Abstract
Motor behaviors are precisely controlled by the integration of sensory and motor systems in the central nervous system (CNS). Proprioceptive sensory neurons, key components of the sensory system, are located in the dorsal root ganglia and project axons both centrally to the spinal cord and peripherally to muscles and tendons, communicating peripheral information about the body to the CNS. Changes in muscle length detected by muscle spindles, and tension variations in tendons conveyed by golgi tendon organs, are communicated to the CNS through group Ia/II, and Ib proprioceptive sensory afferents, respectively. Group Ib proprioceptive sensory neurons connect with motor neurons indirectly via spinal interneurons, whereas group Ia/II axons form both direct (monosynaptic) and indirect connections with motor neurons. Although monosynaptic sensory-motor circuits between spindle proprioceptive sensory neurons and motor neurons have been extensively studied since 1950s, the molecular mechanisms underlying their formation and upkeep have only recently begun to be understood. We will discuss our current understanding of the molecular foundation of monosynaptic circuit development and maintenance involving proprioceptive sensory neurons and motor neurons in the mammalian spinal cord.
Introduction
The activity of motor neurons, which deliver the final instructions to muscles for all motor behaviors, is coordinated, in part, by proprioceptive sensory neurons, which convey peripheral information about muscle contractions to motor neurons as a feedback system to generate appropriate motor responses. Proprioceptive sensory neurons, whose cell bodies are located in the dorsal root ganglia (DRGs), are subdivided into large diameter neurons (groups Ia and Ib) and medium diameter neurons (group II) (Lloyd, 1943; Brown, 1981) (Figure 1). Both group Ia and II proprioceptive sensory neurons detect changes in muscle length and project axons peripherally to muscle spindles, and centrally to the intermediate and ventral spinal cord where they form both direct or indirect connections with motor neurons. In contrast, group Ib proprioceptive sensory neurons, which detect changes in muscle tension, send axons peripherally to golgi tendon organs, and centrally to the intermediate spinal cord where they form only indirect connections with motor neurons via interneurons. We will focus on proprioceptive sensory neurons in DRGs in this review, however, it is worthy to note that cell bodies of a small subset of proprioceptive sensory neurons are also located in the brainstem conveying information to the CNS about jaw and facial muscle movements (Zhang et al., 2012; Stanek et al., 2014).
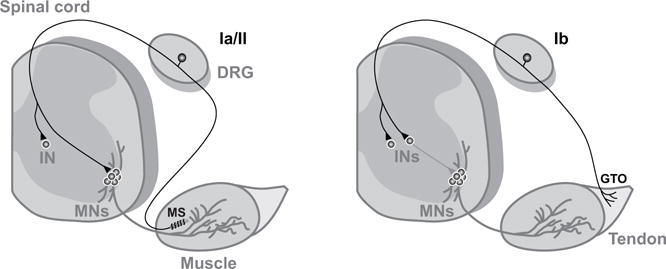
Figure 1.
Schematic drawing of Ia/II and Ib proprioceptive sensory neurons
Ia/II proprioceptive sensory neurons (Ia//II) are located in the dorsal root ganglia (DRGs) and project to specific alpha motor neurons (MNs) which project axons back to the same muscle. Ib Proprioceptive sensory neurons (Ib) innervate golgi tendon organs (GTOs). Group Ia and II proprioceptive sensory neurons form monosynaptic connections with motor neurons, whereas all (Ia, Ib and II) proprioceptive connect with spinal cord interneurons (INs), and then MNs.
In mice, proprioceptive sensory afferents reach the spinal cord and muscles around embryonic day 10.5 (E10.5) (Ozaki and Snider, 1997; Huettl et al., 2011). Then, axons penetrate spinal cord at dorsal root entry zone around E12.5 and form monosynaptic connections with motor neurons by E17 (Mears and Frank, 1997; Ozaki and Snider, 1997). Group Ia/II proprioceptive sensory afferents form monosynaptic connections with specific motor neuron targets that innervate either the same muscle or related muscles that are synergistic, but not antagonistic muscles. Mice lacking proprioceptive sensory neurons or muscle spindles show defects in appropriate muscle activation in vivo (Akay et al., 2014; Takeoka et al., 2014). The simplicity of monosynaptic sensory-motor circuits make them an effective model system to study the molecular mechanisms underlying the different steps of neural circuit formation such as axonal elongation, synaptic specificity, synapse formation, and circuit maintenance. In contrast, much less is known about the formation of the more complex connections between proprioceptive sensory neurons and interneurons in the spinal cord, and we will thus be centering this review on the development of monosynaptic sensory-motor circuits, particularly the establishment of proprioceptive sensory neuron connections with peripheral tissues and the spinal cord, and mechanisms that preserve and maintain these circuits beyond the late embryonic stages.
(1) Interactions between proprioceptive sensory axons and target muscles
Monosynaptic sensory-motor circuit development begins with the proper innervation of limb muscles by proprioceptive sensory afferents. Recent studies have shown that signaling by target limb mesenchyme plays an important role in establishing proprioceptor identity, while molecular communication from proprioceptive sensory afferents directly impacts muscle spindle development.
Poliak and colleagues found that proprioceptive sensory neurons projecting to dorsal limbs and ventral limbs had different molecular identities which were alterable by changing the dorsoventral character of the target limb mesenchyme (Poliak et al., 2016). Dorsal limb-projecting proprioceptors expressed cadherin 13 (Cdh13) and semaphorin 5a (Sema5a), while cartilage acidic protein 1 (Crtac1) was uniquely expressed by ventral limb-projecting proprioceptive sensory neurons. When the limb mesenchyme’s dorsal and ventral character was changed, the expression profiles of Cdh13, Sema5a, and Crtac1 also changed, suggesting that signals from the limb mesenchyme help establish the molecular identities of proprioceptive sensory neurons (Poliak et al., 2016).
