Abstract
Objectives
HIV-positive women have higher human papillomavirus (HPV) prevalence and cervical cancer (CC) incidence than HIV-negative women, partly due to HIV’s modifying effect on HPV pathogenesis. We synthesized the literature on the impact of HIV on HPV natural history.
Design
Systematic review and meta-analysis
Methods
We searched the literature for studies evaluating HPV acquisition and persistence or precancer progression by HIV status. Data on HPV natural history by HIV status, CD4+ cell counts, viral load, and antiretroviral therapy (ART) were summarized using fixed effect models.
Results
Overall, 38 of 1845 abstracts identified met inclusion criteria. HIV-positive women had higher HPV acquisition (relative risk [RRpooled]=2.64, 95% confidence interval [CI] 2.04–3.42) and lower HPV clearance (hazard ratio [HRpooled]=0.72, 95% CI 0.62–0.84) than HIV-negative women. HPV acquisition was higher with declining CD4 and was lower in those virally suppressed on ART. HIV was associated with higher incidence of low-grade squamous intraepithelial lesions (LSIL) (RRpooled=3.73, 95% CI 2.62–5.32) and high-grade squamous intraepithelial lesions (HSIL) (HRpooled=1.32, 95% CI 1.10–1.58), largely due to increased HPV persistence. ART lowered progression from normal cytology to LSIL (HRpooled=0.65, 95% CI 0.52–0.82), but not HSIL. CC incidence was associated with HIV positivity (RR=4.1, 95% CI 2.3–6.6), but not with ART.
Conclusions
HIV-positive women have higher risk of acquiring HPV, with risk inversely associated with CD4 count. ART lowered HPV acquisition, increased clearance, and reduced precancer progression, likely via immune reconstitution. While some of our results are limited by small number of studies, our study can inform screening guidelines and mathematical modeling for CC prevention.
Keywords: HIV, human papillomavirus, HIV/HPV coinfection, precancerous lesions, cervical cancer, antiretroviral therapy
Introduction
Cervical cancer (CC) is the fourth most common cancer in women worldwide and the most common cancer among women in sub-Saharan Africa [1]. Sub-Saharan Africa has high dual burden of human papillomavirus (HPV) and HIV infection [2,3]. HIV is associated with higher rates of HPV acquisition, decreased clearance of HPV and precancerous lesions, and increased risk of CC [4,5]. Compared to HIV-negative women, CC mortality in HIV-positive women is ~2-times higher [6,7]. With increasing life expectancy of HIV-positive women on antiretroviral treatment (ART), there is a growing need for CC prevention, particularly in low resources settings [8]. In the United States, CC screening programs have been highly effective in reducing CC incidence and HPV vaccines have been shown to reduce the prevalence of HPV 16 and 18-attributable precancer lesions [9,10]. However, availability of HPV vaccines and CC screening is extremely limited in developing settings, although efforts to scale up screening are ongoing in Zambia [11,12]. CC screening coverage in the last 3 years among eligible women is less than 6% in Kenya and 23% in South Africa [13].
CC screening is not without harm and research on the optimal screening interval for HIV-positive women is still emerging [14]. In September 2015, the recommended CC screening interval for HIV-positive women with normal cytology was changed from annually to every 3 years in the United States [15]. A better understanding of the impact of HIV on HPV natural history can help inform CC screening and prevention policies for HIV-positive women.
Further, although many studies have evaluated the relationship between HIV and HPV pathogenesis the findings have not been synthesized. While ART decreases the incidence of other AIDS-related cancers, its relationship with CC is unclear [16,17,14]. We conducted a systematic review and meta-analysis to synthesize the literature on HPV natural history in HIV-positive women. Specifically, we evaluate the risk of HPV incidence, persistence, and progression to cervical lesions and cancer among HIV-positive women compare to HIV-negative women. We additionally assess these relationships by CD4+ cell counts, HIV viral load (VL), and ART use.
Methods
Study objectives
We sought to assess the interactions between HIV and HPV on the 1) incidence of HPV infection; 2) persistence/clearance of HPV infection; 3) progression from HPV infection to low-grade squamous intraepithelial lesion (LSIL); 4) progression from LSIL to high-grade squamous intraepithelial lesion (HSIL); 5) regression from LSIL; 6) regression from HSIL; and 7) progression to invasive CC. HPV pathogenesis in HIV-positive women was compared to HIV-negative women. We assess the modifying effect of CD4 count, HIV VL, and ART on interactions between HIV and HPV.
Inclusion/exclusion criteria
Eligible studies examined the acquisition, persistence, or clearance of HPV or the incidence, progression, and regression of cervical lesions by HIV status, CD4 count, HIV VL, and ART using cohort or case-control study design. Studies assessing HPV incidence were included only if they adjusted for confounders identified in previous literature (e.g. age, number of sex partners, etc.) or identified in their data; however we did not restrict on specific confounders (Appendix Table 3). Studies grouping LSIL and HSIL together were only included if proportions of LSIL and HSIL were reported. If >85% of lesions detected were LSIL, we considered the estimates to approximate LSIL natural history.
Search strategy
Following PRISMA guidelines, we conducted a systematic search on PubMed, Embase, Global Health Database, reference list of eligible papers, and conference abstracts (Appendix tables 1 and 2) [18]. Search terms included HIV, HPV, cervical lesion, and disease progression. We included only primary research articles, but did not restrict on language or publication date. Additional details about the search strategy are available in the Appendix.
Study quality assessment
We assessed the risk of bias (low, moderate, high) in studies using modified criteria for non-randomized observational studies across five domains: study population, detection of outcome and exposure, bias related to study design, statistical analysis, and disclosure of conflict of interest [19,20]. See Appendix Figures 1 and 2 for detailed study quality assessment.
Statistical analysis
When possible, effects by HIV status, CD4 count, and ART use were pooled using fixed effect models with inverse variance weighting (SAS 9.4). We combined estimates with the same measures of association (e.g. relative risk, odds ratio, or hazard ratio). We quantified heterogeneity with I2. When the number of studies in a meta-analysis is small, I2 was sometimes a negative value and set to zero.
Results
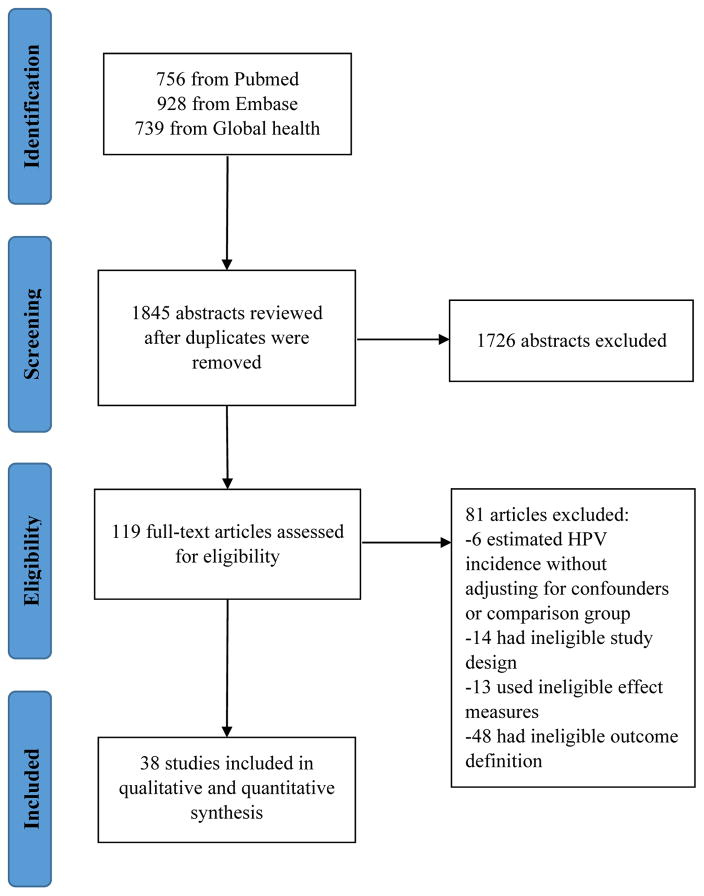
The search was conducted on 5/19/2017 and yielded 1845 studies. We excluded 1726 studies after screening abstracts (Figure 1). Primary reasons for exclusions were ineligible study design and HPV infection site. We further excluded 81 studies after reading the full text. Overall, 38 studies were included (Table 1). Most studies were prospective cohort (n=34); the remaining were retrospective cohort (n=2), nested case-control (n=1), and case-crossover (n=1). Most were conducted in North America (n=18) or Africa (n=13). The US (n=15) and South Africa (n=7) were the most frequently represented countries. One study was conducted in Asia and six in Europe. Half of studies included HIV-negative women as the comparison group (n=23). Papanicolaou (Pap) smear was most commonly used to collect cervical specimens and polymerase chain reaction using L1 consensus primers was most common method for genotype detection.
