Abstract
Objectives
The aim of the study was to compare the intra-individual plasma and intracellular peripheral blood mononuclear cell (PBMC) pharmacokinetics (PK) of tenofovir (TFV) and its intracellular metabolite, tenofovir-diphosphate (TFV-DP) in patients switched from a fixed dose combination (FDC) tablet of tenofovir disoproxil fumarate (TDF)/ emtricitabine (FTC)/ elvitegravir (EVG)/ cobicistat (COBI) to a FDC containing tenofovir alafenamide (TAF)/FTC/EVG/COBI.
Design
A single arm, prospective, non-randomized, cross-over, PK study in patients receiving a TDF-containing regimen (TDF 300mg/ FTC 200mg/ EVG 150mg/ COBI 150mg) switched to a TAF-containing FDC regimen (TAF 10mg/ FTC 200mg/ EVG 150mg/ COBI 150mg).
Methods
Single, sparse plasma and PBMC samples were collected during TDF therapy and 4 to 8 weeks post-switch to the TAF-containing regimen. Plasma TFV and cell associated TFV-DP concentrations were determined with validated liquid chromatography tandem mass spectrometry methods. PBMC cell enumeration was performed by quantification of RNaseP (RPP30) gene copy numbers using a highly sensitive droplet digital PCR (ddPCR) assay. Plasma and PBMC PK were summarized as geometric mean (GSD) and compared as a geometric mean ratio with a Wilcoxon Signed Rank test.
Results
In 30 participants with evaluable data, TFV plasma concentrations decreased 90% [TDF: 99.98 (2.24) ng/mL vs TAF: 10.2 (1.6) ng/mL, p<0.001] after the switch while cell associated TFV-DP increased 2.41 fold [TAF: 834.7 (2.49) vs TDF: 346.85 (3.75) fmol/106 cells, p=0.004].
Conclusions
Intraindividually, plasma TFV concentrations significantly decreased while cell associated TFV-DP concentrations significantly increased after switching from a TDF to a TAF-containing ART regimen.
Keywords: (5-7): HIV infection, tenofovir disoproxil fumarate, tenofovir alafenamide, ART switch, cell associated, pharmacokinetics
Introduction
Tenofovir alafenamide (TAF) is a novel prodrug of the antiretroviral agent tenofovir (TFV). Recently, TAF has been included as a first-line recommended nucleotide reverse transcriptase inhibitor in the treatment of both antiretroviral therapy (ART)-naïve and ART-experienced patients by both the United States Department of Health and Human Services as well as the European AIDS Clinical Society and International Antiviral Society treatment guidelines1,2,3. Two randomized phase 3 studies of TAF compared to an older TFV prodrug, tenofovir disoproxil fumarate (TDF), during initial treatment of HIV infection demonstrated non-inferiority of TAF4,5. A third phase 3 trial in virologically suppressed individuals receiving TDF-containing ART, randomized to either a TAF substitution or continuation of TDF, showed no difference in maintenance of virologic suppression6.
Initial clinical studies suggest TAF possesses a superior safety profile related to renal and bone adverse events when compared with TDF5,7. The improved safety profile of TAF is thought to be due to substantially lower plasma TFV exposure resulting from a lower dose of TAF (either 10mg or 25mg) versus the currently used dose of TDF (300mg), as higher plasma TFV exposure has been shown to correlate with both renal and bone toxicity8. Parallel group pharmacokinetics (PK) from three studies demonstrate 90% lower plasma TFV exposure, while conversely attaining a roughly 4-fold increase in the intracellular active metabolite of TFV, tenofovir diphosphate (TFV-DP), in participants receiving TAF versus TDF9,10. The increased intracellular concentrations of TFV-DP achieved when administered as TAF may be related to the increased plasma stability of the TAF prodrug as compared with TDF, as the prodrugs of TFV more readily enter cells as compared to TFV itself11.
