Supplemental Digital Content is available in the text
Keywords: cost savings, oral medicine, preexposure prophylaxis, prevention of sexual transmission, sexual behaviour, tenofovir
Abstract
Objectives:
To review the main factors influencing the costs of nondaily oral preexposure prophylaxis (PrEP) with tenofovir (±emtricitabine). To estimate the cost reductions possible with nondaily PrEP compared with daily PrEP for different populations (MSM and heterosexual populations).
Design:
Systematic review and data triangulation.
Methods:
We estimated the required number of tablets/person/week for dosing regimens used in the HPTN 067/ADAPT (daily/time-driven/event-driven) and IPERGAY (on-demand) trials for different patterns of sexual intercourse. Using trial data, and behavioural and cost data obtained through systematic literature reviews, we estimated cost savings resulting from tablet reductions for nondaily versus daily oral PrEP, assuming 100% adherence.
Results:
Among different populations being prioritized for PrEP, the median reported number of days of sexual activity varied between 0 and 2 days/week (0–1.5 days/week for MSM, 1–2 days/week for heterosexual populations). With 100% adherence and two or fewer sex-days/week, HPTN 067/ADAPT nondaily regimens reduced the number of tablets/week by more than 40% compared with daily PrEP. PrEP program costs were reduced the most in settings with high drug costs, for example, by 66–69% with event-driven PrEP for French/US populations reporting on average one sex-day/week.
Conclusion:
Nondaily oral PrEP could lower costs substantially (>50%) compared with daily PrEP, particularly in high-income countries. Adherence and efficacy data are needed to determine cost-effectiveness.
Introduction
Preexposure prophylaxis (PrEP) using oral tenofovir (TDF) [±emtricitabine (FTC)] reduces the risk of acquiring HIV [1]. However, PrEP roll-out can be hampered by concerns about expense and cost-effectiveness. As HIV funding has declined in recent years [2], cost-savings in HIV treatment and prevention are urgently needed. Prioritization of PrEP to groups at higher risk of HIV acquisition improves cost-effectiveness for PrEP interventions [3,4]. Another potential cost-saving strategy is the use of nondaily (or intermittent) oral PrEP regimens based on potential HIV exposures, which are expected to require fewer tablets than daily PrEP. Most clinical trials of oral PrEP have used daily dosing, which is currently recommended by the CDC and FDA, but the IPERGAY (Intervention Préventive de l’Exposition aux Risques avec et pour les Gays) trial, amongst MSM in France and Canada, recently showed effectiveness for nondaily PrEP [5].
So far, the safety and adherence of nondaily oral PrEP has been evaluated in MSM, heterosexual sero-discordant couples, female sex workers, and women living in areas with high HIV incidence [5–8]. Different nondaily PrEP regimens, based upon regimens offering protection in animal studies [9], have been proposed and tested. The HPTN 067/ADAPT trial evaluated the feasibility and acceptability of two nondaily PrEP-dosing strategies: ‘time-driven’ dosing (one tablet twice per week with one tablet after sexual intercourse) and ‘event-driven’ dosing (one tablet before and one tablet after sex), as well as daily dosing [10]. IPERGAY prescribed two tablets together before sex, and two tablets after, 24 h apart [5]. An earlier International AIDS Vaccine Initiative (IAVI) trial of nondaily PrEP in Kenya and Uganda also used time-driven dosing [6,7].
Adherence to nondaily oral PrEP requires taking tablets after sexual intercourse, and may require predicting or planning the occurrence of sexual intercourse in advance. Adherence to nondaily PrEP regimens is expected to influence effectiveness. Full cost-effectiveness estimates for nondaily oral PrEP will need to take into account effectiveness (which is influenced by adherence and efficacy), as well as any reductions in costs resulting from the reduced number of tablets required. Nonetheless, much can be learned from deriving the maximum reduction in program costs that could be achieved with nondaily oral PrEP regimens under ideal conditions of 100% adherence.
