Abstract
Background
Current medical treatments for chemotherapy-induced pain (CIP) are either ineffective or have adverse side effects. Acupuncture may alleviate CIP, but its effectiveness against this condition has not been studied. Paclitaxel causes neuropathic pain in cancer patients.
Methods
We evaluated the effects of electroacupuncture (EA) on paclitaxel-induced CIP in a rat model. Paclitaxel (2 mg/kg) or vehicle was injected (i.p.) on alternate days of 0–6. The resulting pain was treated with 10 Hz/2 mA/0.4 ms pulse EA for 30 min at the equivalent of human acupoint GB30 (Huantiao) once every other day between days 14 and 26. For sham control, EA needles were inserted into GB30 without stimulation. Von Frey filaments with bending forces of 2–8 g and 15 g were used to assess mechanical allodynia and hyperalgesia, respectively, on day 13 and once every other day between 14–26 days and then for 2–3 weeks after EA treatment.
Results
Compared to sham control, EA significantly alleviated paclitaxel-induced mechanical allodynia and hyperalgesia, as shown by less frequent withdrawal responses to the filaments. The alleviation of allodynia/hyperalgesia lasted up to 3 weeks after the EA treatment. EA significantly inhibited phosphorylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) in the spinal cord. KN-93, a selective inhibitor of p-CaMKII, inhibited mechanical allodynia/hyperalgesia and p-CaMKII. 5-HT1A receptor antagonist blocked EA inhibition of allodynia/hyperalgesia and p-CaMKII.
Conclusions
EA activates 5-HT 1A receptors in the spinal cord and inhibits p-CaMKII to alleviate both allodynia and hyperalgesia. The data support acupuncture/EA as a complementary therapy for CIP.
Introduction
Chemotherapy, commonly used as adjuvant therapy or treatment of advanced cancer, is one of the most effective therapies for cancer. Chemotherapy-induced pain (CIP) is a major clinical problem (Banach et al., 2017). It represents the chief dose-limiting side effect of the major classes of anti-neoplastic drugs, such as the taxanes, the vinca alkaloids, and the platinum-based drugs, which are used against all of the most common types of cancer. Paclitaxel, one member of taxanes, is one of the most effectively and commonly used anti-neoplastic drugs in the treatment of tumors. It promotes and hyper-stabilizes intracellular microtubular assembly, thereby causing the cell death by disrupting normal microtubule dynamics required for cell division and vital interphase processes (Rowinsky and Donehower 1995). Paclitaxel-induced neurotoxicity typically presents as sensory neuropathy. It is reported that paclitaxel causes neuropathic symptoms in 50%–100% of all treated patients depending on the doses (Postma et al., 1995). Numbness, tingling and burning pain are the most common complaints. These sensory symptoms usually start symmetrically in the feet and sometimes appear simultaneously in hands and feet. Although most cases resolve within months after paclitaxel treatment is discontinued, sensory abnormalities and pain sometimes become chronic (Rowinsky et al., 1993; Chaudhry et al., 1994; Forsyth et al., 1997; Gordon et al., 1997; Cavaletti and Marmiroli 2004), thus affecting the quality of life.
The main treatment for CIP is pharmacotherapy that includes antidepressants, anticonvulsants, topical analgesics, corticosteroids, non-steroidal anti-Inflammatory drugs and opioids (Armstrong et al., 2005). However, these approaches have had limited success (Cavaletti and Zanna 2002; Openshaw et al., 2004; Hausheer et al., 2006), and have resulted in a number of side effects. For example, tricyclic antidepressants result in sedation and various cardiovascular issues (Hempenstall and Rice 2002). Currently, pharmaceutical therapy has been found not to be completely beneficial in preventing or treating chemotherapy-induced neuropathy (Schloss et al., 2016). Therefore, inadequate control and management of CIP remain serious problems (Armstrong et al., 2005; Deng and Cassileth 2005).
Acupuncture, a traditional therapeutic modality, can potentially be used as an effective complementary treatment for CIP. It has been used in China and other Asian countries for thousands of years to treat pain (Cheng 1999), and has few or no adverse effects (Witt et al., 2009). Clinical study showed that acupuncture and reflexology improved symptoms of chemotherapy-induced neuropathy in breast cancer patients (Ben-Horin et al., 2017). Acupuncture combined with methylcobalamin significantly decreased CIP than methylcobalamin alone (Han et al., 2017). Basic study demonstrated that electroacupuncture (EA) significantly inhibits mechanical allodynia and hyperalgesia in nerve injury-induced neuropathic pain rat models (Dai et al., 2001; Hwang et al., 2002). These studies suggest that EA may alleviate CIP. However, there are few investigations of the efficacy of acupuncture/EA for inhibiting CIP (Sui et al., 2016). In this study, we used an established rat model (Hama and Takamatsu 2016), paclitaxel-induced neuropathic pain, to study EA efficacy on such pain.
Our previous study demonstrated that spinal 5-hydroxytryptamine (5-HT) 1A receptors are involved in EA inhibition of inflammatory pain (Zhang et al., 2012). It is not known whether such receptors are involved in EA alleviation of CIP. Further, 5-HT1A receptor agonist, 8-hydroxy-2(di-n-propylamino) tetralin (8-OH-DPAT), significantly reduces the Thr286 phosphorylation state of Ca2+/calmodulin-dependent protein kinase II (CaMKII)(Cai et al., 2002), and the latter is involved in pain at the spinal cord (Chen et al., 2009). We hypothesized that EA will alleviate CIP through activating spinal 5-HT1A receptors and inhibiting phosphorylation of spinal CaMKII.
2. Material and methods
2.1 Animals
Male Sprague Dawley rats (250–270 g body weight, Harlan) was habituated to the plastic chamber (approximately 5″× 8″×11″) and handled gently for two 30-minutes two days before the baseline behavioral test. We produced the paclitaxel-evoked peripheral neuropathy model by i.p. injecting 2 mg/kg paclitaxel (Taxol®, Bristol–Myers–Squibb) on four alternate days (0, 2, 4, and 6) (Flatters and Bennett 2006). Paclitaxel at 6 mg/ml in Cremophor EL was purchased from Bristol-Myers-Squibb and was diluted to 2 mg/ml with saline. None of the animals treated with paclitaxel have shown such signs of ill-health as alopecia, diarrhea or weight loss, and all have gained weight normally. The control rats were produced by i.p. injection of vehicle, a mixture of 1:2 Cremophor EL/saline, as the above. We performed a power analysis using our preliminary data to determine the necessary number of animals needed to provide an 80% chance to achieve statistical significance (two-tailed alpha = .05) between the EA- or chemical-treated rats and the control rats.
