle, or terminal) based on location in the arterial tree relative to the origin of the main cerebral arterial trunk supplying it blood. Consequently, anterior cingulate cortex (which receives blood flow from branches of the proximal anterior cerebral artery) was classified as a proximal volume, and posterior cingulate cortex was classified as a terminal volume (because it is perfused by distal branches of the anterior cerebral artery). In order to minimize multiple comparisons, clusters were created by averaging adjacent volumes or thicknesses as well as homologous volumes or thicknesses across hemispheres. This resulted in twenty-four subcortical and cortical volume clusters and twenty cortical thickness clusters (Table I; available at www.jpeds.com).
Table 1.
Volume and Thickness Clusters
| Distance from arterial trunk | |
|---|---|
| Proximal | |
| B pericalcarine | |
| B caudal anterior cingulate + B rostral anterior cingulate | |
| B superior temporal | |
| Middle | |
| B caudate | (volumes only) |
| B caudal middle frontal | |
| B precentral + postcentral | |
| B superior parietal + B paracentral | |
| B superior frontal + B rostral middle frontal | |
| B inferior parietal | |
| B middle temporal | |
| B pars opercularis | |
| Terminal | |
| B pallidum + putamen | (volumes only) |
| B medial orbitofrontal | |
| B isthmus cingulate + B posterior cingulate | |
| B pars orbitalis + B pars triangularis | |
| B thalamus + B hippocampus | (volumes only) |
| B entorhinal | |
| B precuneus + B cuneus + B lingual | |
| B parahippocampal | |
| B amygdala | (volumes only) |
| B nucleus accumbens + B lateral orbitofrontal | (only B lateral orbitofrontal in thickness cluster) |
| B fusiform + B inferior temporal + B lateral occipital | |
| B insula | |
| B supramarginal |
B: bilateral.
Statistical analyses
Wilcoxon or Fisher exact tests were used to compare d-TGA and control groups with respect to demographic variables and structural MRI findings. For subjects with d-TGA, medical variables measured before or during the arterial switch operation included presence of a ventricular septal defect (VSD), age at surgery (>30 days vs. ≤ 30 days), cooling duration for the first cycle, total duration of deep hypothermic circulatory arrest (DHCA), total time on cardiopulmonary bypass, total support time, and lowest tympanic membrane temperature during surgery. Covariates collected in the postoperative period included history of hospital seizures (electroencephalographic [EEG] or clinical) and number of days in the cardiac intensive care unit (CICU) and hospital. Finally, any cardiac operations after the arterial switch operation were considered.
Linear regression was used to compare volume and thickness between groups. For volume clusters, the regression models included adjustment for age at MRI, sex,, total intracranial volume (TIV), and scanner location (Boston Children’s Hospital or Beth Israel Deaconess Medical Center).30 For thickness clusters, TIV was excluded from the models as previous work has shown that correcting for TIV is inappropriate for cortical thickness analyses.31 In exploratory analyses, partial Spearman correlation coefficients were used to analyze the associations between brain volumes and thicknesses and perioperative medical variables for the patients with d-TGA, adjusting for the same factors as in the regression analyses. The significance level was set at 0.05 for the regression analyses comparing groups and 0.01 for the correlation analyses.
Results
Table II presents the demographic and medical characteristics of the d-TGA and control groups. A total of 111 subjects with d-TGA were scanned; data from 19 were excluded from further analysis due to excessive motion to yield 92 subjects with d-TGA (70 male; mean age 16.2 years, standard deviation 0.7, range 14.9-19.9) in the final analysis. The subjects with d-TGA retained for further analysis had significantly higher median Wechsler Individual Achievement Test Second Edition (WIAT-II), reading composite scores (100.5 vs. 88.0; p=0.02), and median executive function summary scores (9.3 vs. 8.3; p=0.02) than those excluded.32, 33 A total of 55 healthy controls were scanned; data from 6 were excluded due to excessive motion to yield 49 healthy controls (20 male; mean age 15.3 years, standard deviation 1.2, range 13.0-17.0) in the final analysis.