In this study, we aimed to determine for which populations and patterns of sexual behaviour nondaily oral PrEP would substantially reduce the overall costs of PrEP delivery programs, compared with daily regimens, if each regimen was used exactly as prescribed. We reviewed and synthesized studies providing information on the main factors influencing the relative cost of nondaily PrEP programs: dosing requirements for regimens used in two recent nondaily oral PrEP trials (HPTN 067/ADAPT and IPERGAY), frequency of sex in priority populations, and PrEP program costs and the proportion of costs attributable to oral PrEP drugs. We combined this information to estimate potential cost reductions for nondaily compared with daily oral PrEP.
Methods
The study was conducted in four stages, determining: the number of tablets required for different nondaily oral PrEP regimens, sexual activity patterns for populations being considered for nondaily PrEP, and PrEP program costs, in order to estimate potential cost-savings for nondaily oral PrEP.
Number of tablets required for each regimen
First, we examined four TDF/FTC PrEP regimens used in HPTN 067/ADAPT [10] and IPERGAY [5], two recently conducted nondaily oral PrEP trials.
For each regimen, assuming 100% adherence and accurate prediction of sexual activity, we calculated the weekly number of tablets required per person for different patterns of sexual intercourse, which depends on the number and distribution of days per week when sexual intercourse occurs (sex-days/week).
Sexual activity patterns
Second, we determined sexual activity patterns for populations being considered for nondaily PrEP both from HPTN 067/ADAPT trial data, and through a systematic literature review.
Primary data analysis, HPTN 067/ADAPT
HPTN 067/ADAPT was a phase II, randomized, open-label, pharmacokinetic and behavioural equivalence study of daily versus nondaily oral FTC/TDF PrEP [8,11,12]. The study enrolled HIV-uninfected men and transgender women who have sex with men, aged at least 18 years, in Bangkok, Thailand, and Harlem in New York City, USA, and heterosexual women at high risk for HIV infection in Cape Town, South Africa, aged at least 18 years. The study was conducted with the understanding and consent of each participant. Ethics committee approvals were obtained from the Ethical Review Committee for Research in Human Subjects of the Thailand Ministry of Public Health; the Institutional Review Board of the US Centers for Disease Control and Prevention; the Ethics Committee of the Health Science Faculty, University of Cape Town; the Emavundleni Community Advisory Board and the Medicines Control Council of South Africa; and Columbia University Medical Center. The protocol was registered at ClinicalTrials.gov NCT01327651 and is available at https://www.hptn.org/research/studies/82.
For each population, we calculated the median and interquartile range (25th and 75th percentiles) of sex-days per week (counting only days on which participants reported anal or vaginal sex), pooled across PrEP regimens.
Systematic review of sexual behaviour
We searched PubMed and Web of Knowledge databases for articles published between 1 January 2010 and 31 December 2015 (the first oral PrEP trial results were released in 2010 [13]). Conference abstracts for the 2014 and 2015 CROI and International AIDS Society conferences were searched online.
The search terms were (PrEP OR ‘pre-exposure prophylaxis’) AND (intermittent∗ OR non-daily OR nondaily OR coitally-related OR ‘fixed dose’ OR post-coital OR ‘on demand’ OR event-based OR event-driven OR time-driven OR weekly-based OR routine OR periodic).
To be eligible for inclusion in the sexual behaviour analysis, studies had to report sexual activity as frequency of sex-days per unit time. Where different articles referred to the same study population, the most recent data were extracted.
The following data were extracted by one investigator: country, study population and design, number of sex-days per unit time (median or categorical data), spacing of sex-days. The median and interquartile range for the frequency of sex-days was calculated from the data provided. If this information was not available, it was requested from study authors.
Oral preexposure prophylaxis program costs
Third, we systematically searched the literature on PrEP program costs. We searched PubMed and Web of Knowledge databases for articles published between 1 January 2010 and 31 December 2015, and searched 2014 and 2015 CROI and International AIDS Society conference abstracts.
The search terms were HIV AND (PrEP OR ‘pre-exposure prophylaxis’) AND cost.
We additionally included data from a study presented at the 2015 Public Health Science conference [14].
To be included, studies had to give the costs of PrEP programs, specify nondrug and drug costs, and allow us to derive the costs of PrEP/person/year.