2.2 EA treatment
Acupuncture point GB30 was chosen for the EA treatment. We selected GB30 for three reasons: 1) according to the meridian theory of traditional Chinese medicine, it is an appropriate point for treating lower limb pain (Cheng 1999); 2) this point was successfully used to attenuate complete Freund’s adjuvant-induced inflammatory hyperalgesia in a hindpaw inflammatory pain rat model in our previous studies (Lao et al., 2004); 3) based on our point-specificity study using the hindpaw inflammatory pain rat model (Lao et al., 2004), EA at GB30 produced better anti-hyperalgesia than did EA at acupuncture point Waiguan (TE5) on the forepaw or at two non-specific points: one on the abdomen, 3 mm lateral to the umbilicus, another on the quadriceps at the opposite aspect of GB30. In humans, GB30 is located at the junction of the lateral 1/3 and medial 2/3 of the distance between the greater trochanter and the sacral hiatus. In rats, we used the equivalent anatomical landmarks to locate GB30. To determine acupuncture points in animals, the transposition of an acupuncture point from the known human map to the anatomically comparable position in animals was widely used (Lee and Beitz 1993; Lao et al., 2001; Ma et al., 2005; Zhou et al., 2005) and had been demonstrated to be effective (Ulett et al., 1998; Lao et al., 2001). The animals were gently handled for 30 min each day for 2–3 days and habituated to the acupuncture treatment before the experiment. After cleaning the skin with alcohol swabs, two acupuncture needles (gauge # 32, 0.5 inch in length) were swiftly inserted approximately one-half inch deep into each hind limb of the rat bilaterally at GB30 by one investigator while the other gently held the animal. The needles and the electrodes were stabilized with adhesive tape (Lao et al., 2004; Zhang et al., 2008). The procedure typically lasted less than 20 seconds and caused little distress to the animal. Each rat was neither restrained nor given any anesthetic and was placed under an inverted clear plastic chamber. To avoid the possibility of animal tangling in wires and animal biting wires, the wires were pulled out of the inverted clear plastic chamber through a hole on the bottom of the chamber (Lao et al., 2004). The acupuncture needle handles were soldered in advance to one end of electrode, and another end of electrode was connected to the output channel of Electrostimulator (Electrostimulator 8-C, Pantheon Research Inc., Culver City, CA, USA). Then, EA was delivered by the stimulator via two electrodes at 10 Hz, 2 mA, 0.4 ms pulse width for 30 minutes on seven alternate days (14, 16, 18, 20, 22, 24 and 26) post-paclitaxel. These parameters were used because they produced significant relieve of pain in previous study (Zhang et al., 2007). A symmetrical biphasic wave was delivered to the electrodes so that the electrodes were alternately positive and negative and the bilateral GB30 was stimulated alternately. To minimize discomfort, stimulation intensity was gradually increased over a period of two minutes to 2 mA, which we have found to be the maximum level that can be tolerated by unrestrained rats. Mild muscle twitching was observed. During EA treatment, the animals remained awake and still and gave no observable signs of distress. Regarding sham control, since our point-specificity study demonstrated that electrical stimulation of acupuncture point GB30, but not non-specific points produced significant alleviation of hyperalgesia, the sham control was acupuncture needle insertion into bilateral GB30 but with no electrical stimulation or manual needle manipulation (Lao et al., 2004). In our previous study Sham EA showed little anti-hyperalgesia (Lao et al., 2004). All rats in EA-treatment and control groups were handled identically.
2.3 Behavioral tests
The investigators for behavioral tests were blind to treatment assignments. We used von Frey filaments with bending forces of 2, 4, 8 and 15 g to assess mechanical allodynia/hyperalgesia. Previous study demonstrated that the median mechanical threshold is 15 g (Chaplan et al., 1994), thus the chemotherapy-induced responses to 15 g are described as hyperalgesia (exaggerated pain response to noxious stimulus), while the chemotherapy-induced responses to <15 g are described as allodynia (pain from a normally innocuous stimulus) (Flatters and Bennett 2006). In ascending order of force, each filament was applied five times to the mid-plantar area of each hind paw, avoiding the base of the tori. Each application was held for 5 sec. Withdrawal responses to the von Frey filaments from both hind paws were counted and then expressed as an overall percentage response. For example, if a rat withdrew to 2 out of the total 10 von Frey applications, this was recorded as a 20% overall response to that filament.
2.4 Intrathecal drug delivery
Acute lumbar punctures were performed as we did before (Li et al., 2011). Briefly, injection catheter was a PE10 polyethylene tube (Clay Adams, Franklin Lakes, NJ, USA), which was submerged in 70˚C water, and stretched to about 150% of the original length to reduce its diameter. One end of the catheter was connected with a 29-gauge needle to a 10-cm PE10 tube, which was then connected to a 50-μl glass Hamilton syringe with a PE50 tube. The injection catheter was pre-filled with 5 μl of drug or vehicle and 5 μl of saline separated by a small air bubble. The dorsal pelvic area was shaved and swabbed with 70% alcohol under isoflurane anesthesia. A 21-gauge guide needle was inserted between lumbar vertebrae L5 and L6. The catheter was then inserted into the guide needle and rostrally advanced 4 cm from the tip of the needle into the lumber enlargement confirmed by a tail-flick. The drug, or vehicle, was injected and followed by a saline flush. Five minutes after injection the catheter was withdrawn and the needle was removed from the inter-vertebral space.
