Abstract
The methylazoxymethanol acetate (MAM) rodent neurodevelopmental model of schizophrenia exhibits aberrant dopamine system activation attributed to hippocampal dysfunction. Context discrimination is a component of numerous behavioral and cognitive functions and relies on intact hippocampal processing. The present study explored context processing behaviors, along with dopamine system activation, during fear learning in the MAM model.
Male offspring of dams treated with MAM (20mg/kg,i.p.) or saline on gestational day 17 were used for electrophysiological and behavioral experiments. Animals were tested on the immediate shock fear conditioning paradigm, with either different preconditioning contexts or varying amounts of context pre-exposure (0–10 sessions). Amphetamine-induced locomotor activity and dopamine neural activity was measured 1-week after fear conditioning.
Saline, but not MAM animals, demonstrated enhanced fear responses following a single context pre-exposure in the conditioning context. One week following fear learning, saline rats with 2 or 7 minutes of context pre-exposure prior to fear conditioning also demonstrated enhanced amphetamine-induced locomotor response relative to MAM animals. Dopamine neuron recordings showed fear learning-induced reductions in spontaneous dopamine neural activity in MAM rats that was further reduced by amphetamine. Apomorphine administration confirmed that reductions in dopamine neuron activity in MAM animals resulted from over excitation, or depolarization block.
These data show a behavioral insensitivity to contextual stimuli in MAM rats that coincides with a less dynamic dopamine response after fear learning.
Keywords: schizophrenia, fear learning, dopamine, context discrimination, animal models, electrophysiology
Introduction
The hippocampus plays a pivotal role in context discrimination functions, including those necessary for fear learning (Frankland et al., 1998; McDonald et al., 2004) (Frohardt et al., 1999; Holt and Maren, 1999; Quintero et al., 2011; Young et al., 1995). Schizophrenia is a complex psychiatric disorder with known hippocampal dysfunction and context discrimination deficits (Benes, 2015; Guillaume et al., 2015; MacDonald et al., 2005; MacDonald et al., 2003; Schobel et al., 2013; Schobel et al., 2009a; Schobel et al., 2009b; Servan-Schreiber et al., 1996; Siever et al., 2002; Talamini and Meeter, 2009), such as inappropriate memory generalization or an inability to ignore irrelevant stimuli (Gal et al., 2005; Ivleva et al., 2012; Jazbec et al., 2007; Racsmany et al., 2008; Roiser et al., 2009; Shohamy et al., 2010; Warren and Haslam, 2007). Patients are also unable to modulate hippocampal activation during recognition memory, especially in response to novel stimuli (Ivleva et al., 2012; Schott et al., 2015). The hippocampus shows aberrant increases in activity preceding transition to psychosis, and there is a proposed link between hippocampal activation, morphological changes, and severity of positive symptoms (e.g. hallucinations and delusions) (Arnold et al., 2015; Jensen et al., 2008; Narr et al., 2004; Schobel et al., 2009a; Talati et al., 2014; Zierhut et al., 2013).
Abnormal hippocampal activity in schizophrenia likely underlies the pathological alteration of the dopamine system (Abi-Dargham et al., 1998; Abi-Dargham et al., 2004; Abi-Dargham et al., 2009; Breier et al., 1997; Howes et al., 2013; Laruelle and Abi-Dargham, 1999). The methylazoxymethanol acetate (MAM) rodent neurodevelopmental model of schizophrenia has demonstrated that increased dopamine activity measured both electrophysiologically and behaviorally can be attributed to disrupted GABA-mediated inhibition within the ventral hippocampus (Gill and Grace, 2014; Gill et al., 2011; Lodge et al., 2009; Lodge and Grace, 2007). How this hyperactivity of the dopamine system relates to potential context processing deficits is not clear, although there is evidence from patients of an altered dopamine activation in response to contextual novelty (Heinz and Schlagenhauf, 2010). In normal rats, increased dopamine release in limbic brain regions is associated with contextual fear learning(Martinez et al., 2008). Elevated dopamine activation in MAM rats resulting from hippocampal overdrive could obscure fear learning related changes in dopamine release.
We examined context processing deficits in MAM rats using the immediate shock fear conditioning paradigm. This task requires the rapid and accurate retrieval of contextual information acquired during a pre-exposure session (Huff et al., 2006; Matus-Amat et al., 2007; Robinson-Drummer and Stanton, 2015; Rudy et al., 2002). Accurate fear learning in this paradigm requires the hippocampus, a region with known perturbation in the MAM model (Lodge et al., 2009; Lodge and Grace, 2007).
The ability to discriminate between two distinct contexts following fear conditioning, as well as the impact of repeated presentation of contextual stimuli on performance, was assessed in the MAM model. It was anticipated that MAM rats would require more extensive context pre-exposure to produce a similar reduction of fear responses that is observed in normal rats. In contrast, more extensive context exposure may instead be necessary for increasing fear responses in MAM rats to comparable levels accomplished with less context exposure in normal rats due to a malfunctioning ventral hippocampus. Typically, repeated presentation of a discrete stimulus prior to conditioning lessens its associative strength in a process described as latent inhibition. Deficits in latent inhibition in schizophrenia are inconsistent and appear dependent on medication status or disease duration (Gal et al., 2009; Lubow et al., 2000; Rascle et al., 2001; Swerdlow et al., 1996; Vaitl et al., 2002; Williams et al., 1998). However, there is compelling evidence from animal models that latent inhibition results in both increased dopamine release in the nucleus accumbens and requires intact processing in the ventral hippocampus, especially via the primary output of the ventral subiculm(Gray et al., 1995; Peterschmitt et al., 2005). Electrophysiological recordings from dopamine neurons in the ventral tegmental area are an indirect measure of underlying hippocampal hyperactivity in the MAM model(Lodge et al., 2009; Lodge and Grace, 2007) and other constructs (stress (Valenti et al., 2012), pilocarpine model of temporal lobe epilepsy (Cifelli and Grace, 2012), amphetamine (Lodge and Grace, 2008)). Consequently, whether there was a persistent consequence of contextual fear learning on the dopamine system of MAM animals was measured via electrophysiological recordings from the ventral tegmental area or the locomotor response to amphetamine 7–10 days after fear conditioning.
Methods
Experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Animals were housed in a temperature (22°C) and humidity (47%) controlled environment with a 12-hour light/dark cycle (lights on 7 a.m.) with ad libitum access to both food and water. For behavioral experiments, animals were housed in a reverse light cycle room (lights on 7 p.m.) and tested during the lights-off cycle. Behavioral experiments began 7 days after animals were placed in the reverse light cycle room.
