Abstract
Objective
To assess geospatial patterns of HIV antiretroviral therapy (ART) treatment facility use and whether they were impacted by viral load (VL) suppression.
Methods
We extracted data on the location and type of care services utilized by HIV-positive persons accessing ART between February 2015 and September 2016 from the Rakai Community Cohort Study (RCCS) in Uganda. The distance from RCCS households to facilities offering ART was calculated using the open street map road network. Modified Poisson regression was used to identify predictors of distance traveled and, for those traveling beyond their nearest facility, the probability of accessing services from a tertiary care facility.
Results
1554 HIV-positive participants were identified, of whom 68% had initiated ART. The median distance from households to the nearest ART facility was 3.10 km (Interquartile range, IQR, 1.65–5.05), but the median distance traveled was 5.26 km (IQR, 3.00–10.03, p<0.001) and 57% of individuals travelled further than their nearest facility for ART. Those with higher education and wealth were more likely to travel further. 93% of persons on ART were virally suppressed, and there was no difference in the distance traveled to an ART facility between those with suppressed and unsuppressed VLs (5.26 km vs. 5.27 km, p=0.650).
Conclusions
Distance traveled to HIV clinics was increased with higher socioeconomic status, suggesting that wealthier individuals exercise greater choice. However, distance traveled did not vary by those who were or were not virally suppressed.
Keywords: distance to clinic, global positioning systems, HIV/AIDS, sub-Saharan Africa, Uganda
INTRODUCTION
Persons living with HIV need of high quality accessible health care[1] and geographic distance from residence to health facility may impede service utilization in sub-Saharan Africa[2]. Several studies have shown associations between transport barriers and adverse HIV outcomes, including decreased ART adherence, decreased patient retention, and increased mortality[3–5]. However, the dynamics of health-care utilization are complex and several other studies have failed to show this association[6–11]. The discrepancy could be due to inconsistent measurement of transportation barriers across studies, which have included self-reported travel distance[9,10,12,13], self-reported travel time[6,13–16], self-reported travel cost[13,14,17], cost surface distance[18], linear travel distance[13,14,19], and calculated travel distance[13,14].
Choice in healthcare adds an additional complexity to the relationship with health outcomes. In sub-Saharan Africa, diversity of public and private providers allows HIV-positive individuals a choice of health care facilities. Many individuals reside far from a treatment facility and have no choice but to travel a substantial distance for treatment[20–22]; while others have considerable flexibility of access [23] and may choose more distant facilities[23,24]. The extent to which individuals exercise service choice is not well understood, and willingness to travel beyond local services has implications for HIV treatment programs.
We assessed whether longer distance to the nearest ART treatment facilities was associated with lower antiretroviral (ART) coverage, viral suppression, demographics, and health facility choice.
METHODS
Study setting and population
The first AIDS cases in east Africa were identified in Rakai District, Uganda[25] and communities in and around Rakai continue to have among the highest HIV prevalence in Uganda[26]. We used data from the Rakai Community Cohort Study (RCCS), an open population-based census and HIV surveillance cohort of consenting residents aged 15–49 in 38 communities in and near Rakai District. Written informed consent was provided at each visit. The RCCS conducted a household census and subsequently interviewed consenting individuals aged 15–49 using structured questionnaires in the local language (Luganda). Data include sociodemographic characteristics, behavioral and health service information including the location of their care provider. HIV testing used a validated three rapid test algorithm[27], and viral load was determined using Amplicor Monitor Assay, version 1.5 (Roche Diagnostics, Branchburg, NJ, USA, or Abbott Real Time assay). HIV-positive persons were offered same day free HIV counselling and testing and referred for care and treatment. ART was initiated at CD4 counts <500 cells/mL, or at time of diagnosis for HIV-discordant couples, pregnant/lactating women and key populations (sex workers, fishing communities).
The study was reviewed and approved by the Ugandan Virus Research Institute’s Scientific and Ethics Committee, the Uganda Council on Science and Technology, and Western Institutional Review Board, Olympia WA, and the Johns Hopkins School of Medicine Institutional Review Board.
Geospatial distance measurement
Geographic coordinates of RCCS households and all fixed-location HIV care facilities in the study region were assessed with handheld GPS units. Health facilities were classified by level of care (primary or tertiary), managing authority (Government, private, non-governmental organization (NGO), and HIV and general health services offered. The health-care facilities providing ART include public and nonprofit hospitals/clinics [28]. Public facilities providing ART were categorized as Government Health Centre II (HC2) which are smaller clinics which provide outpatient, antenatal and immunization services; ART is available at a limited number of HC2s in conjunction with NGOs. Government Health Centers III providing inpatient care, and Health Centers IV hospitals providing tertiary services.