Figure 1.
Flow chart of the systematic review process.
Table 1.
Summary of 38 studies included in the systematic review and meta-analysis.
| Author | Data collecti on |
Study Design | Location | Population | N | Age | Follow up duration |
Study visit interval |
Question addressed | Refe renc e |
|---|---|---|---|---|---|---|---|---|---|---|
| Abraham, 2013 | 1996–2010 | Prospective; Nested case-control | US | CC cases in HIV positive and negative women | Cohort: 25711 Case-control: 17 |
Median 37 | Median: 4.5 – 5.0 years | N/A | CC incidence | 54 |
| Adler, 2012 | 2003–2010 | Prospective | South Africa | HIV positive women | 1123 | Mean 32.6 | Median: 2.7 years | Median 1.2 years | Progression to LSIL, HSIL; LSIL regression | 17 |
| Ahdieh, 2000 | 1992–1998 | Prospective | US | HIV-positive and HIV-negative women | 187 | Median 36.2 | Median 5–6 visits | 6 months | HPV clearance | 35 |
| Ahdieh-Grant, 2004 | 1994–1995 | Prospective | US | HIV positive women | 312 | Median 36.8–39.2 | ≥7 years | 6 months | LSIL regression | 51 |
| Banura, 2008 | 2004 | Prospective | Uganda | HIV positive and negative pregnant women | 334 | N/A | Range: 3–9 months | Range: 3–9 months | HPV incidence | 21 |
| Banura, 2010 | 2002–2006 | Prospective | Uganda | Sexually active HIV positive and negative women | 380 | Median 20 | 2 years | Median 18.5 months | HPV incidence and clearance | 22 |
| Blitz, 2013 | 1993–2012 | Prospective | Canada | HIV-positive and high-risk HIV-negative women | 1073 | Mean 26–33 | N/A | 6 months | HPV clearance | 33 |
| Clifford, 2016 | 1988–2013 | Nested case control | Switzerland | HIV-positive CIN2/3 and CC cases and controls | 364 CIN2/3 cases 20 CC cases |
CIN2/3: mean 29.7 CC: mean 34.3 |
N/A | N/A | HSIL and CC incidence | 4 |
| Del Mistro, 2004 | 2000–2002 | Prospective | Italy | HIV-positive women | 201 | Median 33 | N/A | 6–12 months | LSIL and HSIL regression | 52 |
| Delmas, 2000 | 1993–1998 | Prospective | Europe | HIV-positive women | 467 | Median 31 | Median 2 years | 6 months | LSIL incidence and regression | 41 |
| Denny, 2008 | 2002–2003 | Prospective | South Africa | HIV-positive women | 400 | Median 29.3 | 18 months | 6 months | HPV and LSIL incidence | 23 |
| Duerr, 2006 | 1993–1995 | Prospective | US | HIV-positive and HIV-negative women | 1199 | N/A | Median 4.4 years | 6 months | LSIL incidence | 38 |
| Ellerbrock, 2000 | 1991–1996 | Prospective | US | HIV-positive and HIV-negative women | 653 | N/A | Mean 29.5–33.3 months | 6 months | LSIL incidence | 39 |
| Firnhaber, 2012 | 2005–2009 | Prospective | South Africa | HIV-positive women | 601 | Median 35 | Median 1.2 years | 6 months | LSIL incidence | 42 |
| Hawes, 2006 | 1994–1998 | Prospective | Senegal | HIV-positive and HIV-negative women | 627 | Median 30.5–34.2 | Mean 2.2–2.7 years | 4 months | HSIL incidence | 45 |
| Heard, 2006 | 1993–2005 | Prospective | France | HIV-positive women | 298 | Median 33.3 | Median 28 months | 6 months | LSIL incidence | 43 |
| Kelly, 2016 | 2011–2012 | Prospective | Burkina Faso and South Africa | HIV-positive women | 1238 | Median 34–36 | Median 16 months | 6 months | HSIL incidence | 49 |
| Kim, 2013 | 1991–2011 | Retrospective | US | HIV-positive women | 245 | Mean 36.5 | Range 2–36 months | Median 11.8 months | LSIL incidence | 44 |
| Koshiol, 2006 | 1993–2000 | Prospective | US | HIV-positive and high risk HIV-negative women | 801 | Mean 35 | Median 4.4 years | 6 months | HPV clearance | 32 |
| Lillo, 2001 | 1995–1997 | Prospective | Italy | HIV-positive women | 163 | Mean 33.6 | Median 15.4 months | 6 months | HPV incidence | 24 |
| Mane, 2016 | 2006–2009 | Prospective | India | HIV-positive women | 215 | Median 31 | 12 months | 12 months | HPV incidence | 25 |
| Massad, 2001 | 1994–1999 | Prospective | US | HIV-positive and high risk HIV-negative women | 2091 | Median 36 | Median 4.0 years | 6 months | LSIL and HSIL incidence | 40 |
| Massad, 2005 | 1994–2003 | Prospective | US | HIV-positive and high risk HIV-negative women | 494 | Median 33.8–37.1 | Median 3.7–4.0 years | 6 months | HSIL incidence | 46 |
| Massad, 2004 | 1994–2002 | Prospective | US | HIV-positive and high risk HIV-negative women | 223 | Mean 3.3 years | 6 months | LSIL regression | 50 | |
| Mbulawa, 2012 | 2006–2009 | Prospective | South Africa | HIV-positive women in heterosexual couples | 486 | Median 35 | N/A | 6 months | HPV incidence and clearance | 59 |
| Minkoff, 2010 | 1994–2009 | Case-crossover | US | HIV-positive HAART recipients | 286 | Median 39 | 5 years | 6 months | HPV incidence and clearance | 27 |
| Moscicki, 2004a | 1996–2000 | Prospective | US | HIV-positive and HIV-negative adolescents | 334 | Mean 16.7 | 5.5–6 visits | 6 months | HPV clearance | 36 |
| Moscicki, 2004b | 1996–2000 | Prospective | US | HIV-positive and HIV-negative adolescents | 256 | Mean 16.7 | Mean 37.8–39.5 months | 6 months | HSIL incidence | 50 |
| Omar, 2011 | 2003–2009 | Prospective | South Africa | HIV-positive women | 1074 | Median 32 | Median 2.5 years | 12 months | HSIL incidence | 54 |
| Phelan, 2009 | 1992–1997 | Prospective | US | HIV-positive and HIV-negative women | 219 | Mean 37 | N/A | N/A | HPV incidence | 28 |
| Rowhani-Rahbar, 2007 | 1994–1998 | Prospective | Senegal | HIV-positive and HIV-negative women | 481 | Mean 31.4 | Median 1.2 years | 4 months | HPV clearance | 37 |
| Safaeian, 2008 | 1998–2007 | Prospective | Uganda | HIV-positive and HIV-negative women | 1055 | Median 28 | Median 3 years | 12 months | HPV incidence and clearance | 29 |
| Shrestha, 2010 | 1996–2000 | Case-crossover | US | HIV-positive and HIV-negative adolescents | 373 | Median 17 | N/A | 6 months | HPV incidence | 30 |
| Six, 1998 | 1993–1995 | Prospective | France | HIV-positive and HIV-negative women | 442 | Median 29–31 | Median 13 months | 6 months | HSIL incidence and LSIL regression | 48 |
| Strickler, 2005 | 1994–1997 | Prospective | US | HIV-positive women | 2362 | N/A | Median 36 months | 6 months | HPV incidence | 5 |
| Whitham, 2017 | 1994–2010 | Prospective | Senegal | HIV-positive and HIV-negative women | 1320 | Median 35 | Mean 2 years | 4 months | HPV clearance and HSIL incidence and regression | 34 |
| Xie, 2013 | 1994–2001 | Prospective | US | HIV-positive and HIV-negative women | 2386 | N/A | N/A | 6 months | HPV incidence | 31 |
| Zeier, 2012 | 1992–2010 | Retrospective | South Africa | HIV-positive and HIV-negative women | 1720 | Median 32.5–35.6 | Median 17.5–31.4 | N/A | HSIL incidence and LSIL regression | 47 |
CC, cervical cancer; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; HPV, human papillomavirus; CIN 2/3, cervical intraepithelial neoplasia grade 2 or 3; HAART, highly active antiretroviral therapy
The majority of studies had low risk of bias in selection of study population (79%), ascertainment of exposure and outcome variables (76%), and statistical analysis (71%) (Appendix figures 2 and 3). Only 32% had low risk of study design-specific bias, due to incomplete ascertainment of ART use or high loss to follow-up.