Methylazoxymethanol Treatment
Timed pregnant female Sprague Dawley rats (Hilltop) were obtained on gestational day (GD) 15. MAM (20 mg/kg, i.p.) or saline (1 ml/kg, i.p.) was administered on GD 17, as described previously(Gill et al., 2011; Lodge et al., 2009; Lodge and Grace, 2007; Moore et al., 2006). Male pups were weaned (day 21) and pair-housed with littermates until use in electrophysiological or behavioral experiments (approximately 3–4 months). Each MAM and Saline litter varied in the overall number of male offspring produced (range:3–7). However, animals from individual MAM and Saline litters were counterbalanced across the fear conditioning treatment groups to avoid a potential litter effect. Therefore within any given behavior or electrophysiological group (e.g. fear conditioned MAM rats with 1 pre-exposure), rats from different litters were represented. In addition, the control MAM and saline animals used for behavioral and electrophysiological comparisons were offspring of MAM- and saline-treated dams that did not undergo any fear conditioning but were exposed to the testing environment.
Exps. 1 and 5: Context pre-exposure during fear conditioning
All animals were handled for a minimum of 2 days (2 min/day) prior to context pre-exposure and training in the immediate shock fear conditioning paradigm (Barrientos et al., 2002; Huff and Rudy, 2004; Rudy et al., 2002). Experiments varied (details below) by the type of context pre-exposure (Exp.1) or the number of context pre-exposures (Exp.2). Animals were randomly assigned to the experimental conditions and counterbalanced (Fig.1).
Fig.1.
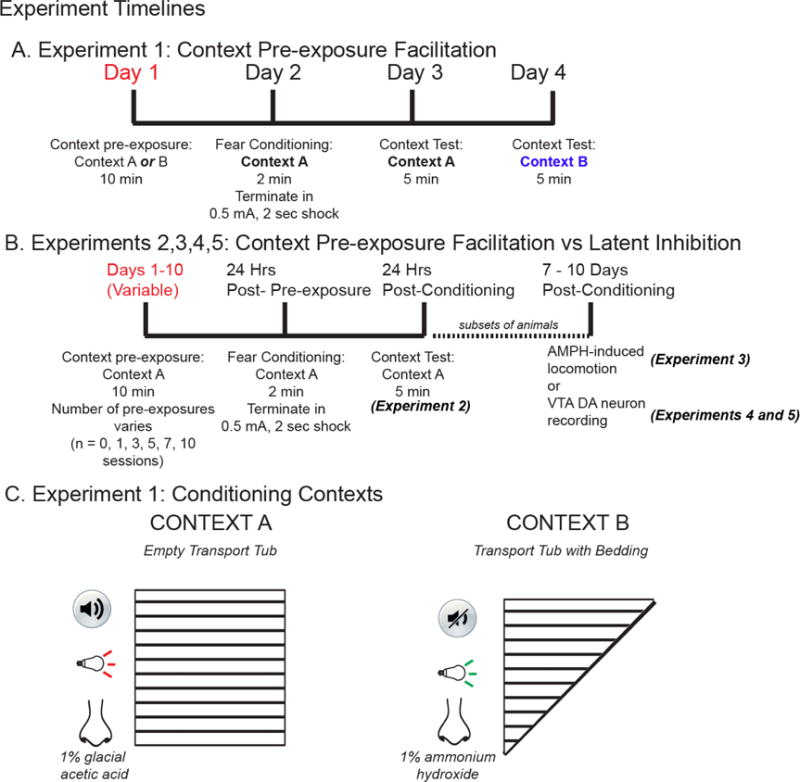
(A) Experimental timeline for context pre-exposure in Context A or B 24hr prior to fear conditioning. (B) Experimental timeline of context pre-exposure (0,1,3, 5,7, or 10 sessions Context A only) prior to fear conditioning and amphetamine-induced locomotor tests or electrophysiological recording. (C) Exp.1, Contexts A or B varied along the dimensions of sound (with or without 75-dB 3000 Hz tone), light cue (red vs green), spatial configuration of testing chamber (square or triangle), and odor (1%glacial acetic acid vs 1%ammonium hydroxide).
Exp.1
Context A and Context B varied along several dimensions (Fig.1C). On Day 1, animals explored one of two conditioning contexts (Fig.1A) for 10 min. 24 hours after pre-exposure, all animals were placed in Context A for 2 min, terminating in a 2-sec, 0.5 mA shock through the grid floor. Animals were immediately returned to the home cage. 24-hours after conditioning, animals were placed in Context A and freezing behavior was measured (5 min). Subsequently, 24-hours after the Context A test, animals were placed in Context B and freezing behavior was measured (5 min).
Exp.2: Amphetamine-induced locomotor activity post-fear conditioning
7–10 days following fear conditioning (Exp.2), animals received acute injections of D-Amphetamine hemisulfate salt (Sigma; 0.5mg/kg, i.p.). This dose typically produces a greater locomotor response in MAM animals relative to saline controls (Gill et al., 2014; Gill et al., 2011; Lodge et al., 2009). Baseline (30min) and post-amphetamine (90min) locomotor activity was measured by beam breaks in the x–y plane of an open field arena (Coulbourn Instruments, TruScan software, Allentown, PA). Total distance travelled (cm) was computed (5min epochs).
Exps. 3 and 4: DA neuron electrophysiological recordings post-fear conditioning
7–10 days following fear conditioning (Exp.2), single-unit electrophysiological recordings were conducted from the ventral tegmental area (VTA) of animals anesthetized with chloral hydrate (See Supplemental methods).
Some animals received a dose of amphetamine (0.5 mg/kg, i.p.) 30 min prior to dopamine recordings. In another subset of animals, the VTA was sampled in both right and left hemispheres pre- and post-apomorphine (Sigma; 20μg/kg, i.v.) administration, respectively. Since D2 auto-receptors are more responsive to dopamine than the post-synaptic receptors, low doses of apomorphine (range of doses applied 20–120μg/kg, i.v.) can preferentially stimulate D2 autoreceptors and inhibit dopamine neuron firing (Akaoka et al., 1992; Bunney and Grace, 1978; Chiodo et al., 1984; Grace and Bunney, 1985; Valenti et al., 2011). The doses of apomorphine used in the present study are consistent with the auto-receptor selectivity.
30 min after apomorphine administration, the contralateral VTA was sampled in an identical manner. (See Supplement for histological methods.)
