Abstract
The ex vivo human skin wound model is a widely accepted model to study wound epithelialization. Due to a lack of animal models that fully replicate human conditions, the ex vivo model is a valuable tool to study mechanisms of wound re-epithelialization, as well as for pre-clinical testing of novel therapeutics. The current standard for assessment of wound healing in this model is histomorphometric analysis, which is labor intensive, time consuming, and requires multiple biological and technical replicates in addition to assessment of different time points. Optical Coherence Tomography (OCT) is an emerging non-invasive imaging technology originally developed for non-invasive retinal scans that avoids the deleterious effects of tissue processing. This study investigated OCT as a novel method for assessing re-epithelialization in the human ex vivo wound model. Excisional ex vivo wounds were created, maintained at air-liquid interface, and healing progression was assessed at days 4 and 7 with OCT and histology. OCT provided adequate resolution to identify the epidermis, the papillary and reticular dermis, and importantly, migrating epithelium in the wound bed. We have deployed OCT as a non-invasive tool to produce, longitudinal “optical biopsies” of ex vivo human wound healing process, and we established an optimal quantification method of re-epithelialization based on en face OCT images of the total wound area. Pairwise statistical analysis of OCT and histology based quantifications for the rate of epithelialization have shown the feasibility and superiority of OCT technology for non-invasive monitoring of human wound epithelialization. Furthermore, we have utilized OCT to evaluate therapeutic potential of allogeneic adipose stem cells revealing their ability to promote re-epithelialization in human ex vivo wounds. OCT technology is promising for its applications in wound healing and evaluation of novel therapeutics in both the laboratory and the clinical settings.
Keywords: Optical Coherence Tomography, epithelialization, human wound healing, histology
INTRODUCTION
Optical coherence tomography (OCT) is a non-invasive optical imaging technology that uses signal interference to rapidly produce cross-sectional as well as three-dimensional images (1). OCT imaging technology produces micron scale images with 1–10 μm resolution and a 2–3 mm depth of imaging (2) and can be performed on ex vivo and in vivo tissue in real time or after fixation, overcoming the limitations of histological examinations (1). An OCT image represents a cross-sectional, micron scale picture of the optical reflectance properties of the tissue (2). This image can either be used to assess tissue features and pathologies qualitatively or to make quantitative measurements objectively. Originally developed for imaging the retina, it is now being explored for its use in other organs, especially for imaging the skin and skin pathology (3–5). It is able to distinguish epidermis, cornified epidermis, papillary dermis, and reticular dermis as well as skin appendages such as hair follicles, blood vessels, and sebaceous glands (3). Newer high-definition (HD) OCT devices can produce real-time three-dimensional images with adequate resolution to visualize individual cells and have been used to assess fibroblasts in culture, recellularization of acellular dermal matrices, and age-related morphological skin changes in vivo (6–8).
Cutaneous wound healing is an evolutionarily conserved process comprised of the overlapping phases of inflammation, proliferation/migration, and remodeling (9). The human ex vivo wound model was developed almost 20 years ago to overcome obvious difficulties in sampling acute wounds in humans due to ethical concerns (10). Since then this model has been successfully utilized to decipher various molecular mechanisms of the epithelialization process such as the role of mircoRNAs, local production of hormones, neuropeptides, neurotransmitters, and even scarring (11–21). The human ex vivo wound model is also well established for evaluating the effects of novel therapies (14–16, 21–26). This model utilizes skin from reduction surgeries in which small partial-thickness wounds are made with punch biopsies to the depth of the papillary dermis. These wounds are excised and the skin is maintained viable at air-liquid interface allowing for full wound healing by epithelialization (22).
The human ex vivo wound model has several distinct advantages. First, it eliminates inter-patient variability because an entire experiment including technical replicates can be done using a single patient’s skin. Furthermore, the ex vivo wound model preserves the integrity of the basement membrane, whereas it is absent in in vitro organotypic skin models (27). In addition, the human ex vivo wound model has been shown to have comparable gene expression patterns to in vivo models (13, 16, 21, 24, 28–32), which increases the translational potential of ex vivo data to clinical care. The gold standard for assessment of re-epithelialization in this wound model is histomorphometric analysis (9). However, this process is time-consuming, labor-intensive, and involves deleterious tissue processing that requires a sample to be sacrificed rather than monitored over time.
Recently, stem cell therapies have been explored as new treatments for difficult to heal wounds (33, 34). Human adipocyte derived stem cells (ASCs) have emerged as viable alternatives to bone marrow-derived stem cells because of their similar differentiation potentials as well as their similar transcriptomic and proteomic profiles (35). As adult stem cells, ASCs avoid any ethical issues. They are found in abundant supply and are relatively easy to obtain. Several studies to date have demonstrated that ASCs promote wound healing through paracrine effects, differentiation, and vasculogenesis (33, 36), however previous studies have not utilized a human ex vivo model for therapeutic evaluation of ASC.
Here we explore the viability of OCT for assessment of re-epithelialization of human ex vivo wounds. In addition, our comparative analyses identified quantification of en face OCT images as a new method with increased sensitivity for wound closure measurement than standard histomorphometric analyses. Lastly, we demonstrate the effectiveness of OCT in evaluating novel therapeutics in the human ex vivo wound model by imaging wounds and quantifying re-epithelialization upon treatment with ASCs.
MATERIALS AND METHODS
Human ex vivo wound culture model
After Institutional Review Board approval (Protocol number UM20070922), human skin was ethically obtained upon consent from five patients aged between 43 and 49 years of age undergoing elective reduction surgeries and was declared non-human subject research. Acute wounds were generated as described previously (22). First, underlying adipose tissue and fascia were cleaned from the interior aspect. Then, a wound was made with sterile 3 mm punches (Integra, Plainsboro, NJ) carefully removing only the epidermis with sterile forceps and surgical shears. A larger full-thickness sterile 8 mm punch (Integra, Plainsboro, NJ) was made around the wound. Lastly, the wounds were excised and placed at air-liquid interface inside 6 cm culture dishes (Corning Inc., Corning, NY) on top of sterile gauze pads. Media consisting of DMEM (Thermo Scientific, Waltham, MA) with 10% FBS (HyClone, Logan, UT) and Anti-Anti Streptomycin sulfate + Penicillin G formulation (Thermo Scientific, Waltham, MA) was applied to the gauze pad and the open wound daily. In addition, wounds were treated with human recombinant epidermal growth factor (rhEGF) 25 ng/ml (Merck Millipore, Billerica, MA). All ex vivo wounds (n=47) were incubated at 37° C with 5% CO2 and collected at representative time points of 0h, 4d, and 7d with technical triplicates at each time point and treatment group.