2.5 Western blot assay
The lumbar spinal cord was removed from the anesthetized and decapitated rats after finishing the behavioral tests. The dorsal horn was separated from the ventral horn and was used for western blot experiment. The membrane proteins were extracted using a ProteoJET™ Membrane Protein Extraction Kit (Fermentas Life Science) according to the protocol provided by manufacturer. The membrane proteins were fractionated on a 4–15% (w/v) SDS-PAGE and transferred onto a polyvinylidine difluoride (PVDF) membrane. The membrane was incubated overnight at 4°C with rabbit polyclonal antibodies against phospho-CaMKII (Thr286, 1:1000; Cell Signaling Technology) and then with goat anti-rabbit horseradish peroxidase-conjugated IgG (1:3000; Cell Signaling Technology). The immunoreactivity of the proteins on the membrane was visualized using the Super Signal chemiluminescence kit (Pierce). Autoradiograms were analyzed and the intensity of the immunoreactive bands of interest was quantified (NIH Image J). The membranes were then incubated in stripping buffer at room temperature for 15 min and reprobed with rabbit polyclonal antibodies against CaMKII (1:100; Cell Signaling Technology).
2.6 Interpretation and analysis
The average of percentage response of EA or drug treatment and control groups were presented in mean ± S.E.M. and was analyzed with repeated measures ANOVA followed by Bonferroni posttests (Graphpad Prism, La Jolla, CA, USA). The western blot data was analyzed with ANOVA followed by Tukey’s multiple comparisons. P<0.05 will be considered significant.
3. Results
3.1 EA alleviated paclitaxel-induced mechanical allodynia and hyperalgesia
Rats were randomly divided into 4 groups (n=6 per group): 1) paclitaxel + EA, 2) paclitaxel + sham EA control, 3) vehicle control + EA, and 4) vehicle control + sham EA control. EA treatment started on day 14 post-paclitaxel injection when rats showed allodynia/hyperalgesia and continued every other day through day 26. Mechanical allodynia and hyperalgesia were assessed before paclitaxel injection for baseline mechanical sensitivity, on day 13 before EA treatment for confirming the development of allodynia and hyperalgesia, once every other day from day 14 through day 26 and then on days 40 and 47 for evaluating the long term efficacy of EA treatment. When EA was applied, tests were performed 30 min after each EA treatment. Figures 1 shows the effect of EA on mechanical response frequency. Before paclitaxel injection, overall mean baseline responses to 4–15 g filament stimulation were similar in four groups of rats (Fig. 1). Compared to vehicle-injected rats, paclitaxel-injected rats showed significantly higher frequencies of responses to 4–15 g, but not 2 g, filament stimulation on day 13 post-paclitaxel injection, demonstrating that paclitaxel caused mechanical allodynia and hyperalgesia. Compared to sham control, EA treatment significantly decreased the frequencies of responses to 4–15 g filament stimulation during EA treatment. This indicates that EA treatment alleviated paclitaxel-caused mechanical allodynia and hyperalgesia. The alleviation lasted up to 3 weeks after the EA treatment (Fig. 1). Additionally, there was no significant difference in the response frequency between vehicle/sham and vehicle/EA rats, suggesting that EA did not influence mechanical sensitivity in healthy rats. These data indicate that paclitaxel-induced persistent allodynia and hyperalgesia were significantly attenuated by EA.
Fig. 1.

Effect of EA on paclitaxel-induced mechanical hyperalgesia and allodynia (n = 6/group). The mechanical response was evoked using von Frey filaments with bending forces 2 (A), 4 (B), 8 (C) and 15 g (D). The EA treatment (10 Hz, 2 mA / 30 min, 0.4 ms pulse) was given on days 14, 16, 18, 20, 22, 24 and 26 after paclitaxel injection. Paclitaxel-injected rats with sham EA treatment (∆) showed significantly higher frequencies of mechanical responses than vehicle-injected ones with sham EA (○) from days 13–47 post-paclitaxel injection. Rats with paclitaxel and EA treatment (▲) showed significantly lower frequencies of mechanical responses than those with paclitaxel and sham EA control (∆). The EA inhibition of mechanical responses persisted up to day 47, the longest time point observed in the experiment. Vehicle-injected rats given EA treatment (●) showed similar mechanical responses as did vehicle-injected rats with sham EA (○). This suggests that EA did not influence the mechanical sensitivity in healthy condition. *P<0.05, **P<0.01, ***P<0.001 vs vehicle + sham; #P<0.05, ##P<0.01, ###P<0.001 vs paclitaxel + EA.
3.2 5-HT1A receptor antagonist blocked EA alleviation of paclitaxel-induced mechanical allodynia and hyperalgesia
As aforementioned in introduction, it is not known whether 5-HT1A receptors are involved in EA inhibition of neuropathic pain. To investigate the roles of 5-HT1A receptors in the effect of EA on paclitaxel-induced pain, rats were injected with paclitaxel and divided into four groups (n=7 per group): 1) WAY-100635 (12.5 nmol / 5 μl, a 5-HT 1A receptor antagonist) + EA, 2) WAY-100635 + sham, 3) saline + EA, and 4) saline + sham. The antagonists, dissolved in saline, were i.t. administered 30 min before each EA treatment. The data showed that the frequencies of mechanical responses to 4–15 g stimulation were significantly decreased in EA + vehicle than those in sham + vehicle, demonstrating that EA significantly inhibited the mechanical allodynia and hyperalgesia (Fig. 2), consistent with aforementioned in section 3.1. However, the intrathecal pretreatment with a 5-HT 1A receptor antagonist WAY-100635 in EA-treated rats significantly increased the mechanical response frequencies compared to the vehicle pretreatment, demonstrating that WAY-100635 blocked EA inhibition of the mechanical allodynia and hyperalgesia. These results suggest that spinal 5-HT1A receptor activation was involved in EA-produced relief of CIP. Way100635 + sham did not significantly influence the frequencies of mechanical response compared to vehicle + sham, indicating that Way100635 did not influence the mechanical sensation in sham control rats.
Fig. 2.