The following data were extracted by one investigator: country of study, study population, year, total costs/person/year, costs of drugs and/or proportion of costs attributable to drugs, currency. Costs were converted from other currencies into US$ for the same year using the CCEMG–EPPI-Centre Cost Converter [15], using Purchasing Power Parity conversion factors from the International Monetary Fund. Because of the large differences seen in costs between high income countries (HIC) and low and middle-income countries (LMICs), we summarized costs separately for HIC, upper middle-income countries (UMIC), lower middle-income countries (L-MIC) and low-income countries (LIC), following World Bank 2017 classifications [16].
Cost-saving estimates
Fourth, using the data collected in the previous three sections, we estimated the range of cost savings possible because of tablet reductions compared with daily PrEP, assuming 100% adherence, as follows:
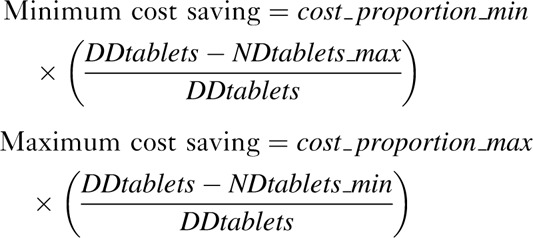 |
where cost_proportion_min/cost_proportion_max = minimum/maximum percentage of costs attributable to PrEP drugs, DDtablets = tablets required per week with daily dosing, and NDtablets_min/NDtablets_max = minimum/maximum tablets required per week with nondaily dosing. The number of tablets was estimated using the median number of sex-days/week. Wherever data on the full distribution of sex-days/week were available, the number of tablets were also estimated summing across the distribution. In sensitivity analysis, we used each of the full distributions of sex-days/week obtained for other settings to assess potential cost-savings for each setting which had both cost and behaviour data. Additionally, we calculated hypothetical cost-savings for different frequencies of sex-days/week for four countries with different estimates of the proportion of costs attributable to PrEP drugs.
Results
Number of tablets required for each regimen
In HPTN 067/ADAPT, participants were randomized to one of three PrEP regimens: daily, time-driven, or event-driven TDF/FTC PrEP. Those randomized to daily PrEP were prescribed one tablet per day. The time-driven arm was prescribed one tablet 2 days per week with one tablet within 2 h after sexual intercourse. The event-driven arm was prescribed one tablet between 24 and 48 h before sexual intercourse and a second tablet within 2 h after sex [10].
IPERGAY used an on-demand regimen. Participants were prescribed two tablets together 2–24 h before sexual intercourse, and a third and fourth tablet 24 and 48 h after the first two, respectively [5].
For further details see Supplementary Appendix 1 and Supplementary Figure 1.
For 0, 1, and 2 weekly sex-days, 0, 2, and 3–4 weekly tablets, respectively, are required with HPTN 067/ADAPT event-driven dosing (Fig. 1a).
Fig. 1.
Tablets required per person in a given week for different number and pattern of sex-days per week assuming 100% regimen adherence and accurate forecasting of sexual behaviour – patterns giving maximum and minimum tablet numbers are shown.
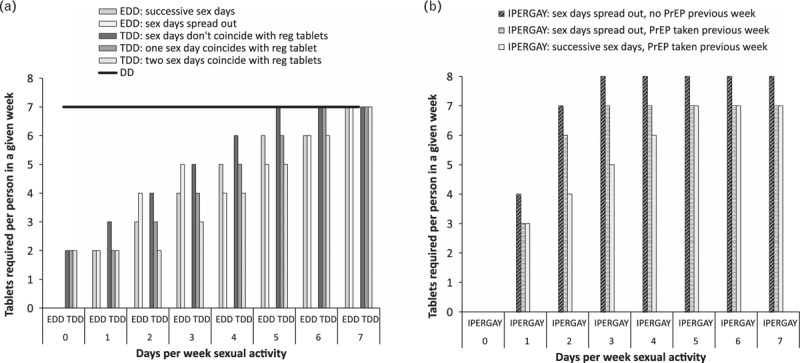
(a) Regimens from HPTN 067/ADAPT: event-driven dosing (EDD), time-driven dosing (TDD), daily dosing (DD); (b) IPERGAY on-demand regimen. Daily dosing is shown as a horizontal line, other regimens as bars.