Taken collectively, these studies present a rationale for switching persons with HIV infection who are virologically suppressed on a TDF-containing regimen to a similar TAF-containing regimen, especially those at higher risk for renal impairment or bone mineral density loss. In 2015 the first TAF containing fixed-dose combination product was approved by the U.S. Food and Drug Administration, and subsequently recommended as a first-line ART regimen by HIV guidelines2,3,12. In this study, we investigated the intra-individual PK of plasma TFV and intracellular peripheral blood mononuclear cell (PBMC) TFV-DP concentrations in patients undergoing a planned switch from a TDF-containing regimen [TDF / emtricitabine (FTC) / elvitegravir (EVG) / cobicistat (COBI)] to a TAF-containing regimen (TAF/FTC/EVG/COBI).
Methods
A single arm, prospective, non-randomized, cross-over PK study was conducted at the University of Nebraska Medical Center. Consecutive patients for whom a switch from TDF/FTC/EVG/COBI to TAF/FTC/EVG/COBI was planned were approached during routine clinic visits and invited to participate. Entry criteria were age >19 years, diagnosis of HIV-infection, receiving TDF 300mg/FTC 200mg/EVG 150mg/COBI 150mg once daily for at least 4 weeks prior to enrollment, and switching to TAF 10mg/FTC 200mg/EVG 150mg/COBI 150mg once daily therapy. Exclusion criteria were HIV RNA >50 copies / mL after 6 months on ART, or after previously being virologically suppressed on ART. Additionally, patients could not be receiving any concomitant medications that are contraindicated with TDF, FTC, EVG, COBI or TAF13,14. Data on demographics, medical history, CD4+ cell count and HIV-RNA at entry were abstracted from participant medical records.
Pharmacokinetic Evaluations
Participants underwent PK sampling at two study visits: the first was during TDF/FTC/EVG/COBI therapy, and the second was six to eight weeks after switching to TAF/FTC/EVG/COBI. The six to eight week cross over period was based on tenofovir and tenofovir-DP half-lives as well as rates of enzyme and transporter synthesis and turnover which have been previously reported to normalize by 28 days post perturbation15–17. Participants were asked to come to clinic at the same time of day for each PK visit, although PK samples were collected irrespective of the time post-dose. Participants were asked to provide the time of the three previous doses of ART taken prior to each PK study visit. If a participant had missed any of the three previous ART doses, their PK sampling visit was rescheduled to a later date where adherence to study medication was ensured. At each of the visits, 5 mL of whole blood was collected by venipuncture into a Vacutainer spray dried K2 ethylenediaminetetraacetic acid (EDTA) tube for plasma TFV concentration determination. Blood was immediately processed upon collection. Plasma was separated by centrifugation (1200 × g for 10 minutes at 4 C) and transferred to labeled polypropylene cryotubes. Plasma was stored at −80 C until analysis. PBMC’s were collected at each of the two PK study visits as previously described18. Briefly, 6-8 mL of blood was collected by venipuncture into cell preparation tubes (CPT) with sodium heparin anticoagulant. The CPT tubes were centrifuged and mononuclear cells collected. Cells were washed via a rapid spin through oil approach prior to placing the cells in 70% methanol for long term storage at −80 C. PBMC cell enumeration was performed by quantification of RNaseP (RPP30) gene copy numbers using a highly sensitive droplet digital PCR (ddPCR) assay as previously described19.
Statistical Analysis
Descriptive statistics (median with range or frequency distributions) were used to summarize all demographic information. Median and interquartile range (IQR) were used to describe the times post dose of the PK blood sampling. PK data were summarized as geometric mean (GSD) and geometric mean ratio (GMR, 90% CI) of TAF:TDF, and compared with a Wilcoxon signed rank test.
Ethics
This study was approved by the UNMC Institutional Review Board and all participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Results
Study Population
Forty-seven participants consented to the study between January 2016 and June 2016, and 30 participants completed both PK sampling visits. The median age of the 30 participants with complete PK data was 39 years (25-58), 4 (13%) were female, 16 (53%) non-Hispanic white, median CD4+ cell count was 632 (429–713) cells/mm3, and all participants had an undetectable (<20 copies/mL) HIV-1 RNA at study entry.
