Abstract
BACKGROUND
An elevated serum level of interleukin-6 (IL-6) in pulmonary arterial hypertension (PAH) patients results in a greater symptom burden and increased mortality; however, the mechanisms underlying these observations remain unclear. Because both pre-clinical and clinical data associate elevated IL-6 levels with impaired cardiac function, we hypothesized that the adverse effects of IL-6 in PAH result, in part, from right ventricular (RV) dysfunction.
METHODS
We analyzed the relationship between IL-6 and RV function in 40 patients with PAH identified in our institutional PAH registry. Serum IL-6 levels was quantified by enzyme-linked immunoassay.
RESULTS
PAH patients had higher IL-6 levels than age- and gender-matched controls. Circulating IL-6 levels correlated inversely with echocardiography-based measures of RV function and RV–pulmonary artery (RV-PA) coupling. When dividing PAH patients by median IL-6 level, patients with higher IL-6 had significantly worse RV function (fractional area change [FAC] 23 ± 12% vs 38 ± 11%, tricuspid annular plane systolic excursion [TAPSE] 1.3 ± 0.3 cm vs 2.1 ± 0.5 cm), impaired RV-PA coupling (0.6 ± 0.5%/mm Hg vs 0.9 ± 0.5%/mm Hg), higher right atrial pressure (13 ± 7 mm Hg vs 9 ± 5 mm Hg), reduced cardiac index (2.0 ± 0.5 liters/min/m2 vs 2.8 ± 1.0 liters/min/m2) and lower stroke volume (48 ± 20 ml vs 70 ± 28 ml). In contrast, the relationships between IL-6 and mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (PVR) and pulmonary arterial compliance (PAC) were not significant. Finally, IL-6 was independently associated with RV function and RV-PA coupling after adjusting for static (PVR) and pulsatile (PAC) after-load on the RV.
CONCLUSIONS
Serum IL-6 levels are independently associated with RV function and RV-PA coupling in PAH. Patients with higher IL-6 levels have more severe RV dysfunction and diminished RV-PA coupling despite a comparable severity of pulmonary vascular disease.
Keywords: inflammation, RV-PA coupling, biomarkers, pulmonary hypertension, interleukins
Pulmonary arterial hypertension (PAH) has a substantial inflammatory phenotype characterized by accumulation of CD68+ macrophages in the pulmonary vasculature, decreased T-regulatory cells and dysregulation of natural killer cells.1 In some PAH patients, such as those with systemic lupus erythematosus, anti-inflammatory therapies can be extremely effective; however, none of the currently approved PAH-targeted therapies are primarily directed at inflammatory mechanisms.1 Thus, a better understanding of the role of inflammation in PAH pathogenesis may offer new biomarkers and therapeutic targets to assist in managing this devastating disease.
Levels of the inflammatory cytokine interleukin-6 (IL-6) are elevated in patients with PAH2,3 and animal models of PAH.4,5 High serum levels of IL-6 are associated with greater symptomatic burden6 and increased mortality in PAH3,7; however, a mechanistic understanding for the negative effects of IL-6 is not well defined. One possible reason for the adverse association between outcomes and IL-6 levels is its ability to promote adverse pulmonary vascular remodeling. This is suggested in pre-clinical data, as transgenic overexpression of IL-6 induces pulmonary vascular remodeling and elevates right ventricular (RV) pressures under normoxic conditions and causes an even more severe PH phenotype in hypoxic conditions.8 Conversely, knockout of IL-6 leads to partial protection against chronic hypoxia-induced pulmonary hypertension.9 Furthermore, transgenic overexpression of a mutant bone morphogenic protein receptor II (BMPRII) increases IL-6 levels in the lungs and renders mice susceptible to PAH.10 Finally, IL-6 reduces BMPRII protein levels through activation of a microRNA 17/92 cluster pathway.11 Although there are substantial data supporting a role for IL-6 in the pathogenesis of pulmonary vascular remodeling in animal models, the contribution of elevated IL-6 levels to pulmonary vascular disease severity in humans with PAH is not well established.
Interestingly, both pre-clinical and clinical data have also identified a link between IL-6 and cardiac dysfunction. IL-6 has an acute negative inotropic effect on isolated hamster and chicken left ventricular cardiomyocytes that is mediated by nitric oxide overproduction.12,13 Furthermore, neonatal rat ventricular cardiomyocytes cultured in the presence of IL-6 have reduced sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) mRNA and protein levels,14 which may explain the impaired calcium-handling kinetics induced by IL-6 in isolated cardiomyocytes.13 Moreover, IL-6 knockout mice exhibit less pathologic left ventricular (LV) remodeling and have improved cardiac function in response to LV pressure overload when compared with control littermates.15 Like-wise, IL-6 knockout mice have shown less LV cardiac fibrosis and improved cardiac function in the streptozotocin-induced diabetic cardiomyopathy model.16
An association between cardiac structure/function and IL-6 exists in clinical studies as well. IL-6 levels have a negative relationship with LV systolic function, as defined by cardiac magnetic resonance imaging, in asymptomatic patients in the Multi-Ethnic Study of Atherosclerosis (MESA).17 IL-6 levels are also related to functional impairment in patients with LV systolic heart failure.18 In addition, cardiac IL-6 mRNA levels are elevated in advanced heart failure patients undergoing cardiac transplantation and they correlate inversely with LV ejection fraction.19 Although less is known about the effects of IL-6 on the RV, IL-6 level was shown to be an independent predictor of RV dilation in the MESA-RV study.20 Because of the substantial data connecting elevated IL-6 to cardiac dysfunction, it is possible that the increased mortality in PAH patients with elevated IL-6 is related to the adverse effects of IL-6 on RV function, which is the major determinant of long-term outcomes in these patients.21–25 Accordingly, in this study, we investigated the hypothesis that elevated IL-6 levels in patients with PAH are associated with RV dysfunction and impaired RV–pulmonary artery (RV-PA) coupling.