Human Adipose Derived Stem Cell Treatment of Ex Vivo Wounds
ASCs were isolated from subcutaneous adipose tissue of patients undergoing inguinal hernia surgery (IRB# 20070922). Subcutaneous adipose tissue was excised, washed in phosphate buffer solution without Ca2+ and Mg2+ (PBS) containing 30% GIBCO® Pen/Strep (Life Technologies, Grand Island, NY) and digested in media containing 0.75% type II collagenase (Sigma-Aldrich, St. Louis, MO). The suspension was centrifuged to separate floating adipocytes from the stromal vascular fraction. The resultant pellet was resuspended and cultured in ADSC™ Growth Medium (Lonza Group Ltd; Basel, Switzerland). Following the initial 24-hr incubation period, non-adherent cells were removed. When confluent, cells were expanded and cryopreserved in RecoveryTM Cell Culture Freezing Medium (Life Technologies, Grand Island, NY) at passage 2 and 3. Cells were assayed for mesenchymal stem cell markers on Flow-assisted cell sorting (FACS) Canto™ II (BD Biosciences; San Jose, CA) as previously described (37). The isolated ASCs demonstrated the well-characterized expression pattern of mesenchymal markers including positivity for CD90 (98.6%), CD105 (99.5%), CD29 (98.7%), while no expression of the hematopoietic and endothelial lineage markers (CD117, CD45, CD34) was observed. Cells were expanded and 105 ASCs were injected into the wound center immediately after wounding.
OCT Imaging
OCT devices create cross-sectional tomographic images using low-coherence light interferometry (1). Measuring the coherence property of light reflected from a sample provides optical information that is capable of discerning signals with a resolution of several micrometers (1). Human ex vivo wounds were scanned with two OCT devices, the VivoSight Dx 1302 (Michelson Diagnostics Ltd., Kent, UK) and Wasatch Photonics OCT systems (Wasatch Photonics, Wasatch, NC) at days 0, 4 and 7. The VivoSight Dx is a multi-beam swept source Fourier domain OCT device with a central wavelength of 1305 nm and sweep range of ~147 nm, giving a depth penetration of ~2 mm and with estimated axial and lateral resolutions of <10 μm and <7.5 μm, respectively. Field of view (FOV) for the VivoSight Dx device is 6 mm × 6 mm. The VivoSight 4.1 software (Michelson Diagnostics Ltd., Kent, UK) allows for surface curve fitting of the OCT images to generate en face views of the tissue surface. The Wasatch Photonics probe is a spectral domain OCT device with a central bandwidth of 850 nm and spectrum cover of 750-930 nm giving a depth of penetration of ~1.9 mm and estimated axial and lateral resolutions of <3 μm and <6μm, respectively. The FOV of the Wasatch Photonics device is 5 mm × 5 mm. En face images were obtained using the Wasatch Photonics’ proprietary device software. Images from both devices were then analyzed with ImageJ (NIH, Bethesda, MD) and Photoshop Elements 12 (Adobe, San Jose, CA).
Ex vivo wounds were imaged with the OCT probe perpendicularly above the wound. Although close, there was never any direct contact between the probe and the wounds, and no conduction medium was used. Wounds that were imaged longitudinally at multiple time points were positioned under the OCT probe under sterile conditions in a hood (Supp. Fig. 1). To reduce motion artifacts in the hood, the handheld OCT probe was placed on a stand that was designed on SketchUp (Google, Mountain View, CA) and 3D-printed to allow it the probe stand on its own without user manipulation. The human ex vivo wound specimen was centered under the imaging lens (Supp. Fig 1). Both cross-sectional and en face images were derived from the visual data of the same scan with no need to reposition the device.
Quantification of re-epithelialization with OCT was performed with Photoshop Elements 12 (Adobe, San Jose, CA) image selection tools. Selection of total wound area and the non-healed wound area allowed for measurement of these two areas in pixel units. Wounds were quantified with the formula [100 × (Total wound area – Non-healed wound area)/Total wound area].
Histomorphometric Assessment of Wound Epithelialization
Ex vivo wounds were fixed in 10% formalin and then embedded in paraffin. 8 μm sections were stained with Hematoxylin & Eosin. Images were acquired using a Nikon Eclipse E400 microscope (Nikon Corp., Tokyo, Japan). Wounds were quantified using Nikon NIS Elements BR 3.2 software (Nikon Corp., Tokyo, Japan). Quantification of re-epithelialization was performed by capturing the tissue 80 μm from the beginning of the paraffin block containing wound tissue until reaching the middle of the wound at its widest point in diameter. Percent re-epithelialization was calculated by the sum of the epithelial tongues’ length (T) divided by total wound width (W) as [100 × (T1 + T2)/W].
Quantification of wound re-epithelialization using OCT images
To quantify extent of re-epithelialization in OCT acquired images, we utilized en face pictures of the wounds. The total wound area was selected and the surface area of the non-epithelialized wound bed delineated by the edge of the migrating epithelial tongue was selected and quantified. The percent of re-epithelialization was calculated using the formula [100 × (Total wound area – Non-healed wound area)/Total wound area], which allowed quantification of the entire wound rather than only quantifying locations at regular intervals throughout the wound using the standard histomorphometric approach.