Studies in mice revealed that proper muscle spindle development is essential for establishment of monosynaptic sensory-motor circuits. Group Ia/II proprioceptive axons innervate the intrafusal muscle fibers of muscle spindles, and transduce changes in intrafusal fiber length into electrical signals (Maier, 1997; Proske and Gandevia, 2012; Bewick and Banks, 2015). Muscle spindle sensitivity is regulated by these gamma motor neurons which do not receive direct connections from proprioceptive sensory neurons (Hunt and Kuffler, 1951; Eccles et al., 1957; Zampieri et al., 2014).
The transcription factor early growth response protein 3 (Egr3) is expressed in both intrafusal fibers and proprioceptive sensory neurons, and regulates muscle spindle development, which impacts their innervation by proprioceptive axons. Egr3 mutant mice show reduced spindle fiber numbers and exhibit gait ataxia (Tourtellotte and Milbrandt, 1998; Akay et al., 2014; Takeoka et al., 2014; Oliveira Fernandes and Tourtellotte, 2015). Targeted deletion of Egr3 in sensory neurons does not cause muscle spindle defects, therefore Egr3 from the intrafusal fibers seems to be the key contributor to proper muscle spindle development (Oliveira Fernandes and Tourtellotte, 2015).
Innervation by proprioceptive sensory neurons also affects muscle spindle development. This influence is regulated by signaling between a splice variant of Neuregulin1 (Ig-Nrg1) containing an Ig domain that is selectively expressed by proprioceptive sensory neurons in the DRG, and its receptor, ErbB2, which is expressed by intrafusal fibers (Andrechek et al., 2002; Hippenmeyer et al., 2002; Leu et al., 2003; Cheret et al., 2013). Deletion of either Nrg1 or ErbB2 in mice causes reduced numbers of muscle spindles and limb coordination deficits (Andrechek et al., 2002; Hippenmeyer et al., 2002; Leu et al., 2003; Shneider et al., 2009; Cheret et al., 2013). Nrg1 has been shown to be a substrate for the membrane-bound protease, Beta-secretase 1 (Bace1), which is expressed in nervous tissues. Bace1 is an amyloid precursor protein (APP) cleavage enzyme that causes accumulation of amyloid β peptide, a hallmark of Alzheimer’s disease (Vassar et al., 1999). Deletion of Bace1 similarly causes a reduction in the number of muscle spindles (Cheret et al., 2013). In a transgenic mouse line that overexpresses Ig-Nrg1, muscle spindle numbers were increased by 50%. Bace1-deletion in these mice eliminates only the augmentation in spindle numbers, indicating that Bace1 cleavage of Nrg1 may be necessary for appropriate muscle spindle formation (Cheret et al., 2013). Interestingly, pharmacological inhibition of Bace1 or conditional deletion of Nrg1 in adult wild-type mice results in defects in muscle spindle numbers and locomotor deficits, suggesting that Bace1 activity is also required for muscle spindle maintenance (Cheret et al., 2013).
Taken together, proprioceptive innervation and the limb mesenchyme and muscle spindles have reciprocal influences during development. This bi-directional signaling between developing proprioceptive sensory neurons and peripheral tissues appear to be integral to the development of appropriate sensory-motor circuits.
(2) Molecular mechanisms directing central axon projections of proprioceptive sensory neurons
Proprioceptive sensory neurons project axons centrally to the spinal cord and invade the grey matter around E12.5 in mice (Ozaki and Snider, 1997). The axons avoid the dorsal spinal cord but project to the intermediate and ventral spinal cord. In contrast, cutaneous sensory neurons in the DRGs project axons directly to the dorsal spinal cord. Recent studies have shown that an array of different molecules—neurotrophic factors, transcription factors, protein kinases, and axon guidance molecules—all influence the central projections of proprioceptive sensory neurons within the spinal cord.
The growth factor neurotrophin 3 (NT3) is an essential trophic factor for proprioceptive sensory neurons, and its receptor TrkC is expressed specifically by proprioceptive sensory neurons in DRGs (Marmigere and Ernfors, 2007; Lallemend and Ernfors, 2012). Interestingly, previous studies show that NT3-TrkC signaling also plays a critical role in targeting axonal projections of proprioceptive sensory neurons in the spinal cord (Ernfors et al., 1994; Klein et al., 1994; Patel et al., 2003). NT3 or TrkC deletion in mice resulted in complete loss of all proprioceptive sensory neurons, rendering it impossible to examine how NT3-TrkC signaling affected proprioceptive axonal projections. However, in mice lacking both NT3 and BCL2-associated protein (Bax) deletion, which prevents cell death in DRG neurons, proprioceptive axons entered the spinal cord but terminated in the intermediate zone (Patel et al., 2003). The mechanism by which NT3-TrkC signaling controls proprioceptive axon projections is not understood, however one pathway currently being explored involves the transcription factor, ETS variant 1 (Etv1, also known as Er81), whose expression is induced by NT3-TrkC signaling. In NT3 mutants, expression of Etv1 is reduced in the DRGs, whereas ectopic NT3 in muscles in the transgenic mice induces Etv1 expression (Patel et al., 2003). Interestingly, overexpression of NT3 largely rescues the defects in proprioceptive axonal projections caused by Etv1 deletion. In Etv1 mutant mice, proprioceptive axons aberrantly terminate in the intermediate spinal cord, similar to what was observed in NT3/Bax mutant mice, although the Etv1 mutant phenotype appears to be weaker than that of NT3/Bax double mutant mice since some proprioceptive sensory neurons project axons to the ventral spinal cord, suggesting that NT3-TrkC signaling regulates proprioceptive axon projections in an Etv1-dependent and independent manner (Arber et al., 2000; Patel et al., 2003; Li et al., 2006; de Nooij et al., 2013).