Exp.5
Animals received varying amounts of Context A pre-exposure (Fig.1B; 0,1,3,5,7,10 10-min sessions; one session per day separated by 24 hours), with conditioning and testing completed 24 and 48 hours after the last pre-exposure, respectively.
Statistics
Electrophysiological analysis was performed using PowerLab (AD instruments) and Nex (NEX Technologies) software. Freezing behavior was quantified using Any-Maze software (San Diego Instruments). Parameters for individual bouts of freezing, initiated by immobility greater than 2 sec, were established in the software. Movements greater than that associated with respiration (e.g. rearing, head movements, locomotion) signaled the end of a bout of freezing. Freezing was then expressed as the total time spent freezing summed across all bouts of freezing. All data are represented as the mean ± SEM. Unless otherwise stated, electrophysiological and behavioral data were assessed with 2-way analysis of variance (ANOVA; SigmaPlot software). Post-hoc comparisons were done using Sidak’s multiple comparisons test after a significance of p < 0.05 for main effects was observed.
Results
Exp1. MAM-treated offspring failed to demonstrate enhancement of contextual fear responses following context pre-exposure
During the Context A Test (5 min), the amount of time spent freezing was assessed for Saline (n=18) and MAM-treated (n=18) offspring that were pre-exposed to either Context A (conditioning context) or Context B (neutral context) 24 hours prior to fear conditioning. There was a significant interaction between type of context pre-exposure (A vs B) and MAM treatment (Fig.2a; F1,32 = 17.58, p<0.05). Saline animals displayed the expected benefit of pre-exposure to the conditioning context such that animals in the Context A (conditioning context) group froze significantly more than animals in the Context B group (neutral context) (p=0.0006). In contrast, there was no difference in the freezing demonstrated by MAM animals between Context A or Context B groups (p = 0.43). This suggests MAM animals cannot utilize previously acquired contextual information in a novel fear learning situation.
Fig.2.

(A) Pre-exposure to the conditioning context (Context A) enhanced freezing responses in Saline-treated offspring (n=18) in comparison to pre-exposure to a neutral context (context B). In contrast, MAM-treated offspring (n=18) did not show enhanced fear responses as a result of pre-exposure to the conditioning context. (B). Both Saline and MAM-treated offspring spent little time freezing when placed in the neutral context after fear conditioning. In addition, the time spent freezing did not differ as a factor of pre-exposure to Context A or Context B. (* denotes p<0.05).
To assess whether freezing behavior was exclusive to the conditioning context, all animals were placed in the neutral context for the Context B test (5 min; Fig 2b). There were comparable levels of freezing in the neutral context demonstrated by all groups with respect to pre-exposure type, MAM treatment, and interaction (F’s1,32 = 0.01, 3.15, and 0.09, respectively; p’s = 0.91, 0.09, 0.77, respectively). Additional direct comparison of the amount of freezing exhibited by MAM rats showed that despite demonstrating less freezing than Saline rats, MAM animals froze more in Context A than Context B (data not shown; F1,32 = 9.51, p <0.05), but their freezing behavior did not vary as a function of Context pre-exposure (F1,32 = 1.73, p = 0.19). This would imply that general fear learning responses are intact in MAM animals, but that those responses are impaired relative to the responses exhibited by normal rats and they are not modulated by contextual information.
Exp.2: Differential locomotor response to amphetamine occurs after fear conditioning in MAM and Saline animals
The previously fear-conditioned MAM (n=24) and Saline rats (n=23), along with separate groups of naïve MAM (n=4) and Saline rats (n=4; see Fig.1b for behavior timeline), were tested for their acute response to amphetamine 7–10 days post-conditioning.
There was a significant interaction between MAM treatment and context pre-exposure prior to fear conditioning on the peak locomotor response to amphetamine (two-way ANOVA; F6.41= 4.88, p = 0.008; main effect MAM F1.41= 2.42, p = 0.13; main effect context pre-exposure F6.41= 0.81, p = 0.57). Consistent with previous studies of the MAM model, post-hoc comparisons showed that naïve MAM animals had enhanced locomotor activity relative to naïve Saline rats (p=0.03). However, following fear conditioning, saline animals had enhanced amphetamine-induced locomotor activity relative to fear conditioned MAM animals in the 0 and 1 context pre-exposure groups (Fig.3; p = 0.005 and 0.04, respectively). Therefore, brief amounts of context exposure in a fear learning situation caused a persistent increase in the level of dopamine system activation in Saline, but not MAM, animals. In contrast, fear conditioning reduced the amount of dopamine system activation in MAM animals. (See Supplemental Results (A and B) for additional linear regression analysis of the fear responses and dopamine system activation in addition to changes in novelty-induced locomotor activity).
Fig.3.

Naïve MAM animals (n=4) demonstrated an enhanced locomotor response to amphetamine (0.5 mg/kg, i.p.) compared to naïve saline rats (n=4; dashed line, 0.5 mg/kg, i.p.). One week after fear conditioning, Saline animals with small amounts of context experience (0 and 1 pre-exposure groups; n=4 per exposure group) demonstrated a peak locomotor response to amphetamine that was significantly greater than MAM fear conditioned animals. Fear conditioning in MAM rats also reduced the peak locomotor response to amphetamine across context exposure groups. (* denotes p<.05).
Exp.3: Electrophysiological evidence for fear-induced reduction in dopamine system activation in MAM rats
Previous studies in the MAM model reported enhanced locomotor response to amphetamine as well as increased spontaneous dopamine neuron activity measured electrophysiologically (Du and Grace, 2013; Gill et al., 2014; Gill et al., 2011; Lodge et al., 2009; Lodge and Grace, 2007; Valenti et al., 2011). Based on the groups showing the greatest differences between MAM and Saline rats in Exp.3, electrophysiological recordings were limited to fear conditioned Saline (n=18) and MAM (n=19) animals that experienced a single context pre-exposure. In addition, the combined effect of fear learning (one week prior to electrophysiology) and AMPH administration (30 min prior to electrophysiological recording) was tested.
There were significant effects of fear learning (F1,30 = 3.68, p<0.05) as well as the interaction between MAM treatment, fear learning, and amphetamine administration (F2,30 = 6.35, p<0.006) on the number of spontaneously active dopamine neurons observed per track (Fig.4a). Consistent with previous studies, control MAM rats exhibited a significantly greater number of active dopamine neurons than control Saline rats (p=0.04). Fear conditioned MAM rats demonstrated significantly fewer spontaneously active dopamine neurons than control MAM rats (p=0.02). In addition, in comparison to naïve MAM animals, the combination of fear conditioning and amphetamine also reduced the number of active dopamine neurons (p=0.002). However, fear conditioning and the combination of fear conditioning with amphetamine did not significantly alter the number of spontaneously active dopamine neurons in Saline rats (p = 0.95 and 0.99, respectively). This would suggest that the activity of dopamine neurons in MAM animals was more sensitive to fear learning and amphetamine manipulations.
