Abstract
Although significant advances in the treatment of breast cancer have been made, in particular in the use of endocrine therapy, de novo and aquired resistance to therapy, and metastatic recurrence continue to be major clinical problems. Given the high prevalence of breast cancer, new life-style or chemotherapeutic approaches are required. In this regard, cholesterol has emerged as a risk factor for the onset of breast cancer, and elevated cholesterol is associated with a poor prognosis. While treatment with cholesterol lowering medication is not associated with breast cancer risk, it does appear to be protective against recurrence. Importantly, the cholesterol axis represents a potential target for both life-style and pharmacological intervention. This review will outline the clinical and preclinical data supporting a role for cholesterol in breast cancer pathophysiology. Specific focus is given to 27-hydroxycholesterol (27-OHC; (3β,25R)-Cholest-5-ene-3,26-diol)), a primary metabolite of cholesterol that has recently been defined as an endogenous Selective Estrogen Receptor Modulator. Future perspectives and directions are discussed.
Keywords: cholesterol, 27-hydroxychoelsterol, estrogen receptor, liver x receptor, breast cancer, selective estrogen receptor modulator
Introduction
With an estimated incidence of one in eight women, breast cancer continues to be the most commonly diagnosed cancer in women (American Cancer Society) [1]1 (1). It can be subdivided based on the presence or absence of the estrogen receptor alpha (ERα), the progesterone receptor (PR) or the epidermal growth factor receptor 2 (HER2). The advent of endocrine therapy such as ER antagonists (for example, tamoxifen) or aromatase inhibitors (for example, exemestane or letrozole) or HER2 based therapy (such as trastuzumab or Lepatinib) has significantly increased survivorship. However, breast cancer remains the second most common cause of cancer-related mortality among women (American Cancer Society). Metastatic spread of endocrine therapy-refractory disease is the major cause of mortality, and there is a growing population of women living with stage IV disease (Mariotto, Etzioni, Hurlbert et al., 2017). Therefore, there is significant interest in identifying those risk and prognostic factors that may also be amenable to therapeutic intervention.
Accounting for ~5–10% of all cases are those breast cancers with mutations in the BCRA1 or BCRA2 genes (Cancer Genome Atlas, 2012,Hall, Lee, Newman et al., 1990) (Shah, Rosso and Nathanson, 2014). Mutations in other genes such as ATM, CHEK2, and PALB2 have also been implicated, but with a low frequency (Michailidou, Hall, Gonzalez-Neira et al., 2013). Other risk factors include previous occurrence of cancer, age of menarche, age of menopause, parity, age of first child, and lifestyle (Colditz and Rosner, 2000,Collaborative Group on Hormonal Factors in Breast, 2012,Endogenous, Breast Cancer Collaborative, Key et al., 2013,Lord, Bernstein, Johnson et al., 2008,McPherson, Steel and Dixon, 2000,Petracci, Decarli, Schairer et al., 2011,Singletary, 2003). It is becoming increasingly recognized that lifestyle is a significant risk and prognostic factor. While exercise regimens appear protective, the metabolic syndrome, obesity, dyslipidemia and type II diabetes mellitus are all associated with increased risk and poor prognosis (Bianchini, Kaaks and Vainio, 2002,Capasso, Esposito, Pentimalli et al., 2011,Ha, Sung and Song, 2009,Hu, La Vecchia, de Groh et al., 2012,Jones and Alfano, 2013,Kitahara, Berrington de Gonzalez, Freedman et al., 2011,Michels, Solomon, Hu et al., 2003,Ronco, De Stefani and Stoll, 2010) (Berrino, Villarini, Traina et al., 2014,Ewertz, Jensen, Gunnarsdottir et al., 2011,Llaverias, Danilo, Mercier et al., 2011,Pasanisi, Berrino, De Petris et al., 2006). Of these risk/prognostic factors, the cholesterol axis is highly amenable to drug intervention, and therefore might offer a therapeutically tractable mechanism for the treatment of breast cancer. This review will highlight recent work investigating the roles of cholesterol and its metabolism in breast cancer pathophysiology, with specific focus on 27-hydroxychoelesterol (27-OHC; (3β,25R)-Cholest-5-ene-3,26-diol)) a primary metabolite which has been found to modulate the activities of the estrogen receptors and liver X receptors (ERs and LXRs respectively).
Clinical evidence supporting a role for cholesterol in the pathophysiology of breast cancer
Evidence for elevated circulating cholesterol concentrations as a risk factor for breast cancer onset are conflicting. It is not clear whether total, LDL or HDL cholesterol impact risk (Danilo and Frank, 2012,Kuzu, Noory and Robertson, 2016,Nelson, Chang and McDonnell, 2014,Silvente-Poirot and Poirot, 2014). However, when adjusted for body-mass-index, it was found that elevated dietary cholesterol was significantly associated with an increased likelihood of being diagnosed with breast cancer in postmenopausal but not in premenopausal women (Hu, La Vecchia, de Groh et al., 2012). The association with increased cholesterol in the diet was confirmed in a separate study of a distinct cohort of women (Ronco, De Stefani and Stoll, 2010). Finally, a recent prospective trial has found that total circulating cholesterol is associated with the onset of breast cancer (Ha, Sung and Song, 2009,Kitahara, Berrington de Gonzalez, Freedman et al., 2011).
Statins, inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), are widely prescribed and have proven to be very effective at lowering circulating cholesterol levels and preventing cardiovascular disease (Chou, Dana, Blazina et al., 2016). Retrospective studies have provided contradictory evidence regarding associations between statin use and breast cancer onset, with some studies reporting statin use as a risk modulator while others report no significant correlations (Beck, Wysowski, Downey et al., 2003,Blais, Desgagne and LeLorier, 2000,Boudreau, Gardner, Malone et al., 2004,Cauley, McTiernan, Rodabough et al., 2006,Cauley, Zmuda, Lui et al., 2003,Desai, Chlebowski, Cauley et al., 2013,Friis, Poulsen, Johnsen et al., 2005,Graaf, Beiderbeck, Egberts et al., 2004,Kaye and Jick, 2004). The largest meta-analysis to date found that there is no significant relationship between statin use and breast cancer onset (Undela, Srikanth and Bansal, 2012). Finally, more recent data from the Nurses’ Health Study indicate that statin use was not associated with the risk of breast cancer onset, a study that investigated 79,518 participants over 12 years (Borgquist, Tamimi, Chen et al., 2016). However, the retrospective nature of these studies presents several potentially confounding factors that may not have been specifically controlled for, such as body-mass-index, baseline cholesterol levels, breast cancer subtype, or even access to primary care and thus the potential for earlier detection (Baek and Nelson, 2016). Future prospective trials in at-risk populations are warranted, in order to definitively assess the impact of cholesterol lowering medication on breast cancer onset.