HPV incidence
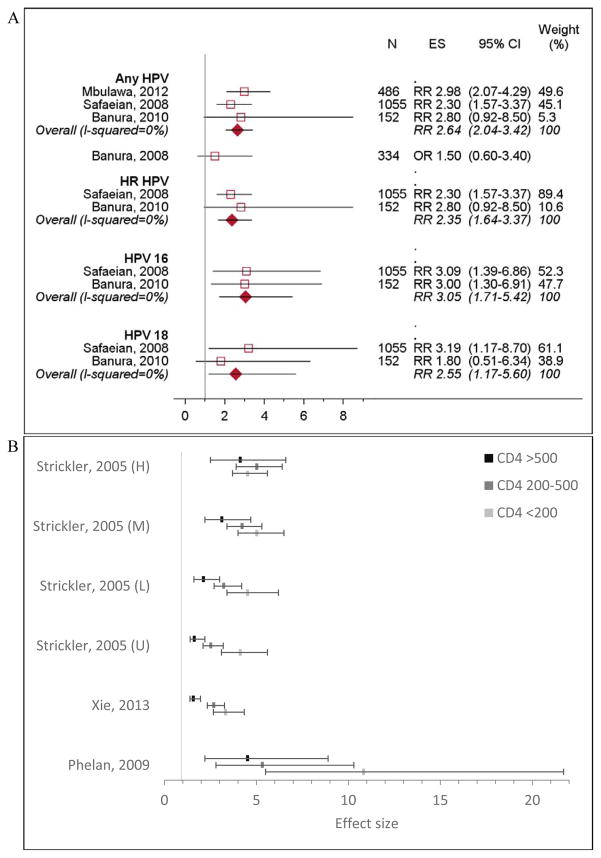
Twelve studies assessed HPV incidence [5,21–31], seven included HIV-negative women as reference [5,21,22,26,28,31]. HIV-positive women had higher risk of acquiring infection with any HPV (RRpooled 2.64, 95% CI 2.04–3.42), as well as high risk (HR) HPV, HPV 16, and HPV 18 (RRpooled 2.35, 95% CI 1.64–3.37; RRpooled 3.05, 95% CI 1.71–5.42; RRpooled 2.55, 95% CI 1.17–5.60, respectively) (Figure 2a).
Figure 2. HPV incidence among HIV-positive women compared to HIV-negative women.
N, sample size; ES, effect size. A). Incidence of HPV infection by HPV type. Banura, 2008 was not included in calculating the overall effect because it reported odds ratio; other studies reported relative risk. B). Relative incidence of any HPV infection, by CD4 count. The effect estimates from Strickler et al, 2005 were stratified HIV RNA load; U = undetectable (<4,000 copies/mL), L = low (4,000–20,000 copies/mL), M = moderate (20,001–100,000 copies/mL), and H = high (>100000 copies/mL). Effect size was measured in odds ratios in Phelan et al, 2009 and hazard ratios in Xie et al, 2003 and Strickler et al, 2005. Effect estimates from Mbulawa et al 2012, Denny et al, 2008, and Mane et al, 2016 were not included in the figure. Mbulawa et al, 2012 used a different CD4 cutoff (>350 vs. <350) and Denny et al, 2008 and Mane et al, 2016 evaluated CD4 as a continuous variable.
CD4 count
Four studies found that CD4 count modified HPV acquisition in HIV-positive women [5,23,28,31]. Women with high CD4 count (>500 cells/mm3) were at greater risk of infection compared to HIV-negative women, and risk dramatically increased with declining CD4 count (Figure 2b) [5,28,31]. One study found the risk of HPV infection decreased by 18% with every 100-cell increase in CD4 (OR 0.82, 95% CI 0.70–0.96) [23]. Two studies found higher CD4 count did not significantly reduce HPV incidence (Appendix Table 3) [25,26].
ART
Three of the four studies that ascertained ART use from medical records or self-reports found no association between ART and HPV incidence (Appendix Table 3) [25,28,30]; while one study found ART reduced HPV 16 and 18 incidence by 72% (RR 0.28, 95% CI 0.09–0.86) but not any HPV incidence (RR 0.79, 95% CI 0.28–2.23) [24]. One study comparing effective ART (defined as reducing HIV VL by >90% or to undetectable), to no treatment found that effective ART decreased incidence of any HPV by 36% (OR 0.64, 95% CI 0.46–0.88) but the impact on HR HPV incidence was not statistically significant (OR 0.62, 95% CI 0.38–1.02) [27].
HIV VL
Two studies evaluated the role of HIV VL on HPV acquisition [5,26]. Compared to HIV-negative women, the risk of any HPV infection was 3.29 times (95% CI 2.18–4.95) higher among HIV-positive women with VL>10,000 copies/mL and 2.31 times (95% CI 1.49–3.58) higher among those with VL<10,000 copies/mL [26]. Further, HPV acquisition was higher with increasing VL among women with CD4 >200 cells/mm3 (Figure 2b), although no pattern in HPV acquisition by VL was observed in women with CD4 <200 cells/mm3 [5].
HPV Reactivation
One study found that new HPV infections were detected in women who reported no sexual activity in the last 18 months, indicating potential reactivation of latent infections. The rate of potential reactivations was 1.4–4.4 times higher among HIV-positive compared to HIV-negative women [5].