Fig.4.

Fear conditioning selectively attenuates VTA activity in MAM rats (A) Control MAM animals (n=5;red bars) demonstrate significantly more spontaneous active dopamine neurons than control Saline (n=5; black bars) animals. One week after fear conditioning, fear conditioned MAM (n=10), but not Saline (n=9), animals showed a reduction in the number of spontaneously active dopamine neurons. Spontaneous VTA activity was reduced further in fear conditioned MAM animals following the administration of amphetamine (0.5 mg.kg, i.p; n=4). (B) The firing rate of spontaneously active dopamine neurons was reduced in fear conditioned MAM animals (n=82 neurons) relative to MAM controls (n=69 neurons). (C) Fear conditioning (n=69 neurons) and the combination of fear conditioning and amphetamine (n=15 neurons) reduced the burst activity of spontaneously active dopamine neurons in MAM rats. (* denotes p<0.05)
There was a significant effect of fear learning on DA neuron firing rate. Fear learning reduced the firing rate of dopamine neurons in MAM rats (Fig.4b; main effect fear learning, F2,325 = 5.18, p<0.007; interaction, F2,325 = 6.30, p<0.003; post-hoc p=0.0002). This reduction was not observed after amphetamine administration (p = 0.99). There were no significant changes in firing rate of dopamine neurons in Saline rats resulting from either fear learning or amphetamine administration (p = 0.99 and 0.70, respectively)
When the % of spikes occurring in bursts was analyzed, there was a significant main effect of fear learning (Fig.4c; F2,325 = 4.62, p < 0.02), but no other effects. Post-hoc comparisons showed that fear learning significantly reduced bursting activity in MAM (p=0.007), but not Saline, rats (p = 0.99), which was no longer significant when amphetamine administration was added (p = 0.46). Additional tests with apomorphine during electrophysiological recordings in Exp.4 show that it might not be as simple as reduced activation of the dopamine system following fear learning in MAM animals.
Exp.4: Fear-induced reductions in VTA dopamine activity in MAM rats likely involves depolarization block
Depolarization block is a condition whereby over-activation of the VTA results in a reduction in the number of spontaneously active dopamine neurons encountered (Blaha and Lane, 1987; Grace and Bunney, 1986; Hausknecht et al., 2013; Hollerman et al., 1992), and can be reversed by administration of autoreceptor-selective doses of the dopamine agonist apomorphine (Gill et al., 2011; Grace and Bunney, 1986; Valenti et al., 2011).
In a different group of MAM animals, following dopamine neuron recordings from the right hemisphere VTA, the contralateral VTA was sampled after administration of a low-dose of apomorphine (range 20–40 μg/kg). Effective doses of apomorphine were determined by first encountering a spontaneously active dopamine neuron in the left hemisphere and then incrementally administering apomorphine (5 μg/kg/increment) until there was an observable change in the baseline firing rate or bursting activity. (See Supplementa Results (C) for representative histology and electrophysiological recording from dopamine recording before and after apomorphine administration) The changes in the number of DA cells/track following apomorphine treatment varied by fear conditioning (repeated measures ANOVA; main effect fear learning, F1,18 = 7.17, p=0.02; main effect pre vs. post-apomorhine, F1,18 = 12.62, p=0.002; interaction time by fear learning (F1,18 = 49.38, p<0.001)).
Post-hoc analysis showed that apomorphine reduces the number of spontaneously active dopamine neurons in naïve MAM animals ((n=8);p<0.001). In contrast, following apomorphine administration, a majority of fear conditioned MAM animals (N=12;75%) demonstrated an increase in the number of dopamine neurons per track observed between the right and left hemispheres, indicative of removal of depolarization block (Fig.5; p=0.02). (See Supplemental Results (D) for effect of apomorphine treatment on DA activity in naïve Saline rats)
Fig.5.

In MAM control animals (n=8), injection of apomorphine reduces the number of spontaneously active dopamine neurons. A majority of fear conditioned MAM animals (n=9/12) demonstrated an increase in cells per track following apomorphine that is characteristic of reversal of depolarization block. (* denotes p<0.05)
Exp.5: MAM-treated offspring failed to demonstrate modulation of fear learning induced by extended context pre-exposure
Typically, following repeated exposure to contextual stimuli there is a transition towards a decrease in fear learning as a result of a proposed latent inhibition process (Holt and Maren, 1999; Maren and Holt, 2000; Rudy, 1994). The number of pre-exposure sessions was varied (0,1,3,5,7 or 10 10-min sessions) in Exp.2 for Saline- (n=24 total; 4 per group) and MAM-treated (n=24 total; 4 per group) offspring. 24 hours after conditioning, freezing behavior was measured upon re- exposure to Context A (5 min). In Saline animals, the amount of freezing exhibited after a single context pre-exposure session was significantly greater than that observed following 0, 3, 7 or 10 sessions (significant interaction between amount of pre-exposure and MAM treatment on time spent freezing during the context test, F5,36 = 3.47, p < 0.05; p=0.23; p’s = 0.02, 0.01, 0.04, and 0.01 for 0, 3,7, and 10 sessions respectively; Fig 6). In particular, the significant difference in freezing behavior between Saline rats that experienced only 2-min exploration of the conditioning context prior to shock on the conditioning day and rats that had an additional single pre-exposure session suggests a threshold by which context pre-exposure is beneficial in enhancing fear learning behaviors in normal rats. In contrast, there were no differences between the different exposure groups in the amount of freezing exhibited by MAM rats. However, consistent with Exp.1, following a single pre-exposure session, Saline animals froze significantly more than MAM animals (p=0.01).
Fig.6.

MAM and Saline rats did not differ in the amount of time spent freezing 24 hours after conditioning when averaged across pre-exposure groups (0, 1, 3, 5, 7, or 10 10-min sessions, n=4 per group). However, there was a significant treatment X context pre-exposure interaction. Saline animals exhibited significantly more freezing than MAM animals following 1 pre-exposure.When comparing between pre-exposure groups, Saline but not MAM animals with 1 pre-exposure demonstrated significantly more freezing than the 0, 3, 7, and 10 pre-exposure groups.. (* denotes p<0.05)
Discussion
During the immediate shock fear learning paradigm, the rapid and accurate hippocampal-dependent processing of contextual information is crucial (Huff et al., 2006; Huff and Rudy, 2004; Rudy et al., 2002; Rudy and O’Reilly, 1999; Strekalova et al., 2003). During this task, subjects must rely on previously acquired context representations to incorporate new aversive experiences for subsequent generation of fear responses. MAM animals were impaired in their use of previously acquired contextual information to modulate future fear responses. In contrast, normal rats displayed enhanced fear responses following a single context pre-exposure that dissipated with additional context exposure, perhaps due to latent inhibition.