Another downstream target of the NT3-TrkC signaling cascade, the synapses of amphids defective (SAD) kinases (SAD-A and –B in mammals), have also been shown to influence proprioceptive axonal projections. In SAD-A/B double mutant mice, proprioceptive axons stop in the intermediate zone similar to what was observed in NT3/Bax and Etv1 mutant mice (Lilley et al., 2013). SAD kinases are regulated positively by the NT3-TrkC signaling cascade through two mechanisms. First, NT3 increases protein levels of SAD kinases post-translationally through the Raf/MEK/ERK pathway, and second, NT3 upregulates SAD kinase activity via inactivation of the inhibitory c-terminal domain of SAD kinases (Lilley et al., 2013). Although it is unknown whether SAD kinases induce Etv1 expression in proprioceptive sensory neurons, the similarities in mutant phenotypes suggest that NT3-TrkC signaling may exert its effects on proprioceptive axonal projections through a pathway involving both SAD kinases and Etv1.
In addition to Etv1, another transcription factor, Runt-related transcription factor 3 (Runx3), is also essential for proprioceptive sensory neuron development (Inoue et al., 2002; Levanon et al., 2002; Chen et al., 2006; Kramer et al., 2006; Nakamura et al., 2008; Appel et al., 2016). In Runx3 mutant mice, proprioceptive sensory afferents failed to reach the ventral spinal cord (Inoue et al., 2002). Neuronal survival was unaffected in these mice (Inoue et al., 2002). A separate analysis of Runx3 mutant mice on a different genetic background, showed that Runx3 is critical for proprioceptive sensory neuron survival (Levanon et al., 2002). In chicks, however, loss- and gain-of-function experiments showed that Runx3 determines the dorsoventral termination points of both proprioceptive and cutaneous sensory neurons without affecting neuronal survival (Chen et al., 2006). Thus, the Runx3 transcription factor regulates survival and axonal projections of proprioceptive sensory neurons.
As mentioned earlier, cutaneous sensory neurons extend axons into the dorsal region of the spinal cord, whereas proprioceptive sensory axons avoid the dorsal area and project to the intermediate and ventral spinal cord regions. Recently, we discovered that repulsive signaling between the axon guidance molecule, semaphorin 6D (Sema6D), and its receptor, plexin A1 (PlexA1) repel axons of proprioceptive sensory neurons away from the dorsal spinal cord (Yoshida et al., 2006; Leslie et al., 2011). Knocking out Sema6D or PlexA1 results in proprioceptive axons invading the dorsal spinal cord region (Yoshida et al., 2006; Leslie et al., 2011). These aberrant proprioceptive axon shafts introduce ectopic oligodendrocytes into the dorsal area, inhibiting synapse formation of cutaneous sensory neurons with spinal interneurons (Yoshida et al., 2006; Leslie et al., 2011). Despite these abnormalities, PlexA1 mutant mice do not show obvious defects in monosynaptic sensory-motor connections by electrophysiological assays (Leslie et al., 2011). Thus Sema6D-PlexA1 signaling in mice does not appear to be involved in sensory-motor synaptogenesis but is crucial for proprioceptive axon shaft positioning in the spinal cord which is required for proper neural circuit formation (Yoshida, 2012). These findings also suggest that oligodendrocytes may be involved in shaping synapse formation in the central nervous system in wild-type mice.
(3) Synaptic specificity and synapse formation of monosynaptic sensory-motor connections during development
It has long been established that Ia proprioceptive afferents form specific monosynaptic connections with particular motor neuron pools (Eccles et al., 1957). Therefore, these circuits have become model systems for understanding the molecular mechanisms underlying synaptic specificity.
Proprioceptive sensory afferents form monosynaptic connections with specific motor neuron targets that innervate either the same muscle (homonymous connections) or related muscles (heteronymous connections). In mice, monosynaptic connections are formed earlier than E17 and were believed to be established independent of neuronal activity (Mendelson and Frank, 1991; Mears and Frank, 1997). However, a recent study showed that heteronymous monosynaptic sensory-motor connections for the related muscle pair, tibialis anterior (TA, ankle flexor muscle) and extensor digitorum longus (EDL, toe extensor muscle), are coordinated through a sensory neuron activity-dependent process (Mendelsohn et al., 2015). In early postnatal mice, TA sensory neurons have weak heteronymous monosynaptic connections with EDL motor neurons and strong homonymous monosynaptic connections with TA motor neurons. Blocking proprioceptive sensory neuron activity enhances the number and density of heteronymous connections without affecting homonymous connections (Mendelsohn et al., 2015). These data suggests that heteronymous (but not homonymous) connections are coordinated by sensory inputs in an activity-dependent manner.
