Abstract
Paclitaxel is a cancer chemotherapy with adverse effects that include peripheral neuropathy, neuropathic pain, and depression of behavior and mood. In rodents, hypersensitive paw-withdrawal reflexes from mechanical stimuli serve as one common measure of paclitaxel-induced pain-related behavior. This study tested the hypothesis that paclitaxel would also depress rates of positively reinforced operant responding as a measure of pain-related behavioral depression. Male and female Sprague Dawley rats were equipped with electrodes targeting the medial forebrain bundle, trained to lever press for electrical brain stimulation in an assay of intracranial self-stimulation (ICSS), and treated with four injections of varying paclitaxel doses (0.67, 2.0, or 6.0 mg/kg/injection x 4 injections on alternate days). Mechanical sensitivity, body weight, and ICSS were evaluated before, during, and for three weeks after paclitaxel treatment. Paclitaxel doses sufficient to produce mechanical hypersensitivity did not reliably depress ICSS in male or female rats. Moreover, the degree of behavioral suppression in individual rats did not correlate with mechanical sensitivity. Paclitaxel treatment regimens commonly used to model chemotherapy-induced neuropathic pain in rats are not sufficient to depress ICSS.
Keywords: Paclitaxel, intracranial self-stimulation, motivation, mechanical allodynia, pain, functional impairment, neuropathy, rat
INTRODUCTION
Paclitaxel is an anti-cancer chemotherapeutic that stabilizes polymerized microtubules in metaphase, preventing the progression to anaphase in rapidly dividing cells (Schiff and Horwitz, 1980; Risinger et al., 2014). It is one of the most commonly administered and effective chemotherapeutics in the United States and throughout the world, and it has been used to improve survival in patients with nasopharyngeal (Miyaushiro et al., 2015), non-small cell lung (Langer et al., 2015), breast (Sparano et al., 2008), and ovarian cancers (Suh et al., 2013). Clinical use of paclitaxel is limited by adverse effects that include emesis, alopecia, and diarrhea, but these effects typically resolve with cessation of treatment (Reeves et al., 2012). Paclitaxel also produces chemotherapy-induced peripheral neuropathy (CIPN) in roughly 60% of patients (Serenty et al., 2014). CIPN manifests clinically as somatosensory deficits such as paresthesia or dysesthesia that can exist in the absence or presence of concurrent neuropathic pain, and unlike the other adverse effects, CIPN can be irreversible and impact patient well-being for decades (Goulan-Vered and Pud, 2013). For example, CIPN is associated with signs of functional and emotional impairment, including decreases in days healthy enough to work (Pike et al., 2012), functional mobility (Davies et al., 2016; Miaskowski et al., 2017), and cognitive function (Ando-Tanabe et al., 2013), and increases in fatigue, hopelessness, and depressive symptoms (Pedersen, et al., 2007). At present, there are no adequate treatments to prevent or reverse paclitaxel-induced neuropathy, neuropathic pain, or pain-related functional impairment (Dworkin et al., 2010; Finnerup et al., 2015). As a result, the emergence of these signs often limits paclitaxel dose ranges that can be used in cancer treatment (Speck et al., 2013).
Preclinical assays have been developed as tools for development of medications to treat paclitaxel-induced neuropathic pain, but translation of results has been poor. In rodents, paclitaxel produces hypersensitive paw-withdrawal reflexes from mechanical and thermal stimuli, and numerous treatments have been identified that reduce expression of this hypersensitivity; however, none of these medications has proven to be reliably effective in clinical treatment of either CIPN or neuropathic pain (Sindrup and Jensen, 1999; Xiao et al., 2008; Hama and Takamatsu, 2016). One factor that may impede preclinical-to-clinical translation is the type of dependent measure used to indicate the presence of “pain,” and novel assays have been developed with the goal of modeling pain-related functional impairment and improving translation (Martin et al., 2004; Negus et al., 2006; Mogil, 2009). For example, operant responding reinforced by electrical brain stimulation or food reward can be depressed in rodents by some noxious stimuli, and pain-related depression of operant responding can be blocked or reversed by clinically effective analgesics but not by non-analgesic drugs that produce motor impairment (Martin et al., 2004; Ewan and Martin, 2014; Negus et al., 2015; Warner et al., 2015).
The goal of the present study was to test the hypothesis that paclitaxel treatment regimens sufficient to produce mechanical hypersensitivity in rats would also produce depression of operant responding maintained by electrical brain stimulation in an assay of intracranial self-stimulation (ICSS). Studies were conducted in both males and females because while paclitaxel is used to treat cancer in both sexes, sex differences have been reported for some pain states in patients (Ruau et al., 2012; Bartley and Filingim, 2013), and preclinical studies have reported sex differences in some paclitaxel effects (Hwang et al., 2012; Naji-Esfahani et al., 2016).
METHODS
Subjects
Studies were conducted in adult male (n=39) and female (n=12) Sprague Dawley rats, with initial weights ranging from 360 to 468 g in males and 236 to 298g in females. Rats were individually housed and maintained on a 12-h light/dark cycle, with lights on from 06:00 to 18:00 h, in an AAALAC International-accredited housing facility. Standard chow diet (Teklad standard diet - 19% protein, Envigo, Madison, WI) and water were freely available in the home cage. Animal-use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and complied with the National Research Council (2013) Guide for the Care and Use of Laboratory Animals.