In terms of breast cancer recurrence however, cholesterol and statin use have emerged as clear prognostic markers. For example, it has been found that elevated circulating cholesterol levels correlate with decreased time to recurrence (Bahl, Ennis, Tannock et al., 2005). Several retrospective studies involving distinct populations have found that recurrence free survival time is significantly extended in breast cancer patients on statin therapy (Ahern, Pedersen, Tarp et al., 2011,Kwan, Habel, Flick et al., 2008,Nielsen, Nordestgaard and Bojesen, 2012). A recent meta-analysis supports the protective role of statin use (Zhong, Zhang, Chen et al., 2015). Furthermore, an analysis of a recent prospective trial aimed at determining outcome differences between patients treated with tamoxifen or letrozole, revealed that statin use increased recurrence free survival time (Borgquist, Giobbie-Hurder, Ahern et al., 2017). In summary, whether cholesterol plays a role in the onset of breast cancer is unclear, while there are clear associations between cholesterol or cholesterol lowering drugs and increased recurrence free survival time.
Preclinical evidence in support of a role for cholesterol in the pathophysiology of breast cancer
It was first hypothesized that cholesterol played a role in tumor progression with a report in 1909 indicating that tumors contained waxy crystals, later determined to be cholesterol (White, 1909). Early studies showed that when C3H mice were made experimentally obese and hyperchoelsterolemic by injection of gold thioglucose, they developed tumors sooner than control injected (lean) mice (Waxler, Tabar and Melcher, 1953). Several studies utilizing in vitro models demonstrate the proliferation-inhibitory effects of choelsterol synthesis inhibitors – either statins or oxidosqualene cyclase inhibitors (Campbell, Esserman, Zhou et al., 2006,Mafuvadze, Liang and Hyder, 2014). Numerous reports utilizing different animal models of breast cancer indicate that when placed on diets containing both high-fat and high-choelsterol (otherwise known as the common ‘Western’ diet), there is decreased time to tumor onset, increased tumor growth rate and increased metastasis (Alikhani, Ferguson, Novosyadlyy et al., 2013,Liu, Xu, Lam et al., 2013, and many others, Llaverias, Danilo, Mercier et al., 2011). Due to the nature of the Western diet, many of these studies have not specifically controlled for the potentially confounding effects of dietary fat on outcome. Furthermore two of the studies cited above make use of ApoE−/− mice which, when placed on a Western diet, exhibit circulating cholesterol levels far above those observed in humans.
To our knowledge, only one study to date has specifically controlled for dietary fat intake while manipulating cholesterol. In this study, it was found that placing mice transgenic for MMTV-PyMT on a diet containing 2% cholesterol displayed decreased time to the detection of a tumor and increased tumor growth (Nelson, Wardell, Jasper et al., 2013). In order to better model human cholesterol biology, the authors then utilized mice where the murine Apoe gene had been replaced with the human APOE3 allele. When placed on a high fat diet, E0771 murine mammary cancer grafts grew faster compared to mice on a control diet. For these studies, dietary cholesterol was controlled for. Importantly, oral administration of a statin (atorvastatin) attenuated the growth-promoting effects of a high fat diet, further supporting a direct role for cholesterol in breast cancer pathophysiology (Nelson, Wardell, Jasper et al., 2013). In a separate study, the tumor promoting effects of a Western diet on human xenografts were reduced when Ezetimibe was used to inhibit intestinal uptake of cholesterol (Pelton, Coticchia, Curatolo et al., 2014). Collectively, the preclinical studies support the clinical evidence indicating that cholesterol promotes the progression of breast cancer.
Mechanisms: cholesterol and breast cancer
Intracellular cholesterol metabolism is normally tightly regulated through the combined actions of sterol regulatory element-binding proteins (SREBPs, in particular SREBP 1c) and the liver x receptors (LXRs) (Baek and Nelson, 2016). When cholesterol levels drop, SREBP 1c undergoes proteolytic cleavage to become an active transcription factor which increases the expression of HMGCR and thus cholesterol synthesis. On the other hand, certain precursors and metabolites of cholesterol are agonists of the LXRs. Thus, when cholesterol levels rise, so too do concentrations of LXR agonists such as oxysterols. LXR activation leads to a transcriptional program which ultimately results in decreased cholesterol synthesis and increased cholesterol efflux (Hong and Tontonoz, 2014). Therefore, considering these homeostatic mechanisms, it was at first unclear how cholesterol might promote cancer progression.
Lessons from T-cell biology
A simple hypothesis as to how cholesterol might impact cancer is that, as a critical component of cellular membranes its presence may be a limiting factor for rapidly proliferating cells. However, due to the SREBP and LXR regulatory control of intracellular cholesterol homeostasis that is normally in place, it was unclear whether excess extracellular cholesterol could be utilized in membrane synthesis (Das, Brown, Anderson et al., 2014).
However, it has been recently shown that antigen-activated T cells have a mechanism in place to uncouple their normal cholesterol homeostasis from the increased cellular demand for cholesterol as they rapidly divide. Specifically, upon T-cell activation, the expression of SULT2B1 is robustly increased. This enzyme acts on oxysterols by adding a sulfating moiety, rendering them inactive in terms of being LXR agonists. Therefore, cholesterol effulux is not initiated as intracellular cholesterol and its metabolites increase within the cell (Bensinger, Bradley, Joseph et al., 2008). While a similar mechanism has not yet been described in cancer cells, it is possible that dysregulated cholesterol homeostasis allows cancer cells to utilize excess extracellular cholesterol as a source for membrane synthesis.
Lipid Rafts and Membrane Initiated Signaling
Cellular membranes are heterogeneous in nature. The lipid raft hypothesis proposes that interactions between cholesterol, saturated lipids and glycosylated lipids form ordered membrane regions that provide functional support for the accumulation of other lipids and proteins; all of which being important for subsequent signal transduction cascades (Sezgin, Levental, Mayor et al., 2017). Since cholesterol is an integral component of lipid rafts, and notwithstanding the normal cholesterol homeostatic mechanisms, it is possible that excess cholesterol within the membrane can drive the formation of additional lipid raft units thereby stimulating signaling events such as through the PI3K cascade. Evidence for this comes from a study where mammary tumors were grown in APOE−/− mice on a Western diet, and thus were severely hyperlipidemic and hypercholesterolemic compared to their wildtype counterparts. Tumors from these mice showed increased PI3K activation as well as phosphorylation of its downstream mediator, AKT (Alikhani, Ferguson, Novosyadlyy et al., 2013). This activity was shown to be pathologically relevant as treatment of these mice with BKM120, a PI3K inhibitor, decreased the tumor growth observed in APOE−/− mice. Therefore, under situations of cholesterol excess, it is possible that cholesterol promotes tumor growth by formation of lipid rafts and stimulation of PI3K signaling. However, since cholesterol concentrations in these mice were super-physiologic, it remains to be seen whether cholesterol exerts these activities under cholesterol concentrations normally observed in breast cancer patients (normo- or hypercholesterolemic).