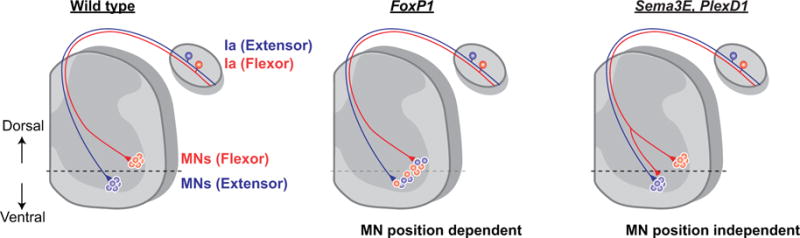
A recent study revealed that the location of motor neurons along the dorsoventral axis plays a key role in establishing monosynaptic sensory-motor specificity (Surmeli et al., 2011; Figure 2). Within the ventral spinal cord, each motor neuron pool is located in stereotypic dorsoventral and mediolateral positions which are determined, at least in part, by the transcription factor, Forkhead box protein P1 (FoxP1) (Dasen et al., 2008; Rousso et al., 2008; Surmeli et al., 2011). In Foxp1 conditional mutant mice, the dorsoventral positions of motor neurons are disrupted causing intermingling of motor neuron pools. Sensory-motor specificity is also perturbed (Surmeli et al., 2011). Interestingly, the sensory axons innervating each muscle in FoxP1 mutants preferentially target motor neuron pools occupying the wild-type dorsoventral positions in the ventral spinal cord, rather than following specific motor neurons to their new, altered locations (Surmeli et al., 2011).
Figure 2.
Molecular mechanisms underlying the formation of monosynaptic sensory-motor connections
In wild type mice (left), flexor Ia proprioceptive sensory (red) and extensor Ia proprioceptive sensory neurons (blue) connect to flexor motor neurons (MNs, red) and extensor MNs (blue), respectively. Sensory-motor connection specificity is determined by MN position-dependent (middle) and MN position-independent mechanisms (right). Abnormal MN positioning causes inappropriate sensory-motor connections in FoxP1 mutants (middle). In Sema3e and PlexD1 mutants (right), the absence of repulsion signaling causes inappropriate connections without MN position defects.
Sensory-motor specificity is also influenced by motor neuron-derived molecules such as the repellant molecule semaphorin 3E (Sema3E) and its receptor plexin D1 (PlexD1) (Figure 2). Sema3E-PlexD1 signaling has been shown to control the specificity of monosynaptic sensory-motor connections (Fukuhara et al., 2013). Motor neurons innervating the gluteus (hip extensor) muscle express Sema3E while PlexD1 is expressed by Ia sensory neurons innervating the hamstrings (hip/knee flexor muscle). The Ia proprioceptive sensory neurons in the hamstrings muscle do not typically form monosynaptic connections with gluteus motor neurons in wild-type mice, however, deletion of either Sema3E or PlexD1 results in direct sensory-motor synapse formation, indicating that under normal circumstances, the Sema3E-PlexD1 repulsion mechanism is necessary to prevent aberrant contacts between Ia proprioceptive sensory neurons and gluteus motor neurons. In Sema3E mutant mice, positiosn and dendritic patterns of motor neurons are not altered, indicating that Sema3E-PlexD1 regulates sensory-motor specificity, independent of motor neuron position (Fukuhara et al., 2013). Sema3E-PlexD1 signaling also precludes the formation of monosynaptic connections by cutaneous maximus (Cm) motor neurons in the cervical spinal cord (Pecho-Vrieseling et al., 2009). Although most proprioceptive sensory neurons form monosynaptic connections with motor neurons, those innervating the Cm muscle do not synapse with Cm motor neurons (Vrieseling and Arber, 2006; Pecho-Vrieseling et al., 2009) due likely to Sema3E being expressed in Cm motor neurons and PlexD1 being expressed by Cm proprioceptive sensory neurons (Pecho-Vrieseling et al., 2009). Knocking out either Sema3E or PlexD1 in mice results in monosynaptic connections forming between Cm proprioceptors and Cm motor neurons (Pecho-Vrieseling et al., 2009). Thus, both at cervical and lumbar levels of the spinal cord, Sema3E-PlxnD1 signaling prevents atypical sensory-motor connections.
Considering that there are over 50 types of hindlimb motor neuron pools and individual motor neuron pool has own specific inputs from particular proprioceptive sensory neurons, a wide array of molecules likely aid in the establishment of monosynaptic sensory-motor circuits (Vanderhorst and Holstege, 1997). For example, members of the cadherin superfamily of adhesion molecules are required for several types of neuronal connections (Takeichi, 2007). In the spinal cord, different type II-cadherin family genes are expressed by different subsets of motor neurons that control a variety of muscles (Price et al., 2002). Another branch of the cadherin superfamily, protocadherins (Pcdhs), are known to be involved in dendritic self-avoidance, and thus, are also good candidates for controlling sensory-motor specificity (Lefebvre et al., 2012; Kostadinov and Sanes, 2015). Since Pcdh mutant mice show upregulation of Ia afferent terminal density, Pcdhs may suppress specific proprioceptive sensory inputs to motor neurons (Prasad and Weiner, 2011; Chen et al., 2012; Hasegawa et al., 2016). Further studies on members of the cadherin superfamily as well as other families of cell surface and signaling molecules will contribute to our understanding of the development of synaptic specificity.