Tenofovir Pharmacokinetics
The participants underwent PK sampling after a mean of 82.8 (38.6) weeks of receiving TDF and 5.9 (1.5) weeks of receiving TAF. Plasma TFV and intracellular TFV-DP PK data are summarized in Table 1 and Figure 1. The time of blood sampling for plasma TFV determination was 11.2 (4.1 – 18.6) hours post dose during TDF-containing ART and 10.8 (2.7 – 17.4) hours post dose during TAF-containing ART. Geometric mean TFV plasma concentrations were 99.98 (2.24) ng/mL during TDF-containing ART and 10.20 (1.60) ng/mL during TAF-containing ART [GMR 0.10; (90% CI 0.06 - 0.18); p<0.001].
Table 1.
Tenofovir plasma and intracellular pharmacokinetic data from TDF and TAF based dosing. Pharmacokinetic data compared with Wilcoxon Signed Rank test.
| Metric | Pre-Switch TFV Plasma Conc. (ng/mL) | Post-Switch TFV Plasma Conc. (ng/mL) | Pre-Switch TFV-DP Intracellular Conc. (fmol / 106 cells) | Post-Switch TFV-DP Intracellular Conc. (fmol / 106 cells) |
|---|---|---|---|---|
| Geometic Mean | 99.98 | 10.20 | 346.85 | 834.70 |
| Geometric Std Dev | 2.24 | 1.60 | 3.75 | 2.49 |
| Time Post Dose, hours (median, IQR) | 11.2 (4.1 – 18.6) | 10.8 (2.7 – 17.4) | ||
| GMR Post : Pre Switch(90% CI) | 0.10; p<0.001 (0.06 – 0.18) |
2.41; p=0.004 (1.23 – 4.73) |
||
GMR=geometric mean ratio; IQR = interquartile range, TFV=tenofovir, TFV-DP= tenofovir diphosphate
Figure 1.
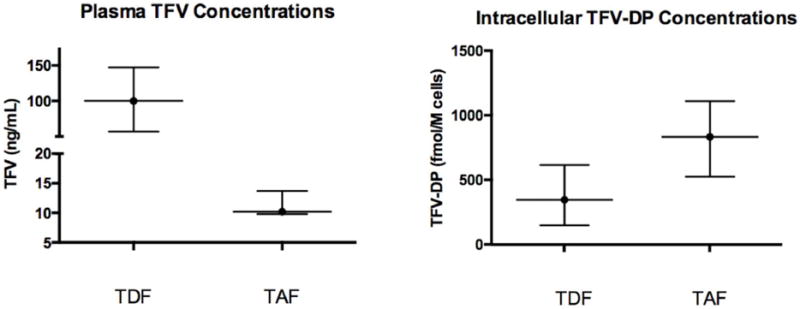
Whisker plot of plasma tenofovir and PBMC tenofovir diphosphate concentrations during TDF and TAF based dosing. Data presented as 25th, 50th and 75th percentiles.
Geometric mean intracellular TFV-DP concentrations measured during the TDF-containing regimen were 346.85 (3.75) fmol/106 cells. After switching, the intracellular TFV-DP concentrations were 834.70 (2.49) fmol/106 cells in participants receiving the TAF-containing regimen [GMR 2.41; (90% CI 1.23 - 4.73); p=0.004].
Discussion
We found plasma TFV concentrations decreased 90% and intracellular TFV-DP concentrations increased 2.41-fold in participants who were switched from TDF/FTC/EVG/COBI to TAF/FTC/EVG/COBI as part of routine clinical care. These intra-individual PK data confirm previous observations of 90% lower plasma tenofovir exposure when comparing TAF to TDF in parallel groups of participants9,10. Additionally, this study confirms the higher intracellular TFV-DP concentrations achieved with TAF compared to TDF.