Drugs
Paclitaxel was obtained as a clinically available 6.0 mg/ml solution (TEVA Pharmaceuticals, North Wales, PA) and diluted in vehicle (8.3% ethanol, 8.3% Cremophor EL, and 83.4% saline) to final concentrations of 0.335, 1.0, and 3.0 mg/ml. All rats were injected i.p. on four alternate days (Days 1, 3, 5, and 7) with vehicle or a given dose of paclitaxel (0.67, 2.0, or 6.0 mg/kg) using an injection volume of 2 ml/kg. These dosing regimens were based on previous studies in rats (Polomano et al., 2001; Pascual et al., 2010; Hwang et al., 2012; Boyette-Davis et al., 2011; Ko et al., 2014) and resulted in cumulative doses of 2.68, 8.0, and 24.0 mg/kg of paclitaxel.
Intracranial self-stimulation (ICSS)
Surgery
Rats were anesthetized with inhaled isoflurane (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ) and implanted with electrodes (Plastics One, Roanoke, VA) in the left medial forebrain bundle at the level of the lateral hypothalamus, using previously published procedures and coordinates (males: 2.8 mm posterior to bregma, 1.7 mm lateral to midsagittal suture, 8.8 mm below skull surface; females: 3.8 mm posterior to bregma, 1.6 mm lateral to midsagittal suture, and 8.7 mm below skull surface) (Lazenka et al., 2016a, b) The electrode was secured to the skull with orthodontic resin and skull screws. Ketoprofen (5 mg/kg i.p.; Spectrum Chemical, New Brunswick, NJ) was administered immediately and 24 hours after surgery as a postoperative analgesic, and rats recovered for 7 days prior to initiation of ICSS training.
Apparatus
Studies were conducted in sound-attenuating boxes containing modular acrylic and metal test chambers (29.2 × 30.5 × 24.1 cm; Med Associates, St Albans, VT). Each chamber contained a response lever, three stimulus lights (red, yellow, and green) centered above the lever, a 2-W house light, and an ICSS stimulator. Electrodes were connected to the stimulator via bipolar cables routed through a swivel commutator (Model SL2C, Plastics One, Roanoke, VA). Control of stimulus delivery in the operant chamber and collection of data on lever presses were accomplished with a computer, interface, and custom software (Med PC-IV, Med Associates).
Training
Rats were trained to respond for electrical brain stimulation using procedures identical to those described previously (Leitl et al., 2014a). Briefly, a white house light was illuminated during behavioral sessions, and responding under a fixed-ratio (FR) 1 schedule produced a 500-msec train of 0.1-msec square-wave cathodal pulses together with 500-msec illumination of stimulus lights over the lever. Responding during brain stimulation had no scheduled consequences. The terminal schedule consisted of sequential 10-min components. Each component consisted of 10 1-min trials, and the available brain-stimulation frequency decreased in 0.05 log Hz increments from one trial to the next (158-56 Hz). Each frequency trial consisted of a 10-s timeout, during which five noncontingent stimulations were delivered at the frequency available during that trial, followed by a 50-s “response” period, during which responding resulted in electrical stimulation. Training continued with presentation of three sequential components per day until the following two criteria for stable responding were met for three consecutive days: (1) ≤5% variability in the maximum rate of reinforcement in any trial, and (2) ≤10% variability in the total number of stimulations per component.
Testing
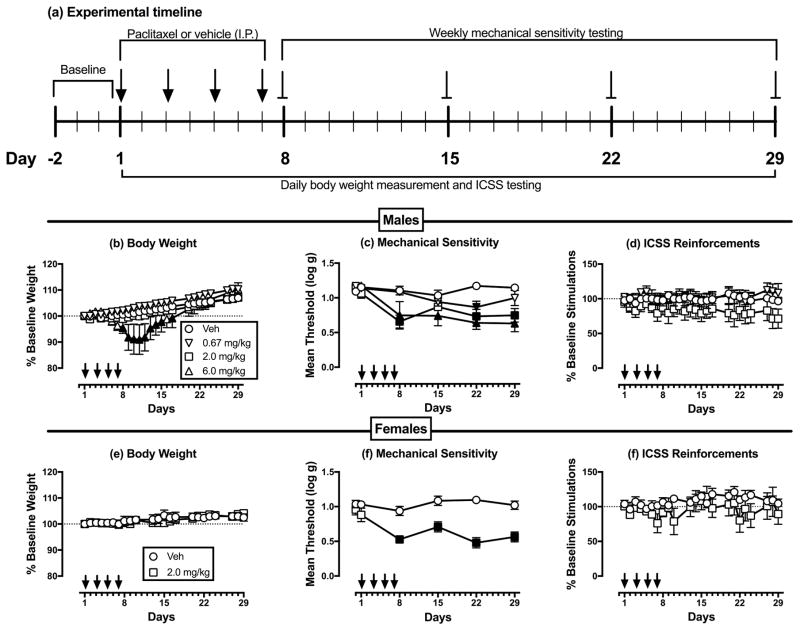
Once responding stabilized, a 29-day testing protocol began (Figure 1a). Three-component ICSS operant behavioral sessions were conducted daily (with occasional exceptions on weekends) throughout the 29-day test period, and vehicle or paclitaxel was administered 2 h before behavioral sessions on Days 1, 3, 5, and 7. Studies were conducted in three phases. First, four groups of male rats (n=6–7 per group) were used to evaluate effects of vehicle and three different paclitaxel doses (0.67, 2.0, or 6.0 mg/kg/day). The initial paclitaxel dose-effect study revealed a small but non-significant decrease in ICSS after treatment with 2.0 mg/kg/day paclitaxel and severe weight loss after 6.0 mg/kg/day paclitaxel. Second, to assess potential sex differences in paclitaxel effects, two groups of female rats (n=6 per group) were treated with vehicle or 2.0 mg/kg/day paclitaxel. The group sizes for these initial studies were based on previous experience to show significant depression of ICSS by other chronic pain stimuli in group sizes of n=6–8 rats (Leitl et al., 2014b, 2016), but the initial studies with paclitaxel failed to show a significant decrease in ICSS despite decreases in some rats. Accordingly, a follow-up study was conducted in males. Mean data and standard deviations for effects of vehicle and 2.0 mg/kg/day paclitaxel on ICSS on Day 29 in the dose-effect study in males were used for power analysis sample-size estimates required to detect significance for the paclitaxel effect size, given an alpha of 0.05, power of 0.8, allocation ratio of 1.5 (i.e. 50% more rats in the paclitaxel treatment group given variability in paclitaxel effects), and use of a one-tailed t-test (given the prediction that paclitaxel would reduce ICSS) (Faul et al., 2007). The computed sample sizes were 12 vehicle-treated and 18 paclitaxel-treated rats. Thus, in the final phase of the study, six vehicle- and 12 paclitaxel-treated rats were added to the initial samples. All rats were weighed before each operant behavioral session. In addition, mechanical sensitivity was assessed before and on Days 8, 15, 22, and 29 after initiation of paclitaxel treatment (methods described below).