Table 2.
Demographic and Medical Characteristics of d-TGA and Control Subjects from Whom Volumetric Data was Acquired at 16 Years of Age
| Variable | d-TGA (n=92) |
Control (n=49) |
P* |
|---|---|---|---|
| Birth weight, kg | 3.5 (3.2-3.9) | 3.5 (3.0-3.8) | 0.34 |
| Gestational age, wk | 40 (39-40) | 40 (40-40) | 0.45 |
| Male, % | 76 | 41 | <0.001 |
| White, % | 88 | 76 | 0.09 |
| Scanner location (BCH), % | 75 | 69 | 0.55 |
| Age at MRI, y | 16.1 (15.8-16.4) | 15.7 (14.2-16.3) | <0.001 |
| Social class at 16 y of age† | 48 (39.5-57.0) | 56 (47-61) | 0.007 |
| VSD diagnosis, % | 22 | — | — |
| Age at surgery >30 days, % | 9 | — | — |
| Cooling duration for the first cycle, min | 15 (13-18) | — | — |
| Duration of DHCA, min | 37.5 (10.5-54.5) | — | — |
| Duration of CPB, min | 108.5 (78.5-126) | — | — |
| Total support time, min | 136 (127.5-150) | — | — |
| Lowest tympanic temperature during surgery, °C | 13.9 (12.4-15.0) | — | — |
| Hospital seizures (EEG or clinical), % | 14 | — | — |
| CICU stay, days | 5 (4-7) | — | — |
| Hospital stay, days | 9 (7.5-11) | — | — |
| Any cardiac operation after ASO, % | 12 | — | — |
BCH: Boston Children’s Hospital; DHCA: deep hypothermic circulatory arrest; CPB: cardiopulmonary bypass; EEG: electroencephalogram; ASO: arterial switch operation.
Values are median (interquartile range) or percent.
Determined by Wilcoxon test or Fisher exact test.
Score on Hollingshead Four Factor Index of Social Status, with higher scores indicating higher social status.
Retained and excluded controls were similar except that the retained subject group comprised 41% males versus 100% males among excluded control subjects (p=0.008).
Gross Structural Imaging Characteristics
Compared with controls, subjects with d-TGA had more anatomic brain abnormalities (36% vs. 4%; p<0.001). Although focal or multifocal abnormalities were more common among adolescents with d-TGA (26% vs. 0%; p<0.001), these abnormalities consisted largely of brain mineralization (23% vs. 0%; p<0.001) rather than focal infarction or atrophy (8% vs. 0%; p=0.10). Among the group with d-TGA, only one patient had an area of infarction that involved a region of brain reduced in volume (left inferior parietal). There were no significant between-group differences in diffuse, generalized, or developmental abnormalities.
Subcortical and Cortical Volume Differences
Total intracranial volume did not differ between groups (median 1,546 cm3 vs. 1,559 cm3; p=0.78). Among the 24 volume clusters tested, four demonstrated significantly smaller volumes in the subjects with d-TGA than in the control group (Figure and Table III). These differences existed bilaterally in caudate (p=0.04), superior parietal-paracentral (p=0.03), superior frontal-rostral middle frontal (p=0.03), and inferior parietal (p<0.001) lobules. No volumes were significantly smaller among controls as compared with subjects with d-TGA.
Figure 1.