27-Hydroxychoelsterol, a Selective Estrogen Receptor Modulator
In 2007 and 2008, two reports emerged which described the oxysterol, 27-hydroxycholesterol (27-OHC) as having properties of a Selective Estrogen Receptor Modulator (SERM) (DuSell, Umetani, Shaul et al., 2008,Umetani, Domoto, Gormley et al., 2007a). 27-OHC ((3β,25R)-Cholest-5-ene-3,26-diol) is a primary metabolite of cholesterol, being a product of the secondary bile acid pathway where cholesterol is hydroxylated through the enzymatic activity of CYP27A1. 27-OHC can then compete with cholesterol for re-entry into the active site of CYP27A1, where it is further converted into an aldehyde and then into 3β-hydroxy-5-cholesteoic acid (Pikuleva, Babiker, Waterman et al., 1998). 27-OHC is also catabolized by CYP7B1 into the bile acid precursor 7α, 25-hydroxycholesterol (Nelson, Wardell and McDonnell, 2013). Of all the oxysterols, 27-OHC circulates at comparable or slightly higher concentrations compared to 7β-OHC, both of which circulating at higher levels than other oxysterols (Karuna, Holleboom, Motazacker et al., 2011,Prunet, Petit, Ecarnot-Laubriet et al., 2006).
SERMs are defined as a class of compounds that possess activity which differentially modulate the activity of ER in a context specific manner (Wardell, Nelson and McDonnell, 2014). The best described SERM is tamoxifen which behaves as an ER antagonist in the breast and breast cancer, but as an ER agonist in the bone or uterus (reviewed by Nelson, Wardell and McDonnell, 2013). The diverse signaling of SERMs has been attributed to the unique pharmacology of nuclear receptors. Upon ligand binding, the receptor undergoes a conformational change allowing it to associate with specific co-regulatory proteins, either co-activators or co-repressors. It turns out that even subtle changes in ligand structure can result in unique conformational changes of nuclear receptors, and thus the recruitment of a specific set of co-regulatory proteins (Norris, Joseph, Sherk et al., 2009,Norris, Paige, Christensen et al., 1999). Thus, it is thought that the presence and/or abundance of different co-regulatory proteins in different tissues drives the tissue-specific activities of SERMs. Indeed, the agonist activity of tamoxifen could be modulated by the artificial expression of Steroid Receptor Coactivator 1 (SRC-1, also known as Nuclear Receptor Coactivator 1) (Smith, Nawaz and 239 O'Malley, 1997).
By using a Gal4-ER co-transfection assay, Umetani et al. screened a library of oxysterols for their ability to stimulate ERα and ERβ activity (Umetani, Domoto, Gormley et al., 2007a). They found that 24S-hydroxycholesterol, 25-hydroxycholesterol and 27-OHC significantly inhibited the effects of estradiol in this system, with IC50 values ranging from 1–5µM. However, 27-OHC was the most potent (IC50 of ~1µM), and was the only oxysterol that circulates at values approaching its IC50 (range of ~0.2–0.9µM in normocholesterolemic people) (Karuna, Holleboom, Motazacker et al., 2011,Pataj, Liebisch, Schmitz et al., 2016). 27-OHC was demonstrated to shift the ER into a conformation unique from that of estradiol or tamoxifen (DuSell, Umetani, Shaul et al., 2008). In models of cardiovascular disease, 27-OHC behaves as an ER antagonist blocking the estradiol mediated increase in nitric oxide and vasodilation, while in osteoblasts and cellular models of ER+ breast cancer it behaves as a partial ER agonist (DuSell, Nelson, Wang et al., 2010,DuSell, Umetani, Shaul et al., 2008,Nelson, DuSell, Wang et al., 2011,Umetani, Domoto, Gormley et al., 2007b). Based on these reports, 27-OHC is now considered an endogenously produced SERM.
27-Hydroxycholesterol, a key biochemical mediator of the tumor promoting effects of cholesterol
27-OHC is a SERM and behaves as a partial agonist in cellular models of ER+ breast cancer, stimulating their proliferation (DuSell, Umetani, Shaul et al., 2008). However, 27-OHC is also a LXR agonist and LXR activation typically results in inhibition of cellular proliferation, secondary to cellular loss of cholesterol through efflux (Baek and Nelson, 2016). This presents an apparent ‘competition’ between the signaling of the ERs versus LXRs. Evidence for this cross-modulation comes from experiments where 27-OHC is administered to cells that have had their LXRs knocked down or antagonized. In these scenarios the induction of ER-target genes is enhanced. On the other hand, when ERs are knocked down or antagonized, 27-OHC treatment results in significant increases in LXR target gene expression (Nelson, DuSell, Wang et al., 2011,Nelson, Wardell, Jasper et al., 2013). Therefore, it appears that in ER+ breast cancer cells, the ‘ER-activity’ of 27-OHC is predominant over the growth inhibitory actions of the LXRs, but the extent of this may be modulated by cellular and microenvironment factors impinging on either the LXR or ER axes. Other reports indicate that 27-OHC reduces P53 transcriptional activity in an ER dependent manner, 27-OHC results in increased reactive oxygen species which activate the STAT-3 pathway in an ER-independent fashion, and that oxysterols may also lead to the activation of CXCR2, all mechanisms that may explain the proliferative activity in the face of LXR activation (Raccosta, Fontana, Maggioni et al., 2013,Raza, Ohm, Dhasarathy et al., 2015,Zhu, Shen, Liu et al., 2016).
Regardless, due to its ability to stimulate the proliferation of ER+ breast cancer cell lines, it was hypothesized that the tumor growth-promoting effects of cholesterol may be mediated through this metabolite. Two independent studies found that administration of exogenous 27-OHC was sufficient to promote the growth of MCF7 xenografts (Nelson, Wardell, Jasper et al., 2013,Wu, Ishikawa, Sirianni et al., 2013). This stimulatory effect could be inhibited when mice were co-treated with the ER antagonist Fulvestrant (ICI 182,780). 27-OHC treatment also increased the tumor growth in transgenic MMTV-PyMT mice and syngeneic grafts of murine E0771 cells. Furthermore, tumors grew faster in MMTV-PyMT mice lacking the expression of CYP7B1, the enzyme responsible for the catabolism of 27-OHC. Importantly, the tumor promoting effects of cholesterol were attenuated in MMTV mice lacking the expression of CYP27A1. Therefore, the growth promoting effects of cholesterol required its conversion to 27-OHC, at least in the MMTV-PyMT model. When human transgenic replacement APOE3 mice were placed on a high fat diet, treatment with a small molecule CYP27A1 inhibitor modestly reduced E0771 tumor growth, again suggesting that 27-OHC is an important mediator of cholesterol action (Nelson, Wardell, Jasper et al., 2013).