Time-driven dosing requires more tablets than event-driven dosing for 0 sex-days, but similar numbers for 1 or 2 weekly sex-days.
IPERGAY dosing requires no tablets for 0 sex-days/week. For at least one sex-day per week, at least as many weekly tablets are required as with either event-driven or time-driven PrEP (Fig. 1b).
Sexual activity patterns
The systematic review identified 207 unique articles and conference abstracts, of which 14 provided sexual behaviour data, describing 10 populations (7 MSM, 3 heterosexual) in seven independent studies (Supplementary Figure 2a, Supplementary Table 1). The median number of sex-days/week was requested and received from authors for two studies [17,18].
The median number of sex-days varied between 0 and 1.5 per week for MSM populations from France, Thailand, Kenya, and the United States [6,11,12,17–20] and between 1 and 2 for heterosexual populations from Uganda and South Africa [7,8,21] (Fig. 2, Supplementary Table 1). Three MSM studies showed variation in number of sex-days/week among respondents, with a few reporting daily sex, but many reporting no sex at all [17,19,20] (Supplementary Table 1).
Fig. 2.
Forest plot showing median and inter-quartile range (IQR; 25th and 75th percentile) number of days per week sexual activity reported in different populations identified in systematic literature search.
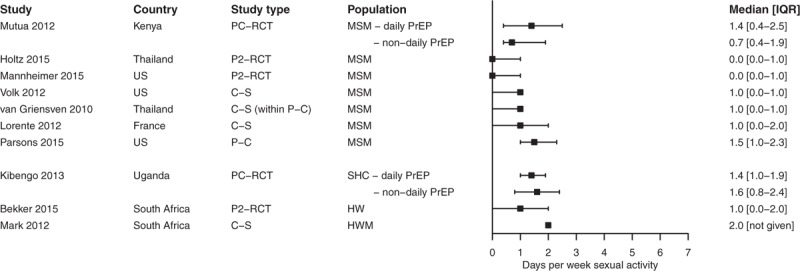
PC-RCT, placebo-controlled randomized controlled trial nondaily versus daily PrEP; P2-RCT, phase II randomized controlled trial nondaily versus daily PrEP; C-S, cross-sectional study; P-C, prospective cohort; SHC, sero-discordant heterosexual couples; HW, heterosexual women; HWM, heterosexual women and men.
Five studies found sex was most likely to be reported at the weekend [17–21], but also frequently occurred on weekdays (Supplementary Table 1).
Oral preexposure prophylaxis program costs
The systematic review identified 222 unique papers and conference abstracts, of which 18, covering 17 independent studies, provided cost data (Supplementary Figure 2b, Table 1). Six studies came from HIC, five from UMIC, five from L-MIC, and one from LIC.
The nondrug costs included varied considerably between studies (Table 1). Most studies (16/17) explicitly costed laboratory tests. Specific tests mentioned usually included HIV testing and serum creatinine. Sexually transmitted infection (STI), hepatitis B, pregnancy, and blood urea tests were included less frequently. Staff costs were mentioned in most (12/17) studies. Specific staff frequently mentioned were physicians, laboratory staff, and nurses. Three studies each included staff training and adherence counselling costs. Other costs mentioned in one or two studies included condom provision, risk reduction counselling, building, and facilities costs (Table 1). Drug costs from some studies included pharmacy-dispensing fees – wherever these were given separately we re-classified them as nondrug costs, but this was not always possible (Table 1).
Table 1.
Estimated costs of preexposure prophylaxis programs, and proportion of costs because of preexposure prophylaxis drugs, from costing and cost-effectiveness studies conducted since 2010, by World Bank country classifications for 2017.