Fear learning also caused a persistent change in the level of dopamine-dependent behavioral activation in normal rats that was dependent on the amount of context exposure. In contrast, MAM animals displayed reduced dopamine system responsiveness in comparison to naïve MAM animals, measured both behaviorally and electrophysiologically, that was not modulated by the amount of context pre-exposure. Subsequent testing with autoreceptor-selective doses of apomorphine in MAM rats suggested that fear conditioning causes additional hyperactivation of DA neurons, leading to depolarization block. Overall, these data support a model whereby the baseline hyperactivity of the dopamine system in MAM rats interfered with any potential dynamic changes in dopamine system activation after fear learning and occurs in tandem with an inability to effectively use context information.
Hippocampal dysfunction in schizophrenia underlies deficits in context processing
There is accumulating evidence that hippocampal dysfunction occurs early on in schizophrenia patients and contributes significantly to the cognitive pathology (Schobel et al., 2013). While learning processes in multiple paradigms are less efficient or impaired in schizophrenia patients, predominately deficits appear to involve altered memory generalization resulting from disrupted context processing (Ivleva et al., 2012; MacDonald et al., 2003; Martins Serra et al., 2001). Indeed, context processing deficits overlap with reduced IQ and working memory performance in schizophrenia patients (MacDonald et al., 2005).
Previous work with the MAM rodent model of schizophrenia has shown behavior deficiencies consistent with impairments observed in schizophrenia, including working memory, pre-attentive sensorimotor gating, latent inhibition in response to discrete stimuli and extra-dimensional set shifting (Flagstad et al., 2004; Gomes et al., 2015; Gourevitch et al., 2004; Lodge and Grace, 2007; Modinos et al., 2015; Moore et al., 2006). However, contextual discrimination deficits in MAM animals has not been addressed previously. Prior studies involving the immediate shock fear conditioning paradigm have shown that context pre-exposure is necessary for conditioned freezing responses to occur, and is specific to the particular context used during pre-exposure (Frankland et al., 2004; Robinson-Drummer and Stanton, 2015; Rudy et al., 2002). In addition, studies employing lesions of the hippocampus have demonstrated the necessity of this structure during the consolidation of the contextual stimuli in this paradigm (Huff and Rudy, 2004; Rudy et al., 2002).
Typically, repeated presentation of contextual stimuli should result in a latent inhibition of subsequent fear learning (Yap and Richardson, 2005; Zhang et al., 2004). The ventral hippocampaus, in particular the ventral subiculum, can modulate latent inhibition-induced dopamine release in the nucleus accumbens (Peterschmitt et al., 2005). There are known perturbations of latent inhibition in schizophrenia patients. Animal models employing prenatal or early postnatal lesions of the ventral hippocampus have demonstrated similar deficits in latent inhibition to those observed in schizophrenia patients (Angst et al., 2007; Grecksch et al., 1999; Meyer et al., 2009; Meyer and Louilot, 2011; Naert et al., 2013; Ouhaz et al., 2014). However, it should be noted that the low number of animals in each pre-exposure condition limits the interpretation of the reliability of impairment in all MAM animals.
Saline rats in the present study demonstrated the expected reduction in fear responses following extensive context pre-exposure. In initially failing to show comparable levels of freezing in saline rats following a single pre-exposure session, it was expected that additional pre-exposure may be necessary in MAM rats to overcome a malfunctioning hippocampus. Likewise, a latent inhibition of fear responses in MAM rats was also expected to have a rightward shift and to occur after a greater number of context pre-exposure sessions than saline rats. Instead, we show that the fear responses of MAM animals are not modulated either by differences in contextual stimuli or amount of context exposure, which is consistent with the known perturbations in ventral hippocampal function exhibited by MAM animals, e.g. altered oscillatory activity and decreased parvalbumin expression (Gill and Grace, 2014; Lodge et al., 2009).
We are uncertain if this deficit in context-dependent processing is due to disruptions in encoding or expression. A failure to consolidate contextual information during encoding would have an indistinguishable behavioral effect as a context discrimination deficit during recall upon re-exposure to the conditioning context.
Dopamine system modulation of contextual processing and depolarization block
Various stressors (e.g. inescapable shock, restraint) can have an activating effect on the dopamine system. Thus, although the aversive shock stimuli employed in the present study was both brief in duration and small in magnitude, it was sufficient to activate the dopamine system in normal rats. However, it was not the shock alone that had the activating effect on the dopamine system in normal rats, since only animals that had received brief bouts of context exposure prior to the shock experience demonstrated enhanced amphetamine-induced locomotor activity one week after conditioning. In addition, in normal animals, amphetamine given acutely does not alter the spontaneous activity of DA neurons when tested 1 hr. post-injection (Belujon et al., 2016). These data are consistent with the behavioral sensitizing effect of dopamine agonists combined with contextual novelty (Badiani et al., 1998; Browman et al., 1998). Changes in dopamine release may be important when a given context is paired with a salient or aversive experience. In normal rats, expression of fear learning is both context specific and associated with increased dopamine release in the nucleus accumbens (Martinez et al., 2008). In addition, the simple repeated pairing of two neutral stimuli also leads to increased dopamine release in the nucleus acumbens.(Young et al., 1998)
Dopamine hyperactivity in the MAM model has been directly attributed to the aberrant output from the ventral hippocampus (Lodge and Grace, 2007). Based on the correlative data from the present study showing persistent alterations in dopamine system activation in MAM rats following fear learning, we propose that the fear-induced activation of the DA system could combine with baseline DA hyperactivity driven by the ventral hippocampus to initiate depolarization block. While the recordings from dopamine neurons in the VTA of MAM animals after fear conditioning initially suggested a “normalization” of dopamine activity, subsequent tests with apomorphine confirmed instead a state of depolarization block. Depolarization block will prevent further activation in response to external stimuli. The contribution of ventral hippocampus to fear-induced depolarization block of dopamine neurons in MAM animals remains an important unresolved question for future studies. Measuring changes in hippocampal activity directly following fear learning could provide a crucial link between fear learning and increased dopamine pathology in MAM animals. A naïve MAM animal typically demonstrates dopamine hyperactivity. However, following fear learning, and depolarization block, there is an overdrive of the dopamine system such that there is a loss in responsivity, or reduction in spontaneous activation (Fig.7). A consequence of this persistent alteration in the dopamine system could be a loss of adequate modulation of dopamine activity in response to behaviorally salient events. This potential model is supported by the observation that when the system is challenged further by amphetamine administration, there is an additional reduction in spontaneous dopamine activity that can also be observed behaviorally as a reduced locomotor response. As a consequence, stimuli that would normally activate the dopamine system in normal rats might instead induce an inactivation and interference of dopamine function in the MAM rats.