Methods
Study population
We studied patients in the Minnesota Pulmonary Hypertension Repository,26 which is a customized patient database that tracks clinical variables on every patient evaluated at the University of Minnesota Pulmonary Hypertension Clinic (March 2014 to present). Data are collected by chart review and entered using an internet-based electronic data capture system. Patients diagnosed before March 2014 were entered retrospectively. Informed consent was obtained before participation in the repository. The institutional review boards of the University of Minnesota approved the Minnesota Pulmonary Hypertension Repository.
For the current study, we identified 40 adult patients (≥18 years of age) with PAH in the Minnesota Pulmonary Hypertension Repository who had a right heart catheterization (RHC), a transthoracic echocardiogram (TTE) and a serum IL-6 level obtained within an 8-month time interval. We included both prevalent and incident World Health Organization (WHO) Group 1 PAH. In brief, the diagnosis of PAH required the following: (1) a mean pulmonary artery pressure (mPAP) ≥25 mm Hg at rest, with a pulmonary capillary wedge pressure of ≤15 mm Hg and a pulmonary vascular resistance (PVR) > 3 Wood units; and (2) the exclusion of other WHO categories of pulmonary hypertension by clinical evaluation and objective tests. Patients were excluded if they had obstructive lung disease diagnosed by reduced expiratory flow rates (forced expiratory volume in 1 second [FEV1] / forced vital capacity [FVC] < 75% predicted); more than mild interstitial lung disease, as diagnosed by reduced total lung capacity < 60%; and chronic pulmonary thromboembolic disease, as diagnosed by high or intermediate probability ventilation perfusion scan or evidence of a pulmonary embolism on a contrast-enhanced chest CT or pulmonary angiogram. Patients with pulmonary venous hypertension, defined as mPAP ≥25 mm Hg at rest with a pulmonary capillary wedge pressure of > 15 mm Hg, were also excluded.
We included 10 age- and gender-matched healthy volunteers with no significant medical history as control subjects. The mean age of the control group was 49 ± 6 years. There were 8 females and 2 males.
Measurement of IL-6 levels
Serum IL-6 levels were measured from the peripheral venous blood samples drawn during routine clinic visits for patients with PAH. Peripheral venous blood samples were collected, kept on ice, and centrifuged within 30 minutes. IL-6 levels were measured using a Quantikine enzyme-linked immunoassay kit (R&D Systems, Minneapolis, MN) at the University of Minnesota Cytokine Reference Laboratory. Control and PAH serum samples were stored at −80°C until analyzed. The normal reference range for this assay is < 3.01 pg/ml.
Hemodynamics
Patients being evaluated for pulmonary hypertension underwent right heart catheterization at the cardiac catheterization laboratory of the University of Minnesota. The following hemodynamic variables were recorded at the end of expiration: right atrial pressure; right ventricular systolic and end-diastolic pressures; systolic pulmonary artery pressure; diastolic pulmonary artery pressure; mPAP; and pulmonary capillary wedge pressure, as an average of 3 beats. Cardiac output was determined as the mean of 3 measurements with the thermodilution method or indirect Fick method based on total body oxygen consumption estimated via the formula of LaFarge and Miettinen.27 PVR was calculated in Wood units as the difference between mPAP measure and pulmonary capillary wedge pressure divided by the cardiac output. Stroke volume (in mm) was calculated from cardiac output and heart rate. Pulmonary arterial compliance (PAC, in mm per mm Hg) was calculated as the ratio of stroke volume to the pulmonary artery pulse pressure, as described elsewhere.28 All formulas used are presented in Table S1 in the Supplementary Material (available online at www.jhltonline.org).
RV function and RV-PA coupling as determined by echocardiography
RV function was quantified using RV fractional area change (RVFAC) and/or tricuspid annular plane systolic excursion (TAPSE), as recommended by the American Society of Echocar-diography.29 If patients did not have M-mode of the tricuspid annulus recorded on their clinical echocardiogram, we measured TAPSE by post-processing of 2-dimensional apical 4-chamber images using a Java-based imaging software program (Image J, National Institutes of Health, Bethesda, MD), as described elsewhere.30 RV-PA coupling was estimated non-invasively by the following formulas (also described elsewhere): RV FAC/mPAP from RHC data (%/mm Hg) or TAPSE/mPAP from RHC data (mm/mm Hg),26,31–35 which were shown to correlate with invasively measured RV-PA coupling.35
Statistical analysis
Categorical data are expressed as frequency and proportion, whereas continuous data are presented as mean ± standard deviation. Unpaired t-tests were used to compare means of 2 groups with continuous variables with equivalent variance. If there was unequal variance, as determined by F-test, we used the Mann–Whitney U-test (serum IL-6 level, TAPSE, RV-PA coupling defined as TAPSE/mPAP, cardiac index, N-terminal pro–brain natriuretic peptide). Either the chi-square or Fisher’s exact test was performed to compare proportions for categorical variables. Pearson's correlation was used to compare IL-6 with invasively measured hemodynamic parameters and RV function as determined by echocardiography. We performed multivariable analysis of 2 models of RV function as defined by RVFAC and TAPSE, and 2 models of RV-PA coupling adjusting for PVR, PAC and the natural logarithmic transformation of serum IL-6. Because of the small sample size, we did not include other variables in the model. Statistical analyses were performed using GRAPHPAD PRISM version 7.01 (GraphPad, Inc., La Jolla, CA) and STATA version 10 (StataCorp LP, College Station, TX). p < 0.05 was considered statistically significant.