After Ia proprioceptive sensory afferents find their appropriate target motor neurons, the synapses mature. A recent study in mice showed that loss of Cdc42, a small GTPase, caused defects in synapse formation in monosynaptic sensory-motor connections (Imai et al., 2016b). Although Cdc42 in post-synaptic neurons has been shown to control synapse formation in other regions of the CNS, knocking out Cdc42 in motor neurons (the post-synaptic neurons in monosynaptic sensory-motor connections) did not affect sensory-motor synaptogenesis (Irie and Yamaguchi, 2002; Murakoshi et al., 2011; Kim et al., 2014; Hedrick et al., 2016). Cdc42 was deleted from sensory neurons (the pre-synaptic neurons), proprioceptive sensory axons appropriately reached the ventral spinal cord and formed monosynaptic connections with their proper motor neuron targets, however, the numbers of contacts were reduced (Imai et al., 2016b). These findings revealed that Cdc42 in proprioceptive sensory neurons does not affect synaptic specificity, but is required for synapse formation. The downstream targets of Cdc42 that ultimately drive synaptogenesis in proprioceptive sensory neurons are undetermined, but some have proposed that molecules involved in cytoskeletal regulation in dendrites, such as actin, are likely candidates (Hedrick and Yasuda, 2017).
Taken together, proprioceptive sensory afferents find their specific target motor neurons through motor neuron-positioning and cues from guidance molecules. Although thus far, only repulsive molecules have been found that influence monosynaptic sensory-motor specificity, it is plausible that attractant molecules also participate in the process. After proprioceptive afferents form initial contacts with their target motor neurons, the synapse formation, in part, through cytoskeletal regulation by intracellular signaling molecules such as Cdc42.
(4) Maintenance of monosynaptic sensory-motor circuits
After monosynaptic sensory-motor connections are established during embryogenesis, these circuits need to be maintained throughout an animal’s lifetime. Recent studies have begun to address mechanisms underlying the support and maintenance of monosynaptic sensory-motor circuits.
In general, neural circuit maintenance can be disrupted by disease and aging. For example, in amyotrophic lateral sclerosis (ALS), a motor neuron disease whereby accumulation of misfolded superoxide dismutase 1 (SOD1) proteins in motor neurons causes neuronal death, recent studies show that sensory neurons are also affected in human patients with ALS and in an ALS mouse line (Hammad et al., 2007; Pugdahl et al., 2007; Sabado et al., 2014; Vaughan et al., 2015). In the ALS mouse model, accumulation of misfolded SOD1 proteins in proprioceptive sensory neurons and degeneration of spiral structures in proprioceptive sensory endings are observed in 90–120 day-old mice without noticeable impact on neuronal survival (Sabado et al., 2014; Vaughan et al., 2015). In addition to peripheral defects, sensory inputs on motor neurons are reduced (Vaughan et al., 2015). Similar structural degeneration of proprioceptive sensory endings occurs during the normal aging process in wild-type mice (11–15 month-old), suggesting that the maintenance program in sensory neurons can be disrupted similarly by disease and aging (Vaughan et al., 2016). Spinal muscular atrophy (SMA) is another disease affecting neurons. A recent study demonstrated that monosynaptic sensory-motor circuits are not maintained in a model SMA mouse line (Mentis et al., 2011). In SMA mice, sensory inputs on motor neurons seem to be normally formed at P0, but their connections are decreased by P4. Additionally, reduction of sensory inputs seems to cause motor neuron dysfunction, suggesting that proprioceptive sensory neurons play a critical role in protecting motor neurons against the cell death that is a hallmark of SMA disease (Mentis et al., 2011; Fletcher et al., 2017).
In addition to disease and aging, sensory-motor maintenance appears to be influenced by the extracellular molecule NT3. Muscle spindle-specific NT3 mutant mice as well as muscle spindle defect mice (Egr3 and Erbb2 mutants) show reduced strength in monosynaptic sensory-motor connections compared to control mice but only in later postnatal stages (Chen et al., 2002; Shneider et al., 2009). This suggests that spindle–derived NT3 is important for the maturation and/or maintenance of sensory-motor connections rather than their initiation or specification. Therefore, these studies indicate that muscle spindles and/or spindle-derived factors such as NT3 may play prominent roles in regulating sensory-motor strength and maintenance.
Recent studies have revealed a molecular mechanism underlying the maintenance of monosynaptic sensory-motor circuits in wild-type mice (Imai et al., 2016a; O’Toole et al., 2017). Loss of Dicer, an RNase essential for processing microRNAs (miRNAs), in proprioceptive sensory neurons causes sensory ataxia (Imai et al., 2016a; O’Toole et al., 2017). Proprioceptive sensory neuron-specific Dicer deletion in mice does not affect specificity or formation of monosynaptic sensory-motor connections in early postnatal mice, however, after postnatal day 21, proprioceptive sensory neurons lose identity markers such as central and peripheral projections, and typical gene expression patterns, suggesting that Dicer is essential for maintenance rather than initiation of synaptic contacts (Imai et al., 2016a; O’Toole et al., 2017). Indeed, in these mutant mice, some mature miRNAs are downregulated and entire mRNA expression profiles are changed, indicating that Dicer-mediated miRNA maturation helps to maintain proprioceptor identities as defined by transcription factors, signaling molecules, channels and other cellular proteins (Imai et al., 2016a; O’Toole et al., 2017). For example, miRNA mir-127 is highly expressed in all of DRG neurons and contains target sequences for F-box only protein2 (Fbxo2) which negatively regulates synapse formation and/or maintenance in hippocampus, suggesting that mir-127 mediated Fbxo2 downregulation could be involved in sensory-motor maintenance (Atkin et al., 2015; Imai et al., 2016a). Future studies will reveal roles of miRNAs and miRNA-related gene regulation on the maintenance of these and other neural circuits, and their possible functions in disease and aging.