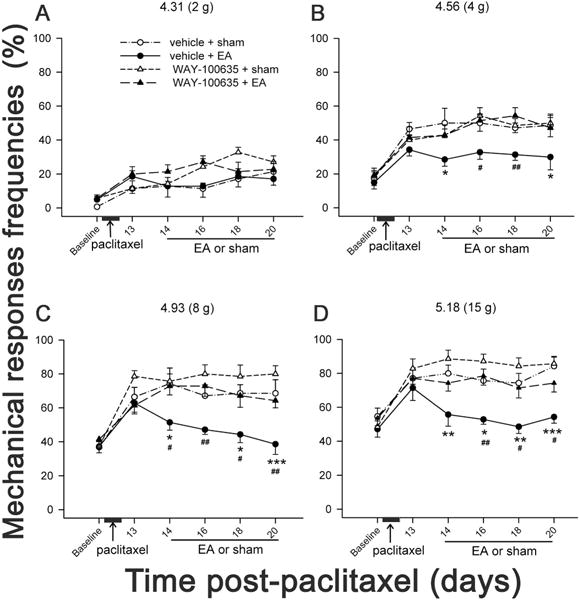
Effect of 5-HT1A receptor antagonist, WAY-100635, on EA inhibition of paclitaxel-induced mechanical hyperalgesia and allodynia. WAY-100635 (12.5 nmol / 5 μl) or vehicle was i.t. given 30 min before each EA treatment in paclitaxel-injected rats (n=7/group). EA treatment was given on days 14, 16, 18 and 20 after paclitaxel injection. EA plus vehicle (●) significantly decreased the frequencies of responses to 4–15 g stimulation compared to sham plus vehicle (○), indicating EA alleviation of mechanical allodynia and hyperalgesia. EA plus WAY-100635 (▲) showed significantly higher frequencies of responses to 4–15 g stimulation than EA plus vehicle (●), and similar response frequencies as sham plus WAY-100635 (∆) or sham plus vehicle (○). This indicates that WAY-100635 blocked EA alleviation of mechanical allodynia and hyperalgesia. * P < 0.05, ** P < 0.01, ***P<0.001 vs sham + vehicle; # P<0.05, ##P<0.01 vs EA + WAY-100635.
3.3 5-HT1A receptor agonist alleviated paclitaxel-induced mechanical allodynia and hyperalgesia
In another two groups of rats (n=7 per group), a 5-HT1A receptor agonist, 8-OH-DPAT (15 μg /5 μl), or vehicle was i.t. administered into the spinal cord on 14, 16, 18 and 20 days post-paclitaxel injection, 30 min prior to assessing mechanical allodynia/hyperalgesia. The data showed that before paclitaxel injection, overall mean baseline responses to 4–15 g filament stimulation were similar in two groups of rats (Fig. 3). The frequencies of mechanical responses to 4–15 g filament were significantly higher on day 13 post-paclitaxel injection than baseline, demonstrating mechanical allodynia and hyperalgesia. The 8-OH-DPAT pretreatment among days 14–20 post-paclitaxel injection significantly decreased the frequencies of mechanical responses to 8–15 g filament stimulation compared to vehicle control. This indicates that activation of 5-HT1A receptors in the spinal cord alleviated paclitaxel-induced mechanical allodynia and hyperalgesia.
Fig. 3.

Effect of intrathecal administration of 5-HT1A agonist 8-OH-DPAT on paclitaxel-induced mechanical hyperalgesia and allodynia (n = 7/group). The 8-OH-DPAT was i.t. administered into the spinal cord on 14, 16, 18 and 20 days post-paclitaxel injection, 30 min prior to assessing mechanical allodynia/hyperalgesia. It significantly decreased the frequencies of mechanical responses to 8–15 filament stimulation compared to vehicle control, indicating that 5-HT1A receptor activation alleviated mechanical allodynia and hyperalgesia. *P<0.05 and **P<0.01 vs vehicle.
3.4 EA inhibited phosphorylation of spinal CaMKII and 5-HT1A receptor antagonist prevented such EA inhibition
To investigate the EA effect on p-CaMKII, the spinal cord was removed from the four groups of rats at day 20 after finishing the behavioral test in 5-HT1A receptor experiment. Another group of 7 rats with vehicle of paclitaxel, i.t. saline and sham EA were used as control. As shown in Fig. 4, the data demonstrate that paclitaxel-injected (i.p.) rats with i.t. saline and sham EA (group 4, Fig. 4) showed significantly higher levels of p-CaMKII than vehicle-injected rats with i.t. saline and sham EA (group 5), indicating higher p-CaMKII levels in the spinal cord during CIP. EA treatment in paclitaxel-injected rats (group 3) significantly decreased p-CaMKII levels compared to sham (group 4), suggesting that EA treatment inhibited the phosphorylation of CaMKII in the spinal cord. Further, pretreatment of intrathecal 5-HT1A receptor antagonist (group 1) increased the p-CaMKII levels in EA-treated rats compared to the vehicle pretreatment (group 3). This demonstrates that 5-HT1A receptor antagonist blocked EA reduction of p-CaMKII levels and suggests that EA may inhibit phosphorylation of CaMKII through activation of 5-HT1A receptors in the spinal cord.
Fig. 4.

Representative Western blot (upper panel) and quantification (lower panel) of relative p-CaMKII levels in the spinal cord of paclitaxel-injected rats (n = 7/group), which was normalized to vehicle of paclitaxel-injected control (100%). Note that p-CaMKII levels are higher in paclitaxel- (group 4) than in vehicle of paclitaxel-injected rats (group 5). EA treatment (group 3) significantly decreased p-CaMKII compared to sham control (group 4). Pretreatment of intrathecal 5-HT1A receptor antagonist (group 1) increased the p-CaMKII levels in EA-treated rats compared to the vehicle pretreatment (group 3). *P < 0.05 and **P < 0.01.