| Cost components | |||||||||||||||||||||
| Reference | Population | Country | ARV drugs | Laboratory tests | HIV tests | STI testing | Hepatitis B test | Blood urea nitrogen | Serum creatinine | Pregnancy test | Staff costs | Physician | Nurse | Laboratory staff | Staff training | Adherence counselling | Other | Cost unitsa | PrEP total cost per person per year | PrEP drug cost per person per year | Percentage of total costs attributable to drugs |
| High-income countries | |||||||||||||||||||||
| Juusola et al. [22] | MSM | USA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | US$ 2010 | 10,237 | 9,441 | 92% | |||||||
| Horberg and Raymond [23] | Not specified | USA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Hepatitis B vaccination, risk reduction counselling | US $ | 17,808–17,939 | 17,125 | 96% | |||
| Ouellet et al. [24] | MSM | Canada | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Condoms, overheads, pharmacist dispensing fees | Can $2012 (US $2012) | 11,593 (9,387) | 9,396 (7,608) | 81% | |||||||
| Schneider et al. [25] | MSM | Australia | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | AU $2013 (US $2013) | 10,362 (7,025) | 9,597b (6,506) | 93% | |||||||
| Ong et al. [14] | MSM | UK | ✓ | Clinic costs | GB £2013/14 (US $2014) | 4,560 (6,514) | 4,311 (6,159) | 95% | |||||||||||||
| Mabileau et al. [26] | Discordant couples | France | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Urine ovulation tests | €2013 (US $2013) | 6,702 (8,055) | 6,437 (7,737) | 96% | ||||||
| Upper-middle income countries | |||||||||||||||||||||
| Gomez et al. [27] | MSM | Peru | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Condoms and lubricants, behaviour change communication, counselling, project level costs | US $2011 | 606–828 | 420–600 | 66–75% | ||||||
| Pretorius et al. [28] | General population | South Africa | ✓ | ✓ | ✓ | ✓ | US $2010 | 150 | 134 | 89% | |||||||||||
| Cremin et al. [29] | General population | South Africa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Facilities, outreach | US $2012 | 252 | 118 | 47% | |||
| Stover et al. [30] | General population | South Africa | ✓ | ✓ | Service delivery | US $ | 95 | 50 | 53% | ||||||||||||
| Walensky et al. [31] | High-risk women | South Africa | ✓ | ✓ | ✓ | Chemistry panels, clinic visits | US $2014 | 148 | 75 | 51% | |||||||||||
| Lower-middle income countries | |||||||||||||||||||||
| Alistar et al. [32] | People who inject drugs | Ukraine | ✓ | ✓ | ✓ | ✓ | ✓ | Monitoring PrEP side effects | US $ | 950 | 450 | 47% | |||||||||
| Nichols et al. [33] | General population | Zambia | ✓ | ✓ | ✓ | ✓ | HIV testing includes outpatient visits | US $2012 | 134 | 127 | 95% | ||||||||||
| Nichols et al. [34] | General population | Zambia | ✓ | ✓ | ✓ | ✓ | ✓ | Outpatient visits | US $ | 137 | 126 | 92% | |||||||||
| Kiragu et al. [35] | MSM and young women | Kenya | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Demand-creation, lab equipment, transport, building costs | US $2012 | 618 1st year; 522 2nd year | 110 | 18% 1st year; 21% 2nd year | ||||||||
| Mitchell et al. [36] | Discordant couples | Nigeria | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Drug delivery costs, mass media, facilities, logistics | US $2012 | 233 | 91 | 39% | ||||||
| Low-income countries | |||||||||||||||||||||
| Ying et al. [37,38] | Discordant couples | Uganda | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Wages for other staff, start-up costs, office supplies, transport, building costs | US $2012 | Cost for 6 months: 408 (Partners Demo project)92 (Ministry of Health estimate) | 30475 | 37%41% | ||
aWhere year is missing, this was not specified in the article.
bThe drug costs include pharmacist markup and dispensing fees.
In studies from high-income countries (n = 6), drug costs were high (US$6160–17 130/person/year), and accounted for 81–96% of PrEP program costs (Table 1) [14,22–26].
In LMICs (n = 11), drug costs varied between US$50–600/person/year [27–37]. Costs were higher in LMICs outside Africa (US$420–600/person/year; n = 2) than in Africa (US$50–134 for ‘real world’ settings, US$304 in a demonstration project; n = 9). Eight LMIC studies estimated that 21–75% of total PrEP costs were attributable to drugs [27,29–32,35–37], whereas three studies in LMICs estimated that drugs accounted for 89–95% of program costs [28,33,34] (Table 1).