Summary and future directions
In this article, we reviewed the recent molecular advancements in the field of sensory-motor circuit development and maintenance. Contemporary studies have revealed how proprioceptive sensory afferents project axons to the spinal cord, how they form appropriate connections with particular motor neuron targets, how sensory-motor synapses mature, and how monosynaptic sensory-motor circuits are maintained in mice. However, many questions still remain. For example, what drives the different proprioceptive sensory afferents to either synapse directly with motor neurons (in the case of group Ia/II proprioceptive sensory neurons) or synapse on interneurons that then connect with motor neurons (group Ib proprioceptors). Although several proprioceptor-specific molecules such as TrkC, Etv1, Parvalbumin and Runx3 have been shown to be expressed in all proprioceptive sensory neurons, unique molecular markers for different proprioceptor subtypes have yet to be identified (de Nooij et al., 2013; Sonner et al., 2017). Single-cell gene expression studies (Chiu et al., 2014; Usoskin et al., 2015) paired with electrophysiological subtyping experiments (Vincent et al., 2017) could identify genes specific to different classes of proprioceptors. Also worthy of note - proprioceptive sensory neurons at cervical levels project additional axons into the external cuneate nucleus (ECN) in the medulla (Niu et al., 2013). Functional analysis of those axons will be another interesting avenue of exploration. Finally, compared to monosynaptic sensory-motor connections, the molecular mechanisms underlying synaptogenesis between proprioceptive sensory neurons and spinal interneurons in disynaptic connections remain virtually unknown. As recent studies uncover more about the molecular identities of different interneuron subtypes (Goulding, 2009; Bikoff et al., 2016; Gabitto et al., 2016; Kiehn, 2016), classification of each subtype and subsequent mapping of proprioceptor-interneuron circuits will help us to better understand the function and development of disynaptic sensory-motor connections within the spinal cord (Bikoff et al., 2016; Gabitto et al., 2016).
Acknowledgments
We thank to D. Ladle (Wright State University) for providing comments on the manuscript.
Funded by National Institute of Neurological Disorders and Stroke: RO1NS093002 and RO1NS100772
References
- Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci U S A. 2014;111:16877–16882. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrechek ER, Hardy WR, Girgis-Gabardo AA, Perry RL, Butler R, Graham FL, Kahn RC, Rudnicki MA, Muller WJ. ErbB2 is required for muscle spindle and myoblast cell survival. Mol Cell Biol. 2002;22:4714–4722. doi: 10.1128/MCB.22.13.4714-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel E, Weissmann S, Salzberg Y, Orlovsky K, Negreanu V, Tsoory M, Raanan C, Feldmesser E, Bernstein Y, Wolstein O, Levanon D, Groner Y. An ensemble of regulatory elements controls Runx3 spatiotemporal expression in subsets of dorsal root ganglia proprioceptive neurons. Genes Dev. 2016;30:2607–2622. doi: 10.1101/gad.291484.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Atkin G, Moore S, Lu Y, Nelson RF, Tipper N, Rajpal G, Hunt J, Tennant W, Hell JW, Murphy GG, Paulson H. Loss of F-box only protein 2 (Fbxo2) disrupts levels and localization of select NMDA receptor subunits, and promotes aberrant synaptic connectivity. J Neurosci. 2015;35:6165–6178. doi: 10.1523/JNEUROSCI.3013-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GS, Banks RW. Mechanotransduction in the muscle spindle. Pflugers Arch. 2015;467:175–190. doi: 10.1007/s00424-014-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikoff JB, Gabitto MI, Rivard AF, Drobac E, Machado TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ, Mentis GZ, Jessell TM. Spinal Inhibitory Interneuron Diversity Delineates Variant Motor Microcircuits. Cell. 2016;165:207–219. doi: 10.1016/j.cell.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG. Organization in the Spinal Cord. New York: Springer; 1981. [Google Scholar]
- Chen AI, de Nooij JC, Jessell TM. Graded activity of transcription factor Runx3 specifies the laminar termination pattern of sensory axons in the developing spinal cord. Neuron. 2006;49:395–408. doi: 10.1016/j.neuron.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Chen HH, Tourtellotte WG, Frank E. Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci. 2002;22:3512–3519. doi: 10.1523/JNEUROSCI.22-09-03512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WV, Alvarez FJ, Lefebvre JL, Friedman B, Nwakeze C, Geiman E, Smith C, Thu CA, Tapia JC, Tasic B, Sanes JR, Maniatis T. Functional significance of isoform diversification in the protocadherin gamma gene cluster. Neuron. 2012;75:402–409. doi: 10.1016/j.neuron.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheret C, Willem M, Fricker FR, Wende H, Wulf-Goldenberg A, Tahirovic S, Nave KA, Saftig P, Haass C, Garratt AN, Bennett DL, Birchmeier C. Bace1 and Neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO J. 2013;32:2015–2028. doi: 10.1038/emboj.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, Lou S, Bryman GS, Roberson DP, Ghasemlou N, Piccoli C, Ahat E, Wang V, Cobos EJ, Stucky CL, Ma Q, Liberles SD, Woolf CJ. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife. 2014;3 doi: 10.7554/eLife.04660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Doobar S, Jessell TM. Etv1 inactivation reveals proprioceptor subclasses that reflect the level of NT3 expression in muscle targets. Neuron. 2013;77:1055–1068. doi: 10.1016/j.neuron.