Fig.7.
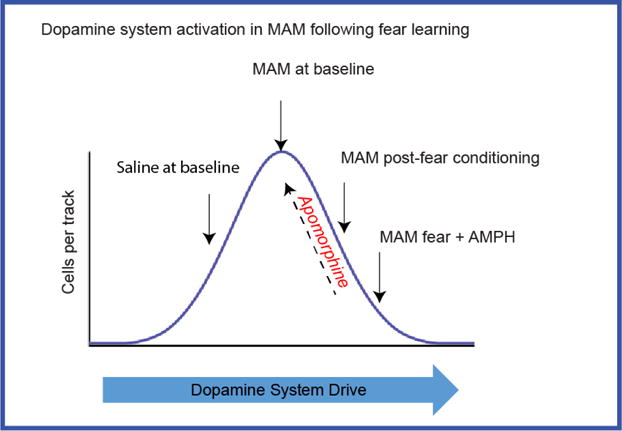
The pattern of dopamine system activation in MAM rats following fear learning (e.g. lowered response to amphetamine, reduced dopamine cells/track, reduced firing rate and bursting activity of dopamine neurons) suggests excitation-induced depolarization block that can be reversed by apomorphine. Dopamine system overdrive in MAM rats following fear learning is exacerbated by further stimulation following amphetamine administration. This could result in reduced responsiveness of the dopamine system to behaviourally-relevant salient stimuli.
Conclusion
Schizophrenia patients are hindered in their ability to flexibly use contextual information. This can have grave consequences in situations requiring fast and accurate behavioral responses based on contextual information. In a more general sense, there is a diminished capacity for contextual associative learning that contributes to the cognitive deficits that plague schizophrenia patients. The present study demonstrates that the blunted contextual processing in the MAM model of schizophrenia is associated with a less dynamic dopamine response after fear learning.
Supplementary Material
Acknowledgments
We thank Drs. Pauline Belujon and Chun-hui Chang for their useful comments pertaining to the experimental design. We also recognize Nicole MacMurdo for her technical assistance.
Role of the Funding Source
This work was supported by the following United States Public Health Service Grants MH57440 (A.A.G.) and MH105782 (K.M.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: K.M. Gill designed the study, conducted behavioral and electrophysiological experiments, executed statistical analysis, and composed the first draft of the manuscript. S.A. Miller performed electrophysiological experiments and immunohistochemical verification. A.A. Grace assisted with study design, data interpretation, and manuscript revisions. All authors contributed to and have approved the final manuscript.
Competing financial interests. Johnson and Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio (A.A.G.). All other authors have no biomedical financial interests or potential conflicts of interest to report.
References
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155(6):761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Kegeles LS, Zea-Ponce Y, Mawlawi O, Martinez D, Mitropoulou V, O’Flynn K, Koenigsberg HW, Van Heertum R, Cooper T, Laruelle M, Siever LJ. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry. 2004;55(10):1001–1006. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry. 2009;65(12):1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Charlety P, Saunier CF, Buda M, Chouvet G. Inhibition of nigral dopamine neurons by systemic and local apomorphine: possible contribution of dendritic autoreceptors. Neuroscience. 1992;49(4):879–891. doi: 10.1016/0306-4522(92)90364-8. [DOI] [PubMed] [Google Scholar]
- Angst MJ, Macedo CE, Guiberteau T, Sandner G. Alteration of conditioned emotional response and conditioned taste aversion after neonatal ventral hippocampus lesions in rats. Brain Res. 2007;1143:183–192. doi: 10.1016/j.brainres.2007.01.093. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Ivleva EI, Gopal TA, Reddy AP, Jeon-Slaughter H, Sacco CB, Francis AN, Tandon N, Bidesi AS, Witte B, Poudyal G, Pearlson GD, Sweeney JA, Clementz BA, Keshavan MS, Tamminga CA. Hippocampal volume is reduced in schizophrenia and schizoaffective disorder but not in psychotic bipolar I disorder demonstrated by both manual tracing and automated parcellation (FreeSurfer) Schizophr Bull. 2015;41(1):233–249. doi: 10.1093/schbul/sbu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18(24):10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, O’Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002;134(1–2):299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Belujon P, Jakobowski NL, Dollish HK, Grace AA. Withdrawal from Acute Amphetamine Induces an Amygdala-Driven Attenuation of Dopamine Neuron Activity: Reversal by Ketamine. Neuropsychopharmacology. 2016;41(2):619–627. doi: 10.1038/npp.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Building models for postmortem abnormalities in hippocampus of schizophrenics. Schizophr Res. 2015;167(1–3):73–83. doi: 10.1016/j.schres.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Lane RF. Chronic treatment with classical and atypical antipsychotic drugs differentially decreases dopamine release in striatum and nucleus accumbens in vivo. Neurosci Lett. 1987;78(2):199–204. doi: 10.1016/0304-3940(87)90633-1. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94(6):2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. Modulatory effect of environmental stimuli on the susceptibility to amphetamine sensitization: a dose-effect study in rats. J Pharmacol Exp Ther. 1998;287(3):1007–1014. [PubMed] [Google Scholar]
- Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978;23(16):1715–1727. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12(1):1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Cifelli P, Grace AA. Pilocarpine-induced temporal lobe epilepsy in the rat is associated with increased dopamine neuron activity. Int J Neuropsychopharmacol. 2012;15(7):957–964. doi: 10.1017/S1461145711001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38(10):1881–1888. doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29(11):2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112(4):863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. Consolidation of CS and US representations in associative fear conditioning. Hippocampus. 2004;14(5):557–569. doi: 10.1002/hipo.10208. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Young SL. Intrahippocampal infusions of a metabotropic glutamate receptor antagonist block the memory of context-specific but not tone-specific conditioned fear. Behav Neurosci. 1999;113(1):222–227. doi: 10.1037//0735-7044.113.1.222. [DOI] [PubMed] [Google Scholar]