Among 31 ART treatment facilities, 22 (71%) were public, 6 (19%) were private/NGO, and 3 (10%) were hospitals (Figure 1A). Five additional tertiary care facilities outside the study region were reported by study participants. Locations of facilities were geocoded using Google Earth. The distance from the participant’s home to facilities offering ART was calculated using travel distance along the open street map road network[29]. The Open Source Routing Machine (osrm) package in R was used to query driving distances from OpenStreetMaps API. Distance to the nearest facility was used to determine the minimum travel distance and compared to the distance actually traveled.
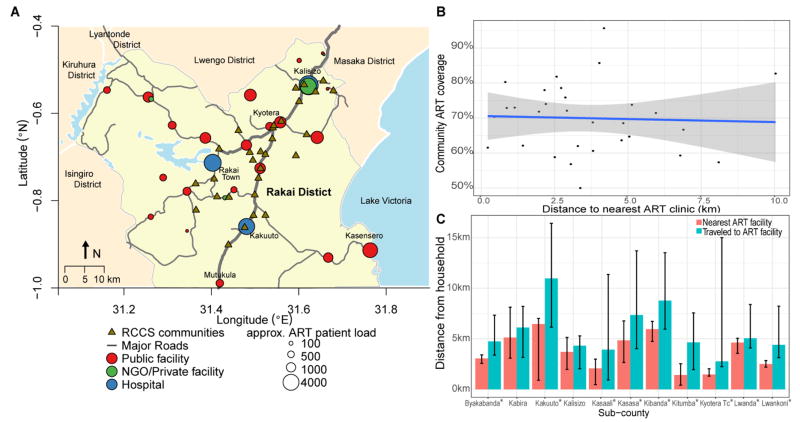
Figure 1. HIV treatment facilities, in the Rakai region.
(A) Map of the Rakai region showing the location of ART treatment facilities and RCCS communities; red circles represent public facilities, green circles represent private or NGO-run clinics, and blue circles hospitals. (B) Community antiretroviral therapy coverage and distance to nearest treatment facility. Distances are from household to their treatment facility in kilometers; (C) Distance to nearest treatment facility and distance traveled to preferred treatment facility by sub-county. Plot show medians and IQRs of distances from household to treatment facility in kilometers. *Indicates Wilcoxon-rank p-value < 0.05 comparing distance traveled and distance to nearest facility.
Statistical analysis
Variables of interest included viral suppression (<400 copies/mL); distance traveled in kilometers; whether or not persons traveled further than the closest ART facility; and the level of service accessed. Socioeconomic status was based on household building materials, with modern construction materials indicating higher wealth [30]. Demographic characteristics were compared between individuals attending the nearest facility and those traveling beyond the nearest facility. Cumulative distribution functions, medians and interquartile ranges summarized distances traveled, differences in travel distance by subgroups and by viral supression, were assessed by Wilcoxon-rank sum tests. RCCS data were aggregated into 11 sub-counties. For those traveling beyond the nearest ART facility, modified Poisson regression was used to estimate prevalence risk ratios (PRR) with 95% CI of attending a Health Center Level 4 for tertiary care versus lower level facilities. Statistical analyses were performed in R statistical software (V3.2).
RESULTS
From February 2015 and September 2016, a total of 1554 HIV-positive persons were identified in the RCCS residing in 30 communities serviced by static HIV clinics. Of these persons, 69%(1076/1554) were on ART and 93%(1002/1076) of those on ART were virally suppressed. 76 persons missing treatment facility information were excluded from the facility choice analysis.
Demographics of ART-treated and virally suppressed populations
Supplement Table 1 shows the demographics of all HIV-infected persons, those on ART, and those virally suppressed. Men were less likely to be on ART (p<0.001). Persons aged 15–24 were less likely to be on ART (p<0.001) and to have suppressed viral load (p<0.001). Persons previously married and those in non-high risk occupations were more likely to be on ART.