The increase in TFV-DP concentrations in our participants is less than that seen in parallel studies. However, to our knowledge, this is the first study to evaluate plasma and intracellular concentrations within participants, and this difference may simply reflect differences in intraindividual versus interindividual PK variability. In addition, it must be noted that the dosing duration of TAF differs between the present study and prior reports. The present study investigated TFV plasma and TFV-DP PBMC concentrations after a mean of 5.9 weeks on TAF, ensuring steady state concentrations were reached after switching regimens, as plasma and intracellular TFV and TFV-DP half-lives have been reported as 17 and 87-150 hours respectively. Previous studies have evaluated TFV-DP after as few as 10 days of TAF therapy20–22. Additionally, both regimens in this study contained TAF 10mg once daily combined with the PK booster, COBI; therefore, the results may only be applicable in patients receiving a COBI-boosted ART regimen. Further work is needed to determine the effect of switching patients to or from other ART regimens containing TAF, or other TAF dosing strategies in combination with PK boosters (eg. TAF 25mg).
There remain differences in analytical methodology that may account for some of this difference. For example, in our present study, we enumerated PBMC cells via a highly precise ddPCR assay19 while previous studies of intracellular TFV-DP concentrations have utilized other less precise or less accurate methods of cell enumeration, such as quantitative DNA based approaches or traditional manual and automated cell counting techniques. Second, we utilized a rapid spin through oil approach when collecting PBMC’s for PK analysis. The benefits of the spin through oil approach have been previously described18. Additionally, our study had the limitation of single, non-timed sample collection which contributes to the PK variability observed in our study. However, intracellular half-lives of TFV-DP of 87 to >150 hours have been reported in the literature, minimizing the effect of differences in sampling times across the intracellular PK samples.
The PK relationships for plasma TFV PK and pharmacodynamic response as well as drug related adverse events have been well described23–26. There remains a need to better understand exposure-response relationships with TFV-DP, the intracellular metabolite of TFV. As TFV-DP is the pharmacologically active component of both TDF and TAF formulations, TFV-DP may offer the best option for efficacy based evaluations. Studies investigating TDF pharmacotherapy as pre-exposure prophylaxis (PrEP) for the prevention of HIV infection have elegantly described relationships between the number of doses taken, intracellular TFV-DP concentrations, and prevention of HIV infection27. Relationships between TFV-DP concentrations and metrics of treatment efficacy (e.g., plasma HIV RNA) for HIV are not as explicitly understood. The data generated via this present study, along with the PK data from previous phase 2 and phase 3 studies of TAF are reassuring in that higher intracellular concentrations are achieved with lower TAF dosing and lower TFV plasma exposure in patients who switch from TDF to TAF. Furthermore, drug-drug interaction studies to date have identified some differences in p-glycoprotein inducers on TAF vs. TDF, based on plasma drug exposure12. These data further inform future drug-drug interaction studies, as intracellular TFV-DP PK data would suggest the threshold for interactions that decrease TAF exposure may be greater with TAF-containing formulations than it is for TDF-containing formulations. Conversely, it remains to be determined whether there is an increased rate of adverse events associated with long-term higher TFV-DP exposure intracellularly, and if so, what the concentration threshold for such adverse events may be. Collectively, these plasma and intracellular PK data of TAF add to the current body of pharmacologic knowledge surrounding the optimal use of TAF in present day antiretroviral therapy.
Acknowledgments
This study was supported by the NIAID and NICHD of the National Institutes of Health: 1R01HD085887-01A1 (to KS) and RO1 AI124965-01 and UM1AI06701 (to CVF). We thank the study participants and the staff at the UNMC Specialty Care Clinic and UNMC Antiviral Pharmacology Laboratory.
Funding Support: We acknowledge support from the following grants from the National Institutes of Health: 1R01HD085887-01A1 (to KS) and RO1 AI124965-01 and UM1AI06701 (to CVF).
Susan Swindells discloses research grants to her institution from ViiV and Merck; Sara Bares discloses research funding to her institution from Gilead Sciences.
Footnotes
Author Contributions
ATP, SHB, CVF, SS and KKS designed the study. ATP and KKS performed the data analysis and wrote the manuscript. SHB, JH, RDS, JO and SL took part in the conduct of the study.