The proportion of costs attributable to drugs tended to be higher in UMIC then in LIC or L-MIC, and was generally higher in countries with high drug costs (Fig. 3). Neither drug costs nor the proportion of costs attributable to PrEP drugs changed significantly over time (not shown).
Fig. 3.
The proportion of PrEP program costs which are attributable to medication costs plotted against the costs of PrEP medication per person per year, from costing or cost-effectiveness studies published during or after 2010, for low-income, lower middle-income, upper middle-income and high-income countries (World Bank 2017 classifications).
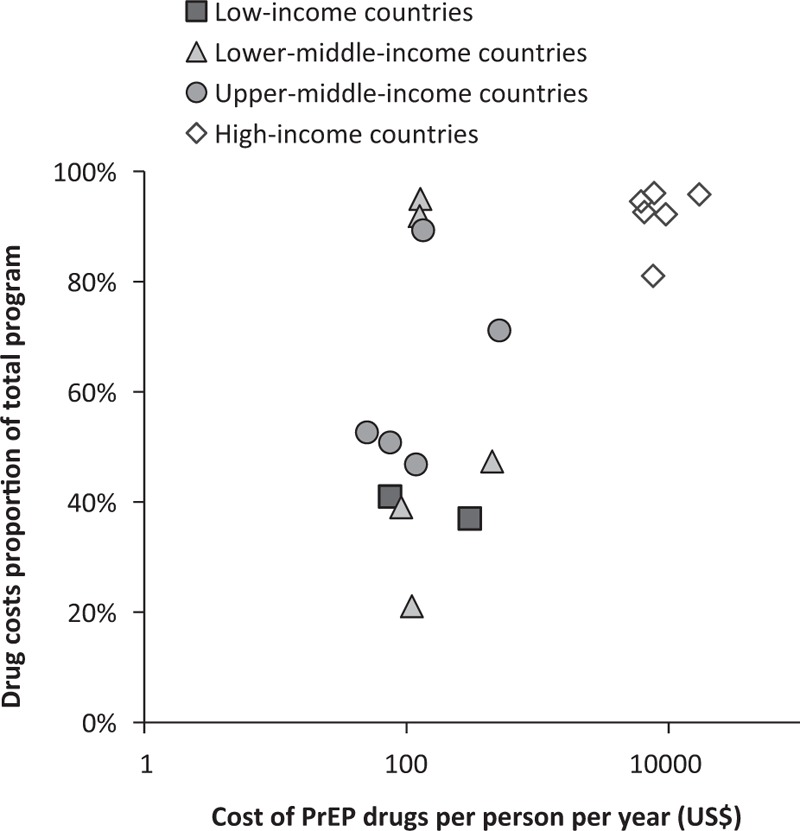
Costs are converted to US$ but not adjusted for year of estimate. Note the log scale used on the x-axis.
Cost-saving estimates
Supplementary Figure 3 shows the hypothetical cost savings for different frequencies of sex-days/week and nondaily PrEP regimens in different settings.
For four settings with both cost and behaviour data (MSM in the United States, France, and Kenya, heterosexuals in South Africa), we estimated the cost savings achievable with nondaily compared with daily oral PrEP.
Using the median number of sex-days/week, program costs were reduced the most – by 66–69% – for lower-activity (median one sex-day/week) US or French MSM taking event-driven PrEP (Table 2). The reduction in costs was more modest in settings where drug costs accounted for a smaller proportion of program costs. For example, only a 9–15% reduction was predicted for MSM in Kenya. Cost reductions were also more modest in populations with more frequent sexual activity. When using median sex-days/week, the IPERGAY regimen gave smaller cost-reductions than the HPTN 067/ADAPT regimens, because of the smaller reductions in tablets required.
Table 2.