Figure 1.
Dose-dependent paclitaxel effects in male and female rats. Panel a shows the experimental timeline for treatment administration and data collection. Panels b–d show effects of different paclitaxel doses on different experimental endpoints in male rats. Panels e–g show effects of 2.0 mg/kg paclitaxel on different experimental endpoints in female rats. Horizontal axes: Time in days relative to initiation of vehicle/paclitaxel treatment on Day 1. Arrows indicate vehicle/paclitaxel treatment days. Vertical axes: (b, e) % baseline body weight, (c, f) mechanical sensitivity expressed as threshold stimulation to elicit paw withdrawal in log g, and (d, g) ICSS performance expressed as % baseline number of brain-stimulation reinforcements earned per 10-min component. All points show mean±SEM for n=6 rats except data for 6.0 mg/kg/day paclitaxel in males (n=3, see text). Filled points indicate a significant difference from vehicle on a given day, as indicated by the Holm-Sidak post hoc test after a significant two-way ANOVA, p<0.05. Statistical results are as follows. (b) Significant main effects of treatment [F(3,17)=3.33; p<0.05] and time [F(24,408)=83.64; p<0.001], and a significant interaction [F(72,408)=6.73; p<0.001]. (c) Significant main effects of treatment [F(3,17)=15.54; p<0.001] and time [F(5,85)=13.68; p<0.001], and a significant interaction [F(15,85)=3.16; p<0.001]. (d) No significant main effects of treatment [F(3,17)=1.14; NS] or time [F(24,408)=0.74; NS], and no significant interaction [F(72,408)=0.87; NS]. (e) No significant main effect of treatment [F(1,10)=0.06; NS], a significant effect of time [F(22,220)=6.08; p<0.001], and no significant interaction [F(22,220)=1.044; NS]. (f) Significant main effects of treatment [F(1,10)=35.63; p<0.001] and time [F(5,50)=7.11; p<0.001], and a significant interaction [F(5,50)=6.37; p<0.001]. (g) No significant main effects of treatment [F(1,10)=1.37; NS] or time [F(22,220)=1.28; NS], and no significant interaction [F(22,220)=0.78; NS].
Data analysis
The first baseline component of each test session was considered to be a “warm up” component, and data were discarded. Data from the remaining two components were analyzed as previously described (Leitl et al., 2014b; Negus and Miller, 2014). The primary dependent measure was the total number of reinforcements per component (i.e. the total number of stimulations delivered across all brain-stimulation frequencies during each component). Data for the final three training days prior to vehicle/paclitaxel treatment were averaged to provide a mean pre-paclitaxel baseline measure of reinforcements per component in each rat. Once paclitaxel treatment was initiated, the number of reinforcements per component was determined daily in each rat and expressed as a percentage of that rat’s pre-paclitaxel baseline using the equation: % Baseline Reinforcements per Component = (Number Reinforcements per Component on a Test Day ÷ Pre-Paclitaxel Baseline Reinforcements per Component) x 100.
Changes in ICSS performance over time were then averaged across rats and evaluated in two ways. In the first approach, data from each day of the study were analyzed by two-way ANOVA, with paclitaxel dose as a between-subjects factor and treatment day as a within-subjects factor. A significant ANOVA was followed by the Holm-Sidak post-hoc test. For this and all other analyses described below, statistical analysis was conducted using Prism 7.0 (Graphpad Software Inc., San Diego, CA), and the criterion for statistical significance was p<0.05. A secondary and more granular measure of ICSS performance was the reinforcement rate in stimulations per frequency trial. Raw reinforcement rates for each rat from each trial were converted to percent maximum control rate (%MCR), with MCR defined as the mean of the maximal rates observed at any trial during the three pre-paclitaxel baseline sessions. Thus, %MCR values for each trial were calculated as {(reinforcement rate during a frequency trial ÷ MCR) × 100}. %MCR values were then averaged across rats and analyzed by repeated-measures two-way ANOVA, with ICSS frequency and treatment day as the two factors. A significant ANOVA was followed by the Holm-Sidak post-hoc test.