Results
We identified 40 patients with PAH who had an echocardio-gram, serum IL-6 level assessment and right heart catheterization all performed within 8 months of each other. Table 1 presents the clinical characteristics of the study group. The mean age of the study group was 55 ± 12 years and there were 35 females (88%). Eighteen patients had connective tissue disease–associated PAH (45%), 11 being scleroder-ma-associated PAH; 14 patients had idiopathic PAH (35%), 3 had portopulmonary hypertension (7.5%), 2 had drug-induced PAH (5%), 1 had human immunodeficiency virus–associated PAH (2.5%), 1 had congenital heart disease–associated PAH (2.5%) and 1 had pulmonary veno-occlusive disease (2.5%). There were 21 incident cases (52.5%) and 19 prevalent cases (47.5%). The cohort had severe PAH with mPAP 48 ± 13 mm Hg, PVR 10 ± 5 Wood units and PAC 1.6 ± 1.0 ml/mm Hg. The mean RVFAC and TAPSE were 31 ± 14% and 1.7 ± 0.6 cm, respectively.
Table 1.
Characteristics of PAH Study Cohort
| Characteristics | Total cohort @(n = 40) |
Low IL-6 group @(n = 20) |
High IL-6 group @(n = 20) |
p-value for @high vs low |
|---|---|---|---|---|
| Age (years) | 55 ± 12 | 56 ± 12 | 55 ± 12 | 0.69 |
| Female gender | 35 (88%) | 18 (90%) | 17 (85%) | 0.64 |
| Incident | 21 (52%) | 13 (65%) | 8 (40%) | 0.12 |
| Prevalent | 19 (48%) | 7 (35%) | 12 (60%) | 0.12 |
| Etiology | ||||
| CTD-associated PAH | 18 (45%) | 8 (40%) | 10 (50%) | 0.54 |
| Idiopathic PAH | 14 (35%) | 9 (45%) | 5 (25%) | 0.19 |
| Portopulmonary PAH | 3 (7.5%) | 2 (10%) | 1 (5%) | 0.56 |
| Drug-induced PAH | 2 (5%) | 0 (0%) | 2 (10%) | 0.15 |
| Congenital heart disease | 1 (2.5%) | 0 (0%) | 1 (5%) | 0.32 |
| HIV-associated PAH | 1 (2.5%) | 0 (0%) | 1 (5%) | 0.32 |
| PVOD | 1 (2.5%) | 1 (5%) | 0 (0%) | 0.32 |
| WHO functional class | 0.035 | |||
| 1 | 2 (5%) | 2 (10%) | 0 (0%) | |
| 2 | 4 (10%) | 1 (5%) | 3 (15%) | |
| 3 | 24 (60%) | 13 (65%) | 11 (55%) | |
| 4 | 5 (12.5%) | 0 (0%) | 5 (25%) | |
| Not available | 5 (12.5%) | 4 (20%) | 1 (5%) | |
| Creatinine (mg/dl) | 0.92 ± 0.29 | 1.04 ± 0.3 | 0.82 ± 0.25 | 0.015 |
| NT pro-BNP (pg/ml) | 2,217 ± 2,129 | 1,560 ± 1,287 | 2,264 ± 2,694 | 0.24 |
| Serum IL-6 (pg/ml) | 15.1 ± 21.1 | 3.1 ± 1.4 | 27.1 ± 24.7 | < 0.0001 |
| PAH-specific therapy at time of IL-6 measurement | ||||
| Endothelin receptor antagonists | 14 (35%) | 9 (45%) | 5 (25%) | 0.19 |
| Phosphodisterase-5 inhibitor | 22 (55%) | 10 (50%) | 12 (60%) | 0.54 |
| Parental prostacylin | 9 (23%) | 4 (20%) | 5 (25%) | 0.71 |
| Inhaled prostacylin | 3 (8%) | 1 (5%) | 2 (10%) | 0.88 |
| Treatment-naive | 11 (27.5%) | 5 (25%) | 6 (30%) | 0.73 |
| Monotherapy | 14 (35%) | 8 (40%) | 6 (30%) | 0.52 |
| Dual therapy | 11 (27.5%) | 5 (25%) | 6 (30%) | 0.73 |
| Triple therapy | 4 (10%) | 2 (10%) | 2 (10%) | 0.99 |
Data presented as mean ± standard deviation.
CTD, connective tissue disease; HIV, human immunodeficiency virus; IL-6, interleukin-6; PAH, pulmonary arterial hypertension; NT pro-BNP, N-terminal pro–brain natriuretic protein; PVOD, pulmonary veno-occlusive disease; WHO, World Health Organization.
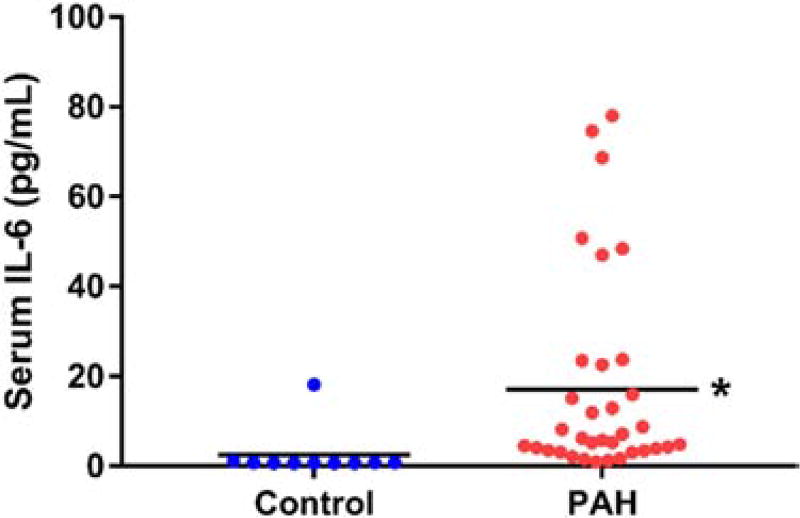
The mean IL-6 level was 15.1 ± 21.1 pg/ml, which was significantly elevated when compared with 10 age- and gender-matched controls (2.5 ± 5.5 pg/ml, p < 0.0001; Figure 1). The median time interval between serum IL-6 measurement and echocardiogram was 1 day (interquartile range 12 to 19 days) and between serum IL-6 measurement and right heart catheterization was 0 day (interquartile range 11 to 18 days). The median time interval between echocardiogram and right heart catheterization was 1 day (interquartile range −29 days to 25 days).