Estimated cost savings with nondaily PrEP.
| Country | Population | Median days/week have sex | Percentage of costs attributable to PrEP drugs | Cost reductions with EDD | Cost reductions with TDD | Cost reductions with IPERGAY regimen |
| United States | MSM (lower activity) | 1 | 92–96% | 66–69% | 53–69% | 39–55% |
| United States | MSM (higher activity) | 1.5 | 92–96% | 39–69% | 39–69% | 0–55% |
| France | MSM | 1 [using distribution] | 96% | 69% [69–73%] | 55–69% [52–64%] | 41–55% [50–64%] |
| Kenya | MSM | 1 | 21% | 15% | 12–15% | 9–12% |
| South Africa | Heterosexual (lower activity) | 1 | 47–53% | 34–38% | 27–38% | 20–30% |
| South Africa | Heterosexual (higher activity) | 2 | 47–53% | 20–30% | 20–38% | 0–23% |
EDD, event-driven PrEP, TDD, time-driven PrEP (regimens from HPTN 067/ADAPT). Figures in italics were estimated using the full distribution of sex-days per week across the population, rather than the median.
However, for MSM in France, using data on the full distribution of sex-days across the week gave slightly different estimated savings from using the median number of sex-days. Using the full distribution suggested slightly greater savings with event-driven PrEP, slightly lower savings with time-driven PrEP, but somewhat larger savings with the IPERGAY regimen than when only the median number of sex-days was used (IPERGAY: 50–64% reduction considering the full distribution versus 41–55% using the median). The full distribution of sex-days/week was not available for the other settings with cost data.
In sensitivity analysis, we used each distribution of sex-days/week found in the literature review, all having median one sex-day/week (Supplementary Table 1), to estimate cost savings for the populations in Table 2, assuming their sexual activity followed these distributions. Using the full distributions rather than the median slightly reduced cost-savings with time-driven PrEP (by up to four percentage points) and somewhat increased cost-savings for event-driven and IPERGAY regimens (up to 5 and 14 percentage points, respectively). Greater increases in cost-savings were seen for distributions with a greater proportion of men reporting no sex-days/week (Supplementary Figure 4). Full distributions were not available for populations reporting more than a median one sex-day/week.
Discussion
In this study, we reviewed the key factors likely to influence what cost reductions could be achieved with nondaily oral PrEP. Our study suggests substantial reductions in required tablets – and therefore costs – could be seen in populations having sexual activity on average 1–2 days a week, a typical level of activity for many populations being prioritized for PrEP around the world. The extent of these cost reductions will partly depend on individuals’ ability to accurately predict or plan sexual activity, and on the proportion of PrEP program costs which are attributable to drug costs. Current evidence suggests that large cost-savings could be made in high-income countries, where generic PrEP drugs are not available, and high drug costs account for the majority (81–96%) of oral PrEP program costs. We predict that costs could be reduced the most – by at least 50% – for MSM in the United States or France reporting sexual activity on average 1 day per week, using any of the nondaily regimens explored here.
Clustering of sex-days on consecutive days reduces the number of tablets required for event-driven and on-demand regimens, but little information on clustering of sex-days has been collected in studies of populations being considered for nondaily PrEP. It is likely that being in a stable partnership is associated with different patterns of sexual behaviour. We were unable to evaluate this here, as published behavioural data was not stratified by partnership status.
Variation between studies in how costs are described and classified makes it difficult to assess whether all costs are being fully accounted for; if nondrug costs have been underestimated, or drug costs over-estimated, then cost-savings associated with nondaily oral PrEP will be reduced. Estimated drug costs for TDF/FTC have recently declined in many LMICs (including Ukraine and many African countries [39,40]), reducing potential future cost-savings for nondaily versus daily oral PrEP. Drug costs have not declined in HIC, but could decline in future, for example, after drug patents expire. We have not specifically looked at the effects of intellectual property rights and price controls on drug costs, making future predictions more uncertain.
We have assumed that PrEP program nondrug costs will not be affected by the PrEP-dosing regimen; that the same frequency of monitoring, and level of adherence counselling, will be required for nondaily as daily PrEP, and that no additional costs are associated with educating patients about nondaily PrEP use. If this is not true, then the potential cost-savings that could be achieved with nondaily PrEP will be affected.