- Gal G, Barnea Y, Biran L, Mendlovic S, Gedi T, Halavy M, Feldon J, Fennig S, Levkovitz Y. Enhancement of latent inhibition in patients with chronic schizophrenia. Behav Brain Res. 2009;197(1):1–8. doi: 10.1016/j.bbr.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Gal G, Mendlovic S, Bloch Y, Beitler G, Levkovitz Y, Young AM, Feldon J, Ratzoni G. Learned irrelevance is disrupted in first-episode but not chronic schizophrenia patients. Behav Brain Res. 2005;159(2):267–275. doi: 10.1016/j.bbr.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Gill KM, Cook JM, Poe MM, Grace AA. Prior Antipsychotic Drug Treatment Prevents Response to Novel Antipsychotic Agent in the Methylazoxymethanol Acetate Model of Schizophrenia. Schizophr Bull. 2014 doi: 10.1093/schbul/sbt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KM, Grace AA. Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. Int J Neuropsychopharmacol. 2014;17(10):1609–1619. doi: 10.1017/S146114571400056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel alpha5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36(9):1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Guimaraes FS, Grace AA. Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int J Neuropsychopharmacol. 2015;18(2) doi: 10.1093/ijnp/pyu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, Krebs MO, Jay TM. Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behav Pharmacol. 2004;15(4):287–292. doi: 10.1097/01.fbp.0000135703.48799.71. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Low doses of apomorphine elicit two opposing influences on dopamine cell electrophysiology. Brain Res. 1985;333(2):285–298. doi: 10.1016/0006-8993(85)91582-3. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: analysis using in vivo intracellular recording. J Pharmacol Exp Ther. 1986;238(3):1092–1100. [PubMed] [Google Scholar]
- Gray JA, Joseph MH, Hemsley DR, Young AM, Warburton EC, Boulenguez P, Grigoryan GA, Peters SL, Rawlins JN, Taib CT, et al. The role of mesolimbic dopaminergic and retrohippocampal afferents to the nucleus accumbens in latent inhibition: implications for schizophrenia. Behav Brain Res. 1995;71(1–2):19–31. doi: 10.1016/0166-4328(95)00154-9. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bernstein HG, Becker A, Hollt V, Bogerts B. Disruption of latent inhibition in rats with postnatal hippocampal lesions. Neuropsychopharmacology. 1999;20(6):525–532. doi: 10.1016/S0893-133X(98)00081-5. [DOI] [PubMed] [Google Scholar]
- Guillaume F, Thomas E, Faget C, Richieri R, Lancon C. Perceptually or conceptually driven recognition: on the specificities of the memory deficit in schizophrenia. Psychiatry Res. 2015;225(3):493–500. doi: 10.1016/j.psychres.2014.11.060. [DOI] [PubMed] [Google Scholar]
- Hausknecht K, Haj-Dahmane S, Shen RY. Prenatal stress exposure increases the excitation of dopamine neurons in the ventral tegmental area and alters their reponses to psychostimulants. Neuropsychopharmacology. 2013;38(2):293–301. doi: 10.1038/npp.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36(3):472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Abercrombie ED, Grace AA. Electrophysiological, biochemical, and behavioral studies of acute haloperidol-induced depolarization block of nigral dopamine neurons. Neuroscience. 1992;47(3):589–601. doi: 10.1016/0306-4522(92)90168-2. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci. 1999;19(20):9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Williams M, Ibrahim K, Leung G, Egerton A, McGuire PK, Turkheimer F. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain. 2013;136(Pt 11):3242–3251. doi: 10.1093/brain/awt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26(5):1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Rudy JW. The amygdala modulates hippocampus-dependent context memory formation and stores cue-shock associations. Behav Neurosci. 2004;118(1):53–62. doi: 10.1037/0735-7044.118.1.53. [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Shohamy D, Mihalakos P, Morris DW, Carmody T, Tamminga CA. Memory generalization is selectively altered in the psychosis dimension. Schizophr Res. 2012;138(1):74–80. doi: 10.1016/j.schres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, Pantelis C, Robbins T, Weickert T, Weinberger DR, Goldberg TE. Intra-dimensional/extra-dimensional set-shifting performance in schizophrenia: impact of distractors. Schizophr Res. 2007;89(1–3):339–349. doi: 10.1016/j.schres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, Crawley AP, Kapur S. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33(3):473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28(31):7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE, Kaplan O, Abramovich P, Rudnick A, Laor N. Visual search in schizophrenia: latent inhibition and novel pop-out effects. Schizophr Res. 2000;45(1–2):145–156. doi: 10.1016/s0920-9964(99)00188-7. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Goghari VM, 3rd, Hicks BM, Flory JD, Carter CS, Manuck SB. A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology. 2005;19(6):814–821. doi: 10.1037/0894-4105.19.6.814. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Pogue-Geile MF, Johnson MK, Carter CS. A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch Gen Psychiatry. 2003;60(1):57–65. doi: 10.1001/archpsyc.60.1.57. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110(1–2):97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Martinez RC, Oliveira AR, Macedo CE, Molina VA, Brandao ML. Involvement of dopaminergic mechanisms in the nucleus accumbens core and shell subregions in the expression of fear conditioning. Neurosci Lett. 2008;446(2–3):112–116. doi: 10.1016/j.neulet.2008.09.057. [DOI] [PubMed] [Google Scholar]
- Martins Serra A, Jones SH, Toone B, Gray JA. Impaired associative learning in chronic schizophrenics and their first-degree relatives: a study of latent inhibition and the Kamin blocking effect. Schizophr Res. 2001;48(2–3):273–289. doi: 10.1016/s0920-9964(00)00141-9. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS, Devan BD. The challenges of understanding mammalian cognition and memory-based behaviours: an interactive learning and memory systems approach. Neurosci Biobehav Rev. 2004;28(7):719–745. doi: 10.1016/j.neubiorev.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Meyer F, Peterschmitt Y, Louilot A. Postnatal functional inactivation of the entorhinal cortex or ventral subiculum has different consequences for latent inhibition-related striatal dopaminergic responses in adult rats. Eur J Neurosci. 2009;29(10):2035–2048. doi: 10.1111/j.1460-9568.2009.06755.x. [DOI] [PubMed] [Google Scholar]