Figure 1.
IL-6 is elevated in PAH patients. Serum IL-6 in 10 control (2.5 ± 5.5 pg/ml) and 40 PAH patients (15.1 ± 21.1 pg/ml) (p < 0.0001). *p < 0.05, as determined by Mann–Whitney U-test.
Relationship between serum IL-6 levels and RV function
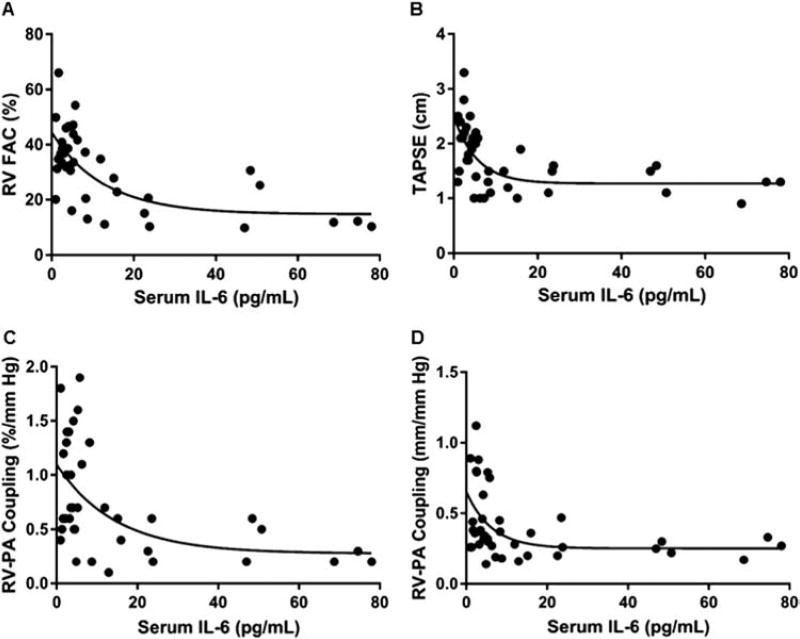
Next, we analyzed the relationship between serum IL-6 levels and RV function and RV-PA coupling. There were negative logarithmic associations between serum IL-6 and RVFAC (r = −0.67), TAPSE (r = −0.61), RV-PA coupling defined as RVFAC/mPAP (r = −0.53) and TAPSE/mPAP (r = −0.48) (Figure 2A–D). Because the relationships between RV function and serum IL-6 were logarithmic, we used the natural logarithmic transformation of serum IL-6 with each measure of RV function to define statistical significance. The natural logarithmic transformation of IL-6 was significantly associated RVFAC (r = −0.64, p < 0.0001), TAPSE (r = −0.55, p = 0.0002), RVFAC/mPAP (r = −0.50, p = 0.001) and TAPSE/mPAP (r = −0.44, p = 0.005). Importantly, the relationships between IL-6 and RV function remained significant, even after excluding patients with connective tissue disease (refer to Figure S1A–D in the Supplementary Material available online at www.jhltonline.org). Finally, although only available for 8 patients, there was a similar negative logarithmic relationship between RV ejection fraction on cardiac MRI and serum IL-6 (r = −0.66) (see Figure S2 online).
Figure 2.
Negative logarithmic relationships between IL-6 and RV function and RV-PA coupling in PAH. Negative logarithmic relationships between IL-6 and RVFAC (r = −0.67) (A), TAPSE (r = 0.61) (B), RV-PA coupling Model 1 (RVFAC/mPAP) (r = −0.53) (C) and RV-PA coupling Model 2 (TAPSE/mPAP) (r = −0.48) (D) exist in PAH patients. The natural logarithmic transformation of IL-6 is significantly associated with RVFAC (r = −0.64, p < 0.0001), TAPSE (r = −0.55, p = 0.0002), RVFAC/mPAP (r = −0.50, p = 0.001) and TAPSE/mPAP (r = −0.44, p = 0.005).