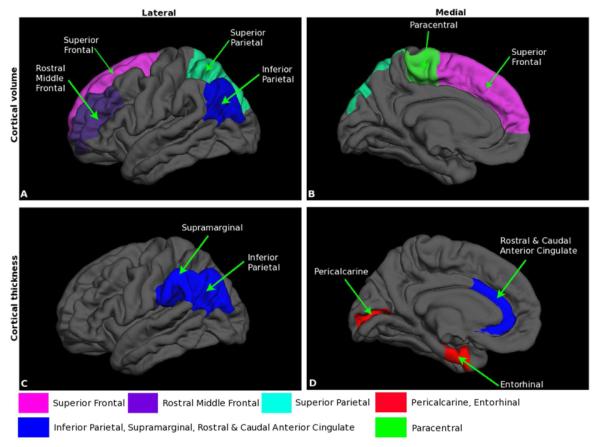
Cortical volume and thickness differences between adolescents with d-TGA and control groups. A, Lateral and B, medial views of the brain showing the cortical locations of decreased volume in d-TGA as compared with control adolescents C, Lateral and D, medial views of the brain showing the locations at which cortical thickness is greater in d-TGA as compared with control groups, as well as locations in which cortical thickness is lower in d-TGA as compared with control groups.
Table 3.
Subcortical and Cortical Regions of Volume and Thickness Differences Between d-TGA and Control Subjects Measured at 16 Years of Age
| Cluster | d-TGA (n=92) |
Control (n=49) |
P* |
|---|---|---|---|
| Volume (mm3) | |||
| B caudate | 3552 (495) | 3803 (466) | 0.04 |
| B superior parietal + B paracentral | 9371 (1061) | 9837 (1318) | 0.03 |
| B superior frontal + B rostral middle frontal |
22,261 (2578) | 23,350 (3228) | 0.03 |
| B inferior parietal | 15,403 (1976) | 16,831 (2844) | <0.001 |
| Thickness (mm) | |||
| B pericalcarine | 1.73 (0.12) | 1.78 (0.13) | 0.02 |
| B caudal ACC + B rostral ACC | 3.02 (0.19) | 2.99 (0.18) | 0.01 |
| B inferior parietal | 2.82 (0.11) | 2.76 (0.14) | 0.002 |
| B entorhinal | 2.90 (0.41) | 3.07 (0.41) | 0.04 |
| B supramarginal | 2.88 (0.12) | 2.82 (0.15) | <0.001 |
B: bilateral; ACC: anterior cingulate cortex.
Determined by linear regression adjusting for age at MRI, sex, scanner location, and total intracranial volume (for volumes only).
Values are unadjusted mean (standard deviation).
Cortical Thickness Differences
Among the 20 thickness clusters tested, five regions were significantly different between the d-TGA and control groups in each hemisphere (Figure and Table III). In two regions, the group d-with TGA demonstrated significantly thinner cortex than controls: bilateral pericalcarine (p=0.02) and bilateral entorhinal (p=0.04) cortices. In three regions, significantly thicker cortex was found in the group with d-TGA as compared with controls: bilateral caudal-rostral anterior cingulate cortices (p=0.01), bilateral inferior parietal lobules (p=0.002), and bilateral supramarginal cortices (p<0.001).
Correlations of Gray Matter Volumes and Cortical Thicknesses of Patients with d-TGA with Medical
Variables
We investigated the relation of preoperative medical variables to the parcellated volumetric and thickness measures we obtained. Volume in the bilateral pericalcarine cortex inversely correlated with age at surgery >30 days (r=−0.28, p=0.007). Bilateral parahippocampal gyrus thickness was significantly negatively correlated with a diagnosis of VSD (r=−0.30, p=0.005) and age at surgery >30 days (r=−0.29, p=0.006).
We explored whether volume and thickness measures were associated with intraoperative variables. First, bilateral thalamus-hippocampus volume was significantly and inversely correlated with total support time (r=−0.33, p=0.002). Second, longer cooling durations were associated with smaller volumes in the bilateral superior frontal-rostral middle frontal (r=−0.28, p=0.009) lobes, bilateral posterior and isthmus of cingulate (r=−0.29, p=0.006), and bilateral thalamus-hippocampus (r=−0.28, p=0.008). Finally, lower tympanic membrane temperature was associated with smaller bilateral amygdala volume (r=0.28, p=0.007).