Treatment with 27-OHC was also found to increase markers of epithelial to mesenchymal transition (EMT) and subsequent lung metastases in MMTV-PyMT mice (Nelson, Wardell, Jasper et al., 2013,Torres, Ramirez, Cruz et al., 2011). Interestingly however, treatment with estradiol did not increase metastasis in this model (Nelson, Wardell, Jasper et al., 2013). On the other hand, since 27-OHC is an oxysterol it can also activate the LXRs. Indeed, treatment with the synthetic LXR agonist did induce EMT-like changes and some metastasis, but failed to recapitulate the robust increase in metastasis observed in mice treated with 27-OHC. Identifying the precise mechanisms by which 27-OHC promotes metastasis continues to be an active area of research.
Clinical evidence for a role of 27-OHC in breast cancer pathophysiology
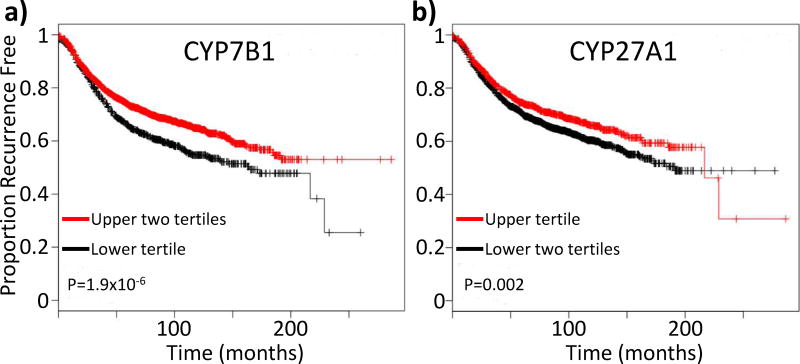
Several lines of evidence support a pathologic role for 27-OHC in breast cancer. First, it has been found that elevated tumoral mRNA expression of CYP7B1, the enzyme responsible for the catabolism of 27-OHC, is associated with increased recurrence free survival. This protective effect of CYP7B1 has been found to be consistent across several distinct databases, and appears to be most pronounced in luminal A (Nelson, Wardell, Jasper et al., 2013,Wu, Ishikawa, Sirianni et al., 2013). A more recent analysis of all subtypes of tumor samples within those databases included in the online Kaplan-Meier Plotter tool (GEO (Affymetrix microarrays only), EGA and TCGA) (Gyorffy, Lanczky, Eklund et al., 2010) continues to support the elevated expression of CYP7B1 mRNA being a beneficial prognostic indicator for breast cancer patients (Fig. 1a). The first rate limiting enzyme in the classic bile acid synthesis pathway is CYP7A1. The actions of CYP7A1 would remove cholesterol from the pool available for 27-OHC synthesis. Interestingly, similar analysis of CYP7A1 expression indicates that elevated expression of this enzyme may also be protective, but not significantly so 311 (P=0.089, N=3951, not shown).
Figure 1.
Tumoral expression of enzymes involved in the metabolism of 27-hydroxychoelsterol (27-OHC) are prognostic of time to recurrence. (a) Elevated mRNA expression of CYP7B1 mRNA, the enzyme responsible for the catabolism of 27-OHC, is associated with an increased recurrence free survival time. (b) Elevated mRNA expression of CYP27A1, the enzyme responsible for conversion of cholesterol to 27-OHC is associated with increased recurrence free survival time. Analysis was performed by the online Kaplan Meier Plotter tool (Gyorffy, Lanczky, Eklund et al., 2010). Patients were parsed into tertiles based on mRNA expression, as indicated on the plots.
On the other hand, tumoral mRNA expression of CYP27A1 was not initially found to correlate with clinical outcome (Nelson, Wardell, Jasper et al., 2013). However, current analysis with the online Kaplan-Meier Plotter tool found, somewhat counterintuitively, that elevated tumoral expression of CYP27A1 is actually associated with increased recurrence free survival time (Fig. 1b) (Gyorffy, Lanczky, Eklund et al., 2010). These results are confirmed by a recent study of 11 merged datasets available in GEO which found that elevated tumoral expression of CYP27A1 mRNA is associated with increased recurrence free- and overall- survival time. However, this association was only apparent in ERα+ cases and in women who were diagnosed at 50 years of age or younger (Kimbung, Chang, Bendahl et al., 2017). Importantly, women under 50 years of age are likely to be pre- or peri-menopausal, with measurable circulating estrogens. It is worth speculating that the protective nature of CYP27A1 among these patients may be due to 27-OHC competing with estrogens for the ER, resulting in decreased ER activity. In support of this notion are findings that although CYP27A1 mRNA is expressed more highly in ER-negative breast tumors, it is not associated with recurrence free survival amongst these patients (Kimbung, Chang, Bendahl et al., 2017).
However, although yet to be formally tested, there is some evidence that CYP27A1 mRNA levels may not accurately reflect protein levels, one study showing an inverse relationship (Kimbung, Chang, Bendahl et al., 2017). When tumors were stained for CYP27A1 protein a positive association between high staining and increased tumor grade at diagnosis was observed (odds ratio of 6.7) (Nelson, Wardell, Jasper et al., 2013). A recent study of a cohort of 42 women treated a the Skåne University Hospital in Sweden showed that CYP27A1 was expressed in 91% of grade 3 tumors while only in 60% of grade 1 and 2 tumors, although this did not reach statistical significance in this small cohort (Kimbung, Chang, Bendahl et al., 2017). Furthermore, myeloid immune cells such as macrophages highly express CYP27A1, and it is well documented that tumor infiltration with these cells is a poor prognostic (Nelson, Wardell, Jasper et al., 2013).
Collectively, expression data for enzymes involved in the metabolism of 27-OHC would suggest that 27-OHC plays an important pathological role in human breast cancer progression. In support of this are data indicating that 27-OHC concentrations within breast tumors are significantly higher than adjacent ‘normal’ breast tissue (Wu, Ishikawa, Sirianni et al., 2013). Interestingly, ‘normal’ adjacent tissue had higher 27-OHC concentrations than breast tissue donated from healthy volunteers. Furthermore, whereas the 27-OHC concentration within normal breast tissue correlated very well with serum 27-OHC levels, this correlation is not evident between tumor tissue and serum 27-OHC levels, likely indicating that in breast tumors there is a dysregulation of 27-OHC metabolism. However, this analysis included only a limited number of samples and thus caution must be taken when interpreting these results. Other cholesterol metabolites also appear to be dysregulated in breast cancer (Silvente-Poirot, de Medina, Record et al., 2016). Therefore, more comprehensive studies are now required to better characterize cholesterol metabolism within breast tumors.