Distance to ART treatment facility
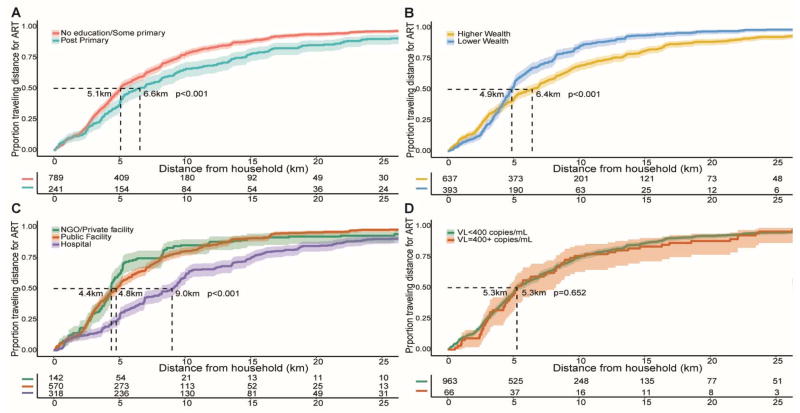
The median distance from households to the nearest ART facility was 3.10 km, interquartile range (IQR, 1.65–5.05), but individuals traveled a median of 5.26 km (IQR, 3.00–10.03) for ART (p<0.001) (Supplemental Table 2), and 57% of patients (589/1030) chose to travel to a facility further than their nearest facility (Supplement Table 2). Figure 1B presents distributions of community ART coverage and distance to nearest treatment facility. There was substantial variability and there was no association between community ART coverage and distance to the nearest ART facility when distance was considered as a linear or as a categorical variable. The distance to the nearest clinic ranged from a median of 1.41 km to 6.45 km across sub-counties, and in all but two sub-counties, individuals traveled significantly further than their nearest facility (range 2.76 km to 10.97 km) (Figure 1C). Figure 2, shows the cumulative proportions of distances traveled for ART by sociodemographic characteristics and facility type. The travel distance was longer in persons with a post-primary school education (6.56 km vs 5.09 km, p<0.001), higher socioeconomic status (6.43 km vs 4.87 km, p=0.001), and use of HC4 tertiary care facilities (9.02 km), compared to HC3 (4.76 km), HC2 (6.97km) or Private/NGO facilities (4.39 km, p=0.001). Supplemental Table 3 shows that the PRRs of travel beyond the nearest ART facility was associated with higher wealth (p=0.038).
Figure 2. Distance traveled to access HIV treatment, in the Rakai Community Cohort Study.
Figure shows the cumulative proportion who traveled particular distances for ART treatment with 95% CI intervals shown as shaded areas. Distances are from their household to their treatment facility in kilometers. (A) Distance to treatment facility by traveled by education level; (B) Distance to treatment facility by traveled by wealth level; (C) Distance to treatment facility by traveled by health facility type; (D) Distance to treatment facility by traveled by viral load.
Virologic and health outcomes
94% (963/1030) of persons on ART were virally suppressed and there were no differences in travel distance between those with or without a detectable viral load (5.27 vs 5.26 km, p=0.650). There were no differences in viral suppression between persons using or not their nearest facility (adjPRR 0.99, 95% CI 0.70–1.37, Supplemental Table 3). Viral suppression did not differ by type of facility. Forty-one percent (241/589) of those not attending their nearest ART facility used a tertiary care facility (Supplemental Table 4).
DISCUSSION
In rural south-central Uganda, there is substantial heterogeneity in distance to the nearest ART facility, however, this distance was not predictive of community ART coverage or viral suppression. More than half HIV-infected individuals traveled beyond their nearest facility, which corroborates a recent study in Uganda which found that people living with HIV tended to bypass nearer ART sites and sought care in higher-tiered ART sites[23].
Those with lower education and wealth were less likely to travel further than their nearest ART facility compared to persons of higher education and wealth. This may reflect avoidance of stigma as reported in other studies[21]. It is possible that patients failed to use the nearest facility because they considered care at this local service to be of lower quality than a more distant facility. The greater use of tertiary-level services by the more affluent and educated suggests that this perception of the inferiority of local services may have affected choice.
Multiple studies have reported conflicting effects of self-reported transportation barriers on HIV outcomes negative[3–5], null[6–8,13], and positive[9–11]effects of self- reported transportation barriers on HIV associated outcomes. Studies utilizing GPS measurements found no effects of distance on adherence [14] but negative effects on visit attendance[13,18,19]. This study measured travel distance on a road network a more accurate estimate of travel distance[22,23] than Euclidean (straight line) distance[3,8,24]and is associated with use of health services[25]. Irrespective of distance traveled, most ART patients achieved viral suppression, suggesting that they are likely to be highly motivated and adherent.