Within the group with d-TGA, we investigated the relationship of the volume and thickness measures to postoperative course. More days in the CICU were correlated with higher bilateral insula volume (r=0.28, p=0.008), but lower bilateral superior temporal gyral thickness (r=−0.35, p<0.001) and bilateral middle temporal gyral thickness (r=−0.30, p=0.005). More days in the hospital were significantly correlated with lower thickness in bilateral superior temporal (r=−0.30, p=0.004), bilateral middle temporal (r=−0.28, p=0.007), bilateral pars opercularis (r=−0.29, p=0.006), and bilateral fusiform-inferior temporal-lateral occipital cortices (r=−0.30, p=0.004).
Discussion
Using volumetric MRI, we identified parcellated gray matter regions with significant reduction of volumes among a large cohort of adolescents with d-TGA and corrected with the arterial switch operation by 3 months of age compared with a healthy control group of similar age. In addition, the d-TGA and control groups differed significantly in several regions in cortical gyral thickness. Gyral thicknesses of adolescents with d-TGA significantly exceeded those of control subjects for 3 of 5 clusters, and control subjects demonstrated greater thicknesses for the remaining two. Taken together, these regions of volume and thickness differences were distributed across hemispheres and involved basal ganglia, medial fronto-parietal regions, and both parietal and temporal lobes. A varied picture of cortical alteration emerged regarding the relationship between medical features of the patients with d-TGA and the structural features of their gray matter. Older age at surgery (>30 days), as well as longer cooling duration, total support time, lowest tympanic temperature, and longer hospital stay were associated with reduced cortical volume and thickness in discrete regions. Finally, bilateral superior frontal and rostral middle frontal gyri were significantly smaller in the group with d-TGA, and smaller volumes were associated with longer cooling duration.
Few studies have investigated gray matter changes in adolescents with CHD. Schaer et al studied 61 adolescents with 22q11.2 deletion syndrome and compared cerebral volumetric measures in those with (44%) and without (56%) CHD.34 Children with both this genetic deletion and CHD were found to have striking reduction in parahippocampal, superior frontal, and middle temporal volumes compared with control children as well as children with 22q11.2 deletion but no CHD. This finding suggests that, although some regional brain volumes may be related to the genetic mutation, other regional reductions in volume may be related to the presence of structural heart disease, its hemodynamic consequences, or treatment. Von Rhein et al found significant volume reductions for overall cortical gray matter, cerebellum, and bilateral thalami in an adolescent cohort composed of patients with a variety of types of CHD.13 Using a surface-based approach to volumetric analyses, the same investigators found that a heterogeneous group of adolescents born with either cyanotic or acyanotic CHD demonstrated significant reduction, compared with control subjects, in whole brain-white matter and cortical gray matter volumes, as well as in summed bihemispheric surface areas of frontal, temporal, parietal, and limbic lobes. Further, adolescents with cyanotic CHD demonstrated significant reduction in total, bihemispheric volumes of corpus callosum, white matter, thalamic temporal lobe, and hippocampal volumes as compared with adolescents born with acyanotic CHD.35 Our data extend previously reported observations in several ways.
We employed a surface-based analytic approach with full parcellation that, to the best of our knowledge, has not been employed previously in this clinical population. Analysis of brain structure in this homogeneous group allowed us to examine the relationship of brain structural characteristics to medical variables without confounding by other characteristics such as type of congenital heart disease, consequent fetal hemodynamics, or differences in management. We found several discrete cortical and subcortical gray matter regions of significantly decreased volume in a homogeneous group of adolescents born with and treated for d-TGA, compared with a group of control adolescents. These regions include not only subcortical gray matter in basal ganglia, but also superior frontal gyrus, inferior parietal lobule, and superior parietal lobule.