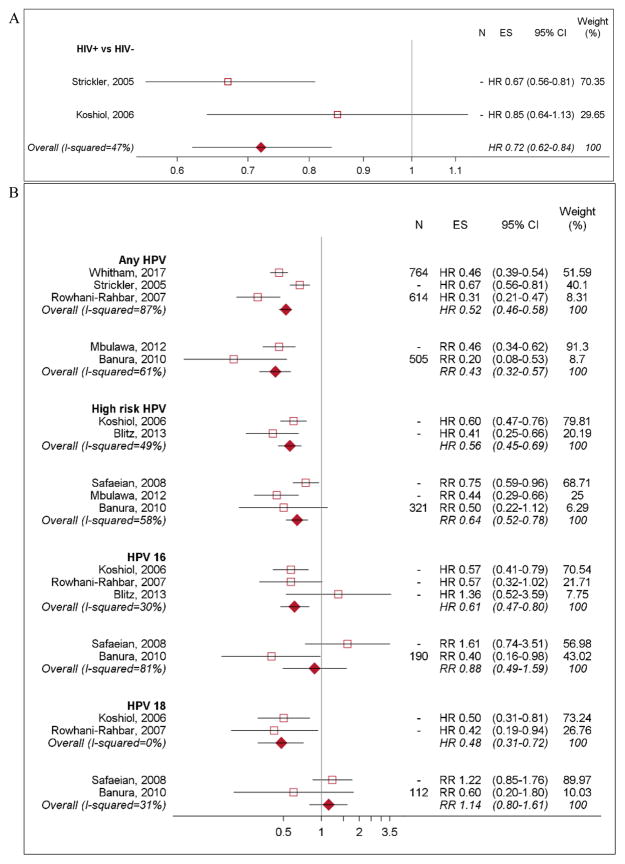
HPV persistence and clearance
Two studies evaluated clearance among women with incident HPV infection [5,32]. HIV-positive women were 28% less likely than HIV-negative women to clear infection with any HPV (HRpooled 0.72, 95% CI 0.62–0.84; Figure 3a). Eleven studies evaluated clearance of incident and prevalent HPV infections together [22,25–27,29,32–37] and found HIV-positive women were 41–46% less likely to clear any HPV infection (HRpooled 0.54, 95% CI 0.48–0.60; RRpooled 0.59, 95% CI 0.49–0.71) and 36–44% less likely to clear HR HPV infection (HRpooled 0.56, 95% CI 0.45–0.69; RRpooled 0.64, 95% CI 0.52–0.78) (Figure 3b) [22,26,32,33]. However, pooled effects based on two studies indicate that HPV 16 and 18 clearance were not statistically different by HIV status (RRpooled 0.88, 95% CI 0.49–1.59; RRpooled 1.14, 95% CI 0.80–1.61, respectively) [22,29]; while the pooled effects based on three studies indicate that HIV-positive women had lower clearance of both types compared to HIV-negative women (HRpooled 0.61, 95% CI 0.47–0.80 for HPV 16; HRpooled 0.48, 95% CI 0.31–0.72 for HPV 18) (Figure 3b) [21,33,37].
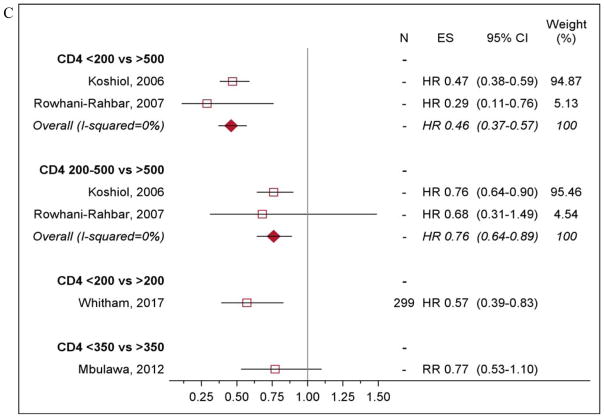
Figure 3. Clearance of newly detected and prevalent HPV infections by HIV status and CD4 count.
N: sample size, ES: effect size. N= “-” Indicates that the study did not report sample size associated with their estimates. A). Clearance of newly detected HPV infection among HIV-positive women compared to HIV-negative women. B). Clearance of prevalent and newly detected HPV infection among HIV-positive women compared to HIV-negative women, by HPV type. C). Clearance of HPV infection among HIV-positive women by CD4 count. Estimates from Mane et al, 2016 and Ahdieh et al, 2000 were not included in the forest plot. Mane et al, 2016 evaluated CD4 count as a continuous variable. And Ahdieh et al, 2000 used HIV-negative women was their reference group.
Women with low CD4 count (<200 cells/mm3) had much lower likelihood of HPV clearance compared to women with higher CD4 count (Figure 3c) or HIV-negative women [26,32,34,35,37]. However, two studies found no difference in HPV clearance by CD4 count (Appendix Table 5) [25,26]. In one study, clearance rates did not differ by HIV VL (RR 0.77, 95% CI 0.51–1.16) but another found that every one log increase in VL resulted in a 23% reduction in likelihood of clearance (HR 0.77, 95% CI 0.64–0.91) [26,27]. ART did not impact clearance of any HPV infection, regardless of adherence or effectiveness (Appendix Table 5) [25,27,33]. However, one study found clearance of HR HPV infection other than HPV 16 or 18 was higher among women on ART compared to those not on ART (HR 2.20, 95% CI 1.22–3.98) [33].
Progression to LSIL
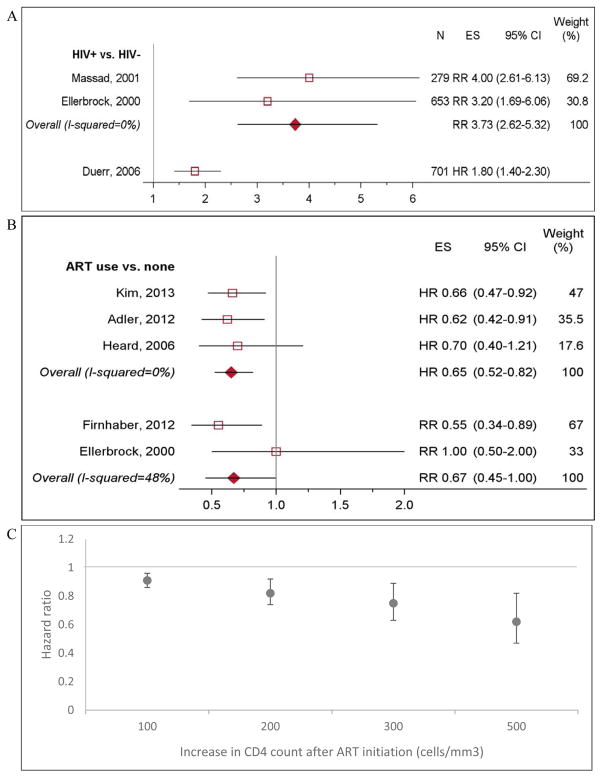
Eight studies evaluated progression to LSIL from normal cytology [17,37–43] and one study evaluated progression to LSIL from normal cytoloy or atypical squamous cells of undetermined significance (ASCUS) [23]. Among women with normal cytology at baseline, HIV-positive women had 3.73 times higher risk of progression to ASCUS or LSIL compared to HIV-negative women (95% CI 2.62–5.32; Figure 4a) [38–40,44]. Among HIV-positive women, LSIL incidence in those with persistent HPV infection (detected at ≥2 visits 3–12 months apart) was 11 times higher with HPV 16/18 (95% CI 1.4–88.7) and 8.9 times higher with non-HPV 16/18 types (95% CI 1.2–66.2), compared to women without persistent HPV [39]. In one study, CD4 count and ART were no longer associated with LSIL incidence after adjusting for persistent HPV infection (Appendix Table 6) [39].
Figure 4. LSIL incidence by HIV status and ART use.
N: Sample size, ES: effect size. A). LSIL incidence among HIV-positive women compared to HIV-negative women. Duerr et al, 2006 reported its effect size in hazard ratios, and was not included in calculating the overall estimate. B). LSIL incidence among HIV-positive women on ART compared to HIV-positive women not on ART. Estimates from Adler et al, 2012, Firnhaber et al, 2012, and Ellerbrock et al, 2000 adjusted for CD4 count. C). LSIL incidence by absolute increase in CD4 count after ART initiation. Data extracted from Kim, 2013.