Endocrine therapy such as tamoxifen or aromatase inhibition has dramatically improved survivorship for ERα+ breast cancer patients. However, de novo or development of resistance to therapy continues to be a significant problem. Using a clinically relevant model of MCF7 tumors resistant to tamoxifen therapy, it was demonstrated that 27-OHC administration at 40mg/kg stimulates their growth slightly more than estradiol pellet (0.72mg, 60 day release), which is in contrast to parental (tamoxifen-naïve) tumors where the estradiol pellet was significantly more growth-promoting than 27-OHC (Nelson, Wardell, Jasper et al., 2013). Clinically, it has been found that in tumors from patients that had relapsed on tamoxifen therapy, there was significantly more tumor associated macrophages, cells known to express high levels of CYP27A1 (Nelson, Wardell, Jasper et al., 2013,Xuan, Wang, Nanding et al., 2014). On the other hand, recent results from the OXYTAM prospective study found that while tamoxifen treatment reduced circulating levels of 24-hydroxychoelsterol, 7α-hydroxycholesterol and 25-hydroxychoelsterol, it had no effect on circulating 27-OHC concentrations (Dalenc, Iuliano, Filleron et al., 2016). However, based on a previous report indicating that circulating 27-OHC concentations were not necessarily a good indicator of intra-tumoral concentrations (Wu, Ishikawa, Sirianni et al., 2013), a role for 27-OHC in the development of tamoxifen resistance remains possible.
Intriguingly though, when patients were treated with an aromatase inhibitor (exemestane or letrozole) for one month, circulating concentrations of 27-OHC were elevated (Dalenc, Iuliano, Filleron et al., 2016). This is despite the fact that both of these drugs were found to also be weak inhibitors of CYP27A1 (Mast, Lin and Pikuleva, 2015). In culture, cells that model chronic exposure to aromatase inhibitiors (long term estrogen deprived cells) exhibit epigenetic reprogramming leading to an upregulation of cholesterol biosynthesis and metabolic pathways, including CYP27A1, ultimately leading to the constitutive activation of the ERα at least in part through 27-OHC (Nguyen, Barozzi, Faronato et al., 2015). Importantly, the reprogrammed epigenetic signature identified in this model was associated with a poor recurrence free survival among breast cancer patients. Intriguingly, the expression of certain genes within the signature (such as Squalene Epoxidase) could independently predict response to aromatase inhibitor therapy, but not tamoxifen therapy. The induction of genes associated with cholesterol homeostasis in cell lines deprived of estrogens was confirmed in an independent study (Simigdala, Gao, Pancholi et al., 2016). Interestingly, in this study, some of the estrogen deprived lines lost ER expression, and in these lines the cholesterol biosynthesis pathway does not appear to be altered. Thus, altered cholesterol homeostasis in response to estrogen withdrawal continues to require ER expression. However, in this study, while estrogen-deprived cells were sensitized to 27-OHC and 25-OHC, the proliferative properties of these oxysterols on either wildtype or estrogen-deprived cells were negligible compared to previous reports (DuSell, Umetani, Shaul et al., 2008,Lappano, Recchia, De Francesco et al., 2011,Nelson, Wardell, Jasper et al., 2013,Wu, Ishikawa, Sirianni et al., 2013). The reasons behind this discrepancy are unclear. In summary, while more extensive studies are required, 27-OHC may be one mediator of resistance to anti-estrogen and aromatase inhibition therapies.
Future Perspectives and Conclusions
Based on the clear associations between elevated cholesterol and poor prognosis, and statin therapy and increased recurrence free survival (Bahl, Ennis, Tannock et al., 2005) (Zhong, Zhang, Chen et al., 2015), there is significant interest in developing prospective studies to investigate the potential beneficial effects of cholesterol lowering medication in breast cancer patients. The BIG 1–98 (Breast International Group 1–98) study was a double blind phase III trial comparing tamoxifen to letrozole, as well as tamoxifen for 2 years followed by letrozole for 3 years and letrozole for 2 years followed by tamoxifen for 3 years (Borgquist, Giobbie-Hurder, Ahern et al., 2017). Interestingly, patients under these arms who also initiated cholesterol lowering medications demonstrated an increased disease free survival time, breast cancer free interval and distant recurrence free interval, thereby demonstrating the potential use of statins in combination with standard endocrine therapy. Collectively, the robust retrospective associations between patients taking cholesterol lowering medication and recurrence free survival provide strong rationale for future, blinded prospective studies, which can control for potential confounding factors such as BMI and access to care.
Although oral statin therapy significantly reduces circulating 27-OHC (Kimbung, Chang, Bendahl et al., 2017,Thelen, Lutjohann, Vesalainen et al., 2006) and will likely be beneficial in extending recurrence free survival, its main effects are inhibiting hepatic production of cholesterol, not synthesis in peripheral tissues. In fact, the post-hepatic steady state concentrations for statins are a log or more below their IC50 for HMGCR (Corsini, Maggi and Catapano, 1995,Lennernas, 2003). Thus, it is possible that local tumoral levels of cholesterol and/or 27-OHC remain unchanged by oral statin therapy. Indeed, in a recent ‘window of opportunity’ trial, it was found that although tumoral expression of the proliferation marker Ki67 was decreased, HMGCR was upregulated in women after only two weeks of dose atorvastatin therapy (Bjarnadottir, Romero, Bendahl et al., 2013). In another study examining samples from the same cohort, it was found that statin therapy significantly increased intratumoral expression of CYP27A1 protein, although this increase was not associated with changes in ki67 (Kimbung, Chang, Bendahl et al., 2017). Therefore, while changes in CYP27A1 expression are unlikely to explain the altered Ki67 observed in these tumors, considering that both CYP27A1 and HMGCR are induced, tumoral adaptation to environments of low circulating cholesterol may decrease the long-term efficiency of statin therapy. Orally administered statin therapy is fairly well tolerated with minimal side-effects, likely due to their very low post-hepatic circulating concentrations. However, increasing the prescribed dose or administering statins systematically (to bypass first pass metabolism) may lead to increased toxicity. Thus, prospective trials investigating the impact of statin therapy on breast cancer outcomes are highly warranted. Furthermore, alternate approaches such as the use of PCSK9 inhibitors or the development of specific CYP27A1 inhibitors should also be considered. To reiterate, prospective trials testing the impact of cholesterol lowering medication on recurrence free- and overall- survival are required to formally address the strong correlations emerging from retrospective analysis.
Clinical and preclinical evidence strongly suggest that cholesterol promotes breast cancer progression. One important mediator of the effects of cholesterol is its primary metabolite, 27-OHC. 27-OHC increases the growth of ERα+ mammary tumors in several different preclinical models, and increases the metastatic burden in MMTV-PyMT mice. Clinical evidence in support of a role for 27-OHC include (1) increased intratumoral concentrations of 27-OHC compared to normal tissue, (2) higher CYP27A1 staining in high grade tumors, (3) increased CYP27A1 expression post-statin therapy, and (4) increased circulating 27-OHC levels post aromatase inhibitor therapy. Moving forward, it is important to keep in mind that other metabolites of cholesterol may also impact breast cancer pathophysiology. For example, although they circulate at lower concentrations compared to 27-OHC, 24S-hydroxycholesterol and 25-hydroxycholesterol can also activate the ER (Lappano, Recchia, De Francesco et al., 2011,Umetani, Domoto, Gormley et al., 2007a). On the other hand, tamoxifen has been shown to inhibit cholesterol-5,6-epoxide hydrolase while stimulating lipo-peroxidation, resulting in the accumulation of 5,6α- and 5,6β- epoxy-cholesterol, compounds that induce breast cancer cell differentiation and death (Segala, de Medina, Iuliano et al., 2013,Sola, Poirot, de Medina et al., 2013). Furthermore, 5,6α epoxy-cholesterol has recently been found to react with histamine to form dendrogenin A, a process requiring an as yet to be identified enzyme. Dendrogenin A does not modulate the ER, but does behave as a selective inhibitor of cholesterol epoxide hydrolase resulting in tumor re-differentiation and reduced growth (de Medina, Paillasse, Segala et al., 2013). Importantly, the concentrations of dendrogenin A are reduced in breast cancer tumors compared to normal tissue. Therefore, it appears that breast tumors display multiple levels of dysregulated cholesterol homeostasis and metabolism, all of which being ripe for therapeutic exploitation.