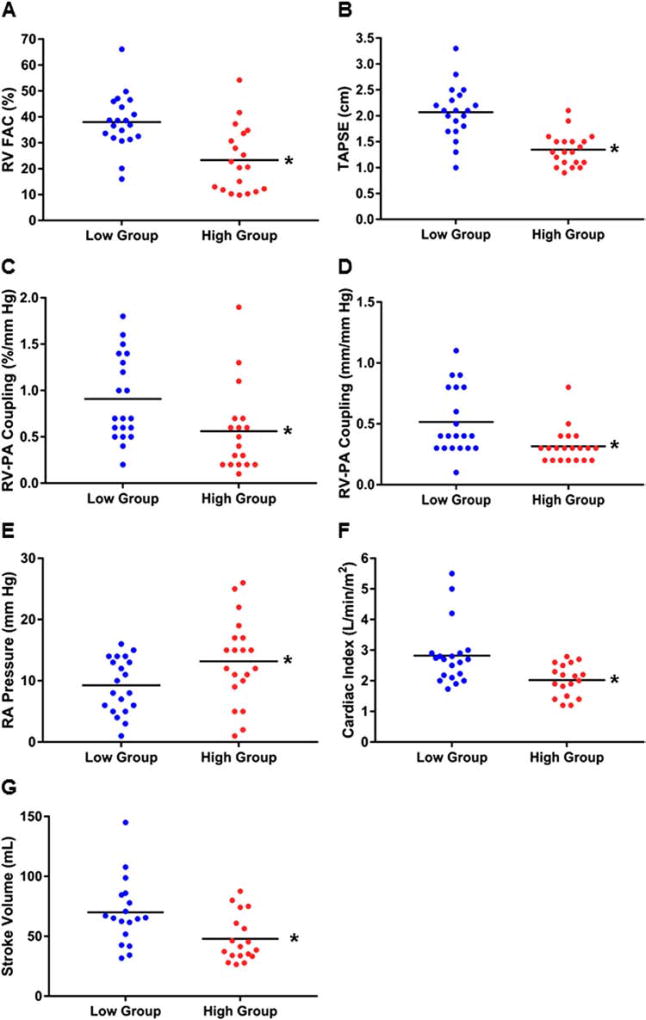
To further define the association between IL-6 and RV function in PAH, we separated our cohort by median IL-6 level and compared echocardiography- and hemodynamics-based assessments of RV function. Higher levels of IL-6 were associated with reduced RV function, as defined by RVFAC (23 ± 12% vs 38 ± 11%, p = 0.0004) (Figure 3A) and TAPSE (1.3 ± 0.3 cm vs 2.1 ± 0.5 cm, p < 0.0001) (Figure 3B). RV-PA coupling defined as RVFAC/mPAP (0.6 ± 0.5%/mm Hg vs 0.9 ± 0.5%/mm Hg, p = 0.03) and TAPSE/mPAP (0.3 ± 0.1 mm/mm Hg vs 0.5±0.3 mm/mm Hg, p = 0.003) was reduced in the patients with higher IL-6 (Figure 3C and D). Moreover, hemodynamics-based analysis of RV function revealed higher RA pressure (13 ± 7 mm Hg vs 9 ± 5 mm Hg, p = 0.04), lower cardiac index (2.0 ± 0.5 liters/min/m2 vs 2.8 ± 1.0 liters/min/m2, p = 0.002) and lower stroke volume (48 ± 20 ml vs 70 ± 28 ml, p = 0.009) (Figure 3E–G) in the group with higher IL-6 levels. There was a trend for higher N-terminal pro–brain natriuretic peptide (NT pro-BNP) in patients with higher IL-6, but this was not statistically significant (Table 1). Finally, patients with higher IL-6 had a greater frequency of WHO Functional Class IV (Table 1).
Figure 3.
Elevated levels of IL-6 are associated with worse RV function in PAH. Patients with higher levels of IL-6 have significantly reduced RVFAC (23 ± 12% vs 38 ± 11%, p = 0.0004) (A), lower TAPSE (1.3 ± 0.3 cm vs 2.1 ± 0.5 cm, p < 0.0001) (B) and worse RV-PA coupling, as defined by RVFAC/mPAP (0.6 ± 0.5%/mm Hg vs 0.9 ± 0.5%/mm Hg, p = 0.03) (C) and TAPSE/mPAP (0.3 ± 0.1 mm/mm Hg vs 0.5 ± 0.3 mm/mm Hg, p = 0.003) (D) when compared to patients with lower IL-6 levels. High IL-6 is associated with higher RA pressure (13 ± 7 mm Hg vs 9 ± 5 mm Hg, p = 0.04) (E), lower cardiac index (2.0 ± 0.5 liters/min/m2 vs 2.8 ± 1.0 liters/min/m2, p = 0.002) (F) and lower stroke volume (48 ± 20 ml vs 70 ± 28 ml, p = 0.009) (G). *p < 0.05, as determined by Student’s t-test or Mann–Whitney U-test.
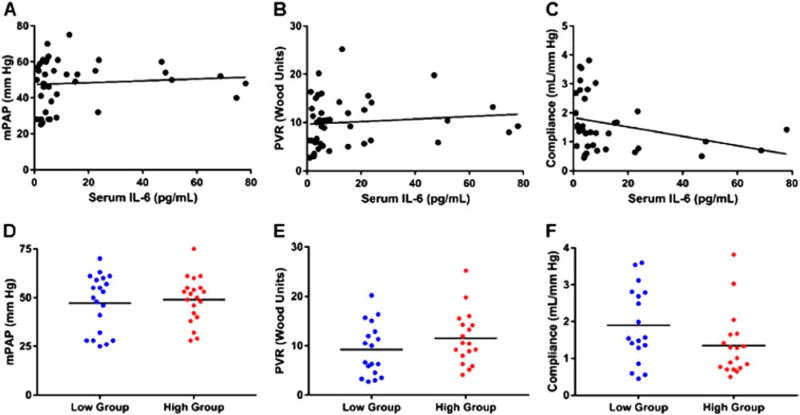
Relationship between serum IL-6 levels and pulmonary vascular disease
Next, we investigated whether serum levels of IL-6 were associated with invasively measured hemodynamics markers of pulmonary vascular disease. There were no significant relationships between serum IL-6 levels and mPAP (r = 0.09, p = 0.60), PVR (r = 0.11, p = 0.50) and PAC (r = −0.31, p = 0.07) (Figure 4A–C). Furthermore, there was no difference in the severity of pulmonary vascular disease when patients were separated by median serum IL-6 levels: mPAP (low 47 ± 15 mm Hg, high 49 ± 12; p = 0.69); PVR (low 9 ± 5 Wood units, high 12 ± 5 Wood units; p = 0.20); and PAC (low 1.9 ± 1.0 ml/mm Hg, high 1.4 ± 0.9 ml/mm Hg; p = 0.09) (Figure 4D–F). Thus, it appears that differences in pulmonary vascular disease did not explain the reduced RV function in patients with high serum IL-6 levels.