This analysis has several limitations. We did not directly measure travel distances or travel times, and the road network analysis may overestimate the actual travel distance. However, the shortest-route is a reasonable conservative estimate. The study excluded regions with mobile HIV treatment services and findings may not be generalizable to areas with mobile services. Our study was restricted to individuals engaged in the local healthcare system and did not capture the extent to which geospatial barriers limit access to HIV care or ART initiation. However, additional information on those not on ART in this study population is reported elsewhere[31]. Our findings also may not be generalizable to other rural settings as Rakai district has substantial and diverse ART facilities so greater patient choice may minimize the impact of travel distance on ART and viral suppression.
In conclusion, ART coverage and viral suppression are not associated with distance traveled to services in Rakai, Uganda. Distance traveled, and level of services used were associated with higher socioeconomic status, so affordability of travel costs may provide an advantage to those with higher relative wealth. Our findings have implications for improving access to care in rural resource-limited settings. Ministry of Health and health system planners must consider location as well as service type, individual preferences, and costs when seeking to provide access to treatment services.
Supplementary Material
Acknowledgments
Sources of Support: This study was supported by the National Institute of Allergy and Infectious Diseases, in part (SJR) by the Division of Intramural Research, NIAID, the National Institute of Mental Health, the National Institute of Child Health and Human Development, the Bill & Melinda Gates Foundation, and Centers for Disease control and Prevention cooperative agreement of PEPFAR non-research clinical records.
This research was supported by the National Institute of Allergy and Infectious Diseases (RO1AI114438, RO1AI110324, UO1AI10031, in part (SJR) by the Division of Intramural Research, NIAID), the National Institute of Mental Health (RO1MH107275), the National Institute of Child Health and Human Development (RO1HD070769), the Bill & Melinda Gates Foundation (22006.03), and Centers for Disease control and Prevention cooperative agreement of PEPFAR non-research clinical records (USGPS000971). We also thank the Rakai Community Cohort Study participants and study team.
References
- 1.World Health Organization. Global Health Sector Strategy on HIV 2016–2021. Geneva, Switzerland: 2016. [Google Scholar]
- 2.Moyer CA, Mustafa A. Drivers and deterrents of facility delivery in sub-Saharan Africa: a systematic review. doi: 10.1186/1742-4755-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingle S, May M, Uebel K, Kotze E, Bachmann M, Sterne JAC, et al. Outcomes in Patients Waiting for Antiretroviral Treatment in the Free State Province, South Africa: Prospective Linkage Study. AIDS. 2011;24:2717–2725. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempf M-C, Allen S, Zulu I, Kancheya N, Stephenson R, Brill I, et al. Enrollment and retention of HIV discordant couples in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47:116–25. doi: 10.1097/QAI.0b013e31815d2f3f. [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe CG, Bolton-Moore C, van Dijk JH, Cotham M, Tambatamba B, Moss WJ. Secular trends in pediatric antiretroviral treatment programs in rural and urban Zambia: a retrospective cohort study. BMC Pediatr. 2010;10:54. doi: 10.1186/1471-2431-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, Ele P, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS One. 2010;5:e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12:687–94. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk JH, Sutcliffe CG, Munsanje B, Sinywimaanzi P, Hamangaba F, Thuma PE, et al. HIV-infected children in rural Zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. PLoS One. 2011;6:e19006. doi: 10.1371/journal.pone.0019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook RE, Ciampa PJ, Sidat M, Blevins M, Burlison J, Davidson MA, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambézia, Mozambique. J Acquir Immune Defic Syndr. 2011;56:e104–9. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng EH, Glidden DV, Bwana MB, Musinguzi N, Emenyonu N, Muyindike W, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS One. 2011;6:e21797. doi: 10.1371/journal.pone.0021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haberer JE, Kiwanuka J, Nansera D, Ragland K, Mellins C, Bangsberg DR. Multiple measures reveal antiretroviral adherence successes and challenges in HIV-infected Ugandan children. PLoS One. 2012;7:e36737. doi: 10.1371/journal.pone.0036737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taiwo BO, Idoko JA, Welty LJ, Otoh I, Job G, Iyaji PG, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr. 2010;54:85–92. doi: 10.1097/01.qai.0000371678.25873.1c. [DOI] [PubMed] [Google Scholar]