LSIL risk was >30% lower in women on ART (HRpooled 0.65, 95% CI 0.52–0.82 and RRpooled 0.67, 95% CI 0.45–1.00; Figure 4b [17,39,42–44]). Risk of LSIL decreased with CD4 count gains after ART initiation (Figure 4c) [44]. Controlling for CD4 count, women on ART had 34% lower risk of LSIL incidence compared to those not on ART (RR 0.66, 95% CI 0.47–0.92) [44].
Progression to HSIL
Ten studies assessed progression to HSIL [4,17,34,40,45–50], seven compared progression by HIV status [34,40,45–48,50]. HIV-positive women had higher risk of developing HSIL compared to HIV-negative women (HRpooled 1.32, 95% CI 1.10–1.58; Appendix figure 3) [34,45–47]. Two studies found persistence of HPV and LSIL were stronger predictors of progressing to HSIL than HIV status alone [45,50]. Risk of progression to HSIL from HPV infection with normal cytology was 3 times higher among HIV-positive compared to HIV-negative women [34,45]; risk of progression from LSIL to HSIL was not significantly different by HIV status [40,46,47,50].
Two studies in HIV-positive women with LSIL or normal cytology did not find an association between ART and HSIL incidence (ORpooled 0.88, 95% CI 0.69–1.12) (Appendix Figure 3) [4,17]. However, the risk of progression to HSIL was 34% lower among women initiating ART before LSIL diagnosis and 36% lower among women on ART for ≥2 years [4,47]. Risk of progression was higher in women with lower CD4 count at HSIL diagnosis regardless of ART use [17]. CD4 count, HIV status, and VL were not associated with HSIL incidence after adjusting for persistent HR HPV infection (Appendix Table 6) [46,49].
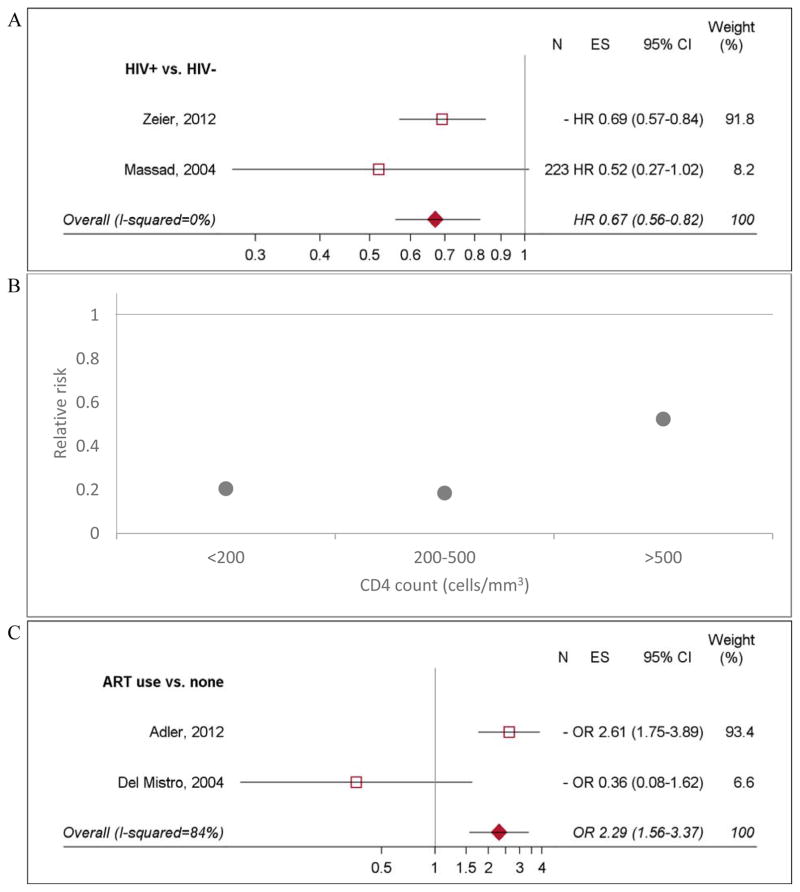
LSIL regression
Eight studies evaluated LSIL regression [17,41,47,48,51–54]; three compared regression by HIV status [47,48,51]. The likelihood of regression was 33% lower among HIV-positive women (HRpooled 0.67, 95% CI 0.56–0.82; Figure 5a) and varied by age, CD4 count, ART, and treatment of lesions [47,51]. One study found that for women not on ART, lower CD4 count was associated with reduced LSIL regression (Figure 5b) [45]. Likelihood of regression from LSIL was 2.29 times higher among women receiving ART compared to those not on treatment (95% CI 1.56–3.37; Figure 5c) [17,53]. Longer duration ART use was associated with increased LSIL regression [47].
Figure 5. LSIL regression by HIV status, CD4 count, and ART use.
N: Sample size, ES: effect estimate. N= “-” Indicates that the study did not report sample size associated with their estimates. A). LSIL regression among HIV-positive women compared to HIV-negative women. B). LSIL regression among HIV-positive women compared to HIV-negative women, by CD4 count. Data extracted from Six et al, 1998. Confidence intervals for these estimates were not included in the study. C). LSIL regression among HIV-positive women on ART compared to HIV-positive women not on ART. Data from Ahdieh-Grant et al, 2004 was not included in the figure. Because LSIL regression was not observed in any of the HIV-positive women not on ART, the study could not obtain an estimate for the effect of ART use on LSIL regression.
HSIL regression
One study evaluating HSIL regression in 173 HIV-positive and negative women with HSIL in Senegal found that HIV-positive women were 43% less likely to regress from HSIL to normal cytology (HR 0.57, 95% CI 0.26–1.29) and equally as likely to regress from HSIL to HPV infection (HR 1.06, 95% CI 0.71–1.59) [34]. Among HIV-positive women, likelihood of HSIL regression was 65% lower in women with HPV 16/18 compared to women infected with other types (HR 0.35, 95% CI 0.23–0.54) [34].
Progression to cervical cancer
CC incidence was assessed in two studies [4,55]. In the US, the age-standardized CC incidence among HIV-positive women from cohorts in North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) was 4.1 times (95% CI 2.3–6.6) higher than expected in Surveillance, Epidemiology, and End Results registry for the general population [55]. A case-control study nested in NA-ACCORD, which compared HIV-positive women to HIV-negative women, found that relative risk of CC increased with decreasing CD4 count (Appendix Figure 4) [55]. Further, among HIV-positive women on ART, CD4 reconstitution halted in CC cases 3 years after initiating ART and 60 months before CC diagnosis, while matched controls (women who never developed CC) continued to gain CD4 cells [55]. A nested case-control study of the Swiss HIV Cohort Study found that ART was not associated with lower CC incidence, regardless of duration (Appendix Table 9) [4].
Discussion
HIV infection was associated with higher incidence of and reduced clearance of HPV infection. Women with low CD4 count and high HIV VL had elevated risk of HPV acquisition, but risk was reduced among ART-adherent women. Low CD4 count was also associated with decreased HPV clearance. While ART reduced LSIL incidence, its impact on HSIL incidence depended on the duration of use. HIV-positive women have lower likelihood of LSIL regression, and ART and high CD4 count were associated with increased LSIL regression. In contrast, HSIL regression was not associated with HIV status. However, the study that examined HSIL regression had a small sample size and should be interpreted with caution.
While the relative risk of acquiring HPV 16/18 was higher among HIV-positive women, HPV 16/18 clearance was not found to differ by HIV status. This may be due to a lack of power or it may be that HPV 16 and 18 are better at evading the host immune system and are less impacted by HIV-associated immunosuppression [56,57]. Studies have found that HPV 16 in particular is more weakly associated with the immune system compared to other types [58,59]. This is consistent with studies that find HIV-positive women have a greater diversity of HPV types in normal cytology and low-grade lesions, but the relative prevalence of HPV 16 and 18 increases with severity of lesions and CC and the proportion of CC attributable to HPV 16/18 is similar between HIV positive and negative women [57,60].