Mechanical sensitivity testing with von Frey filaments
On days when mechanical sensitivity was assessed, testing was conducted approximately 1 h after conclusion of the operant behavioral session on that day. Rats were first placed on an elevated mesh galvanized steel platform in individual chambers with a hinged lid and allowed to acclimate for at least 20 mins before exposure to the mechanical stimuli. Subsequently, von Frey filaments (ranging from 0.4 to 15.0g and increasing in ~0.25 log increments; North Coast Medical, Morgan Hill, CA) were applied to the plantar surface of each paw, and the threshold stimulus to elicit paw withdrawal was determined in log grams using the “up-down” method as previously described (Chaplan et al., 1994; Leitl et al., 2014b). On each test day, data were averaged across paws within each rat and then across rats. Changes in threshold over time were analyzed by two-way ANOVA, with paclitaxel dose and treatment day as the two factors, and a significant ANOVA was followed by the Holm-Sidak post-hoc test. Additionally, mechanical sensitivity data were correlated to ICSS data for all rats using results from the last day of the study (Day 29).
RESULTS
Paclitaxel effects on body weight, mechanical sensitivity, and ICSS in male rats
For male rats used in ICSS studies, the baseline body weight was 411.2 ± 13.3 g, the baseline mechanical sensitivity threshold was 1.14 ± 0.02 log g, and baseline measures of ICSS performance were 153.5 ± 16.4 stimulations per component with maximum control rates (MCR) of 56.3 ± 4.0 stimulations per trial. Figure 1b–d shows the time course of changes in body weight, mechanical sensitivity, and ICSS performance during and after repeated treatment with vehicle or different doses of paclitaxel (0.67, 2.0, or 6.0 mg/kg/day). Body weight increased over time in the vehicle-treated group, and similar weight gain was observed in rats treated with 0.67 and 2.0 mg/kg/day paclitaxel. Seven rats were treated with 6.0 mg/kg/day paclitaxel, but four of these rats lost ≥ 20% of their baseline body weight during the initial week of paclitaxel treatment and were euthanized in accordance with moribundity criteria in the animal use protocol. Data from these four rats were excluded from all subsequent analyses, and their data are not included in Figure 1. The remaining three rats also lost weight, and body weight in these rats was significantly lower than in vehicle-treated rats for Days 7–14 and Day 16; however, the magnitude of weight loss in these rats did not reach the 20% criterion for euthanasia, and their weights recovered to control levels by the end of the 29-day study.
Paclitaxel also produced dose- and time-dependent decreases in mechanical sensitivity thresholds, and paclitaxel was both more potent and longer acting to produce mechanical hypersensitivity than weight loss. Thus, mechanical hypersensitivity was significant on Day 22 in rats treated with 0.67 mg/kg/day paclitaxel, Days 8, 22 and 29 in rats treated with 2.0 mg/kg/day paclitaxel, and all days of testing (Days 8, 15, 22 and 29) in rats treated with 6.0 mg/kg/day paclitaxel. Despite producing significant dose-dependent weight loss and mechanical hypersensitivity, no dose of paclitaxel was sufficient to significantly decrease ICSS responding.
Paclitaxel effects on body weight, mechanical sensitivity, and ICSS in female rats
For all female rats used in ICSS studies, the baseline body weight was 263.8 ± 12.7 g, the baseline mechanical sensitivity threshold was 1.00 ± 0.10 log g, and baseline measures of ICSS performance were 124.5 ± 24.5 stimulations per component with maximum control rates (MCR) of 53.4 ± 6.5 stimulations per trial. T-test analysis indicated that at baseline, females had significantly lower body weights (p<0.0001), mechanical sensitivity thresholds (p=0.001), and total ICSS stimulations/component (p=0.038) but not MCRs (p=0.400) compared to males.
Figure 1e–g shows the time course of changes in body weight, mechanical sensitivity, and ICSS performance during and after repeated treatment with vehicle or 2.0 mg/kg/day paclitaxel. Body weight increased over time in both the vehicle- and the paclitaxel-treated groups, and there was no difference between groups. Mechanical sensitivity did not change in the vehicle-treated group, but relative to the vehicle group, paclitaxel significantly reduced mechanical sensitivity thresholds on Days 8, 15, 22, and 29 following initiation of paclitaxel treatment. As in the males, two-way ANOVA indicated that ICSS performance did not change over time in either the vehicle- or paclitaxel-treated groups, and there was no difference in ICSS between groups.
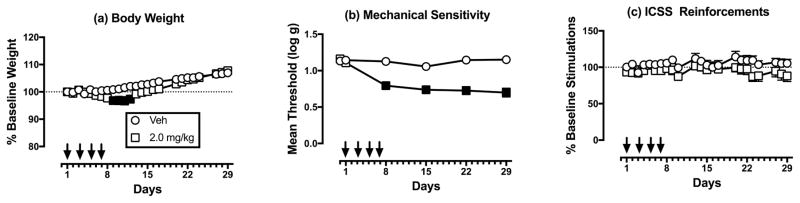
Paclitaxel effects on body weight, mechanical sensitivity, and ICSS in larger sample of male rats
To further explore paclitaxel effects on ICSS in male rats, a follow-up study was conducted to increase the number of subjects to N=12 for vehicle treatment and to N=18 for 2.0 mg/kg/day paclitaxel. Figure 2a–c shows results from all male rats treated with vehicle and 2.0 mg/kg/day paclitaxel. In this larger sample, 2.0 mg/kg/day paclitaxel produced significant but modest weight loss from Days 9–12 (Figure 2a) and significant and sustained mechanical hypersensitivity throughout testing (Figure 2b); however, paclitaxel still failed to significantly alter mean ICSS performance as assessed by two-way ANOVA (Figure 2c).
Figure 2.