Statistical Analyses
Combined OCT and histomorphometric data for re-epithelialization at three time points (0d, 4d, 7d) with triplet measurements was collected from ex vivo wounds of five human donors. A fixed-effects regression model for repeated measurements of the combined data was fitted taking into account the correlation amongst time points with triplet measurements in each time point. Agreement between the two techniques was assessed using the modified Bland-Altman method by taking into account repeated measurements due to the study design (38). In this method, bias represents consistent over or underestimation of one modality as compared to another, while the 95% confidence interval, known as the limits of agreement, describes the predicted limits of discrepancy between the modalities. A Bland-Altman assessment for agreement was used to compare the OCT and HE methods with repeated measured fixed effects models. A range of 95% limits of agreement was defined as mean bias with two standard deviations away from the mean. Mean square error from the repeated measurement fixed-effects regression model is used for the standard deviation. To evaluate differences in epithelialization between ASC-treated and media-treated wounds, a Mann-Whitney U test was implemented. All graphs and analyses were generated using Prism 6 (GraphPad, La Jolla, CA), SAS v9.4 (SAS Institute, Cary, NC), and Excel 2013 (Microsoft, Redmond, WA).
RESULTS
Identification of epithelialization and differentiation of cutaneous tissue compartments with OCT
The ability of OCT to identify key features of re-epithelialization of human wounds was assessed and compared to the standard histology based approach. After generating human ex vivo wounds using established methods (22), OCT images of ex vivo control wounds as well as wounds treated with topical rhEGF to promote epithelialization were assessed at days 4 and 7 post wounding and compared to H&E staining of the same wounds (Fig. 1). In this analysis, cross sectional OCT images were found to be comparable to traditional histological techniques in identifying initial wound edges and the migrating tongue of the epithelium in these wounds (Fig. 1). Even though the migrating tongue can be composed of only 1-2 layers of keratinocytes, the image contrast between epidermis and dermis was identifiable in all ex vivo wounds analyzed by OCT regardless of the extent of wound closure. In addition, the initial wound edge can be distinguished by OCT using the difference in morphology and the disruption of the cornified layer of the epidermis (Fig. 1).
Figure 1. Comparison of OCT vertical sections with histology.
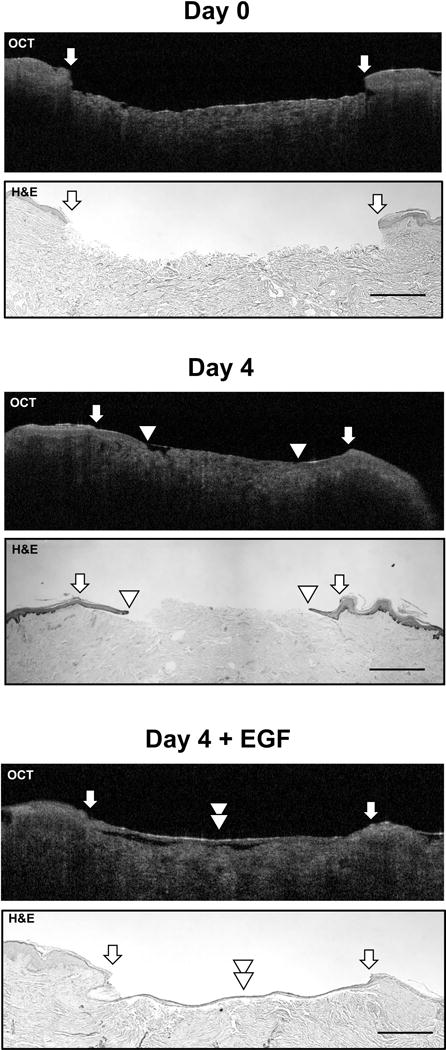
OCT images collected by the VivoSight Dx 1302 (Michelson Diagnostics Ltd., Kent, UK) at 1305 nm wavelength immediately after wounding (Day 0), 4 days after wounding (Day 4), and 4 days after daily treatment with rhEGF accompanied by H&E staining. Arrows indicate the initial wound edge; arrowheads indicate the edge of the migrating epithelial tongue. Scale bar= 500 μm.
OCT imaging of ex vivo wounds in both the cross-sectional and top down (“en face”) aspects was able to provide adequate resolution for differentiation of the cornified epidermis, non-cornified epidermis, papillary and reticular dermis (Fig. 2A&B). In cross-sectional view, the non-cornified migrating epithelial tongue is seen as a hypo-intense homogeneous region that extends from the wound edge without disruption above and parallel to the more disorganized dermis. The differentiated layers of the upper epidermis including the cornified layer can be seen as a hyper-intense region and can be used to determine the initial wound edge of an ex vivo wound. The papillary dermis appears as a heterogeneous layer that is hazy and more loosely associate than the epidermis. OCT also distinguished between papillary and reticular dermis. In addition to cross-sectional images, en face OCT images can also differentiate cornified and non-cornified epidermis, as well as dermis from the top down perspective (Fig. 2B). Furthermore, longitudinal imaging of wounds provides a new perspective on the wound healing process as OCT imaging of ex vivo wounds does not require tissue to be sacrificed to gather data as when processed for histology. This allows for the acquisition of visual data longitudinally at multiple time points in the same wound (Fig. 2C, Supplemental video). In our analysis, after measuring the extent of re-epithelialization over time at days 4 and 7 post-wounding, we found that the OCT technology was able to identify progression of re-epithelialization in the human ex vivo wound model at all time points with the acquisition time of ~1 minute per wound (Supplemental video).
Figure 2. Differentiation of tissue compartments in ex vivo human wounds with OCT and longitudinal imaging of wound healing process with OCT.