elevated cholesterol is associated with poor prognosis in breast cancer patients
cholesterol lowering medication is associated with increased disease free survival
27-hydroxycholesterol (27-OHC) behaves as a selective estrogen receptor modulator
27-OHC promotes tumor growth
cholesterol homeostasis likely represents a potential therapeutic target
Acknowledgments
Funding: National Institutes of Health R00CA172357. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures. Atlanta: American Cancer Society, Inc.; 2017. [Google Scholar]
- 2.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sorensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103:1461–8. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alikhani N, Ferguson RD, Novosyadlyy R, Gallagher EJ, Scheinman EJ, Yakar S, LeRoith D. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene. 2013;32:961–7. doi: 10.1038/onc.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek AE, Nelson ER. The Contribution of Cholesterol and Its Metabolites to the Pathophysiology of Breast Cancer. Horm Cancer. 2016;7:219–28. doi: 10.1007/s12672-016-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahl M, Ennis M, Tannock IF, Hux JE, Pritchard KI, Koo J, Goodwin PJ. Serum lipids and outcome of early-stage breast cancer: results of a prospective cohort study. Breast Cancer Res Treat. 2005;94:135–44. doi: 10.1007/s10549-005-6654-9. [DOI] [PubMed] [Google Scholar]
- 6.Beck P, Wysowski DK, Downey W, Butler-Jones D. Statin use and the risk of breast cancer. J Clin Epidemiol. 2003;56:280–5. doi: 10.1016/s0895-4356(02)00614-5. [DOI] [PubMed] [Google Scholar]
- 7.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berrino F, Villarini A, Traina A, Bonanni B, Panico S, Mano MP, Mercandino A, Galasso R, Barbero M, Simeoni M, Bassi MC, Consolaro E, Johansson H, Zarcone M, Bruno E, Gargano G, Venturelli E, Pasanisi P. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147:159–65. doi: 10.1007/s10549-014-3076-6. [DOI] [PubMed] [Google Scholar]
- 9.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–74. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 10.Bjarnadottir O, Romero Q, Bendahl PO, Jirstrom K, Ryden L, Loman N, Uhlen M, Johannesson H, Rose C, Grabau D, Borgquist S. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res Treat. 2013;138:499–508. doi: 10.1007/s10549-013-2473-6. [DOI] [PubMed] [Google Scholar]
- 11.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–8. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 12.Borgquist S, Giobbie-Hurder A, Ahern TP, Garber JE, Colleoni M, Lang I, Debled M, Ejlertsen B, von Moos R, Smith I, Coates AS, Goldhirsch A, Rabaglio M, Price KN, Gelber RD, Regan MM, Thurlimann B. Cholesterol, Cholesterol-Lowering Medication Use, and Breast Cancer Outcome in the BIG 1–98 Study. J Clin Oncol. 2017;35:1179–1188. doi: 10.1200/JCO.2016.70.3116. [DOI] [PubMed] [Google Scholar]
- 13.Borgquist S, Tamimi RM, Chen WY, Garber JE, Eliassen AH, Ahern TP. Statin Use and Breast Cancer Risk in the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev. 2016;25:201–6. doi: 10.1158/1055-9965.EPI-15-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer. 2004;100:2308–16. doi: 10.1002/cncr.20271. [DOI] [PubMed] [Google Scholar]
- 15.Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, Baehner F, Kumar AS, Adduci K, Marx C, Petricoin EF, Liotta LA, Winters M, Benz S, Benz CC. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–14. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capasso I, Esposito E, Pentimalli F, Crispo A, Montella M, Grimaldi M, De Marco M, Cavalcanti E, D'Aiuto M, Fucito A, Frasci G, Maurea N, Esposito G, Pedicini T, Vecchione A, D'Aiuto G, Giordano A. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol Ther. 2011;10:1240–3. doi: 10.4161/cbt.10.12.13473. [DOI] [PubMed] [Google Scholar]
- 18.Cauley JA, McTiernan A, Rodabough RJ, LaCroix A, Bauer DC, Margolis KL, Paskett ED, Vitolins MZ, Furberg CD, Chlebowski RT Women's Health Initiative Research, G. Statin use and breast cancer: prospective results from the Women's Health Initiative. J Natl Cancer Inst. 2006;98:700–7. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- 19.Cauley JA, Zmuda JM, Lui LY, Hillier TA, Ness RB, Stone KL, Cummings SR, Bauer DC. Lipid-lowering drug use and breast cancer in older women: a prospective study. J Womens Health (Larchmt) 2003;12:749–56. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 20.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;316:2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 21.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. Am J Epidemiol. 2000;152:950–64. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 22.Collaborative Group on Hormonal Factors in Breast, C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–51. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 1995;31:9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 24.Dalenc F, Iuliano L, Filleron T, Zerbinati C, Voisin M, Arellano C, Chatelut E, Marquet P, Samadi M, Roche H, Poirot M, Silvente-Poirot S. Circulating oxysterol metabolites as potential new surrogate markers in patients with hormone receptor-positive breast cancer: Results of the OXYTAM study. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Danilo C, Frank PG. Cholesterol and breast cancer development. Curr Opin Pharmacol. 2012;12:677–82. doi: 10.1016/j.coph.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. Elife (Cambridge) 2014:e02882. doi: 10.7554/eLife.02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Medina P, Paillasse MR, Segala G, Voisin M, Mhamdi L, Dalenc F, Lacroix-Triki M, Filleron T, Pont F, Saati TA, Morisseau C, Hammock BD, Silvente-Poirot S, Poirot M. Dendrogenin A arises from cholesterol and histamine metabolism and shows cell differentiation and anti-tumour properties. Nat Commun. 2013;4:1840. doi: 10.1038/ncomms2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai P, Chlebowski R, Cauley JA, Manson JE, Wu C, Martin LW, Jay A, Bock C, Cote M, Petrucelli N, Rosenberg CA, Peters U, Agalliu I, Budrys N, Abdul-Hussein M, Lane D, Luo J, Park HL, Thomas F, Wactawski-Wende J, Simon MS. Prospective analysis of association between statin use and breast cancer risk in the women's health initiative. Cancer Epidemiol Biomarkers Prev. 2013;22:1868–76. doi: 10.1158/1055-9965.EPI-13-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuSell CD, Nelson ER, Wang X, Abdo J, Modder UI, Umetani M, Gesty-Palmer D, Javitt NB, Khosla S, McDonnell DP. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology. 2010;151:3675–85. doi: 10.1210/en.2010-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endogenous H, Breast Cancer Collaborative, G. Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoff man-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009–19. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 33.Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, Sorensen HT, Olsen JH. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–7. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 34.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–94. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–31. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 36.Ha M, Sung J, Song YM. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control. 2009;20:1055–60. doi: 10.1007/s10552-009-9301-7. [DOI] [PubMed] [Google Scholar]