Effects of vehicle and 2.0 mg/kg/day paclitaxel in a larger sample of males treated with vehicle (n=12) or 2.0mg/kg/day paclitaxel (n=18). Horizontal axes: Time in days relative to initiation of vehicle/paclitaxel treatment on Day 1. Arrows indicate vehicle/paclitaxel treatment days. Vertical axes: (a) % baseline body weight, (b) mechanical sensitivity expressed as threshold stimulation to elicit paw withdrawal in log g, and (c) ICSS performance expressed the % baseline number of brain-stimulation reinforcements earned per 10-min component. All points show mean±SEM, and filled points indicate a significant difference from vehicle on a given day, as indicated by the Holm-Sidak post hoc test after a significant two-way ANOVA, p<0.05. Statistical results are as follows. (a) No significant main effect of treatment [F(1,28)=3.58;p=0.069], but a significant effect of time [F(24,672)=47.68; p<0.001], and a significant interaction [F(24,672)=4.33; p<0.001]. (b) Significant main effects of treatment [F(1,28)=51.52; p<0.001] and time [F(5,140)=17.83; p<0.001], and a significant interaction [F(5,140)=15.32; p<0.001]. (c) No significant main effect of treatment [F(1,28)=2.36; NS], a significant effect of time [F(22,616)=1.76; p<0.02], and no significant interaction [F(22,616)=0.90; NS].
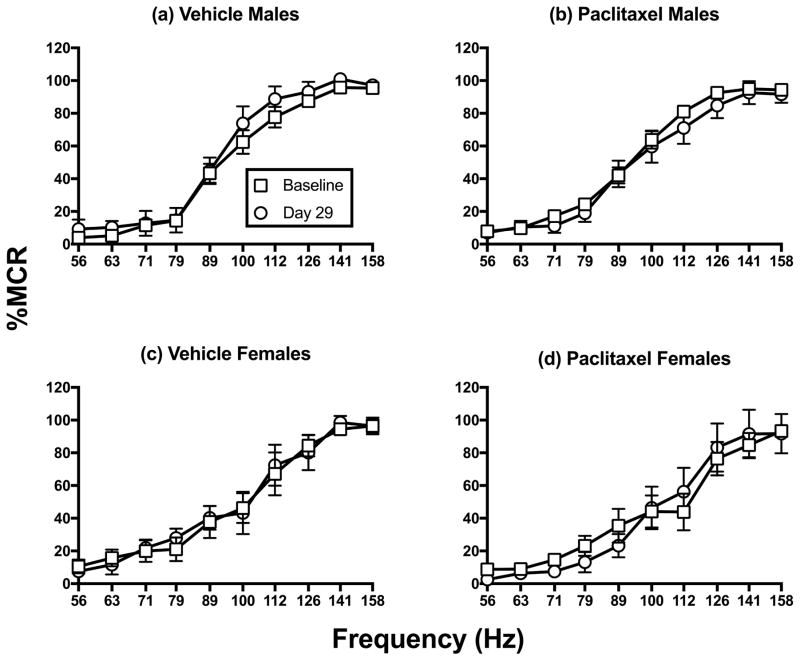
Lack of paclitaxel effects on ICSS frequency-rate curves
As one additional indication of the weak effects of paclitaxel treatment on ICSS performance, Figure 3 compares full ICSS frequency-rate curves at baseline and on Day 29 in all males (Fig. 3a, b) and females (Fig. 3c, d) treated with vehicle or 2.0 mg/kg/day paclitaxel. Two-way ANOVA did not indicate a significant main effect of treatment or an interaction between frequency and treatment for either vehicle or paclitaxel in either sex.
Figure 3.
Comparison of pre-paclitaxel baseline and Day 29 ICSS frequency-rate curves for all rats treated with vehicle or 2.0 mg/kg/day paclitaxel. Panels a–b show the effects of vehicle (n=12) or paclitaxel treatment (n=18) in males, and panels c–d show effects of vehicle (n=6) or paclitaxel (n=6) in females. Horizontal axes: frequency of brain stimulation (Hz). Vertical axes: ICSS rate expressed as percent maximum control rate (%MCR). All points show mean±SEM. Statistical results are as follows. (a) No significant main effect of treatment [F(1,11)=1.44; NS], a significant effect of frequency [F(9,99)=118.30; p<0.001], and no significant interaction [F(9,99)=0.40; NS]; (b) No significant main effect of treatment [F(1,17)=0.54; NS], a significant effect of frequency [F(9,153)=167.4; p<0.001], and no significant interaction [F(9,153)=0.44; NS]. (c) No significant main effect of treatment [F(1,5)=0.01; NS], a significant effect of frequency [F(9,45)=35.78; p<0.001], and no significant interaction [F(9,45)=0.29; NS]. (d) No significant main effect of treatment [F(1,5)=0.02; NS], a significant effect of frequency [F(9,45)=36.14; p<0.001], and no significant interaction [F(9,45)=0.89; NS].
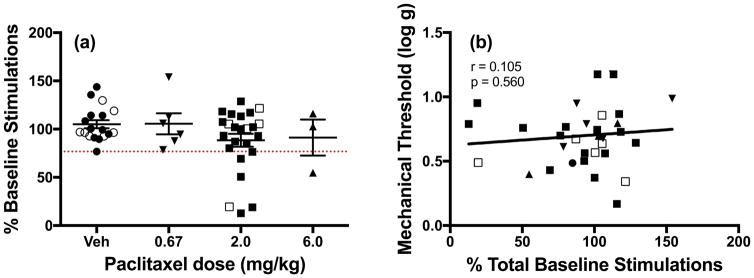
Lack of correlation between ICSS depression and mechanical hypersensitivity
Figure 4a shows individual ICSS data for all male and female rats on the last day of the 29-day study. One-way ANOVA did not reveal a significant effect of paclitaxel dose on ICSS performance, but this analysis did reveal individual variability in rats treated with paclitaxel. In particular, 6 of the 24 rats treated with 2.0 mg/kg/day paclitaxel and one of three rats treated with 6.0 mg/kg/day paclitaxel had ICSS rates below those of the lowest vehicle-treated rat. To evaluate the degree to which ICSS depression might be related to the magnitude of mechanical hypersensitivity, the ICSS and mechanical sensitivity data from individual paclitaxel-treated rats were submitted to correlational analysis. Figure 4b shows that magnitude of ICSS depression did not correlate with magnitude of mechanical hypersensitivity in individual rats treated with paclitaxel.