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Fletcher EV, Simon CM, Pagiazitis JG, Chalif JI, Vukojicic A, Drobac E, Wang X, Mentis GZ. Reduced sensory synaptic excitation impairs motor neuron function via Kv2.1 in spinal muscular atrophy. Nat Neurosci. 2017;20:905–916. doi: 10.1038/nn.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara K, Imai F, Ladle DR, Katayama K, Leslie JR, Arber S, Jessell TM, Yoshida Y. Specificity of monosynaptic sensory-motor connections imposed by repellent Sema3E-PlexinD1 signaling. Cell Rep. 2013;5:748–758. doi: 10.1016/j.celrep.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabitto MI, Pakman A, Bikoff JB, Abbott LF, Jessell TM, Paninski L. Bayesian Sparse Regression Analysis Documents the Diversity of Spinal Inhibitory Interneurons. Cell. 2016;165:220–233. doi: 10.1016/j.cell.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad M, Silva A, Glass J, Sladky JT, Benatar M. Clinical, electrophysiologic, and pathologic evidence for sensory abnormalities in ALS. Neurology. 2007;69:2236–2242. doi: 10.1212/01.wnl.0000286948.99150.16. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Kumagai M, Hagihara M, Nishimaru H, Hirano K, Kaneko R, Okayama A, Hirayama T, Sanbo M, Hirabayashi M, Watanabe M, Hirabayashi T, Yagi T. Distinct and Cooperative Functions for the Protocadherin-alpha, -beta and -gamma Clusters in Neuronal Survival and Axon Targeting. Front Mol Neurosci. 2016;9:155. doi: 10.3389/fnmol.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick NG, Harward SC, Hall CE, Murakoshi H, McNamara JO, Yasuda R. Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature. 2016;538:104–108. doi: 10.1038/nature19784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick NG, Yasuda R. Regulation of Rho GTPase proteins during spine structural plasticity for the control of local dendritic plasticity. Curr Opin Neurobiol. 2017;45:193–201. doi: 10.1016/j.conb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- Huettl RE, Soellner H, Bianchi E, Novitch BG, Huber AB. Npn-1 contributes to axon-axon interactions that differentially control sensory and motor innervation of the limb. PLoS Biol. 2011;9:e1001020. doi: 10.1371/journal.pbio.1001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, Kuffler SW. Stretch receptor discharges during muscle contraction. J Physiol. 1951;113:298–315. doi: 10.1113/jphysiol.1951.sp004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai F, Chen X, Weirauch MT, Yoshida Y. Requirement for Dicer in Maintenance of Monosynaptic Sensory-Motor Circuits in the Spinal Cord. Cell Rep. 2016a;17:2163–2172. doi: 10.1016/j.celrep.2016.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai F, Ladle DR, Leslie JR, Duan X, Rizvi TA, Ciraolo GM, Zheng Y, Yoshida Y. Synapse Formation in Monosynaptic Sensory-Motor Connections Is Regulated by Presynaptic Rho GTPase Cdc42. J Neurosci. 2016b;36:5724–5735. doi: 10.1523/JNEUROSCI.2146-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Ozaki S, Shiga T, Ito K, Masuda T, Okado N, Iseda T, Kawaguchi S, Ogawa M, Bae SC, Yamashita N, Itohara S, Kudo N, Ito Y. Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat Neurosci. 2002;5:946–954. doi: 10.1038/nn925. [DOI] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci. 2002;5:1117–1118. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016;17:224–238. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Wang H, Soderling SH, Yasuda R. Loss of Cdc42 leads to defects in synaptic plasticity and remote memory recall. Elife. 2014;3 doi: 10.7554/eLife.02839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Kostadinov D, Sanes JR. Protocadherin-dependent dendritic self-avoidance regulates neural connectivity and circuit function. Elife. 2015;4 doi: 10.7554/eLife.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012;35:373–381. doi: 10.1016/j.tins.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488:517–521. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie JR, Imai F, Fukuhara K, Takegahara N, Rizvi TA, Friedel RH, Wang F, Kumanogoh A, Yoshida Y. Ectopic myelinating oligodendrocytes in the dorsal spinal cord as a consequence of altered semaphorin 6D signaling inhibit synapse formation. Development. 2011;138:4085–4095. doi: 10.1242/dev.066076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu M, Bellmunt E, Schwander M, Farinas I, Brenner HR, Muller U. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 2003;130:2291–2301. doi: 10.1242/dev.00447. [DOI] [PubMed] [Google Scholar]
- Levanon D, Bettoun D, Harris-Cerruti C, Woolf E, Negreanu V, Eilam R, Bernstein Y, Goldenberg D, Xiao C, Fliegauf M, Kremer E, Otto F, Brenner O, Lev-Tov A, Groner Y. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21:3454–3463. doi: 10.1093/emboj/cdf370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Wang Z, Sedy J, Quazi R, Walro JM, Frank E, Kucera J. Neurotrophin-3 ameliorates sensory-motor deficits in Er81-deficient mice. Dev Dyn. 2006;235:3039–3050. doi: 10.1002/dvdy.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley BN, Pan YA, Sanes JR. SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron. 2013;79:39–53. doi: 10.1016/j.neuron.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DPC. Neuron patterns controlling transmission of ipsilateral hind limb reflexes in cat. J Neurophysiol. 1943;6:293–315. [Google Scholar]