- Meyer FF, Louilot A. Latent inhibition-related dopaminergic responses in the nucleus accumbens are disrupted following neonatal transient inactivation of the ventral subiculum. Neuropsychopharmacology. 2011;36(7):1421–1432. doi: 10.1038/npp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38(3):129–138. doi: 10.1016/j.tins.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60(3):253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert A, Gantois I, Laeremans A, Vreysen S, Van den Bergh G, Arckens L, Callaerts-Vegh Z, D’Hooge R. Behavioural alterations relevant to developmental brain disorders in mice with neonatally induced ventral hippocampal lesions. Brain Res Bull. 2013;94:71–81. doi: 10.1016/j.brainresbull.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21(4):1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ouhaz Z, Ba-M’hamed S, Bennis M. Haloperidol treatment at pre-exposure phase reduces the disturbance of latent inhibition in rats with neonatal ventral hippocampus lesions. Comptes rendus biologies. 2014;337(10):561–570. doi: 10.1016/j.crvi.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Peterschmitt Y, Hoeltzel A, Louilot A. Striatal dopaminergic responses observed in latent inhibition are dependent on the hippocampal ventral subicular region. Eur J Neurosci. 2005;22(8):2059–2068. doi: 10.1111/j.1460-9568.2005.04366.x. [DOI] [PubMed] [Google Scholar]
- Quintero E, Diaz E, Vargas JP, de la Casa G, Lopez JC. Ventral subiculum involvement in latent inhibition context specificity. Physiol Behav. 2011;102(3–4):414–420. doi: 10.1016/j.physbeh.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Racsmany M, Conway MA, Garab EA, Cimmer C, Janka Z, Kurimay T, Pleh C, Szendi I. Disrupted memory inhibition in schizophrenia. Schizophr Res. 2008;101(1–3):218–224. doi: 10.1016/j.schres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Rascle C, Mazas O, Vaiva G, Tournant M, Raybois O, Goudemand M, Thomas P. Clinical features of latent inhibition in schizophrenia. Schizophr Res. 2001;51(2–3):149–161. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Stanton ME. Using the context preexposure facilitation effect to study long-term context memory in preweanling, juvenile, adolescent, and adult rats. Physiol Behav. 2015;148:22–28. doi: 10.1016/j.physbeh.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Stephan KE, den Ouden HE, Barnes TR, Friston KJ, Joyce EM. Do patients with schizophrenia exhibit aberrant salience? Psychol Med. 2009;39(2):199–209. doi: 10.1017/S0033291708003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Ontogeny of context-specific latent inhibition of conditioned fear: implications for configural associations theory and hippocampal formation development. Dev Psychobiol. 1994;27(6):367–379. doi: 10.1002/dev.420270605. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116(4):530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113(5):867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, Harkavy-Friedman J, Malaspina D. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr Res. 2009a;114(1–3):110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009b;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Voss M, Wagner B, Wustenberg T, Duzel E, Behr J. Frontolimbic novelty processing in acute psychosis: disrupted relationship with memory performance and potential implications for delusions. Front Behav Neurosci. 2015;9:144. doi: 10.3389/fnbeh.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53(12):1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Mihalakos P, Chin R, Thomas B, Wagner AD, Tamminga C. Learning and generalization in schizophrenia: effects of disease and antipsychotic drug treatment. Biol Psychiatry. 2010;67(10):926–932. doi: 10.1016/j.biopsych.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ, Koenigsberg HW, Harvey P, Mitropoulou V, Laruelle M, Abi-Dargham A, Goodman M, Buchsbaum M. Cognitive and brain function in schizotypal personality disorder. Schizophr Res. 2002;54(1–2):157–167. doi: 10.1016/s0920-9964(01)00363-2. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Zorner B, Zacher C, Sadovska G, Herdegen T, Gass P. Memory retrieval after contextual fear conditioning induces c-Fos and JunB expression in CA1 hippocampus. Genes Brain Behav. 2003;2(1):3–10. doi: 10.1034/j.1601-183x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Hartston H, Perry W, Geyer MA. Latent inhibition in schizophrenia. Schizophr Res. 1996;20(1–2):91–103. doi: 10.1016/0920-9964(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Talamini LM, Meeter M. Dominance of objects over context in a mediotemporal lobe model of schizophrenia. PLoS One. 2009;4(8):e6505. doi: 10.1371/journal.pone.0006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue MJ, Heckers S. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin. 2014;5:359–364. doi: 10.1016/j.nicl.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Vaitl D, Lipp O, Bauer U, Schuler G, Stark R, Zimmermann M, Kirsch P. Latent inhibition and schizophrenia: Pavlovian conditioning of autonomic responses. Schizophr Res. 2002;55(1–2):147–158. doi: 10.1016/s0920-9964(01)00250-x. [DOI] [PubMed] [Google Scholar]
- Valenti O, Cifelli P, Gill KM, Grace AA. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosci. 2011;31(34):12330–12338. doi: 10.1523/JNEUROSCI.2808-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Gill KM, Grace AA. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci. 2012;35(8):1312–1321. doi: 10.1111/j.1460-9568.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren Z, Haslam C. Overgeneral memory for public and autobiographical events in depression and schizophrenia. Cogn Neuropsychiatry. 2007;12(4):301–321. doi: 10.1080/13546800601066142. [DOI] [PubMed] [Google Scholar]
- Williams JH, Wellman NA, Geaney DP, Cowen PJ, Feldon J, Rawlins JN. Reduced latent inhibition in people with schizophrenia: an effect of psychosis or of its treatment. Br J Psychiatry. 1998;172:243–249. doi: 10.1192/bjp.172.3.243. [DOI] [PubMed] [Google Scholar]
- Yap CS, Richardson R. Latent inhibition in the developing rat: an examination of context-specific effects. Dev Psychobiol. 2005;47(1):55–65. doi: 10.1002/dev.20074. [DOI] [PubMed] [Google Scholar]
- Young AM, Ahier RG, Upton RL, Joseph MH, Gray JA. Increased extracellular dopamine in the nucleus accumbens of the rat during associative learning of neutral stimuli. Neuroscience. 1998;83(4):1175–1183. doi: 10.1016/s0306-4522(97)00483-1. [DOI] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. Scopolamine impairs acquisition and facilitates consolidation of fear conditioning: differential effects for tone vs context conditioning. Neurobiol Learn Mem. 1995;63(2):174–180. doi: 10.1006/nlme.1995.1018. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Murphy CA, Feldon J. Behavioural and cardiovascular responses during latent inhibition of conditioned fear: measurement by telemetry and conditioned freezing. Behav Brain Res. 2004;154(1):199–209. doi: 10.1016/j.bbr.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Zierhut KC, Grassmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain. 2013;136(Pt 3):804–814. doi: 10.1093/brain/aws335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