HIV was also associated with higher LSIL and HSIL incidence, although the relative impact on HSIL was smaller. This suggests that immunosuppression related to HIV infection may play a greater role earlier in the course of HPV natural history, namely acquisition, persistence, and progression to low-grade lesions, while later stage carcinogenicity may be less dependent on immune function. This is consistent with cohort studies that find that, although HIV-positive women have a high burden of abnormal cytology, the vast majority of lesions are low-grade, with only a small increased prevalence in HSIL [45,61]. Therefore, HIV-positive women may be at risk for overtreatment in screening programs as they have a high prevalence of low-grade lesions that infrequently progress to HSIL. The US has recently changed CC screening guidelines in HIV-positive women from annual screening to every 3 years after 3 consecutive normal screens [15].
CD4 cell count in HIV-positive women is inversely associated with risk of HPV infection and cervical lesion progression. Further, several studies showed that women with high CD4 count (>500 cells/mm3) had similar risk of HPV disease progression as HIV-negative women. Similar associations between immunosuppression and HPV risk have been observed in HIV-negative populations with impaired immune system [62–65]. A meta-analysis of cancer incidence in HIV-positive persons and transplant recipients found both groups were at higher risk of cancers with infectious etiology compared to the general population [65]. The destruction of CD4 cells by HIV may increase the likelihood of HPV establishing infection [66]. Recent evidence also suggests that immunosuppression leads to higher probability of HPV reactivation, potentially due to incomplete clearance of HPV DNA [67].
HPV persistence likely plays an important role in the interactions between HIV and HPV. HIV-positive women with persistent HPV infection have dramatically higher incidence of precancerous lesions compared to those infected with HPV for <6 months [36,39,46]. Studies find after controlling for HPV persistence, CD4 count and ART use are no longer associated with increased risk of HPV disease progression. This is likely because HIV acts through HPV persistence to increase risk of precancerous lesions. Women infected with HPV for longer have a greater chance of developing cellular changes that lead to precancer or cancer.
The effect of ART on HPV-related disease is less clear. An equal number of studies find ART is associated with reduced HPV disease progression and find no association between ART and HPV pathogenesis. The equivocal results may be due to changes in ART regimens over time or varying durations of ART use. The majority of studies showing no effect of ART used cohorts formed in the early days of ART. Due to high toxicity of the older ART drugs, healthier HIV-infected individuals likely delayed treatment initiation and had poor adherence. Therefore, the women who were on ART likely already had more advanced HIV disease at treatment initiation. Studies show that HIV disease state at ART initiation significantly impacts morbidity and mortality [68–70]. Further, many studies finding no association approximated ART use with medical records or self-report. Studies using more accurate measures of ART adherence (e.g. viral suppression), found ART reduces HPV disease progression. One study found significantly lower incidence of HPV infection and cervical lesions in women who were virologically suppressed on ART compared to women who not on ART or not virologically suppressed [27]. A study showed CC cases had higher odds of CD4 decline on ART prior to CC diagnosis compared to controls who did not subsequently develop CC, which may indicate a disruption in ART use prior to the development of CC [71]. A longer duration on ART is also associated with reduced HSIL incidence [4,47], which may indicate immune system recovery [72].
In addition to the limitations of including studies that ascertained ART status through self-reports and chart reviews, another potential limitation of this review is the heterogeneity of studies included. While majority of studies were longitudinal, variation in analysis, effect measures, and exposures definition limited our ability to perform quantitative analysis. Additionally, due to small number of studies eligible for some objectives, we could not use I2 to quantify heterogeneity in many cases. High loss to follow up was an issue for some of the studies we included. While this limitation was ameliorated with survival analysis methods that censored individuals lost to follow up, effect estimates may be biased in studies that did not address this limitation in their analysis. The magnitude and direction of bias would depend on whether the lost to follow up was differential by HIV, ART, CD4 or VL status.
This review identified gaps in the research on HPV and cervical pre-cancer among HIV-positive women. There is a lack of studies investigating impact of ART on HPV disease progression using objective measures of adherence (e.g. viral suppression). Due to influence of HIV-related disease severity, evaluating HPV progression in by nadir CD4 count would be useful. Further, studies comparing HPV progression between HIV-positive women on ART with HIV-negative women can provide insight into CC risk of virally suppressed HIV-positive women. Finally, accurate estimates of CC incidence by HIV status are needed; national cancer registries do not currently document HIV status of CC cases.
Supplementary Material
Acknowledgments
Funding: This work is funded by the National Cancer Institute [U01 CA199334]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank the CISNET Cervical Cancer Modelling Consortium and UW librarian, Sarah Safranek, for her guidance in designing the search strategy for abstracts.
Footnotes
Competing interests
The authors have no competing interests to declare.
Authors’ contributions
All authors have contributed to the conception and design of the study. GL and MS acquired, analyzed, and interpreted the data. GL, MS, and RB drafted the manuscript and revised it critically for intellectual content. All authors approved the final version to be published.
Appendix.docx
References
- 1.American Cancer Society. Global cancer facts and figures. 3. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.de Vuyst H, Alemany L, Lacey C, Chibwesha CJ, Sahasrabuddhe V, Banura C, et al. The burden of human papillomavirus infections and related diseases in Sub-Saharan Africa. Vaccine. 2013;31(Suppl 5):F32–F46. doi: 10.1016/j.vaccine.2012.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, et al. Human papillomavirus and related diseases in the world. Summary report. [Accessed July 6, 2017];ICO Information Centre on HPV and Cancer (HPV Information Centre) 2017 [Google Scholar]
- 4.Clifford GM, Franceschi S, Keiser O, Schöni-Affolter F, Lise M, Dehler S, et al. Immunodeficiency and the risk of cervical intraepithelial neoplasia 2/3 and cervical cancer: A nested case-control study in the Swiss HIV cohort study. Int J Cancer. 2016;138(7):1732–40. doi: 10.1002/ijc.29913. [DOI] [PubMed] [Google Scholar]