Figure 4.
Paclitaxel effects in individual rats on the last day of the study (Day 29). Closed symbols denote individual male rats and open symbols denote individual female rats. (a) Effects of repeated vehicle (n=18), 0.67 mg/kg (n=6), 2.0 mg/kg (n=24), or 6.0 mg/kg (n=3) paclitaxel on ICSS responding in all male and female rats. Horizontal axis: paclitaxel dose in mg/kg/day. Vertical axis: ICSS performance expressed as % baseline number of brain-stimulation reinforcements earned per 10-min component. Group data show mean±SEM. One-way ANOVA indicated no significant effect of paclitaxel dose [F(3,47)=1.52, NS]. The dotted line indicates the lowest value for a vehicle-treated rat, and points below this line suggest paclitaxel-induced ICSS depression in some rats. (b) Correlation of ICSS responding and mechanical sensitivity. Horizontal axis: ICSS performance expressed as % baseline number of brain-stimulation reinforcements earned per 10-min component. Vertical axis: mechanical sensitivity expressed as threshold stimulation to elicit paw withdrawal in log g. The correlation was not significant (r=0.105; NS).
DISCUSSION
This study compared effects of paclitaxel treatment on mechanical sensitivity and positively reinforced operant responding in rats. There were two main findings. First, paclitaxel doses sufficient to produce mechanical hypersensitivity did not reliably depress ICSS in male or female rats. Second, analysis of data from individual rats indicated that the degree of behavioral suppression in ICSS did not correlate with mechanical sensitivity. The lack of correlation between mechanical sensitivity and behavioral suppression suggests that mechanical hypersensitivity does not cause behavioral suppression, may have different underlying mechanisms from behavioral suppression, and may not serve as a useful surrogate measure for clinically relevant signs of behavioral depression in neuropathic pain.
Effects of paclitaxel on body weight and mechanical sensitivity
The effects of paclitaxel reported here agree with previous studies in rodents that examined the time course and extent of mechanical hypersensitivity following paclitaxel treatment (Polomano et al., 2001; Pascual et al., 2010; Hwang et al., 2012; Boyette-Davis et al., 2011; Ko et al., 2014; Toma et al., 2017). For example, Polomano et al. 2001, found that four injections of 2.0 mg/kg/day paclitaxel on alternating days produced significant mechanical hypersensitivity for four weeks. With regard to sex differences in paclitaxel effects, one previous study found that female mice were more sensitive than males to paclitaxel-induced cold hypersensitivity (Naji-Esfahani et al., 2016); however, as in previous studies, paclitaxel-induced mechanical hypersensitivity was similar in males and females (Hwang et al., 2012; Naji-Esfahani et al., 2016). The present study extends on these results by showing that a 0.5 log unit higher paclitaxel dose (four injections of 6.0 mg/kg/day) produced sufficient weight loss to require euthanasia in most rats in accordance with moribundity criteria in the animal use protocol.
Effects of paclitaxel on intracranial self-stimulation (ICSS)
Behavioral depression is a cardinal sign of clinically relevant pain (Dworkin et al., 2008) and the importance of pain-depressed behaviors in guiding diagnosis and treatment of human pain is growing, given concerns about reliance on verbal pain reports (Sullivan and Ballantyne, 2016). ICSS is one type of behavioral baseline that can be used to evaluate preclinical expression and treatment of pain-related behavioral depression and functional impairment in rats (Pereira Do Carmo et al., 2009; Negus et al., 2010, 2013), and the principal goal of this study was to test the working hypothesis that paclitaxel treatment regimens sufficient to produce mechanical hypersensitivity would also depress ICSS. Our results do not support this hypothesis.
The weak effects of paclitaxel on ICSS cannot be attributed to a general lack of ICSS sensitivity to putative pain states. Consistent with the expression of both depressed behavior and depressed mood in many human pain states, ICSS in rats can be depressed transiently (hours to days) by inflammatory noxious stimuli that include i.p. injection of dilute acid, paw incision, and intraplantar administration of complete Freund’s adjuvant (Pereira Do Carmo et al., 2009; Ewan and Martin, 2014; Leitl et al., 2014b; Brust et al., 2016). Moreover, these examples of pain-related depression of ICSS can be reversed by clinically effective analgesics (e.g. opioids and nonsteroidal anti-inflammatory drugs) but not by drugs (e.g. centrally acting kappa opioid receptor agonists) that produce motor impairment and appear as false positives in conventional preclinical assays (Negus, 2013). However, the effectiveness of neuropathic manipulations to decrease ICSS has been less consistent. For example, intraplantar formalin administration produced a sustained and analgesic-reversible depression of ICSS for up to two weeks (Leitl and Negus, 2016), but spinal nerve ligation as a surgical neuropathy model failed to alter ICSS (Ewan and Martin, 2014, 2017). Consistent with the effects of spinal nerve ligation, paclitaxel treatments sufficient to produce mechanical sensitivity in the present study failed to produce significant or reliable decreases in ICSS in either male or female rats. It remains possible that other paclitaxel treatment regimens might be effective to decrease ICSS; however, given the severe weight loss produced by repeated 6.0 mg/kg/day paclitaxel in this study, there is a narrow window for more intensive treatments, and pilot studies conducted by us (e.g. a second round of repeated 4×2.0 mg/kg/day paclitaxel) were also not effective (data not shown).