3.5 CaMKII phosphorylation inhibitor inhibited paclitaxel-induced mechanical allodynia and hyperalgesia
Since EA inhibited phosphorylation of spinal CaMKII, it is necessary to investigate whether the spinal p-CaMKII is involved in the CIP. We tested the effects of a selective CaMKII phosphorylation inhibitor, KN-93, and its inactive analog, KN-92, on mechanical allodynia and hyperalgesia in two groups of rats (n=7 per group). KN-93 or KN-92 (10 nmol / 5 μl, respectively) were i.t. administrated in paclitaxel-injected rats on 18 and 20 days post-paclitaxel injection, 60 min prior to assessing mechanical allodynia/hyperalgesia. Before paclitaxel injection, overall mean baseline responses to 4–15 g filament stimulation were similar in the two groups of rats (Fig. 5). The frequencies of mechanical responses to 4–15 g filaments were significantly higher on day 13 post-paclitaxel injection than baseline, demonstrating mechanical allodynia and hyperalgesia. KN-93 treatment on days 18 and 20 post-paclitaxel injection significantly decreased the frequencies of mechanical responses to 4–15 g stimulation compared to KN-92 (Fig. 5). This indicates that KN-93 may inhibit pain through suppressing the p-CaMKII.
Fig. 5.
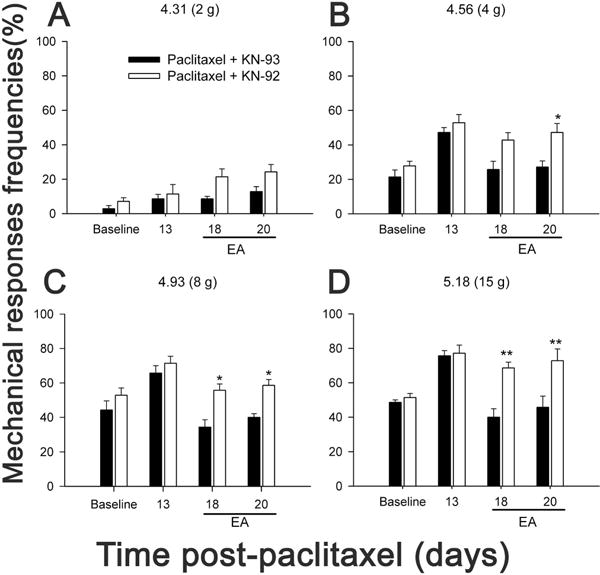
Effect of intrathecal administration of KN-93 or KN-92 (10 nmol / 5 μl, respectively) on paclitaxel-induced mechanical hyperalgesia and allodynia (n = 7/group). KN-93 or KN-92 was i.t. administered into the spinal cord on 18 and 20 days post-paclitaxel injection, 60 min prior to assessing mechanical allodynia/hyperalgesia. Rats showed significantly higher frequencies of responses to 4–15 g filament stimulation on day 13 post-paclitaxel injection, demonstrating that paclitaxel caused mechanical allodynia and hyperalgesia. KN-93 treatment significantly decreased the frequencies of mechanical responses to 4–15 g stimulation compared to KN-92, indicating KN-93 alleviation of the mechanical hyperalgesia and allodynia * P < 0.05 and ** P < 0.01 vs KN-93 treatment.
3.6 CaMKII phosphorylation inhibitor decreased spinal p-CaMKII
To investigate the effect of KN-93 on spinal p-CaMKII, the spinal cord was removed from the two groups of rats at day 20 after finishing the behavioral test in KN-93 behavioral experiment. Another group of 7 rats with vehicle of paclitaxel and i.t. saline were used as control. As shown in Fig. 6, vehicle of paclitaxel-injected rats showed significantly lower levels of p-CaMKII than paclitaxel-injected rats, which suggests that paclitaxel significantly upregulated the levels of p-CaMKII compared to vehicle control. KN-93-treated rats showed significantly lower levels of p-CaMKII than KN-92 treated rats, confirming the involvement of CaMKII phosphorylation in CIP. These results suggest that inhibition of p-CaMKII led to alleviation of paclitaxel-induced mechanical allodynia and hyperalgesia.
Fig. 6.

Representative Western blot (upper panel) and quantification (lower panel) of relative p-CaMKII levels in the spinal cord of paclitaxel-injected rats (n = 7/group.), which was normalized to vehicle of paclitaxel-injected control (100%). Note that p-CaMKII levels are significantly higher in paclitaxel (Lanes 2 and 3)- than in vehicle of paclitaxel-injected (Lane 1) rats. KN-93 treatment significantly decreased p-CaMKII levels compared to KN-92 control. *P < 0.05 and **P < 0.01.
4. Discussion and conclusions
The present study demonstrates that EA treatment significantly alleviated both allodynia and hyperalgesia and allayed them for three weeks after the completion of the treatment in the CIP rat model. A previous case study reported that acupuncture treatment improved CIP-patients’ experience in sensation, gait, and balance and decreased their analgesic dosages, and the benefits of acupuncture were maintained for six months for four of the five patients involved (Wong and Sagar 2006). No adverse effect was observed following acupuncture treatment. A recent study showed that acupuncture combined with methylcobalamin produced a better outcome including pain score than methylcobalamin alone in the treatment of chemotherapy-induced peripheral neuropathy (Han et al., 2017). To date, no drugs have been proven to prevent chemotherapy-induced peripheral neuropathy (Wolf et al., 2008). CIP is often difficult to manage; available treatment options rarely provide total relief (Kaley and Deangelis 2009; Zhou et al., 2009) and the current pharmacological therapy also produces many adverse effects (Hempenstall and Rice 2002). It has been reported that 30% patients with neuropathy have turned to acupuncture due to inadequate control of neuropathic pain (Brunelli and Gorson 2004). Our data, produced using both treatment and control groups, and the previous case studies clearly support that acupuncture could be one of the alternative cares in pain management in patients with CIP.
Further, the present study showed that the spinal 5-HT1A receptor antagonist WAY-100635 blocked the EA inhibition of CIP, and 5-HT1A receptor agonist 8-OH-DPAT alleviated CIP. Our previous studies demonstrated that EA activates the serotonergic neurons in the medulla, and the activated neurons projects to the spinal cord to inhibit pain at the spinal level (Li et al., 2007). The present data suggest that the spinal cord-released serotonin may activate 5-HT1A receptors to inhibit pain. Consistently, previous study demonstrated that 5-HT1A receptor antagonist, WAY-100635, blocked the antinociceptive action of 5-HT (50μg/rat, i.t.) in both phases of biting/licking behaviour in formalin pain model (Bonnefont et al., 2005). 5-HT1A receptor agonist attenuated mechanical allodynia in a rat model of trigeminal neuropathic pain (Deseure et al., 2002), and inhibited formalin-induced Fos expression (Buritova et al., 2005), nociceptive pinch-evoked responses, nociceptive neuron wind-up and C-fiber-mediated late responses (You et al., 2005). It has also been shown that the 5-HT1A receptor antagonists blocked EA-induced analgesic effects in formalin-induced (Chang et al., 2004) and collagen-induced arthritis (Baek et al., 2005) pain model. All these data support that EA-induced spinal serotonin inhibits pain through action of 5-HT1A receptors, which are mainly present in the dorsal horn of the spinal cord (Marlier et al., 1991). However, since 5-HT3 receptors were involved in EA inhibition of formalin-induced (Chang et al., 2004) and collagen-induced arthritis pain (Baek et al., 2005), it is possible that other 5-HT receptor sub-types are also involved in EA inhibition of CIP.