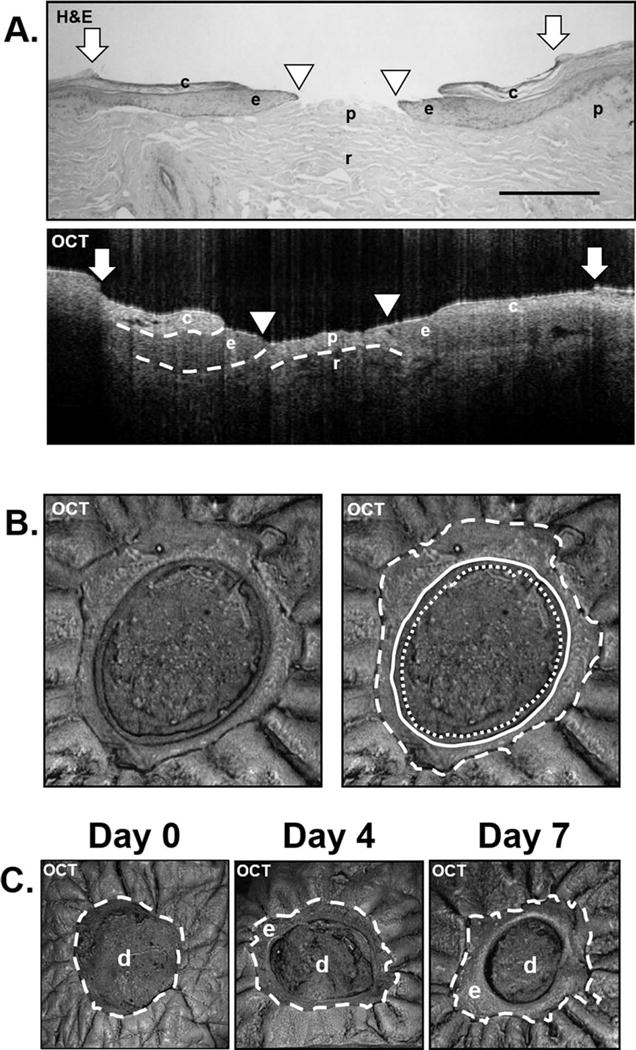
(A.) OCT images collected by the Wasatch Photonics OCT system (Wasatch Photonics, Wasatch, NC) at 850 nm wavelength 7 days post-wounding accompanied by H&E staining demonstrating the ability of OCT to differentiate tissue types. Depicted are c= cornified epidermis; e= non-cornified epithelial tongue; p= papillary dermis; r= reticular dermis. White arrows indicate initial wound edge; white arrowheads indicate the edge of the migrating tongue. Dashed lines indicate the dermal-epidermal junction and the cornified epidermis as well as the border between papillary and reticular dermis. Scale bar= 500 μm. (B.) En face OCT images can also differentiate tissue types from the top down perspective. White dashed line indicates original wound edge. Solid white line indicates the edge of the cornified portion of the newly-formed epidermis. White dotted line indicates the non-cornified portion of the newly-formed epidermis. (C.) En face OCT images at days 0, 4 and 7. Depicted are e= epidermis; d= dermis. White dashed line indicates initial wound edge. En face OCT images in (B) and (C) were collected by the VivoSight Dx 1302 (Michelson Diagnostics Ltd., Kent, UK).
OCT en face image’s quantification of the entire wound is equivalent and superior to histological analysis
We utilized en face images of the wounds acquired with OCT to quantify extent of re-epithelialization and perform comparative analyses with standard histomorphomertic method. The delineation of total wound area and the surface area of the re-epithelialized wound is shown in Fig. 3A&B. While the OCT approach assured unbiased quantification, the percent of closure quantified varied using histology depending on the location of the wound that is chosen for histomorphometric quantification. Depicted in Fig. 3A are two locations that could be judged as the middle of the wound. However, OCT imaging of the same wound showed that the quantification data is vastly different with the histomorphometric approach. Location 1 is only about 50% re-epithelialized while location 2 is completely re-epithelialized (Fig. 3A). Subsequent sectioning of the entire preserved tissue in the paraffin block results in precise quantification of wound closure. However this process is costly and not time effective, and limits the options for further investigations. In addition to providing precise quantification of wound closure in a non-invasive manner, OCT can also guide further immunohistochemistry analyses by identifying optimal locations for sectioning, therefore preserving valuable human biomaterial.
Figure 3. Comparison of quantification methods with OCT and histology.
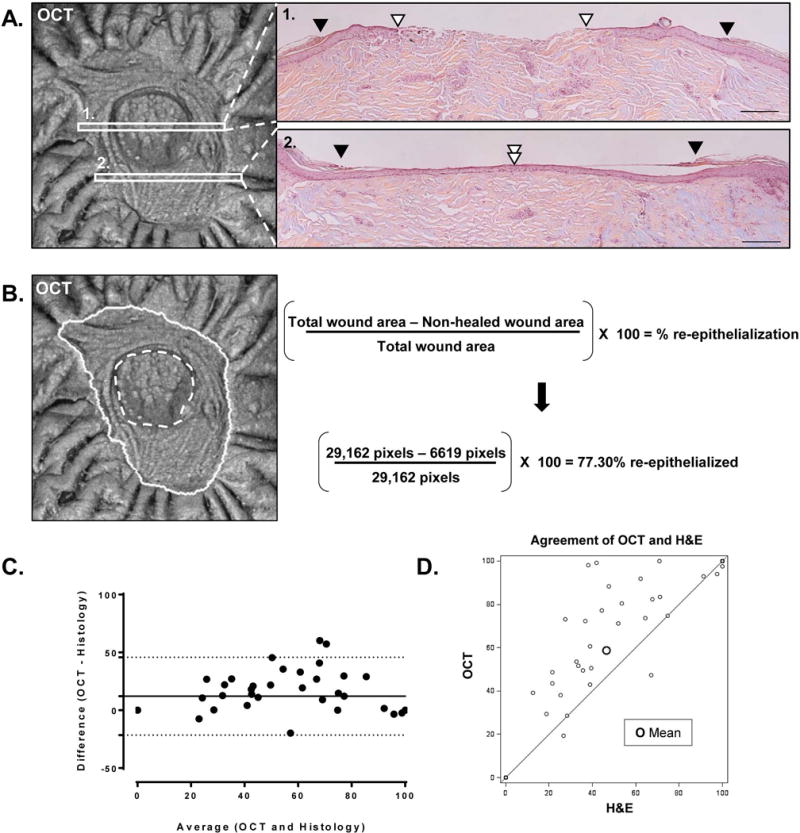
(A.) Quantification of re-epithelialization by histological analysis at two different locations of the same wound; scale bar= 200 μm. Black arrowhead depicts initial wound edge; white arrowhead depicts the edge of the migrating epithelial tongue. (B.) Quantification of re-epithelialization by image processing software of an en face OCT image of an ex-vivo wound. Solid white line= initial wound edge; dashed white line= edge of the epithelial tongue. Using selection tools, the initial wound edge and the inner edge of the epithelial tongue can be traced and their total area measured in pixels. Percent re-epithelialization can be quantified according to the formula shown. This method quantifies the healing of the entire wound area while greatly eliminating histology-dependent variability. The en face OCT images in (A.) and (B.) was collected by the VivoSight Dx 1302 (Michelson Diagnostics Ltd., Kent, UK). (C.) Bland-Altman plot for re-epithelialization of ex vivo wounds comparing OCT and histology-based methods of quantification. Depicted is the agreement between OCT and histomorphometric analysis for the quantification of re-epithelialization in human ex vivo wounds (n=47). Solid horizontal line depicts mean bias. Dotted lines depict the 95% limits of agreement. (D.) Agreement plot for OCT and histology-based methods of quantification. Depicted is the correlation between OCT and histomorphometric analysis for the quantification of re-epithelialization in human ex vivo wounds (n=47). Spearman correlation coefficient R= 0.9198.