- 5.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97(8):577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 6.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33(21):2376–83. doi: 10.1200/JCO.2014.59.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, Efstathiou JA, Grover S, Chiyapo S, et al. HIV infection and survival among women with cervical cancer. J Clin Oncol. 2016;34(31):3749–3757. doi: 10.1200/JCO.2016.67.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS. [Accessed June 30, 2017];Global AIDS update 2016. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 9.Hariri S, Bennett NM, Niccolai LM, Schafer S, Park IU, Bloch KC, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States - 2008–2012. Vaccine. 2015;33(13):1608–13. doi: 10.1016/j.vaccine.2015.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49(15):3262–73. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob Health. 2016;4(7):e453–63. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 12.Parham GP, Mwanahamuntu MH, Kapambwe S, Muwonge R, Bateman AC, Blevins M, Chibwesha CJ, Pfaendler KS, Mudenda V, Shibemba AL, Chisele S, Mkumba G, Vwalika B, Hicks ML, Vermund SH, Chi BH, Stringer JS, Sankaranarayanan R, Sahasrabuddhe VV. Population-level scale-up of cervical cancer prevention services in a low-resource setting: development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLoS One. 2015 Apr 17;10(4):e0122169. doi: 10.1371/journal.pone.0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: Low average levels and large inequalities. PLoS Med. 2008;5(6):e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habbema D, Weinmann S, Arbyn M, Kamineni A, Williams AE, de Kok MCMI, van Kemenade F, Field TS, van Rosmalen J, Brown ML. Harms of cervical cancer screening in the United States and the Netherlands. Int J Cancer. 2017 Mar 1;140(5):1215–1222. doi: 10.1002/ijc.30524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America; [Accessed (Nov. 22, 2017)]. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_OI003407.pdf. [Google Scholar]
- 16.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, Seage GR, 3rd, Suneja G, Kayembe MK, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS One. 2015;10(8):e0135602. doi: 10.1371/journal.pone.0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler DH, Kakinami L, Modisenyane T, Tshabangu N, Mohapi L, De Bruyn G, et al. Increased regression and decreased incidence of human papillomavirus-related cervical lesions among HIV-infected women on HAART. AIDS. 2012;26(13):1645–52. doi: 10.1097/QAD.0b013e32835536a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanderson S, Tatt ID, Higgins JPT. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–76. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. Robins-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016:355. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banura C, Franceschi S, van Doorn LJ, Arslan A, Kleter B, Wabwire-Mangen F, et al. Prevalence, incidence and clearance of human papillomavirus infection among young primiparous pregnant women in Kampala, Uganda. Int J Cancer. 2008;123(9):2180–7. doi: 10.1002/ijc.23762. [DOI] [PubMed] [Google Scholar]
- 22.Banura C, Sandin S, Van Doorn LJ, Quint W, Kleter B, Wabwire-Mangen F, et al. Type-specific incidence, clearance and predictors of cervical human papillomavirus infections (HPV) among young women: A prospective study in Uganda. Infect Agent Cancer. 2010;5(1) doi: 10.1186/1750-9378-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denny L, Boa R, Williamson AL, Allan B, Hardie D, Stan R, et al. Human papillomavirus infection and cervical disease in Human Immunodeficiency Virus-1-infected women. Obstet Gynecol. 2008;111(6):1380–7. doi: 10.1097/AOG.0b013e3181743327. [DOI] [PubMed] [Google Scholar]
- 24.Lillo FB, Ferrari D, Veglia F, Origoni M, Grasso MA, Lodini S, et al. Human papillomavirus infection and associated cervical disease in Human Immunodeficiency Virus-infected women: Effect of highly active antiretroviral therapy. J Infect Dis. 2001;184(5):547–51. doi: 10.1086/322856. [DOI] [PubMed] [Google Scholar]
- 25.Mane A, Sahasrabuddhe VV, Nirmalkar A, Risbud AR, Sahay S, Bhosale RA, et al. Rates and determinants of incidence and clearance of cervical HPV genotypes among HIV-seropositive women in Pune, India. J Clin Virol. 2016;88:26–32. doi: 10.1016/j.jcv.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbulawa ZZ, Marais DJ, Johnson LF, Coetzee D, Williamson AL. Impact of Human Immunodeficiency Virus on the natural history of human papillomavirus genital infection in South African men and women. J Infect Dis. 2012;206(1):15–27. doi: 10.1093/infdis/jis299. [DOI] [PubMed] [Google Scholar]
- 27.Minkoff H, Zhong Y, Burk RD, Palefsky JM, Xue X, Watts DH, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in Human Immunodeficiency Virus-positive women. J Infect Dis. 2010;201(5):681–90. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelan DF, Gange SJ, Ahdieh-Grant L, Mehta SH, Kirk GD, Shah K, et al. Determinants of newly detected human papillomavirus infection in HIV-infected and HIV-uninfected injection drug using women. Sex Transm Dis. 2009;36(3):149–56. doi: 10.1097/OLQ.0b013e31818d3df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safaeian M, Kiddugavu M, Gravitt PE, Gange SJ, Ssekasanvu J, Murokora D, et al. Determinants of incidence and clearance of high-risk human papillomavirus infections in rural Rakai, Uganda. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1300–7. doi: 10.1158/1055-9965.EPI-07-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha S, Sudenga SL, Smith JS, Bachmann LH, Wilson CM, Kempf MC. The impact of highly active antiretroviral therapy on prevalence and incidence of cervical human papillomavirus infections in HIV-positive adolescents. BMC Infect Dis. 2010;10:295. doi: 10.1186/1471-2334-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie X, Strickler HD, Xue X. Additive hazard regression models: An application to the natural history of human papillomavirus. Comput Math Methods Med. 2013;2013:796270. doi: 10.1155/2013/796270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshiol JE, Schroeder JC, Jamieson DJ, Marshall SW, Duerr A, Heilig CM, et al. Time to clearance of human papillomavirus infection by type and Human Immunodeficiency Virus serostatus. Int J Cancer. 2006;119(7):1623–9. doi: 10.1002/ijc.22015. [DOI] [PubMed] [Google Scholar]
- 33.Blitz S, Baxter J, Raboud J, Walmsley S, Rachlis A, Smaill F, et al. Evaluation of HIV and highly active antiretroviral therapy on the natural history of human papillomavirus infection and cervical cytopathologic findings in HIV-positive and high-risk HIV-negative women. J Infect Dis. 2013;208(3):454–62. doi: 10.1093/infdis/jit181. [DOI] [PubMed] [Google Scholar]
- 34.Whitham HK, Hawes SE, Chu H, Oakes JM, Lifson AR, Kiviat NB, et al. A comparison of the natural history of HPV infection and cervical abnormalities among HIV-positive and HIV-negative women in Senegal, Africa. Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-16-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahdieh L, Munoz A, Vlahov D, Trimble CL, Timpson LA, Shah K. Cervical neoplasia and repeated positivity of human papillomavirus infection in Human Immunodeficiency Virus-seropositive and -seronegative women. Am J Epidemiol. 2000;151(12):1148–57. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 36.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004 Jul 1;190(1):37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 37.Rowhani-Rahbar A, Hawes SE, Sow PS, Toure P, Feng Q, Dem A, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among senegalese women. J Infect Dis. 2007;196(6):887–94. doi: 10.1086/520883. [DOI] [PubMed] [Google Scholar]
- 38.Duerr A, Paramsothy P, Jamieson DJ, Heilig CM, Klein RS, Cu-Uvin S, et al. Effect of HIV infection on atypical squamous cells of undetermined significance. Clin Infect Dis. 2006;42(6):855–61. doi: 10.1086/500404. [DOI] [PubMed] [Google Scholar]
- 39.Ellerbrock TV, Chiasson MA, Bush TJ, Sun XW, Sawo D, Brudney K, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283(8):1031–7. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 40.Massad LS, Ahdieh L, Benning L, Minkoff H, Greenblatt RM, Watts H, et al. Evolution of cervical abnormalities among women with HIV-1: Evidence from surveillance cytology in the women’s interagency HIV study. J Acquir Immune Defic Syndr. 2001;27(5):432–42. doi: 10.1097/00126334-200108150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Delmas MC, Larsen C, van Benthem B, Hamers FF, Bergeron C, Poveda JD, et al. Cervical squamous intraepithelial lesions in HIV-infected women: Prevalence, incidence and regression. European study group on natural history of HIV infection in women. AIDS. 2000;14(12):1775–84. doi: 10.1097/00002030-200008180-00013. [DOI] [PubMed] [Google Scholar]