One interpretation of these results is that ICSS in rats is not useful for assessment of any neuropathic pain produced by these paclitaxel treatment regimens. Electrical brain stimulation can function as an extremely efficacious reinforcer (Negus and Miller, 2014), and it is possible that other behaviors (e.g. operant responding for food) may be more susceptible than ICSS to depression by paclitaxel. Consistent with this possibility, 4 injections of 2.0 mg/kg/day produced significant if transient decreases in body weight for males in the present study. However, ICSS was used here for two reasons, in addition to its previously demonstrated utility in other studies of pain-depressed behavior. First, different frequencies of electrical brain stimulation can be used to efficiently maintain a range of low-to-high rates of responding that are stable over time and sensitive to perturbation by a variety of treatments (Negus and Miller, 2014). Second, ICSS relies on direct stimulation of neural circuits that underlie reinforcement, independent of common sensory modalities (e.g. taste), and as a result, the procedure has also been used to examine effects of experimental manipulations on reward system function and neurobiology of motivation (Carlezon and Chartoff, 2007). Perhaps the most common use of ICSS in this regard has been to evaluate abuse liability of drugs, and drugs of abuse typically increase (or “facilitate”) responding suggestive of enhanced reward system function; however, ICSS has also been used to examine effects of manipulations that impair reward system function and contribute to affective signs of anhedonia and depression (Carlezon and Chartoff, 2007; Negus and Miller, 2014). Notably, paclitaxel failed to reduce even low rates of ICSS maintained by low brain-stimulation frequencies that function as weak reinforcers. As such, these results provide no evidence for an effect of paclitaxel treatment on reward system function.
An alternative and more controversial interpretation of these findings is that conventional paclitaxel treatment regimens produce little or no pain in rodents. These treatment regimens were initially validated behaviorally by their effectiveness to produce hypersensitive withdrawal responses from thermal and mechanical stimuli (e.g. Polomano et al., 2001), but hypersensitive withdrawal responses are not a common sign of human chronic pain in general or chemotherapy-induced neuropathic pain in particular (Dworkin et al., 2008; Golan-Vered and Pud 2013). Moreover, although thermal and/or mechanical allodynia is observed in a subset of human neuropathy patients, it is measured not as hypersensitivity of withdrawal responses but as hypersensitivity of verbal endorsement of subjective pain. Use of a common term “allodynia” to describe these different behaviors is problematic for translational research, as preclinical and clinical studies are measuring very different endpoints, despite using the same stimulus. More generally, it may be inappropriate to interpret hypersensitive withdrawal responses as evidence of pain, and the present results suggest that even if paclitaxel-induced hypersensitivity of withdrawal responses is associated with a pain experience in rodents, that experience is not of a sufficient type or intensity to depress ICSS.
Acknowledgments
The authors have no conflicts of interest to disclose.
Supported by NIH grants R01NS070715 and F30CA213956.
References
- Ando-Tanabe N, Iwamitsu Y, Kuranami M, Okazaki S, Yasuda H, Nakatani Y, et al. Cognitive function in women with breast cancer receiving adjuvant chemotherapy and healthy controls. Breast Cancer. 2014;21:453–62. doi: 10.1007/s12282-012-0405-7. [DOI] [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB. Sex differences in pain: A brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–8. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis J, Xin W, Zhang H, Dougherty P. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2013;152:308–13. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, et al. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal. 2016;117:1–12. doi: 10.1126/scisignal.aai8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Pharmacol Exp Ther. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Davies CC, Colon G, Geyer H, Pfalzer L, Fisher MI. Oncology EDGE task force on prostate cancer outcomes: a systematic review of outcome measures for functional mobility. Rehabil Oncol. 2016;34:82–96. [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85:3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats. Anesth Analg. 2014;118:854–62. doi: 10.1213/ANE.0000000000000119. [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. Rewarding electrical brain stimulation in rats after peripheral nerve injury: decreased faciliation by commonly abused prescription opioids. Anesthesiology. 2017;115:1271–80. doi: 10.1097/ALN.0b013e3182330448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, Moore A, Raja SN, Rice ASC. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14:162–73. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan-Vered Y, Pud D. Chemotherapy-induced neuropathic pain and its relation to cluster symptoms in breast cancer patients treated with paclitaxel. Pain Pract. 2013;13:46–52. doi: 10.1111/j.1533-2500.2012.00554.x. [DOI] [PubMed] [Google Scholar]
- Hama A, Takamatsu H. Chemotherapy-induced peripheral neuropathic pain and rodent models. CNS Neurol Disord Drug Targets. 2016;15:7–19. doi: 10.2174/1871527315666151110125325. [DOI] [PubMed] [Google Scholar]