Analysis of combined re-epithelialization quantification data in wounds assayed by both techniques, utilizing the modified Bland-Altman method and a pairwise comparison approach, revealed a mean bias of 12.15 using the Bland-Altman method of agreement, with OCT consistently revealing more re-epithelialization as compared with histomorphometric analysis. The 95% limits of agreement ranged from −74.6 to 98.9 (Fig. 3C). A standard correlation plot shows a strongly positive correlation between OCT and histomorphometric analysis with a Spearman correlation coefficient of R= 0.9198 (Fig. 3D). There was almost perfect agreement in all wounds that were found to be 100% re-epithelialized and perfect agreement in wounds that were not re-epithelialized. However the two modalities differed to a greater extent in wounds with intermediate rates of re-epithelialization, with higher re-epithelialization rates identified by the OCT approach utilizing en face images and quantification of total wound area. In summary, the modified Bland-Altman analysis by taking into account the replicated measurements indicates that the 95% limits of agreement between the two methods is from −74.6 to 98.9. The two methods (OCT and H&E) do consistently provide similar measures because there is a level of agreement up to 12.15.
Evaluation of ASC stem cell therapy in human ex vivo wounds using OCT
We have employed OCT to analyze therapeutic potential of allogeneic human adipose derided stem cells (ASC) in human ex vivo wounds. OCT imaging and quantification of total wound area was performed after monitoring the wounds with OCT for a period of 4 days which is the exponential phase of re-epithelization in this model (18). Quantification of wound closure revealed that all ASC-treated wounds completely re-epithelialized, while media treated control wounds showed 50.07% wound closure (Fig. 4 A, B). The increase in re-epithelialization of ASC-treated wounds at 4 days was found to be statistically significant (*p<0.05) (Fig. 4C). Cross-sectional OCT images have identified both single layer and differentiated layers of epidermis in ASC treated wounds confirming enhancement of wound closure (Fig. 4B).
Figure 4. Assessment of stem cell treatment with OCT in the human ex-vivo acute wound model.
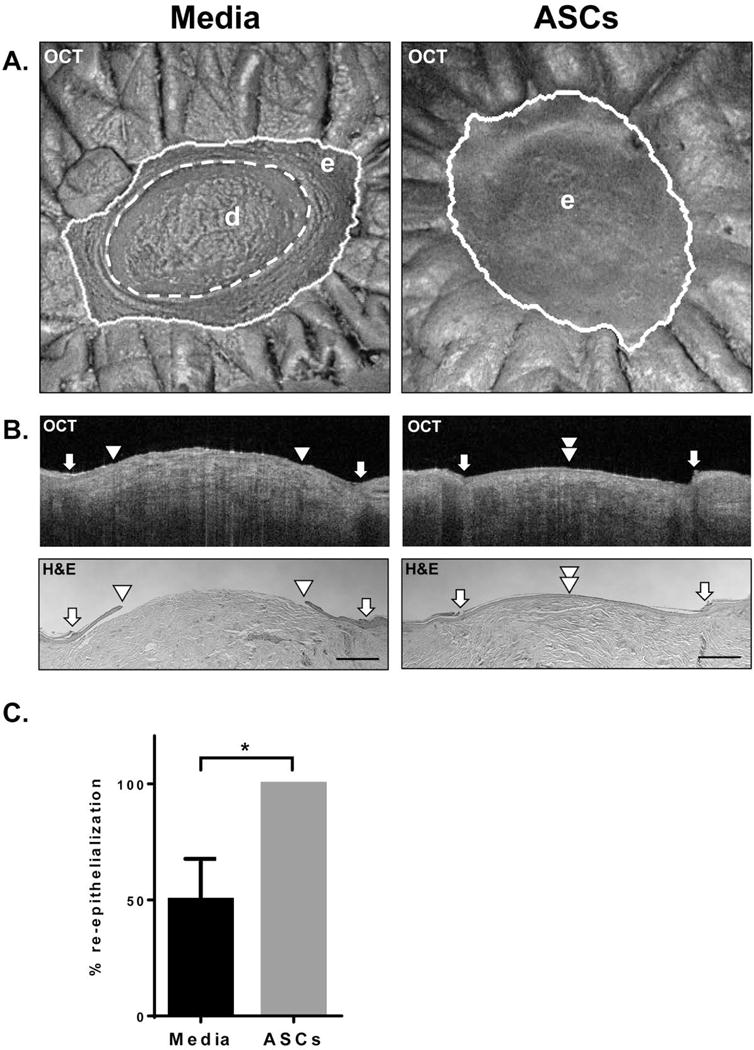
(A.) Representative en face OCT images after 4 days of a media-treated control and a wound treated with human ASCs. Depicted are e=epidermis; d=dermis. ASC treatment enhanced epithelialization that was readily apparent after scanning with OCT. (B.) Representative cross-sectional OCT images and corresponding histology of representative media and ASC-treated wounds. White arrows indicate initial wound edge; white arrowheads indicate the edge of the migrating tongue. Scale bar= 500 μm. Cross-sectional and en face OCT images in (A.) and (B.) were collected by the VivoSight Dx 1302 (Michelson Diagnostics Ltd., Kent, UK). (C.) Bar graph comparing the healing of media and ASC treated wounds (n=7) 4 days post-wounding. Error bar= standard deviation. All ASC treated wounds healed completely compared to media treated control wounds, which was found to be statistically significant using a Mann-Whitney U test (*p<0.05).