Figure 4.
IL-6 is not associated with invasively measured markers of pulmonary vascular disease and patients with higher levels of IL-6 do not have more severe pulmonary vascular disease. Correlational analysis of IL-6 with mPAP (r = 0.09, p = 0.60) (A), PVR (r = 0.11, p = 0.50) (B), and pulmonary arterial compliance (r = −0.31, p = 0.07) (C). There are no significant relationships between IL-6 and measures of pulmonary vascular disease. When patients are separated by mean IL-6 level, there are no significant differences in mPAP (low 47 ± 15 mm Hg, high 49 ± 12 mm Hg; p = 0.69) (D), PVR (low 9 ± 5 Wood units, high 12 ± 5 Wood units; p = 0.20) (E) and compliance (low 1.9 ± 1.0 ml/mm Hg, high 1.4 ± 0.9 ml/mm Hg; p = 0.09) (F) on comparison of the 2 groups.
Relationship between serum IL-6 levels and RV function after adjusting for RV after-load
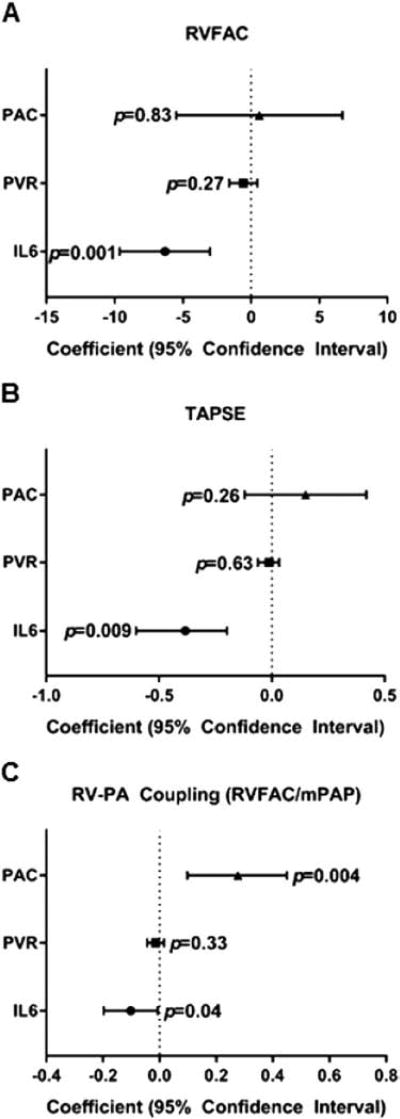
Finally, to determine whether serum IL-6 levels were related to RV function, independent of pulmonary vascular disease (RV after load), we performed multivariable analysis using PVR, PAC and IL-6 levels as the independent variables and RV function and RV-PA coupling as the dependent variables. The relationships between IL-6 and RVFAC, TAPSE and RV-PA coupling (RVFAC/mPAP) remained significant after adjusting for the static (PVR) and pulsatile after-loads (PAC) of the RV (Figure 5A–C). The second model of RV-PA coupling (TAPSE/mPAP) did not show a significant relationship with IL-6 after adjusting for PVR and PAC.
Figure 5.
Forest plot of multivariable analysis of RV function and RV-PA coupling in PAH. IL-6 is significantly associated with RVFAC (A) and TAPSE (B), and RV-PA coupling (RVFAC/mPAP) (C) after adjusting for PVR and PAC. The p-values are shown on Forest plots. Data are presented as coefficient with 95% confidence interval.
Discussion
Our results identify a novel relationship between IL-6 and RV function in PAH. We have shown that patients with higher levels of serum IL-6 have worse RV function and RV-PA coupling despite having similar pulmonary vascular disease severity. Although pre-clinical data suggest IL-6 has a role in pulmonary vascular remodeling, we did not observe statistically significant relationships between serum IL-6 levels and mPAP, PVR and PAC. Finally, we found that serum IL-6 levels were independently associated with RV function and RV-PA coupling after adjusting for the static and pulsatile after-loads of the RV. Taken together, these data document a novel relationship between RV function and serum IL-6 levels in PAH, and may provide mechanistic insight into the increased mortality associated with elevated IL-6 in PAH.
Two reports have shown that elevated serum IL-6 levels are associated with poor survival in PAH. However, neither study evaluated RV function and its relationship to serum IL-6 levels. When we used the logarithmic equation derived from the relationship between TAPSE and serum IL-6 in our cohort to predict RV function based on serum IL-6 levels that were associated with increased mortality in earlier work by Heresi et al7 and Soon et al,3 we encountered some notable findings. First, using the results of Heresi et al, with the serum IL-6 level of 4.7 pg/ml associated with reduced survival,7 our equation predicts a TAPSE of 1.79 cm. Forfia et al showed that a TAPSE of < 1.8 cm was associated with increased mortality in PAH.24 Then, if we predict TAPSE based on a serum IL-6 level of 15.19 pg/ml, which Soon et al showed portended the worst prognosis in their PAH population,3 then the TAPSE value is 1.5 cm. Interestingly, a TAPSE of 1.5 cm identified patients with the highest mortality rates in the study by Ghio et al.36 In summary, using our data to predict TAPSE, based on serum IL-6 levels that are associated with reduced survival in previous published reports, led to TAPSE values that could also predict high mortality rates.