It has been reported that WAY-100635 also acts as full agonist at dopamine 4 (D4) receptors (Chemel et al., 2006). D2-like receptors includes D2, D3 and D4 receptors and they generally exert inhibitory effects. One study demonstrated that spinal D4 receptor antagonist partially blocked a D2-like agonist, quinpirole-produced antinociception (Almanza et al., 2015). The data suggest that D4 receptor agonist (e.g. WAY-100635) may produce analgesia. In our study, WAY-100635 in both sham and EA-treated rats did not alleviate allodynia and hyperalgesia; in contrast, it abolished EA alleviation of pain. This indicates that spinal WAY-100635 in the present study mainly acted on 5-HT1A receptors. This is further supported by the evidences that 1) the Way-100635 showed higher selectivity for serotonin 5-HT1A than for D4 receptors (Martel et al., 2007), and 2) In situ hybridization found that D4 receptors are expressed more in the ventral horns than in the superficial dorsal horn (Zhu et al., 2008).
It has also been known that 8-OH-DPAT may act as 5-HT7 receptor agonist (Sprouse et al., 2004). Previous studies showed that spinal 5-HT7 receptor activation attenuated mechanical allodynia in rats with neuropathy (Lin et al., 2015). It is possible that both 5-HT1A and 5-HT7 receptor activation was involved with the 8-OH-DPAT alleviation of CIP in the present study. This suggests that EA-induced spinal serotonin may alleviate pain through both 5-HT1A and 5-HT7 receptors. The 5-HT1A receptor involvement in EA alleviation of pain is demonstrated with our data that 8-OH-DPAT blocked the EA inhibition of CIP. Whether 5-HT7 receptors are involved in EA alleviation of pain warrants further study.
Moreover, we showed that EA decreased p-CaMKII levels in the spinal cord, which was blocked by 5-HT1A receptor antagonist pretreatment. This indicates that 5-HT1A receptors were involved in the EA-produced inhibition of the p-CaMKII in the spinal cord. Previous studies demonstrated that 5-HT1A receptor-knockout mice show an increase in p-CaMkII (Lo Iacono and Gross 2008), and the selective 5-HT1A receptor agonist 8-OH-DPAT significantly reduces the phosphorylation state of CaMKII (Cai et al., 2002). These studies support that EA-induced 5-HT may activate 5-HT1A receptors in the spinal cord, which in turn inhibits the phosphorylation of CaMKII.
At last, we demonstrated that KN93, an inhibitor of p-CaMKII, not only decreased the p-CaMKII levels in the spinal cord but also inhibited mechanical allodynia and hyperalgesia. It has been demonstrated that KN93 treatment significantly reversed spinal nerve ligation-induced thermal hyperalgesia and mechanical allodynia (Chen et al., 2009). KN-93 significantly reduced the excitability of capsaicin-sensitive small and medium trigeminal ganglion neurons (Liang et al., 2012). The data suggest that inhibition of p-CaMKII may lead to alleviation of neuropathic pain.
KN-93 is the most widely used inhibitor for study of cellular and in vivo functions of CaMKII. Although it is considered as specific inhibitor of CaMKII, it is also a direct extracellular open channel blocker of voltage-gated potassium channels (Rezazadeh et al., 2006). The effect of KN-93 on Kv channels was shown to be independent of CaMKII because KN-92, an inactive form of KN-93, resulted in a similar inhibition of ionic currents (Rezazadeh et al., 2006). Since KN-93, but not KN-92 inhibited paclitaxel-induced mechanical allodynia and hyperalgesia and decreased P-CaMKII, it is believed that KN-93 inhibition of pain is underpinned by KN-93 suppression of p-CaMKII, not blockade of Kv channels.
Taken together, our data suggest that EA activates 5-HT1A receptors in the spinal cord to inhibit p-CaMKII, leading to alleviation of chemotherapy-induced neuropathic pain. The findings strongly support acupuncture/EA as a complementary therapy for CIP.
Additionally, it was observed that high frequency (100 Hz) EA did not produce antinociceptive effect in the CIP rat model. Previous studies also found that EA at 2 Hz, but not 100 Hz, was effective in the treatment of spinal nerve ligation (SNL)-induced neuropathic pain (Xing et al., 2007). It has further been shown that 2Hz EA induced long-term depression (LTD) of C-fiber-evoked potentials while 100 Hz EA induced long-term potentiation (LTP) in SNL rats (Xing et al., 2007). The functional activities of certain brain areas might be correlated with the effect of EA-induced analgesia, in a frequency-dependent dynamic (Zhang et al., 2003). The data suggest that low and high frequency EAs have different effects under a given condition.
In summary, the EA treatment significantly alleviates CIP compared to sham control. EA inhibited such pain through activation of spinal 5-HT 1A receptors and suppression of p-CaMKII. In combination of some clinical case series, the data strongly support acupuncture/EA as a complementary therapy for CIP. It should be noted that there is gender difference of pain sensation. Whether EA alleviates CIP in female rats and whether it acts through the same mechanism warrant further study.
Significance.
EA activates spinal 5-HT1A receptors to inhibit p-CaMKII to alleviate paclitaxel-induced pain. Acupuncture/EA may be used as a complementary therapy for CIP.
Acknowledgments
Funding sources: NIH grants R21 AT004113 and R21 AT008467-01A1.
Footnotes
We do not have any conflicts of interest.