Conflicts of Interest
The remaining authors have no conflicts of interest to declare.
References
- 1.AIDSinfo A. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2017 [Google Scholar]
- 2.Günthard HF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA panel. Jama. 2016;316:191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European AIDS Clinical Society. (EACS) Guidelines v8.2. 2017 Jan; [Google Scholar]
- 4.Sax PE, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;67:52–58. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 5.Wohl D, et al. Brief report: a randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016;72:58–64. doi: 10.1097/QAI.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 6.Gallant JE, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. The lancet HIV. 2016;3:e158–e165. doi: 10.1016/S2352-3018(16)00024-2. [DOI] [PubMed] [Google Scholar]
- 7.Sax PE, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. The Lancet. 2015;385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 8.Van Rompay KK, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrobial agents and chemotherapy. 2008;52:3144–3160. doi: 10.1128/AAC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruane PJ, et al. Antiviral Activity, Safety, and Pharmacokinetics/Pharmacodynamics of Tenofovir Alafenamide as 10-Day Monotherapy in HIV-1–Positive Adults. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013;63:449–455. doi: 10.1097/QAI.0b013e3182965d45. [DOI] [PubMed] [Google Scholar]
- 10.Custodio J, Garner W, Callebaut C. The pharmacokinetics of tenofovir and tenofovir diphosphate following administration of tenofovir alafenamide versus tenofovir disoproxil fumarate (Abstract 6) 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. 2015:26–28. [Google Scholar]
- 11.Lee WA, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrobial agents and chemotherapy. 2005;49:1898–1906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AIDSinfo A. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2013 [Google Scholar]
- 13.Stribild® [package insert] Foster City, CA: Gilead Sciences, Inc; Jul, 2015. [Google Scholar]
- 14.Genvoya® [package insert] Foster City, CA: Gilead Sciences, Inc; Nov, 2015. [Google Scholar]
- 15.Reitman M, et al. Rifampin’s Acute Inhibitory and Chronic Inductive Drug Interactions: Experimental and Model‐Based Approaches to Drug–Drug Interaction Trial Design. Clinical Pharmacology & Therapeutics. 2011;89:234–242. doi: 10.1038/clpt.2010.271. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, et al. Cobicistat (GS-9350): a potent and selective inhibitor of human CYP3A as a novel pharmacoenhancer. ACS medicinal chemistry letters. 2010;1:209–213. doi: 10.1021/ml1000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, et al. Cytochrome p450 turnover: regulation of synthesis and degradation, methods for determining rates, and implications for the prediction of drug interactions. Current drug metabolism. 2008;9:384–393. doi: 10.2174/138920008784746382. [DOI] [PubMed] [Google Scholar]
- 18.Cory TJ, Winchester LC, Robbins BL, Fletcher CV. A rapid spin through oil results in higher cell-associated concentrations of antiretrovirals compared with conventional cell washing. Bioanalysis. 2015;7:1447–1455. doi: 10.4155/bio.15.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shetty Ravi Dyavar. K. K. S., Courtney V Fletcher, Anthony T Podany. American Association of Pharmaceutical Scientists Annual Meeting [Google Scholar]
- 20.Viread® [package insert] Foster City, CA: Gilead Sciences, Inc; Apr, 2017. [Google Scholar]
- 21.Baheti G, Kiser JJ, Havens PL, Fletcher CV. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrobial agents and chemotherapy. 2011;55:5294–5299. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins T, et al. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2005;39:406–411. doi: 10.1097/01.qai.0000167155.44980.e8. [DOI] [PubMed] [Google Scholar]
- 23.Barditch-Crovo P, et al. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrobial agents and chemotherapy. 2001;45:2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper RD, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clinical Infectious Diseases. 2010;51:496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 25.Jullien V, et al. Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy. Antimicrobial agents and chemotherapy. 2005;49:3361–3366. doi: 10.1128/AAC.49.8.3361-3366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate. Clinical pharmacokinetics. 2004;43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 27.Anderson PL, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science translational medicine. 2012;4:151ra125–151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]