DISCUSSION
OCT is emerging as a viable non-invasive imaging modality for the assessment of cutaneous anatomy and pathology (39). Recently, this technology has been used to assess diverse cutaneous conditions such as fibrotic skin diseases (40), skin aging (7, 41), and non-melanoma skin cancers (42) among many other applications. Several recent studies have explored re-epithelialization in vivo with OCT (3–5, 43). Although all these studies concluded that OCT is a viable tool for monitoring wound healing, its application in this field of study is still limited by a lack of standardized methods of quantitative assessment. Furthermore, to the best of authors’ knowledge, no studies have utilized OCT in pre-clinical or ex vivo wound models.
In this study, the viability of the OCT technique in the assessment of re-epithelialization in a human ex vivo cutaneous wound model has been demonstrated. OCT provided adequate resolution to identify the epidermis (both cornified and non-cornified layers), the papillary dermis, reticular dermis, and importantly, migrating epidermis in the wound bed. In addition, en face OCT images were found to be adequate for quantification of re-epithelialization in the human ex vivo wound model. Pairwise statistical analysis including the modified Bland-Altman method of agreement validated the viability and superior precision of en face (top down) OCT imaging for monitoring the wound healing process in this model. Also, we have demonstrated the validity of using OCT to evaluate novel therapies in a human wound model by demonstrating the ability of allogeneic human ASC to promote re-epithelialization. A single ASC treatment of human ex vivo wounds resulted in significant stimulation of re-epithelization as documented by OCT. These results are in agreement with previous studies that utilized human ex vivo wounds to document differentiation of mesenchymal stem cells into both keratinocyte and fibroblast lineage (19). The capability of OCT to quantify the extent of wound closure in real time in a human model has the potential to greatly facilitate pre-clinical testing and bring novel therapeutics to clinical application. The utility of OCT in human ex vivo wounds clearly extends beyond the study of the normal acute wound healing process. It can be used to accurately and efficiently dissect and quantify mechanisms of stem cell action and epidermal migration during re-epithelialization in addition to evaluation of topical or injected therapies.
The advantages of OCT are numerous. Foremost, it is non-invasive and free from deleterious side effects, using only light in the infrared range. This means that tissue samples such as ex vivo wounds do not need to be sacrificed at each time point of interest, but rather the same wounds can be repeatedly scanned at multiple time points to more effectively monitor the progression of wound healing longitudinally while simultaneously reducing variability due to comparing different tissue samples from potentially different topographical skin locations. Also, wounds can be rapidly scanned, with results produced in a matter of seconds to minutes in contrast to the time consuming process of tissue fixation, embedding, sectioning, and staining. Furthermore, using en face OCT images, one can quantify the epithelialization of an entire wound area rather than at specific locations within the wound as is done with histomorphometric analyses. As the OCT imaging approach documented, these wounds often heal unevenly due to the micro-environment of the wound bed at the cellular level. In addition, ex vivo wounds, as with many wound models, are embedded in paraffin, which can lead to an over- or underestimation of the wound closure depending on sample processing. The OCT quantification method is advantageous because it measures closure of the entire wound in units of area while histological methods measure in units of length that are then extrapolated to wound area. Thus, OCT greatly reduces data variability due to uneven healing, sample processing, and technical error in the quantification of wound re-epithelialization, and it rapidly produces data that can reduce the number of tissue samples required for a given experiment.
OCT devices used in this study have certain limitations. Although the VivoSight Dx is already in clinical use, the lack of high-definition of its OCT images does not provide adequate resolution to differentiate between individual cells or all five strata of epidermis as traditional H&E histology. However, newer improved HD-OCT devices can provide real-time three-dimensional images with higher resolution that can visualize individual cells and have been successfully utilized to assess skin in vitro and in vivo (6–8). The depth at which an OCT device can reach as well as the resolution that an OCT device can provide depends on wavelength (1). Shorter wavelengths provide higher resolution and differentiation between different tissue types, while longer wavelengths provide deeper penetration into the dermis (1). Thus, wavelength is an important consideration when designing a project. Additionally, it is difficult for coherence light to penetrate hemostatic material, thus limiting visualization of the epidermis in wounds of in vivo animal studies that are covered with significant crust. The devices used in this study range from $60,000 to $120,000 USD. Therefore, current costs could also be a limitation of the OCT technology in cutaneous studies. Although histological tissue processing and microscopes are not without significant costs, the price of an OCT device remains a consideration.
In all, OCT is a viable and useful method for quantifying re-epithelialization of the human ex vivo wound model. It generates reproducible results quite rapidly, and it has the potential to further optimize pre-clinical wound healing research with improved turnaround time and accuracy. OCT offers the advantage of providing an alternative for rapid tissue screening and as an adjunct to histology when larger areas of tissue need to be investigated. Well established for its safety and utility in clinical and pre-clinical ophthalmologic studies, this technology is now garnering acceptance in cutaneous research as well. In vivo viability of OCT in skin (7, 8), skin diseases, and more recently in acute wounds has already been demonstrated (3–5). However, this technology should also be further explored for its application to pre-clinical research, especially in other pre-clinical models including animal wound models. Further optimization of the current devices and utilization of HD-OCT, combined with increased experience and expertise by clinical and research personnel alike may add further value to the use of the OCT technology in the study of more complicated cutaneous pathology such as chronic wounds both in vivo and in the laboratory setting.