- 37.Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, King MC. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250:1684–9. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 38.Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13:433–44. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 39.Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L Canadian Cancer Registries Epidemiology Research, G. Dietary cholesterol intake and cancer. Ann Oncol. 2012;23:491–500. doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 40.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol. 2013;52:195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- 41.Karuna R, Holleboom AG, Motazacker MM, Kuivenhoven JA, Frikke-Schmidt R, Tybjaerg-Hansen A, Georgopoulos S, van Eck M, van Berkel TJ, von Eckardstein A, Rentsch KM. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis. 2011;214:448–55. doi: 10.1016/j.atherosclerosis.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 42.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635–7. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimbung S, Chang C, Bendahl PO, Dubois L, Thompson WJ, McDonnell DP, Borgquist S. Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr Relat Cancer. 2017 doi: 10.1530/ERC-16-0533. [DOI] [PubMed] [Google Scholar]
- 44.Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–8. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuzu OF, Noory MA, Robertson GP. The Role of Cholesterol in Cancer. Cancer Res. 2016;76:2063–70. doi: 10.1158/0008-5472.CAN-15-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109:573–9. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lappano R, Recchia AG, De Francesco EM, Angelone T, Cerra MC, Picard D, Maggiolini M. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor alpha-mediated signaling in cancer cells and in cardiomyocytes. PLoS One. 2011;6:e16631. doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lennernas H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42:1141–60. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Xu A, Lam KS, Wong NS, Chen J, Shepherd PR, Wang Y. Cholesterol-induced mammary tumorigenesis is enhanced by adiponectin deficiency: role of LDL receptor upregulation. Oncotarget. 2013;4:1804–18. doi: 10.18632/oncotarget.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llaverias G, Danilo C, Mercier I, Daumer K, Capozza F, Williams TM, Sotgia F, Lisanti MP, Frank PG. Role of cholesterol in the development and progression of breast cancer. Am J Pathol. 2011;178:402–12. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lord SJ, Bernstein L, Johnson KA, Malone KE, McDonald JA, Marchbanks PA, Simon MS, Strom BL, Press MF, Folger SG, Burkman RT, Deapen D, Spirtas R, Ursin G. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:1723–30. doi: 10.1158/1055-9965.EPI-07-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mafuvadze B, Liang Y, Hyder SM. Cholesterol synthesis inhibitor RO 48–8071 suppresses transcriptional activity of human estrogen and androgen receptor. Oncol Rep. 2014;32:1727–33. doi: 10.3892/or.2014.3332. [DOI] [PubMed] [Google Scholar]
- 53.Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-16-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mast N, Lin JB, Pikuleva IA. Marketed Drugs Can Inhibit Cytochrome P450 27A1, a Potential New Target for Breast Cancer Adjuvant Therapy. Mol Pharmacol. 2015;88:428–36. doi: 10.1124/mol.115.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology. risk factors, and genetics. BMJ. 2000;321:624–8. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, Schmidt MK, Chang-Claude J, Bojesen SE, Bolla MK, Wang Q, Dicks E, Lee A, Turnbull C, Rahman N, Breast, Ovarian Cancer Susceptibility, C. Fletcher O, Peto J, Gibson L, Dos Santos Silva I, Nevanlinna H, Muranen TA, Aittomaki K, Blomqvist C, Czene K, Irwanto A, Liu J, Waisfisz Q, Meijers-Heijboer H, Adank M, Hereditary B, Ovarian Cancer Research Group, N. van der Luijt RB, Hein R, Dahmen N, Beckman L, Meindl A, Schmutzler RK, Muller-Myhsok B, Lichtner P, Hopper JL, Southey MC, Makalic E, Schmidt DF, Uitterlinden AG, Hofman A, Hunter DJ, Chanock SJ, Vincent D, Bacot F, Tessier DC, Canisius S, Wessels LF, Haiman CA, Shah M, Luben R, Brown J, Luccarini C, Schoof N, Humphreys K, Li J, Nordestgaard BG, Nielsen SF, Flyger H, Couch FJ, Wang X, Vachon C, Stevens KN, Lambrechts D, Moisse M, Paridaens R, Christiaens MR, Rudolph A, Nickels S, Flesch-Janys D, Johnson N, Aitken Z, Aaltonen K, Heikkinen T, Broeks A, Veer LJ, van der Schoot CE, Guenel P, Truong T, Laurent-Puig P, Menegaux F, Marme F, Schneeweiss A, Sohn C, Burwinkel B, Zamora MP, Perez JI, Pita G, Alonso MR, Cox A, Brock IW, Cross SS, Reed MW, Sawyer EJ, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45:353–61. 361e1–2. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, Manson JE, Nurses' Health S. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses' Health Study. Diabetes Care. 2003;26:1752–8. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 58.Nelson ER, Chang CY, McDonnell DP. Cholesterol and breast cancer pathophysiology. Trends Endocrinol Metab. 2014;25:649–55. doi: 10.1016/j.tem.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson ER, DuSell CD, Wang X, Howe MK, Evans G, Michalek RD, Umetani M, Rathmell JC, Khosla S, Gesty-Palmer D, McDonnell DP. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver X receptors. Endocrinology. 2011;152:4691–705. doi: 10.1210/en.2011-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–8. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson ER, Wardell SE, McDonnell DP. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: implications for the treatment and prevention of osteoporosis. Bone. 2013;53:42–50. doi: 10.1016/j.bone.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen VT, Barozzi I, Faronato M, Lombardo Y, Steel JH, Patel N, Darbre P, Castellano L, Gyorffy B, Woodley L, Meira A, Patten DK, Vircillo V, Periyasamy M, Ali S, Frige G, Minucci S, Coombes RC, Magnani L. Differential epigenetic reprogramming in response to specific endocrine therapies promotes cholesterol biosynthesis and cellular invasion. Nat Commun. 2015;6:10044. doi: 10.1038/ncomms10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 64.Norris JD, Joseph JD, Sherk AB, Juzumiene D, Turnbull PS, Rafferty SW, Cui H, Anderson E, Fan D, Dye DA, Deng X, Kazmin D, Chang CY, Willson TM, McDonnell DP. Differential presentation of protein interaction surfaces on the androgen receptor defines the pharmacological actions of bound ligands. Chem Biol. 2009;16:452–60. doi: 10.1016/j.chembiol.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP. Peptide antagonists of the human estrogen receptor. Science. 1999;285:744–6. doi: 10.1126/science.285.5428.744. [DOI] [PubMed] [Google Scholar]