- Maier A. Development and regeneration of muscle spindles in mammals and birds. Int J Dev Biol. 1997;41:1–17. [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- Mears SC, Frank E. Formation of specific monosynaptic connections between muscle spindle afferents and motoneurons in the mouse. J Neurosci. 1997;17:3128–3135. doi: 10.1523/JNEUROSCI.17-09-03128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AI, Simon CM, Abbott LF, Mentis GZ, Jessell TM. Activity Regulates the Incidence of Heteronymous Sensory-Motor Connections. Neuron. 2015;87:111–123. doi: 10.1016/j.neuron.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson B, Frank E. Specific monosynaptic sensory-motor connections form in the absence of patterned neural activity and motoneuronal cell death. J Neurosci. 1991;11:1390–1403. doi: 10.1523/JNEUROSCI.11-05-01390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L, Alvarez FJ, Sumner CJ, O’Donovan MJ. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69:453–467. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Senzaki K, Yoshikawa M, Nishimura M, Inoue K, Ito Y, Ozaki S, Shiga T. Dynamic regulation of the expression of neurotrophin receptors by Runx3. Development. 2008;135:1703–1711. doi: 10.1242/dev.015248. [DOI] [PubMed] [Google Scholar]
- Niu J, Ding L, Li JJ, Kim H, Liu J, Li H, Moberly A, Badea TC, Duncan ID, Son YJ, Scherer SS, Luo W. Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J Neurosci. 2013;33:17691–17709. doi: 10.1523/JNEUROSCI.3429-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole SM, Ferrer MM, Mekonnen J, Zhang H, Shima Y, Ladle DR, Nelson SB. Dicer maintains the identity and function of proprioceptive sensory neurons. J Neurophysiol. 2017;117:1057–1069. doi: 10.1152/jn.00763.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira Fernandes M, Tourtellotte WG. Egr3-dependent muscle spindle stretch receptor intrafusal muscle fiber differentiation and fusimotor innervation homeostasis. J Neurosci. 2015;35:5566–5578. doi: 10.1523/JNEUROSCI.0241-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S, Snider WD. Initial trajectories of sensory axons toward laminar targets in the developing mouse spinal cord. J Comp Neurol. 1997;380:215–229. [PubMed] [Google Scholar]
- Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Norovich AL, Yamagata M, Sanes JR, Jessell TM. Muscle-type Identity of Proprioceptors Specified by Spatially Restricted Signals from Limb Mesenchyme. Cell. 2016;164:512–525. doi: 10.1016/j.cell.2015.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad T, Weiner JA. Direct and Indirect Regulation of Spinal Cord Ia Afferent Terminal Formation by the gamma-Protocadherins. Front Mol Neurosci. 2011;4:54. doi: 10.3389/fnmol.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Pugdahl K, Fuglsang-Frederiksen A, de Carvalho M, Johnsen B, Fawcett PR, Labarre-Vila A, Liguori R, Nix WA, Schofield IS. Generalised sensory system abnormalities in amyotrophic lateral sclerosis: a European multicentre study. J Neurol Neurosurg Psychiatry. 2007;78:746–749. doi: 10.1136/jnnp.2006.098533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousso DL, Gaber ZB, Wellik D, Morrisey EE, Novitch BG. Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron. 2008;59:226–240. doi: 10.1016/j.neuron.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabado J, Casanovas A, Tarabal O, Hereu M, Piedrafita L, Caldero J, Esquerda JE. Accumulation of misfolded SOD1 in dorsal root ganglion degenerating proprioceptive sensory neurons of transgenic mice with amyotrophic lateral sclerosis. Biomed Res Int. 2014;2014:852163. doi: 10.1155/2014/852163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider NA, Mentis GZ, Schustak J, O’Donovan MJ. Functionally reduced sensorimotor connections form with normal specificity despite abnormal muscle spindle development: the role of spindle-derived neurotrophin 3. J Neurosci. 2009;29:4719–4735. doi: 10.1523/JNEUROSCI.5790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner MJ, Walters MC, Ladle DR. Analysis of Proprioceptive Sensory Innervation of the Mouse Soleus: A Whole-Mount Muscle Approach. PLoS One. 2017;12:e0170751. doi: 10.1371/journal.pone.0170751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek Et, Cheng S, Takatoh J, Han BX, Wang F. Monosynaptic premotor circuit tracing reveals neural substrates for oro-motor coordination. Elife. 2014;3:e02511. doi: 10.7554/eLife.02511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell. 2014;159:1626–1639. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet. 1998;20:87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vaughan SK, Kemp Z, Hatzipetros T, Vieira F, Valdez G. Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J Comp Neurol. 2015;523:2477–2494. doi: 10.1002/cne.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan SK, Stanley OL, Valdez G. Impact of Aging on Proprioceptive Sensory Neurons and Intrafusal Muscle Fibers in Mice. J Gerontol A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JA, Gabriel HM, Deardorff AS, Nardelli P, Fyffe REW, Burkholder T, Cope TC. Muscle proprioceptors in adult rat: mechanosensory signaling and synapse distribution in spinal cord. J Neurophysiol. 2017;118:2687–2701. doi: 10.1152/jn.00497.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Yoshida Y. Semaphorin signaling in vertebrate neural circuit assembly. Front Mol Neurosci. 2012;5:71. doi: 10.3389/fnmol.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–788. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri N, Jessell TM, Murray AJ. Mapping sensory circuits by anterograde transsynaptic transfer of recombinant rabies virus. Neuron. 2014;81:766–778. doi: 10.1016/j.neuron.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Luo P, Ro JY, Xiong H. Jaw muscle spindle afferents coordinate multiple orofacial motoneurons via common premotor neurons in rats: an electrophysiological and anatomical study. Brain Res. 2012;1489:37–47. doi: 10.1016/j.brainres.2012.10.021. [DOI] [PubMed] [Google Scholar]