- Hutsell BA, Negus SS, Banks ML. Effects of 21-day d-amphetamine and risperidone treatment on cocaine vs food choice and extended-access cocaine intake in male rhesus monkeys. Drug Alcohol Depend. 2016;168:36–44. doi: 10.1016/j.drugalcdep.2016.08.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BY, Kim ES, Kim CH, Kwon JY, Kim HK. Gender differences in paclitaxel-induced neuropathic pain behavior and analgesic response in rats. Korean J Anesthesiol. 2012;62:66–72. doi: 10.4097/kjae.2012.62.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MH, Hu ME, Hsieh YL, Lan CT, Tseng TJ. Peptidergic intraepidermal nerve fibers in the skin contribute to the neuropathic pain in paclitaxel-induced peripheral neuropathy. Neuropeptides. 2014;48:109–17. doi: 10.1016/j.npep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Langer CJ, Hirsh V, Okamoto I, Lin F-J, Wan Y, Whiting S, et al. Survival, quality-adjusted survival, and other clinical end points in older advanced non-small-cell lung cancer patients treated with albumin-bound paclitaxel. Br J Cancer. 2015;113:20–29. doi: 10.1038/bjc.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Blough BE, Negus SS. Preclinical abuse potential assessment of Flibanserin: effects on intracranial self-stimulation in female and male Rats. J Sex Med. 2016a;13:338–49. doi: 10.1016/j.jsxm.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Legakis LP, Negus SS. Opposing effects of dopamine D1- and D2-like agonists on intracranial self-stimulation in male rats. Exp Clin Psychopharmacol. 2016b;24:193–205. doi: 10.1037/pha0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Negus SS. Pharmacological modulation of neuropathic pain-related depression of behavior: effects of morphine, ketoprofen, bupropion, and Δ9-tetrahydrocannabinol on formalin-induced depression of intracranial self-stimulation in rats. Behav Pharmacol. 2016;27:364–76. doi: 10.1097/FBP.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Banks ML, Negus SS. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids. Neuropsychopharmacology. 2014a;39:614–24. doi: 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Negus SS. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain. 2014b;10:62. doi: 10.1186/1744-8069-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Mastick J, Paul SM, Topp K, Smoot B, Abrams G, et al. Chemotherapy-induced neuropathy in cancer survivors. J Pain Symptom Manage. 2017;54:204–18. doi: 10.1016/j.jpainsymman.2016.12.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaushiro S, Kitanaka A, Kubuki Y, Hidaka T, Shide K, Kameda T, et al. Nasopharyngeal carcinoma with bone marrow metastasis: positive response to weekly paclitaxel chemotherapy. Intern Med. 2015;54:1455–9. doi: 10.2169/internalmedicine.54.3917. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–94. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Motulsky H, Christopoulous A. A Practical Guide to Curve Fitting. San Diego: Graphpad Software; 2013. Fitting models to biological data using linear and nonlinear regression. [Google Scholar]
- Naji-Esfahani H, Vaseghi G, Safaeian L, Pilehvarian AA, Abed A, Rafieian-Kopaei M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab Anim. 2016;50:15–20. doi: 10.1177/0023677215575863. [DOI] [PubMed] [Google Scholar]
- Negus SS. Expression and treatment of pain-related behavioral depression. Lab Anim. 2013;42:292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–14. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Negus SS, Bilsky EJ, Do Carmo GP, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods Mol Biol. 2010;617:79–91. doi: 10.1007/978-1-60327-323-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156:1153–60. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Burgos E, Martin MI. Antinociceptive effect of three common analgesic drugs on peripheral neuropathy induced by paclitaxel in rats. Pharmacol Biochem Behav. 2010;95:331–37. doi: 10.1016/j.pbb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Pedersen SS, Denollet J, Daemen J, van de Sande M, de Jaegere PT, Serruys PW, et al. Fatigue, depressive symptoms, and hopelessness as predictors of adverse clinical events following percutaneous coronary intervention with paclitaxel-eluting stents. J Psychosom Res. 2007;62:455–61. doi: 10.1016/j.jpsychores.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–77. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract. 2012;2012:1–10. doi: 10.1155/2012/913848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, et al. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer. 2012;118:5171–78. doi: 10.1002/cncr.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger AL, Riffle SM, Lopus M, Jordan MA, Wilson L, Mooberry SL. The taccalonolides and paclitaxel cause distinct effects on microtubule dynamics and aster formation. Mol Cancer. 2014;13:41. doi: 10.1186/1476-4598-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012;13:228–34. doi: 10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980;77:1561–5. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–70. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck RM, Sammel MD, Farrar JT, Hennessy S, Mao JJ, Stineman MG, DeMichele A. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract. 2013;9:234–40. doi: 10.1200/JOP.2012.000863. [DOI] [PubMed] [Google Scholar]
- Suh DH, Kim JW, Kang S, Kim HJ, Lee KH. Major clinical research advances in gynecologic cancer in 2013. J Gynecol Oncol. 2014;25:236–248. doi: 10.3802/jgo.2014.25.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD, Ballantyne JC. Must we reduce pain intensity to treat chronic pain? Pain. 2016;157:65–69. doi: 10.1097/j.pain.0000000000000336. [DOI] [PubMed] [Google Scholar]
- Toma W, Kyte SL, Bagdas D, Alkhlaif Y, Alsharari SD, Lichtman AH, et al. Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology. 2017;117:305–15. doi: 10.1016/j.neuropharm.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner E, Krivitsky R, Cone K, Atherton P, Pitre T, Lanpher J, et al. Evaluation of a postoperative pain-like state on motivated behavior in rats: effects of plantar incision on progressive-ratio food-maintained responding. Drug Dev Res. 2015;76:432–41. doi: 10.1002/ddr.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Naso L, Bennett GJ. Experimental studies of potential analgesics for the treatment of chemotherapy-evoked painful peripheral neuropathies. Pain Med. 2008;9:505–17. doi: 10.1111/j.1526-4637.2007.00301.x. [DOI] [PubMed] [Google Scholar]