Supplementary Material
(A.) Left, VivoSight Dx 1302 probe scanning human ex vivo wounds under sterile conditions in a biosafety cabinet. Right, VivoSight Dx 1302 device (Michelson Diagnostics Ltd., Kent, UK) in its entirety. (B.) Wasatch Photonics OCT device (Wasatch Photonics, Wasatch, NC).
Videos are taken in the cross-sectional perspective and depict continuous acquisition from one wound edge to the opposite edge in a series of OCT vertical sections. Frame rate was 22 fps (frames per second), and each frame was taken at a distance of 9.765 μm further into the wound. Depth of penetration was 1.9 mm. OCT allows for rapid cross-sectional analysis of the entire wound area while avoiding tissue processing. Cross sectional OCT images were acquired with the Wasatch Photonics OCT device (Wasatch Photonics, Wasatch, NC).
Acknowledgments
We are thankful to Wasatch Photonics and Michelson Diagnostics for facilitating the OCT devices used in our study. IP acknowledges funding for the University of Miami SAC award UM016. MTC acknowledges funding from the National Institutes of Health for financial support (NR015649; NR013881).
References
- 1.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung W, Boppart SA. Optical coherence tomography for rapid tissue screening and directed histological sectioning. Anal Cell Pathol (Amst) 2012;35(3):129–43. doi: 10.3233/ACP-2011-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greaves NS, Benatar B, Whiteside S, Alonso-Rasgado T, Baguneid M, Bayat A. Optical coherence tomography: a reliable alternative to invasive histological assessment of acute wound healing in human skin? Br J Dermatol. 2014;170(4):840–50. doi: 10.1111/bjd.12786. [DOI] [PubMed] [Google Scholar]
- 4.Singer AJ, Wang Z, McClain SA, Pan Y. Optical coherence tomography: a noninvasive method to assess wound reepithelialization. Acad Emerg Med. 2007;14(5):387–91. doi: 10.1197/j.aem.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Kuck M, Strese H, Alawi SA, Meinke MC, Fluhr JW, Burbach GJ, et al. Evaluation of optical coherence tomography as a non-invasive diagnostic tool in cutaneous wound healing. Skin Res Technol. 2014;20(1):1–7. doi: 10.1111/srt.12077. [DOI] [PubMed] [Google Scholar]
- 6.Boone MA, Draye JP, Verween G, Aiti A, Pirnay JP, Verbeken G, et al. Recellularizing of human acellular dermal matrices imaged by high-definition optical coherence tomography. Exp Dermatol. 2015;24(5):349–54. doi: 10.1111/exd.12662. [DOI] [PubMed] [Google Scholar]
- 7.Boone MA, Suppa M, Marneffe A, Miyamoto M, Jemec GB, Del Marmol V. High-definition optical coherence tomography intrinsic skin ageing assessment in women: a pilot study. Arch Dermatol Res. 2015;307(8):705–20. doi: 10.1007/s00403-015-1575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boone M, Jemec GB, Del Marmol V. High-definition optical coherence tomography enables visualization of individual cells in healthy skin: comparison to reflectance confocal microscopy. Exp Dermatol. 2012;21(10):740–4. doi: 10.1111/j.1600-0625.2012.01569.x. [DOI] [PubMed] [Google Scholar]
- 9.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, et al. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle) 2014;3(7):445–64. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kratz G. Modeling of wound healing processes in human skin using tissue culture. Microsc Res Tech. 1998;42(5):345–50. doi: 10.1002/(SICI)1097-0029(19980901)42:5<345::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Meier NT, Haslam IS, Pattwell DM, Zhang GY, Emelianov V, Paredes R, et al. Thyrotropin-releasing hormone (TRH) promotes wound re-epithelialisation in frog and human skin. PLoS One. 2013;8(9):e73596. doi: 10.1371/journal.pone.0073596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syed F, Bagabir RA, Paus R, Bayat A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Lab Invest. 2013;93(8):946–60. doi: 10.1038/labinvest.2013.82. [DOI] [PubMed] [Google Scholar]
- 13.Pastar I, Khan AA, Stojadinovic O, Lebrun EA, Medina MC, Brem H, et al. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem. 2012;287(35):29324–35. doi: 10.1074/jbc.M112.382135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286(12):10265–75. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vukelic S, Stojadinovic O, Pastar I, Vouthounis C, Krzyzanowska A, Das S, et al. Farnesyl pyrophosphate inhibits epithelialization and wound healing through the glucocorticoid receptor. J Biol Chem. 2010;285(3):1980–8. doi: 10.1074/jbc.M109.016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomic-Canic M, Mamber SW, Stojadinovic O, Lee B, Radoja N, McMichael J. Streptolysin O enhances keratinocyte migration and proliferation and promotes skin organ culture wound healing in vitro. Wound Repair Regen. 2007;15(1):71–9. doi: 10.1111/j.1524-475X.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 17.Pullar CE, Grahn JC, Liu W, Isseroff RR. Beta2-adrenergic receptor activation delays wound healing. FASEB J. 2006;20(1):76–86. doi: 10.1096/fj.05-4188com. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo AE, Beckett LA, Baier BS, Isseroff RR. The linear excisional wound: an improved model for human ex vivo wound epithelialization studies. Skin Res Technol. 2012;18(1):125–32. doi: 10.1111/j.1600-0846.2011.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivamani RK, Schwartz MP, Anseth KS, Isseroff RR. Keratinocyte proximity and contact can play a significant role in determining mesenchymal stem cell fate in human tissue. FASEB J. 2011;25(1):122–31. doi: 10.1096/fj.09-148775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastar I, Stojadinovic O, Sawaya AP, Stone RC, Lindley LE, Ojeh N, et al. Skin Metabolite, Farnesyl Pyrophosphate, Regulates Epidermal Response to Inflammation, Oxidative Stress, and Migration. J Cell Physiol. 2016;231(11):2452–63. doi: 10.1002/jcp.25357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jozic I, Vukelic S, Stojadinovic O, Liang L, Ramirez HA, Pastar I, et al. Stress Signals, Mediated by Membranous Glucocorticoid Receptor, Activate PLC/PKC/GSK-3beta/beta-catenin Pathway to Inhibit Wound Closure. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stojadinovic O, Tomic-Canic M. Human ex vivo wound healing model. Methods Mol Biol. 2013;1037:255–64. doi: 10.1007/978-1-62703-505-7_14. [DOI] [PubMed] [Google Scholar]