Cortical thickness reflects the arrangement, size, and density of gray matter components. In particular, it reflects the size of the neuropil composed of axo-dendritic ramifications, which in turn is a reflection of inputs from and outputs to underlying white matter. Thus, cortical thickness constitutes an appealing metric for inter-group comparison. Interestingly, cortical thickness was significantly reduced in medial temporal lobe cortices bilaterally in the adolescents with d-TGA, whereas it was increased in other gyral locations compared with control adolescents.
Throughout typical development, cortical thickness and volume share a pattern of increase early in childhood followed by decline in adolescence, leading to a period of comparative stability in young adulthood.36-46 Different brain regions may follow this biphasic pattern of development at different time points.36, 43-45 Indeed, in a cohort of 45 typically developing children, Sowell et al. found that gray matter thickness increases were restricted to language-associated regions of the frontal, temporal, and parietal lobes, and more widespread thinning was observed elsewhere in right frontal, bilateral parietal, and occipital lobes.44 We speculate that differences in cortical thickness found between adolescents with d-TGA and controls derive from disruption of the continuous, long-term, biphasic, and regionally-specific pattern of gray matter development.
Previously, we reported on a subset of the current adolescent cohort with d-TGA studied with diffusion tensor imaging (DTI). The group with d-TGA showed reduced white matter microstructure in a distribution involving deep frontal, parietal, temporal, and peri-basal ganglionic regions corresponding to robust white matter tracts including superior and inferior longitudinal fasciculi, inferior fronto-occipital fasciculus, and uncinate fasciculus.10, 47 These long-range tracts ramify in and broadly interconnect multiple cortical gray matter regions. Interestingly, the regions of gray matter difference that we report here juxtapose the regions of white matter microstructure alteration. Similar white matter injury seen in the premature infant has been demonstrated to exert secondary consequences upon cortical gray matter development reflected in altered thickness.11, 12 Consequently, the quantitative differences in regional gray matter features we report now — in conjunction with the previously reported white matter microstructural alterations in this group — may reflect different consequences of the same central nervous system developmental disturbance that began with white matter injury and extended to adjacent and overlying gray matter through Wallerian degeneration as well as injury to subplate neurons and ascending afferent fibers to cortex.48, 49 Such a process could be reflected in adolescence as cortical regions with aberrant thickness.
Infants with CHD have brain immaturity similar to that of preterm infants.5, 8 Adolescents born prematurely demonstrate significantly lower cortical volumes and thickness bilaterally in the temporal, parietal, central, and prefrontal regions, and lower subcortical volumes in the caudate, hippocampus, and thalamus as compared with healthy controls.50, 51 Further, a group of adolescents born at very low birth weight had significantly thinner cortex in parietal, temporal, and occipital lobes, but thicker cortex in the frontal lobe, compared with healthy controls.52 Notably, many of these regions overlap with those we have identified in the group of adolescents with d-TGA that we report here, lending further support for a developmental origin of structural brain differences found in children with congenital heart disease.
Gray matter volumes and thicknesses were significantly related to several perioperative medical variables and risk factors. Gray matter volume in subcortical and midline cortical structures significantly declined as cooling duration and total support time during surgery increased. Additionally, subjects with longer hospital stays tended to have lower cortical thicknesses in temporal and lateral brain regions, likely reflecting the effects of more complicated intra- and post-operative courses. Interestingly, these same variables have been found to correlate significantly with white matter microstructure in adolescents with d-TGA.10
Our study has some limitations. Our findings may not generalize to children with types of CHD other than d-TGA and arise from a sample obtained at one center that received surgical treatment two decades prior. The group with d-TGA contained a higher percentage of males than the control group. To account for this difference, we included sex as a covariate in the regression analyses, though previous reports showed that males and females demonstrate similar patterns in cortical thickness.53, 54 The use of two scanners for data acquisition in this study could have introduced bias in brain volume measurements due to intrinsic hardware differences. However, we accounted for this by including a covariate for scan location in our regression analyses, and subjects with d-TGA and controls were similarly balanced across scan location. In addition, previous reports have shown that measurements are similar across scan site, even when using scanners from different manufacturers and different magnetic field strengths.55-57 Finally, we view our correlation analysis as exploratory, given the large number of associations tested.