Although our results are hypothesis-generating, more studies are required to understand how IL-6 impacts RV function in PAH. Although IL-6 acutely reduces contractility in healthy isolated cardiomyocytes, the mechanism proposed is excessive nitric oxide production.12,13 This is counterintuitive to RV dysfunction in PAH, as increasing nitric oxide improves contractility of hypertrophied RV myocardium.37 Another possible explanation for the negative inotropic activity of IL-6 pertains to its ability to negatively regulate SERCA2a.14 This would fit well with previously published data, as SERCA2a levels are reduced in the RV of human PAH patients38 and animal models of PAH.39–41 Future studies investigating the link between IL-6 and SERCA2a could provide further insight into our findings.
Although our data suggest a role of IL-6 in RV dysfunction, there are many questions that remain unanswered. First, our data cannot definitively determine whether elevated serum IL-6 levels are the cause or the consequence of RV dysfunction. It is possible that serum IL-6 levels rise because of RV dysfunction. However, the direct negative inotropic effect of IL-6 on cardiomyocytes in in-vitro experiments12,13 and attenuation of pressure-overload–induced LV hypertrophy fibrosis and dysfunction in IL-6 knockout mice15 together strongly suggest a causal relationship between elevated IL-6 levels and RV dysfunction. Previous work has shown that, as the degree of chronic kidney disease worsens, average serum IL-6 levels increase.42 Thus, another possibility is that renal dysfunction is responsible for increased IL-6 in some PAH patients. However, serum creatinine in patients with lower IL-6 levels in our cohort was significantly higher than in patients with higher IL-6 (see Table 1). Therefore, it is less likely that renal dysfunction contributes to elevated IL-6 levels in PAH. Furthermore, the cell type responsible for secreting excess levels of IL-6 in PAH remains undefined, but an intriguing possibility is that cardiomyocytes and/or cardiac fibroblasts release IL-6. This was supported by findings showing that IL-6 mRNA levels were elevated in failing RV and LV samples from cardiac transplant patients.19,43 Likewise, isolated rat cardiomyocytes exposed to hypoxia release IL-6.44 More studies are required to elucidate the role of IL-6 in RV dysfunction in PAH.
We did not observe a relationship between serum IL-6 levels and pulmonary vascular disease, which is consistent with the results of Soon et al, as they also did not observe significant relationships between serum IL-6 levels and invasively measured parameters of pulmonary vascular disease.3 Although our group and Soon et al did not identify relationships between pulmonary vascular disease severity and serum IL-6 levels in PAH patients,3 there are plausible mechanisms to explain these observations. First, it is possible that IL-6 has a more important role in initiation of pulmonary vascular remodeling, but not in continued progression of disease phenotype. This is supported by the fact that, although IL-6 knockout mice are resistant to pulmonary vascular remodeling in hypoxia, they still show signs of pulmonary hypertension.9 Furthermore, IL-6 overexpression induces only mild pulmonary hypertension but requires hypoxia to promote a more severe form.8 Yet another explanation could be that there are species-specific differences in pulmonary vascular disease remodeling when comparing rodent models with humans. Certainly, this was observed previously, as mice with human mutations of BMPRII exhibited a less severe phenotype than some human patients harboring the same mutations.45 It is possible that serum levels of IL-6 do not accurately reflect IL-6 levels in the lung.
In conclusion, an ongoing clinical trial may provide insight into how inhibition of the IL-6 pathway can either reverse or stop the progression of RV dysfunction in PAH. The TRANSFORM-UK investigation (NCT02676947) will use tocilizumab, a monoclonal antibody to the IL-6 receptor, to treat PAH. The primary outcome in this study will be change in PVR, but one of the secondary outcomes is change in NT pro-BNP, which could provide indirect information about alterations in RV function. Thus, this trial will provide further insights into the role of IL-6 in PAH pathogenesis and could shed more light on the effects of IL-6 on RV dysfunction in PAH.
Limitations
Our study was limited by its retrospective nature and single-center design as well as the small number of patients. However, this is a hypothesis-generating study and a larger, confirmatory investigation is still needed. We were also limited by using both incident and prevalent cases, and thus the different treatment approaches implemented in our patients may have altered RV function.
Conclusions
We have shown that elevated serum IL-6 levels in patients with PAH are associated with RV dysfunction independent of the burden of pulmonary vascular disease. The association between serum IL-6 levels and RV dysfunction may explain the increased mortality among PAH patients with elevated serum IL-6 levels compared to those with normal serum IL-6 levels.
Supplementary Material
Acknowledgments
T.T. received a modest honorarium from Gilead Sciences and Actelion for advisory board participation. This study was supported by the National Institutes of Health (NIH RO1 HL113003 and ROI HL071115, and NIH F32 HL129554 to S.A.), the Canada Foundation for Innovation (229252 and 33012 to S.A.), a Tier 1 Canada Research Chair in Mitochondrial Dynamics and Translational Medicine (950-229252), the William J Henderson Foundation and the American Heart Association (Scientist Development Grant 15SDG25560048 to T.T.).
Footnotes
Disclosure statement
The remaining authors have no conflicts of interest to disclose.
Supplementary Materials associated with this article can be found in the online version at www.jhltonline.org.