- 23.Emanuelsson P, Kratz G. Characterization of a new in vitro burn wound model. Burns. 1997;23(1):32–6. doi: 10.1016/s0305-4179(96)00073-3. [DOI] [PubMed] [Google Scholar]
- 24.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6(1):e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza-Garcia J, Sebastian A, Alonso-Rasgado T, Bayat A. Optimization of an ex vivo wound healing model in the adult human skin: Functional evaluation using photodynamic therapy. Wound Repair Regen. 2015;23(5):685–702. doi: 10.1111/wrr.12325. [DOI] [PubMed] [Google Scholar]
- 26.Hodgkinson T, Bayat A. Ex vivo evaluation of acellular and cellular collagen-glycosaminoglycan flowable matrices. Biomed Mater. 2015;10(4):041001. doi: 10.1088/1748-6041/10/4/041001. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Jong Hong S, Jia S, Zhao Y, Galiano RD, Mustoe TA. Application of a partial-thickness human ex vivo skin culture model in cutaneous wound healing study. Lab Invest. 2012;92(4):584–99. doi: 10.1038/labinvest.2011.184. [DOI] [PubMed] [Google Scholar]
- 28.Roupe KM, Nybo M, Sjobring U, Alberius P, Schmidtchen A, Sorensen OE. Injury is a major inducer of epidermal innate immune responses during wound healing. J Invest Dermatol. 2010;130(4):1167–77. doi: 10.1038/jid.2009.284. [DOI] [PubMed] [Google Scholar]
- 29.Andrew Chan KL, Zhang G, Tomic-Canic M, Stojadinovic O, Lee B, Flach CR, et al. A coordinated approach to cutaneous wound healing: vibrational microscopy and molecular biology. J Cell Mol Med. 2008;12(5B):2145–54. doi: 10.1111/j.1582-4934.2008.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stojadinovic O, Lee B, Vouthounis C, Vukelic S, Pastar I, Blumenberg M, et al. Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-gamma pathway, and wound healing along with promotion of terminal differentiation. J Biol Chem. 2007;282(6):4021–34. doi: 10.1074/jbc.M606262200. [DOI] [PubMed] [Google Scholar]
- 31.Lee B, Vouthounis C, Stojadinovic O, Brem H, Im M, Tomic-Canic M. From an enhanceosome to a repressosome: molecular antagonism between glucocorticoids and EGF leads to inhibition of wound healing. J Mol Biol. 2005;345(5):1083–97. doi: 10.1016/j.jmb.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167(1):59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int J Mol Sci. 2015;16(10):25476–501. doi: 10.3390/ijms161025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumberg SN, Berger A, Hwang L, Pastar I, Warren SM, Chen W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2012;96(1):1–9. doi: 10.1016/j.diabres.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 35.Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22(3):313–25. doi: 10.1111/wrr.12173. [DOI] [PubMed] [Google Scholar]
- 36.Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20(2):205–16. doi: 10.3727/096368910X520065. [DOI] [PubMed] [Google Scholar]
- 37.Tashiro J, Elliot SJ, Gerth DJ, Xia X, Pereira-Simon S, Choi R, et al. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl Res. 2015;166(6):554–67. doi: 10.1016/j.trsl.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 39.Paul DW, Ghassemi P, Ramella-Roman JC, Prindeze NJ, Moffatt LT, Alkhalil A, et al. Noninvasive imaging technologies for cutaneous wound assessment: A review. Wound Repair Regen. 2015;23(2):149–62. doi: 10.1111/wrr.12262. [DOI] [PubMed] [Google Scholar]
- 40.Babalola O, Mamalis A, Lev-Tov H, Jagdeo J. Optical coherence tomography (OCT) of collagen in normal skin and skin fibrosis. Arch Dermatol Res. 2014;306(1):1–9. doi: 10.1007/s00403-013-1417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trojahn C, Dobos G, Richter C, Blume-Peytavi U, Kottner J. Measuring skin aging using optical coherence tomography in vivo: a validation study. J Biomed Opt. 2015;20(4):045003. doi: 10.1117/1.JBO.20.4.045003. [DOI] [PubMed] [Google Scholar]
- 42.Reggiani C, Manfredini M, Mandel VD, Farnetani F, Ciardo S, Bassoli S, et al. Update on non-invasive imaging techniques in early diagnosis of non-melanoma skin cancer. G Ital Dermatol Venereol. 2015;150(4):393–405. [PubMed] [Google Scholar]
- 43.Greaves NS, Iqbal SA, Hodgkinson T, Morris J, Benatar B, Alonso-Rasgado T, et al. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen. 2015;23(4):483–94. doi: 10.1111/wrr.12308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A.) Left, VivoSight Dx 1302 probe scanning human ex vivo wounds under sterile conditions in a biosafety cabinet. Right, VivoSight Dx 1302 device (Michelson Diagnostics Ltd., Kent, UK) in its entirety. (B.) Wasatch Photonics OCT device (Wasatch Photonics, Wasatch, NC).
Videos are taken in the cross-sectional perspective and depict continuous acquisition from one wound edge to the opposite edge in a series of OCT vertical sections. Frame rate was 22 fps (frames per second), and each frame was taken at a distance of 9.765 μm further into the wound. Depth of penetration was 1.9 mm. OCT allows for rapid cross-sectional analysis of the entire wound area while avoiding tissue processing. Cross sectional OCT images were acquired with the Wasatch Photonics OCT device (Wasatch Photonics, Wasatch, NC).