Author contributions
Y. Zhang: Behavioral tests, western blot, data acquisition, data analysis and drafting the article. A. Li and J Xin: EA treatment, drug administration, behavioral test, western blot. K. Ren, B.M. Berman, L. Lao: conception and design, interpretation of data. R.-X. Zhang: conception and design, interpretation of data, and drafting the article. All authors discussed the results and commented on the manuscript.
References
- Almanza A, Simón-Arceo K, Coffeen U, Fuentes-García R, Contreras B, Pellicer F, Mercado F. A D2-like receptor family agonist produces analgesia in mechanonociception but not in thermonociception at the spinal cord level in rats. Pharmacol Biochem Behav. 2015;137:119–125. doi: 10.1016/j.pbb.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Armstrong T, Almadrones L, Gilbert MR. Chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum. 2005;32:305–311. doi: 10.1188/05.ONF.305-311. [DOI] [PubMed] [Google Scholar]
- Baek YH, Choi DY, Yang HI, Park DS. Analgesic effect of electroacupuncture on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by cholinergic and serotonergic receptors. Brain Res. 2005;1057:181–185. doi: 10.1016/j.brainres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Banach M, Juranek JK, Zygulska AL. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav. 2017;7:e00558. doi: 10.1002/brb3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Horin I, Kahan P, Ryvo L, Inbar M, Lev-Ari S, Geva R. Acupuncture and reflexology for chemotherapy-induced peripheral neuropathy in breast cancer. Integr Cancer Ther. 2017;3:258–262. doi: 10.1177/1534735417690254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont J, Chapuy E, Clottes E, Alloui A, Eschalier A. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114:482–490. doi: 10.1016/j.pain.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Brunelli B, Gorson KC. The use of complementary and alternative medicines by patients with peripheral neuropathy. J Neurol Sci. 2004;218:59–66. doi: 10.1016/j.jns.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Buritova J, Larrue S, Aliaga M, Besson J, Colpaert F. Effects of the high-efficacy 5-HT1A receptor agonist, F 13640 in the formalin pain model: a c-Fos study. Eur J Pharmacol. 2005;514:121–130. doi: 10.1016/j.ejphar.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002;277:36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Expert Opin Drug Saf. 2004;3:535–546. doi: 10.1517/14740338.3.6.535. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Zanna C. Current status and future prospects for the treatment of chemotherapy-induced peripheral neurotoxicity. Eur J Cancer. 2002;38:1832–1837. doi: 10.1016/s0959-8049(02)00229-0. [DOI] [PubMed] [Google Scholar]
- Chang FC, Tsai HY, Yu MC, Yi PL, Lin JG. The central serotonergic system mediates the analgesic effect of electroacupuncture on ZUSANLI (ST36) acupoints. J Biomed Sci. 2004;11:179–185. doi: 10.1007/BF02256561. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: Clinical and electrophysiological studies. Ann Neurol. 1994;35:304–311. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Luo F, Yang C, Kirkmire CM, Wang ZJ. Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J Pharmacol Exp Ther 3. 2009;30:650–659. doi: 10.1124/jpet.109.152165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X. Chinese Acupuncture and Moxibustion. Beijing: Foreign Languages Press; 1999. [Google Scholar]
- Dai Y, Kondo E, Fukuoka T, Tokunaga A, Miki K, Noguchi K. The effect of electroacupuncture on pain behaviors and noxious stimulus-evoked Fos expression in a rat model of neuropathic pain. J Pain. 2001;2:151–159. doi: 10.1054/jpai.2001.19964. [DOI] [PubMed] [Google Scholar]
- Deng G, Cassileth BR. Integrative oncology: complementary therapies for pain, anxiety, and mood disturbance. CA Cancer J Clin. 2005;55:109–116. doi: 10.3322/canjclin.55.2.109. [DOI] [PubMed] [Google Scholar]
- Deseure K, Koek W, Colpaert FC, Adriaensen H. The 5-HT1A receptor agonist F 13640 attenuates mechanical allodynia in a rat model of trigeminal neuropathic pain. Eur J Pharmacol. 2002;456:51–57. doi: 10.1016/s0014-2999(02)02640-7. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth PA, Balmaceda C, Peterson K, Seidman AD, Brasher P, DeAngelis LM. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J Neurooncol. 1997;35:47–53. doi: 10.1023/a:1005805907311. [DOI] [PubMed] [Google Scholar]
- Gordon AN, Stringer CA, Matthews CM, Willis DL, Nemunaitis J. Phase I dose escalation of paclitaxel in patients with advanced ovarian cancer receiving cisplatin: rapid development of neurotoxicity is dose-limiting. J Clin Oncol. 1997;15:1965–1973. doi: 10.1200/JCO.1997.15.5.1965. [DOI] [PubMed] [Google Scholar]
- Hama A, Takamatsu H. Chemotherapy-induced peripheral neuropathic pain and rodent models. CNS Neurol Disord Drug Targets. 2016;15:7–19. doi: 10.2174/1871527315666151110125325. [DOI] [PubMed] [Google Scholar]
- Han X, Wang L, Shi H, Zheng G, He J, et al. Acupuncture combined with methylcobalamin for the treatment of chemotherapy-induced peripheral neuropathy in patients with multiple myeloma. BMC Cancer. 2017;17:40. doi: 10.1186/s12885-016-3037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33:15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Hempenstall K, Rice AS. Current treatment options in neuropathic pain. Curr Opin Investig Drugs. 2002;3:441–448. [PubMed] [Google Scholar]
- Hwang BG, Min BI, Kim JH, Na HS, Park DS. Effects of electroacupuncture on the mechanical allodynia in the rat model of neuropathic pain. Neurosci Lett. 2002;320:49–52. doi: 10.1016/s0304-3940(02)00027-7. [DOI] [PubMed] [Google Scholar]
- Kaley TJ, Deangelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol. 2009;145:3–14. doi: 10.1111/j.1365-2141.2008.07558.x. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang G, Wei F, Berman BM, Ren K. Electroacupuncture attenuates behavioral hyperalgesia and selectively reduces spinal Fos protein expression in rats with persistent inflammation. J Pain. 2001;2:111–117. doi: 10.1054/jpai.2001.19575. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang RX, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- Lee J, Beitz A. The distribution of brain-stem and spinal cord nuclei associated with different frequencies of electroacupuncture analgesia. Pain. 1993;52:11–28. doi: 10.1016/0304-3959(93)90109-3. [DOI] [PubMed] [Google Scholar]