References
- 1.Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–75. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–31. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 3.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–7. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 4.Bhargava A, Kumar A, Yuan N, et al. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis. 1999;1:126–32. [PubMed] [Google Scholar]
- 5.Miyata M, Sakuma F, Yoshimura A, et al. Pulmonary hypertension in rats. 2. Role of interleukin-6. Int Arch Allergy Immunol. 1995;108:287–91. doi: 10.1159/000237166. [DOI] [PubMed] [Google Scholar]
- 6.Matura LA, Ventetuolo CE, Palevsky HI, et al. Interleukin-6 and tumor necrosis factor-α are associated with quality of life-related symptoms in pulmonary arterial hypertension. Ann Am Thorac Soc. 2015;12:370–5. doi: 10.1513/AnnalsATS.201410-463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heresi GA, Aytekin M, Hammel JP, et al. Plasma interleukin-6 adds prognostic information in pulmonary arterial hypertension. Eur Respir J. 2014;43:912–4. doi: 10.1183/09031936.00164713. [DOI] [PubMed] [Google Scholar]
- 8.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–44. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savale L, Tu L, Rideau D, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagen M, Fagan K, Steudel W, et al. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1473–9. doi: 10.1152/ajplung.00197.2006. [DOI] [PubMed] [Google Scholar]
- 11.Brock M, Trenkmann M, Gay RE, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104:1184–91. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 12.Finkel MS, Oddis CV, Jacob TD, et al. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–9. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 13.Kinugawa K, Takahashi T, Kohmoto O, et al. Nitric oxide-mediated effects of interleukin-6 on [Ca2+]i and cell contraction in cultured chick ventricular myocytes. Circ Res. 1994;75:285–95. doi: 10.1161/01.res.75.2.285. [DOI] [PubMed] [Google Scholar]
- 14.Villegas S, Villarreal FJ, Dillmann WH. Leukemia inhibitory factor and interleukin-6 downregulate sarcoplasmic reticulum Ca2+ ATPase (SERCA2) in cardiac myocytes. Basic Res Cardiol. 2000:47–54. doi: 10.1007/s003950050007. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Cheng G, Jin R, et al. Deletion of interleukin-6 attenuates pressure overload-induced left ventricular hypertrophy and dysfunction. Circ Res. 2016;118:1918–29. doi: 10.1161/CIRCRESAHA.116.308688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Wang JH, Zhang YY, et al. Deletion of interleukin-6 alleviated interstitial fibrosis in streptozotocin-induced diabetic cardiomyopathy of mice through affecting TGFβ1 and miR-29 pathways. Sci Rep. 2016;6:23010. doi: 10.1038/srep23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan AT, Yan RT, Cushman M, et al. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2010;31:875–82. doi: 10.1093/eurheartj/ehp454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torre-Amione G, Kapadia S, Benedict C, et al. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–6. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 19.Plenz G, Song ZF, Tjan TD, et al. Activation of the cardiac interleukin-6 system in advanced heart failure. Eur J Heart Fail. 2001;3:415–21. doi: 10.1016/s1388-9842(01)00137-4. [DOI] [PubMed] [Google Scholar]
- 20.Harhay MO, Tracy RP, Bagiella E, et al. Relationship of CRP, IL-6, and fibrinogen with right ventricular structure and function: the MESA-Right Ventricle Study. Int J Cardiol. 2013;168:3818–24. doi: 10.1016/j.ijcard.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thenappan T, Shah SJ, Rich S, et al. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–87. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 23.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–72. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 24.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 25.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–9. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 26.Prins KW, Weir EK, Archer SL, et al. Pulmonary pulse wave transit time is associated with right ventricular-pulmonary artery coupling in pulmonary arterial hypertension. Pulm Circ. 2016;6:576–85. doi: 10.1086/688879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. doi: 10.1093/cvr/4.1.23. [DOI] [PubMed] [Google Scholar]
- 28.Mahapatra S, Nishimura RA, Sorajja P, et al. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 29.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Steiner J, Wu WC, Jankowich M, et al. Echocardiographic predictors of mortality in patients with pulmonary hypertension and cardiopulmonary comorbidities. PLoS One. 2015;10:e0119277. doi: 10.1371/journal.pone.0119277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melenovsky V, Hwang SJ, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–62. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen MJ, Hwang SJ, Kane GC, et al. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:542–50. doi: 10.1161/CIRCHEARTFAILURE.114.002114. [DOI] [PubMed] [Google Scholar]
- 33.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–81. doi: 10.1152/ajpheart.00157.2013. [DOI] [PubMed] [Google Scholar]
- 34.Guazzi M, Naeije R, Arena R, et al. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest. 2015;148:226–34. doi: 10.1378/chest.14-2065. [DOI] [PubMed] [Google Scholar]
- 35.Guazzi M, Dixon D, Labate V, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. doi: 10.1016/j.jcmg.2016.12.024. in press. [DOI] [PubMed] [Google Scholar]
- 36.Ghio S, Klersy C, Magrini G, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140:272–8. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 37.Nagendran J, Archer SL, Soliman D, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–48. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 38.Rain S, Bos Dda S, Handoko ML, et al. Protein changes contributing right ventricular cardiomyocyte diastolic dysfunction in pulmonary arterial hypertension. J Am Heart Assoc. 2014;3:e000716. doi: 10.1161/JAHA.113.000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon MR, Aziz A, Lee AM, et al. Differential calcium handling in two canine models of right ventricular pressure overload. J Surg Res. 2012;178:554–62. doi: 10.1016/j.jss.2012.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kögler H, Hartmann O, Leineweber K, et al. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ Res. 2003;93:230–7. doi: 10.1161/01.RES.0000085042.89656.C7. [DOI] [PubMed] [Google Scholar]
- 41.Xie YP, Chen B, Sanders P, et al. Sildenafil prevents and reverses transverse-tubule remodeling and Ca(2+) handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension. 2012;59:355–62. doi: 10.1161/HYPERTENSIONAHA.111.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550–6. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]
- 43.Plenz G, Eschert H, Erren M, et al. The interleukin-6/interleukin-6-receptor system is activated in donor hearts. J Am Coll Cardiol. 2002;39:1508–12. doi: 10.1016/s0735-1097(02)01791-6. [DOI] [PubMed] [Google Scholar]
- 44.Yamauchi-Takihara K, Ihara Y, Ogata A, et al. Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation. 1995;91:1520–4. doi: 10.1161/01.cir.91.5.1520. [DOI] [PubMed] [Google Scholar]
- 45.West J, Fagan K, Steudel W, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–14. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.