- 13.Iroha E, Esezobor CI, Ezeaka C, Temiye EO, Akinsulie A. Adherence to antiretroviral therapy among HIV-infected children attending a donor-funded clinic at a tertiary hospital in Nigeria. Afr J AIDS Res. 2010;9:25–30. doi: 10.2989/16085906.2010.484543. [DOI] [PubMed] [Google Scholar]
- 14.Carlucci JG, Kamanga A, Sheneberger R, Shepherd B, Jenkins CA, Spurrier J, et al. Predictors of Adherence to Antiretroviral Therapy in Rural Zambia. J Acquir Immune Defic Syndr. 2008;47:615–622. doi: 10.1097/QAI.0b013e318165dc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88:681–8. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramadhani HO, Thielman NM, Landman KZ, Ndosi EM, Gao F, Kirchherr JL, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45:1492–8. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 17.Crawford KW, Wakabi S, Magala F, Kibuuka H, Liu M, Hamm T. Evaluation of treatment outcomes for patients on first-line regimens in US President’s Emergency Plan for AIDS Relief (PEPFAR) clinics in Uganda: Predictors of virological and immunological response from RV288 analyses. HIV Med. 2015;16:95–104. doi: 10.1111/hiv.12177. [DOI] [PubMed] [Google Scholar]
- 18.Munyaneza F, Ntaganira J, Nyirazinyoye L, Birru E, Nisingizwe M, paul Gupta N, et al. Community-based accompaniment and the impact of distance for HIV patients newly initiated on antiretroviral therapy: early outcomes and clinic visit adherence in rural Rwand. AIDS Behav. 2016:1–20. doi: 10.1007/s10461-016-1658-5. [DOI] [PubMed] [Google Scholar]
- 19.Conley NJ, Pavlinac PB, Guthrie BL, Mackelprang RD, Muiru AN, Choi RY, et al. Distance from home to study clinic and risk of follow-up interruption in a cohort of HIV-1-discordant couples in Nairobi, Kenya. PLoS One. 2012:7. doi: 10.1371/journal.pone.0043138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duff P, Kipp W, Wild TC, Rubaale T, Okech-Ojony J. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS Soc. 2010;13:37. doi: 10.1186/1758-2652-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation Costs Impede Sustained Adherence and Access to HAART in a Clinic Population in Southwestern Uganda: A Qualitative Study. AIDS Behav. 2010;14:778–784. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muchedzi A, Chandisarewa W, Keatinge J, Stranix-Chibanda L, Woelk G, Mbizvo E, et al. Factors associated with access to HIV care and treatment in a prevention of mother to child transmission programme in urban Zimbabwe. J Int AIDS Soc. 2010:38. doi: 10.1186/1758-2652-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akullian AN, Mukose A, Levine GA, Babigumira JB. People living with HIV travel farther to access healthcare: A population-based geographic analysis from rural Uganda. J Int AIDS Soc. 2016;19:1–8. doi: 10.7448/IAS.19.1.20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav. 2014;18:1199–223. doi: 10.1007/s10461-014-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serwadda D, Mugerwa RD, Sewankambo NK, Lwegaba A, Carswell JW, Kirya GB, et al. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet. 1985;2:849–52. doi: 10.1016/S0140-6736(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 26.Grabowski MK, Lessler J, Redd AD, Kagaayi J, Laeyendecker O, Ndyanabo A, et al. The Role of Viral Introductions in Sustaining Community-Based HIV Epidemics in Rural Uganda: Evidence from Spatial Clustering, Phylogenetics, and Egocentric Transmission Models. PLoS Med. 2014:11. doi: 10.1371/journal.pmed.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galiwango RM, Musoke R, Lubyayi L, Ssekubugu R, Kalibbala S, Ssekweyama V, et al. Evaluation of current rapid HIV test algorithms in Rakai, Uganda. J Virol Methods. 2013;192:25–7. doi: 10.1016/j.jviromet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uganda Ministry of Health. Health Sector Strategic Plan 3. Gov Uganda. 2014:16–18. [Google Scholar]
- 29.OpenStreetMap Project Wiki. [accessed 1 Sep2016];OpenStreetMap Wiki. http://wiki.openstreetmap.org/wiki/Main_Page.
- 30.Makumbi F, Nakigozi G, Lutalo T, Kagayi J, Sekasanvu J, Settuba A, et al. Use of HIV-Related Services and Modern Contraception among Women of Reproductive Age, Rakai Uganda. African J Reprod Heal. 2010 Dec;:14. [PubMed] [Google Scholar]
- 31.Billioux VG, Chang LW, Reynolds SJ, Nakigozi G, Ssekasanvu J, Grabowski MK, et al. Human immunodeficiency virus care cascade among sub-populations in Rakai, Uganda: an observational study. J Int AIDS Soc. 2017;20:1–9. doi: 10.7448/IAS.20.1.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.