- Li A, Wang Y, Xin J, Lao L, Ren K, Berman BM, Zhang RX. Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res. 2007;1186:171–179. doi: 10.1016/j.brainres.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Zhang Y, Lao L, Xin J, Ren K, Berman BM, Zhang RX. Serotonin Receptor 2A/C is Involved in Electroacupuncture Inhibition of Pain in an Osteoarthritis Rat Model. Evid Based Complement Alternat Med. 2011;2011:619650. doi: 10.1093/ecam/neq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Liu X, Wei L, Wang W, Zheng P, et al. The modulation of the excitability of primary sensory neurons by Ca-CaM-CaMKII pathway. Neurol Sci. 2012;33:1083–1093. doi: 10.1007/s10072-011-0907-7. [DOI] [PubMed] [Google Scholar]
- Lin H, Heo BH, Kim WM, Kim YC, Yoon MH. Antiallodynic effect of tianeptine via modulation of the 5-HT7 receptor of GABAergic interneurons in the spinal cord of neuropathic rats. Neurosci Lett. 2015;598:91–95. doi: 10.1016/j.neulet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci. 2008;28:6250–6257. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SX, Ma J, Moise G, Li XY. Responses of neuronal nitric oxide synthase expression in the brainstem to electroacupuncture Zusanli (ST 36) in rats. Brain Res. 2005;1037:70–77. doi: 10.1016/j.brainres.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Martel JC, Leduc N, Ormière AM, Faucillon V, Danty N, et al. WAY-100635 has high selectivity for serotonin 5-HT(1A) versus dopamine D(4) receptors. Eur J Pharmacol. 2007;574:15–9. doi: 10.1016/j.ejphar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Openshaw H, Beamon K, Synold TW, Longmate J, Slatkin NE, et al. Neurophysiological Study of Peripheral Neuropathy after High-Dose Paclitaxel: Lack of Neuroprotective Effect of Amifostine. Clin Cancer Res. 2004;10:461–467. doi: 10.1158/1078-0432.ccr-0772-03. [DOI] [PubMed] [Google Scholar]
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6:489–494. doi: 10.1093/oxfordjournals.annonc.a059220. [DOI] [PubMed] [Google Scholar]
- Rezazadeh S, Claydon TW, Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther. 2006;317:292–299. doi: 10.1124/jpet.105.097618. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Donehower RC. Paclitaxel (Taxol) N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- Schloss JM, Colosimo M, Vitetta L. Chemotherapy-induced peripheral neuropathy management. J Clin Oncol. 2016;34:154–154. doi: 10.4103/2347-5625.170977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology. 2004;46:52–62. doi: 10.1016/j.neuropharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Sui M, Lessans S, Yan T, Cao D, Lao L, Dorsey SG. Mechanism of electroacupuncture on “Zusanli (ST 36)” for chemotherapy-induced peripheral neuropathy. Zhongguo Zhen Jiu. 2016;36:512–516. [PubMed] [Google Scholar]
- Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- Witt CM, Pach D, Brinkhaus B, Wruck K, Tag B, Mank S, Willich SN. Safety of Acupuncture: Results of a Prospective Observational Study with 229,230 Patients and Introduction of a Medical Information and Consent Form. Forsch Komplementmed. 2009;16:91–97. doi: 10.1159/000209315. [DOI] [PubMed] [Google Scholar]
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Wong R, Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy–a case series. Acupunct Med. 2006;24:87–91. doi: 10.1136/aim.24.2.87. [DOI] [PubMed] [Google Scholar]
- Xing GG, Liu FY, Qu XX, Han JS, Wan Y. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol. 2007;208:323–332. doi: 10.1016/j.expneurol.2007.09.004. [DOI] [PubMed] [Google Scholar]
- You HJ, Colpaert FC, Arendt-Nielsen L, You HJ, Colpaert FC, Arendt-Nielsen L. The novel analgesic and high-efficacy 5-HT1A receptor agonist F 13640 inhibits nociceptive responses, wind-up, and after-discharges in spinal neurons and withdrawal reflexes. Exp Neurol. 2005;191:174–183. doi: 10.1016/j.expneurol.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, et al. Electroacupuncture attenuates bone cancer pain and inhibits spinal interleukin-1 beta expression in a rat model. Anesth Analg. 2007;105:1482–1488. doi: 10.1213/01.ane.0000284705.34629.c5. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Xin J, et al. Electroacupuncture attenuates bone-cancer-induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur J Pain. 2008;12:870–878. doi: 10.1016/j.ejpain.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WT, Jin Z, Cui GH, Zhang KL, Zhang L, et al. Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging study. Brain Res. 2003;982:168–178. doi: 10.1016/s0006-8993(03)02983-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang RX, Zhang M, Shen XY, Li A, et al. Electroacupuncture inhibition of hyperalgesia in an inflammatory pain rat model: involvement of distinct spinal serotonin and norepinephrine receptor subtypes. Br J Anaesth. 2012;109:245–252. doi: 10.1093/bja/aes136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Tjen-A-Looi SC, Longhurst JC. Brain stem mechanisms underlying acupuncture modality-related modulation of cardiovascular responses in rats. J Appl Physiol. 2005;99:851–60. doi: 10.1152/japplphysiol.01365.2004. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Garcia MK, Chang D, Chiang J, Lu J, et al. Multiple Myeloma, Painful Neuropathy, Acupuncture? Am J Clin Oncol. 2009;32:319–325. doi: 10.1097/COC.0b013e318173a520. [DOI] [PubMed] [Google Scholar]
- Zhu H, Clemens S, Sawchuk M, Hochman S. Unaltered D1, D2, D4, and D5 dopamine receptor mRNA expression and distribution in the spinal cord of the D3 receptor knockout mouse. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:957–962. doi: 10.1007/s00359-008-0368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