In conclusion, adolescents with d-TGA repaired in early infancy, compared with control subjects, demonstrate an altered pattern of subcortical and cortical gray matter volume and thickness in several brain regions known to play important roles in higher cognition. Cortical or subcortical volumes or thicknesses correlated with several demographic and medical indicators of more prolonged surgical and hospital courses — factors known to be associated with greater neurocognitive impairment. Importantly, the discrete regions of significant cortical and subcortical gray matter volume and thickness alteration appear to coincide with previously documented changes in white matter microstructure. These findings suggest that the cognitive profile of this important clinical group derives from alteration of brain development early in life that affects both white matter and gray matter and evolves into adolescence.15
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (R01 HL77681 and HL41786), The Children’s Heart Foundation (Chicago), the Farb Family Fund, and the Kostin Family Innovation Fund.
Abbreviations
- CHD
congenital heart disease
- CICU
cardiac intensive care unit
- d-TGA
d-transposition of the great arteries
- MRI
magnetic resonance imaging
- TIV
total intracranial volume
- VSD
ventricular septal defect
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of the study were presented as a poster at the meeting of the Child Neurology Society, <city, state>, <dates>, 2012.
References
- [1].Dearani JA, Connolly HM, Martinez R, Fontanet H, Webb GD. Caring for adults with congenital cardiac disease: successes and challenges for 2007 and beyond. Cardiol Young. 2007;17(Suppl 2):87–96. doi: 10.1017/S1047951107001199. [DOI] [PubMed] [Google Scholar]
- [2].Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. The Journal of Thoracic and Cardiovascular Surgery. 2003;126:1397–403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- [3].Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathologica. 2005;110:563–78. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- [4].Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery. 2004;128:841–9. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- [5].McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- [6].Watanabe K, Matsui M, Matsuzawa J, Tanaka C, Noguchi K, Yoshimura N, et al. Impaired neuroanatomic development in infants with congenital heart disease. The Journal of Thoracic and Cardiovascular Surgery. 2009;137:146–53. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- [7].Ortinau C, Beca J, Lambeth J, Ferdman B, Alexopoulos D, Shimony JS, et al. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. The Journal of Thoracic and Cardiovascular Surgery. 2012;143:1264–70. doi: 10.1016/j.jtcvs.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery. 2009;137:529–37. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. New England Journal of Medicine. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- [10].Rivkin MJ, Watson CG, Scoppettuolo LA, Wypij D, Vajapeyam S, Bellinger DC, et al. Adolescents with d-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. The Journal of Thoracic and Cardiovascular Surgery. 2013;146:543–9. doi: 10.1016/j.jtcvs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- [12].Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Annals of Neurology. 1999;46:755–60. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [13].von Rhein M, Buchmann A, Knirsch W, Klaver P, Latal B. Morphometric MRI findings in adolescents with congenital heart disease. Neuropediatrics. 2011;42:V15. [Google Scholar]
- [14].von Rhein M, Scheer I, Loenneker T, Huber R, Knirsch W, Latal B. Structural brain lesions in adolescents with congenital heart disease. The Journal of Pediatrics. 2011;158:984–9. doi: 10.1016/j.jpeds.2010.11.040. [DOI] [PubMed] [Google Scholar]
- [15].Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Dunbar-Masterson C, et al. Adolescents With d-Transposition of the Great Arteries Corrected With the Arterial Switch Procedure Neuropsychological Assessment and Structural Brain Imaging. Circulation. 2011;124:1361–9. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban K, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. New England Journal of Medicine. 1993;329:1057–64. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- [17].Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. New England Journal of Medicine. 1995;332:549–55. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- [18].Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–32. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- [19].Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. The Journal of Thoracic and Cardiovascular Surgery. 