- 66.Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119:236–8. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 67.Pataj Z, Liebisch G, Schmitz G, Matysik S. Quantification of oxysterols in human plasma and red blood cells by liquid chromatography high-resolution tandem mass spectrometry. J Chromatogr A. 2016;1439:82–8. doi: 10.1016/j.chroma.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 68.Pelton K, Coticchia CM, Curatolo AS, Schaffner CP, Zurakowski D, Solomon KR, Moses MA. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am J Pathol. 2014;184:2099–110. doi: 10.1016/j.ajpath.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petracci E, Decarli A, Schairer C, Pfeiffer RM, Pee D, Masala G, Palli D, Gail MH. Risk factor modification and projections of absolute breast cancer risk. J Natl Cancer Inst. 2011;103:1037–48. doi: 10.1093/jnci/djr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pikuleva IA, Babiker A, Waterman MR, Bjorkhem I. Activities of recombinant human cytochrome P450c27 (CYP27) which produce intermediates of alternative bile acid biosynthetic pathways. J Biol Chem. 1998;273:18153–60. doi: 10.1074/jbc.273.29.18153. [DOI] [PubMed] [Google Scholar]
- 71.Prunet C, Petit JM, Ecarnot-Laubriet A, Athias A, Miguet-Alfonsi C, Rohmer JF, Steinmetz E, Neel D, Gambert P, Lizard G. High circulating levels of 7beta- and 7alpha-hydroxycholesterol and presence of apoptotic and oxidative markers in arterial lesions of normocholesterolemic atherosclerotic patients undergoing endarterectomy. Pathol Biol (Paris) 2006;54:22–32. doi: 10.1016/j.patbio.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, Chiricozzi E, Trincavelli ML, Daniele S, Martini C, Gustafsson JA, Doglioni C, Feo SG, Leiva A, Ciampa MG, Mauri L, Sensi C, Prinetti A, Eberini I, Mora JR, Bordignon C, Steffensen KR, Sonnino S, Sozzani S, Traversari C, Russo V. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711–28. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raza S, Ohm JE, Dhasarathy A, Schommer J, Roche C, Hammer KD, Ghribi O. The cholesterol metabolite 27-hydroxycholesterol regulates p53 activity and increases cell proliferation via MDM2 in breast cancer cells. Mol Cell Biochem. 2015;410:187–95. doi: 10.1007/s11010-015-2551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ronco AL, De Stefani E, Stoll M. Hormonal and metabolic modulation through nutrition: towards a primary prevention of breast cancer. Breast. 2010;19:322–32. doi: 10.1016/j.breast.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Segala G, de Medina P, Iuliano L, Zerbinati C, Paillasse MR, Noguer E, Dalenc F, Payre B, Jordan VC, Record M, Silvente-Poirot S, Poirot M. 5,6-Epoxy-cholesterols contribute to the anticancer pharmacology of tamoxifen in breast cancer cells. Biochem Pharmacol. 2013;86:175–89. doi: 10.1016/j.bcp.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 76.Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition: regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah R, Rosso K, Nathanson SD. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J Clin Oncol. 2014;5:283–98. doi: 10.5306/wjco.v5.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silvente-Poirot S, de Medina P, Record M, Poirot M. From tamoxifen to dendrogenin A: The discovery of a mammalian tumor suppressor and cholesterol metabolite. Biochimie. 2016;130:109–114. doi: 10.1016/j.biochi.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 79.Silvente-Poirot S, Poirot M. Cancer. Cholesterol and cancer, in the balance. Science. 2014;343:1445–6. doi: 10.1126/science.1252787. [DOI] [PubMed] [Google Scholar]
- 80.Simigdala N, Gao Q, Pancholi S, Roberg-Larsen H, Zvelebil M, Ribas R, Folkerd E, Thompson A, Bhamra A, Dowsett M, Martin LA. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016;18:58. doi: 10.1186/s13058-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237:474–82. doi: 10.1097/01.SLA.0000059969.64262.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–66. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 83.Sola B, Poirot M, de Medina P, Bustany S, Marsaud V, Silvente-Poirot S, Renoir JM. Antiestrogen-binding site ligands induce autophagy in myeloma cells that proceeds through alteration of cholesterol metabolism. Oncotarget. 2013;4:911–22. doi: 10.18632/oncotarget.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thelen KM, Lutjohann D, Vesalainen R, Janatuinen T, Knuuti J, von Bergmann K, Lehtimaki T, Laaksonen R. Effect of pravastatin on plasma sterols and oxysterols in men. Eur J Clin Pharmacol. 2006;62:9–14. doi: 10.1007/s00228-005-0068-9. [DOI] [PubMed] [Google Scholar]
- 85.Torres CG, Ramirez ME, Cruz P, Epunan MJ, Valladares LE, Sierralta WD. 27-hydroxycholesterol induces the transition of MCF7 cells into a mesenchymal phenotype. Oncol Rep. 2011;26:389–97. doi: 10.3892/or.2011.1284. [DOI] [PubMed] [Google Scholar]
- 86.Umetani M, Domoto H, Gormley A, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator that inhibits the cardiovascular effects of estrogen. Nature Med. 2007a;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 87.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007b;13:1185–92. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 88.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat. 2012;135:261–9. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- 89.Wardell SE, Nelson ER, McDonnell DP. From empirical to mechanism-based discovery of clinically useful Selective Estrogen Receptor Modulators (SERMs) Steroids. 2014;90:30–8. doi: 10.1016/j.steroids.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waxler SH, Tabar P, Melcher LR. Obesity and the time of appearance of spontaneous mammary carcinoma in C3H mice. Cancer Res. 1953;13:276–8. [PubMed] [Google Scholar]
- 91.White CP. On the occurrence of crystals in tumours. Journal of Pathology and Bacteriology. 1909;13:3–10. [Google Scholar]
- 92.Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA, Umetani M, Euhus DM, Xie Y, Shaul PW. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–45. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xuan QJ, Wang JX, Nanding A, Wang ZP, Liu H, Lian X, Zhang QY. Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol Oncol Res. 2014;20:619–24. doi: 10.1007/s12253-013-9740-z. [DOI] [PubMed] [Google Scholar]
- 94.Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41:554–67. doi: 10.1016/j.ctrv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Zhu D, Shen Z, Liu J, Chen J, Liu Y, Hu C, Li Z, Li Y. The ROS-mediated activation of STAT-3/VEGF signaling is involved in the 27-hydroxycholesterol-induced angiogenesis in human breast cancer cells. Toxicol Lett. 2016;264:79–86. doi: 10.1016/j.toxlet.2016.11.006. [DOI] [PubMed] [Google Scholar]