- 42.Firnhaber C, Westreich D, Schulze D, Williams S, Siminya M, Michelow P, et al. Highly active antiretroviral therapy and cervical dysplasia in HIV-positive women in south africa. J Int AIDS Soc. 2012;15(2):17382. doi: 10.7448/IAS.15.2.17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heard I, Potard V, Costagliola D. Limited impact of immunosuppression and haart on the incidence of cervical squamous intraepithelial lesions in HIV-positive women. Antivir Ther. 2006;11(8):1091–6. [PubMed] [Google Scholar]
- 44.Kim SC, Messing S, Shah K, Luque AE. Effect of highly active antiretroviral therapy (haart) and menopause on risk of progression of cervical dysplasia in human immune-deficiency virus- (HIV-) infected women. Infect Dis Obstet Gynecol. 2013;2013:784718. doi: 10.1155/2013/784718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawes SE, Critchlow CW, Sow PS, Toure P, N’Doye I, Diop A, et al. Incident high-grade squamous intraepithelial lesions in Senegalese women with and without Human Immunodeficiency Virus type 1 (HIV-1) and HIV-2. J Natl Cancer Inst. 2006;98(2):100–9. doi: 10.1093/jnci/djj010. [DOI] [PubMed] [Google Scholar]
- 46.Massad LS, Evans CT, Strickler HD, Burk RD, Watts DH, Cashin L, et al. Outcome after negative colposcopy among Human Immunodeficiency Virus-infected women with borderline cytologic abnormalities. Obstet Gynecol. 2005;106(3):525–32. doi: 10.1097/01.AOG.0000172429.45130.1f. [DOI] [PubMed] [Google Scholar]
- 47.Zeier MD, Botha MH, van der Merwe FH, Eshun-Wilson I, van Schalkwyk M, la Grange M, et al. Progression and persistence of low-grade cervical squamous intraepithelial lesions in women living with Human Immunodeficiency Virus. J Low Genit Tract Dis. 2012;16(3):243–50. doi: 10.1097/LGT.0b013e3182403d18. [DOI] [PubMed] [Google Scholar]
- 48.Six C, Heard I, Bergeron C, Orth G, Poveda JD, Zagury P, et al. Comparative prevalence, incidence and short-term prognosis of cervical squamous intraepithelial lesions amongst HIV-positive and HIV-negative women. AIDS. 1998;12(9):1047–56. [PubMed] [Google Scholar]
- 49.Kelly HA, Sawadogo B, Chikandiwa A, Segondy M, Gilham C, Lompo O, et al. Epidemiology of high-risk human papillomavirus and cervical lesions in African women living with HIV/AIDS: Effect of anti-retroviral therapy. AIDS. 2016 doi: 10.1097/QAD.0000000000001301. [DOI] [PubMed]
- 50.Moscicki AB, Ellenberg JH, Crowley-Nowick P, Darragh TM, Xu J, Fahrat S. Risk of high-grade squamous intraepithelial lesion in HIV-infected adolescents. J Infect Dis. 2004 Oct 15;190(8):1413–21. doi: 10.1086/424466. [DOI] [PubMed] [Google Scholar]
- 51.Massad LS, Evans CT, Minkoff H, Watts DH, Strickler HD, Darragh T, et al. Natural history of grade 1 cervical intraepithelial neoplasia in women with Human Immunodeficiency Virus. Obstet Gynecol. 2004;104(5 Pt 1):1077–85. doi: 10.1097/01.AOG.0000143256.63961.c0. [DOI] [PubMed] [Google Scholar]
- 52.Ahdieh-Grant L, Li R, Levine AM, Massad LS, Strickler HD, Minkoff H, et al. Highly active antiretroviral therapy and cervical squamous intraepithelial lesions in Human Immunodeficiency Virus-positive women. J Natl Cancer Inst. 2004;96(14):1070–6. doi: 10.1093/jnci/djh192. [DOI] [PubMed] [Google Scholar]
- 53.Del Mistro A, Bertorelle R, Franzetti M, Cattelan A, Torrisi A, Giordani MT, et al. Antiretroviral therapy and the clinical evolution of human papillomavirus-associated genital lesions in HIV-positive women. Clin Infect Dis. 2004;38(5):737–42. doi: 10.1086/381681. [DOI] [PubMed] [Google Scholar]
- 54.Omar T, Schwartz S, Hanrahan C, Modisenyane T, Tshabangu N, Golub JE, et al. Progression and regression of premalignant cervical lesions in HIV-infected women from Soweto: A prospective cohort. AIDS. 2011;25(1):87–94. doi: 10.1097/QAD.0b013e328340fd99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abraham AG, D’Souza G, Jing Y, Gange SJ, Sterling TR, Silverberg MJ, et al. Invasive cervical cancer risk among HIV-infected women: A North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013;62(4):405–13. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahdieh L, Klein RS, Burk R, Cu-Uvin S, Schuman P, Duerr A, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in Human Immunodeficiency Virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184(6):682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 57.Clifford GM, de Vuyst H, Tenet V, Plummer M, Tully S, Franceschi S. Effect of HIV infection on human papillomavirus types causing invasive cervical cancer in Africa. J Acquir Immune Defic Syndr. 2016;73(3):332–9. doi: 10.1097/QAI.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strickler HD, Palefsky JM, Shah KV, Anastos K, Klein RS, Minkoff H, et al. Human papillomavirus type 16 and immune status in Human Immunodeficiency Virus-seropositive women. J Natl Cancer Inst. 2003;95(14):1062–71. doi: 10.1093/jnci/95.14.1062. [DOI] [PubMed] [Google Scholar]
- 59.Massad LS, Xie X, Burk RD, D’Souza G, Darragh TM, Minkoff H, et al. Association of cervical precancer with human papillomavirus types other than 16 among HIV co-infected women. Am J Obstet Gynecol. 2016;214(3):354e1–6. doi: 10.1016/j.ajog.2015.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mbulawa ZZ, Coetzee D, Williamson AL. Human papillomavirus prevalence in South African women and men according to age and Human Immunodeficiency Virus status. BMC Infect Dis. 2015;15:459. doi: 10.1186/s12879-015-1181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massad LS, Xie X, D’Souza G, Darragh TM, Minkoff H, Wright R, et al. Incidence of cervical precancers among HIV-seropositive women. Am J Obstet Gynecol. 2015;212(5):606e1–8. doi: 10.1016/j.ajog.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. 2013;13(12):3202–9. doi: 10.1111/ajt.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown MR, Noffsinger A, First MR, Penn I, Husseinzadeh N. HPV subtype analysis in lower genital tract neoplasms of female renal transplant recipients. Gynecol Oncol. 2000;79(2):220–4. doi: 10.1006/gyno.2000.5942. [DOI] [PubMed] [Google Scholar]
- 64.Dugué P-A, Rebolj M, Garred P, Lynge E. Immunosuppression and risk of cervical cancer. Expert Rev Anticancer Ther. 2013;13(1):29–42. doi: 10.1586/era.12.159. [DOI] [PubMed] [Google Scholar]
- 65.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 66.Nicol AF, Fernandes AT, Bonecini-Almeida Mda G. Immune response in cervical dysplasia induced by human papillomavirus: The influence of Human Immunodeficiency Virus-1 co-infection -- review. Mem Inst Oswaldo Cruz. 2005;100(1):1–12. doi: 10.1590/s0074-02762005000100001. [DOI] [PubMed] [Google Scholar]
- 67.Doorbar J. Latent papillomavirus infections and their regulation. Curr Opin Virol. 2013;3(4):416–21. doi: 10.1016/j.coviro.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 68.Grant PM, Zolopa AR. When to start art in the setting of acute AIDS-related opportunistic infections: The time is now! Curr HIV/AIDS Rep. 2012;9(3):251–8. doi: 10.1007/s11904-012-0126-8. [DOI] [PubMed] [Google Scholar]
- 69.Siegfried N, Uthman OA, Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev. 2010;(3):Cd008272. doi: 10.1002/14651858.CD008272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: A collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rohner E, Sengayi M, Goeieman B, Michelow P, Firnhaber C, Maskew M, et al. Cervical cancer risk and impact of PAP-based screening in HIV-positive women on antiretroviral therapy in Johannesburg, South Africa. Int J Cancer. 2017 Aug 1;141(3):488–496. doi: 10.1002/ijc.30749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van den Dries L, Claassen MAA, Groothuismink ZMA, van Gorp E, Boonstra A. Immune activation in prolonged cart-suppressed HIV patients is comparable to that of healthy controls. Virology. 2017;509:133–9. doi: 10.1016/j.virol.2017.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.