2003;126:1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- [20].Almli CR, Rivkin M, McKinstry R, Group BDC. The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. Neuroimage. 2007;35:308–25. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- [21].Evans AC, Group BDC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- [22].Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- [23].Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- [24].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- [25].Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- [27].Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- [28].Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of human brain: brainstem and cerebellum. Neurology. 1996;47:1125–35. doi: 10.1212/wnl.47.5.1125. [DOI] [PubMed] [Google Scholar]
- [29].Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- [30].Group BDC. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cerebral Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barnes J, Ridgway GR, Bartlett J, Henley S, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–55. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- [32].Wechsler D. Individual Achievement Test-II (WIAT-II) The Psychological Corporation; 2001. [Google Scholar]
- [33].Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS) Psychological Corporation; 2001. [Google Scholar]
- [34].Schaer M, Glaser B, Ottet M-C, Schneider M, Cuadra MB, Debbané M, et al. Regional cortical volumes and congenital heart disease: a MRI study in 22q11. 2 deletion syndrome. Journal of Neurodevelopmental Disorders. 2010;2:224–34. doi: 10.1007/s11689-010-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain: A Journal of Neurology. 2014;137:268–76. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- [36].Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–49. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- [38].Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- [39].Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children A volumetric imaging study. Brain. 1996;119:1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- [40].Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- [41].Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal Brain Development and Aging: Quantitative Analysis at in Vivo MR Imaging in Healthy Volunteers 1. Radiology. 2000;216:672–82. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- [42].Kennedy DN, Makris N, Herbert MR, Takahashi T, Caviness VS. Basic principles of MRI and morphometry studies of human brain development. Developmental Science. 2002;5:268–78. [Google Scholar]
- [43].Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- [44].Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24:8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rollins CK, Watson CG, Asaro LA, Wypij D, Vajapeyam S, Bellinger DC, et al. White matter microstructure and cognition in adolescents with congenital heart disease. The Journal of Pediatrics. 2014;165:936–44. e2. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Volpe JJ. Encephalopathy of Congenital Heart Disease–Destructive and Developmental Effects Intertwined. The Journal of Pediatrics. 2014;164:962–5. doi: 10.1016/j.jpeds.2014.01.002. [DOI] [PubMed] [Google Scholar]
- [50].Nagy Z, Ashburner J, Andersson J, Jbabdi S, Draganski B, Skare S, et al. Structural correlates of preterm birth in the adolescent brain. Pediatrics. 2009;124:e964–e72. doi: 10.1542/peds.2008-3801. [DOI] [PubMed] [Google Scholar]
- [51].Nagy Z, Lagercrantz H, Hutton C. Effects of preterm birth on cortical thickness measured in adolescence. Cerebral Cortex. 2011;21:300–6. doi: 10.1093/cercor/bhq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Martinussen M, Fischl B, Larsson H, Skranes J, Kulseng S, Vangberg T, et al. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain. 2005;128:2588–96. doi: 10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- [53].Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, et al. How does your cortex grow? The Journal of Neuroscience. 2011;31:7174–7. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lv B, Li J, He H, Li M, Zhao M, Ai L, et al. Gender consistency and difference in healthy adults revealed by cortical thickness. Neuroimage. 2010;53:373–82. doi: 10.1016/j.neuroimage.2010.05.020. [DOI] [PubMed] [Google Scholar]
- [55].Pardoe H, Pell GS, Abbott DF, Berg AT, Jackson GD. Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy. Neuroimage. 2008;42:611–6. doi: 10.1016/j.neuroimage.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Stonnington CM, Tan G, Kloppel S, Chu C, Draganski B, Jack CR, Jr, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer’s disease. Neuroimage. 2008;39:1180–5. doi: 10.1016/j.neuroimage.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]