Two of the behavioural studies identified collected prospective sexual forecasting data [18,41]. In both studies, 57% of those saying they would have sex the following day actually did so. A substantial number of unnecessary tablets were taken by participants prescribed event-driven PrEP in HPTN 067/ADAPT, for example, 1293 for United States MSM, versus 2573 prescribed. If United States MSM prescribed event-driven oral PrEP took 1–1.4 unnecessary tablets for every two prescribed, as implied by these data, this would reduce the cost-reduction in our best-case scenario (prescribing event-driven PrEP for United States MSM having sex on average on 1 day/week) from 66–69% to 47–55%.
In the studies identified, both prospective and retrospective reporting suggested substantial underestimation of when sex would occur [18–21,41]. In HPTN 067/ADAPT and the earlier IAVI trial of nondaily PrEP, adherence was almost always better for daily than nondaily PrEP [6–8,11,12], the only exception being MSM in Bangkok, for whom adherence was comparable in the daily and time-driven arms. Thus effectiveness for nondaily oral PrEP may be lower than that seen with daily dosing. Notably, low adherence to postsex tablets was found for nondaily regimens in both trials [6–8,11,12,42]. However, MSM in the IPERGAY trial had high reductions in HIV incidence [5], demonstrating that nondaily oral PrEP can be effective.
One limitation of this analysis is the small number of studies reporting sexual activity in terms of sex-days per week. This sexual activity metric is the most relevant for nondaily PrEP use but it is not routinely measured (e.g. it was not measured in IPERGAY). In addition, because of a lack of data on the full distribution of sex-days, we used the median frequency of sex-days/week in our cost-reduction estimates to produce comparable estimates across different settings, which may have led to inaccuracies. Sensitivity analysis suggests that this may have underestimated the cost-savings possible with the IPERGAY regimen because of the large proportion of men not reporting any sex in the last week who required no tablets.
This study reviewed the available data on sexual activity and forecasting relevant for nondaily PrEP, and analysed the cost-reductions of nondaily oral regimens in comparison with daily oral PrEP with 100% regimen adherence. It showed that large cost-reductions are possible for populations in high-income countries, although these might be partially attenuated for regimens requiring forecasting of sexual activity, if additional tablets unnecessary for protection are taken. Smaller cost-reductions were predicted for LMICs in Africa, because of the much lower cost of drugs. A lack of cost and behavioural data from the same countries precluded us from drawing conclusions for LMICs in other regions, such as Thailand.
The next step necessary for estimating cost-effectiveness of nondaily oral PrEP is to evaluate the effectiveness of these regimens, for which efficacy as well as adherence to nondaily PrEP will need to be taken into account [43,44].
Estimates for MSM suggest that four doses of oral PrEP per week are sufficient to provide a 96% reduction in HIV risk [45]. As higher regimen adherence is often seen for daily compared with nondaily PrEP, one way to reduce costs for MSM might be to provide blister packs alternating PrEP and nonactive tablets for daily use, akin to the female contraceptive pill. Alternatively, they could be prescribed two tablets on 2 days per week. These regimens could reduce program costs – and improve cost-effectiveness – without reducing effectiveness.
Trials of nondaily oral PrEP amongst women vaginally exposed to HIV have not been powered to detect efficacy, and daily dosing trials have suggested that six to seven PrEP doses per week are needed to provide 94% protection against HIV after vaginal sex [46,47]. Pharmacokinetic/pharmacodynamic modelling suggests that the IPERGAY PrEP regimen should provide good protection for women at the time of sex and for 72 h afterwards, but that drug concentrations in the female genital tract then decline rapidly, meaning that further postsex doses may be required for full protection [47]. Further studies are therefore required to determine whether, and under which regimens, nondaily PrEP could be protective for women.
In conclusion, we have shown that nondaily oral PrEP regimens could theoretically substantially reduce program costs compared with daily PrEP, particularly for populations in high-income countries having sex on average 1 day/week or less. However, lower adherence reported with nondaily regimens, and imperfect prediction of future sexual activity, may reduce PrEP effectiveness and/or increase costs. Full cost-effectiveness analysis will be necessary to evaluate the full benefits of nondaily oral PrEP regimens.
Acknowledgements
Author contributions: M.C.B. conceived the study; K.M.M., D.D., and J.P.H. conceived additional analyses; K.M.M. calculated required pill numbers, carried out systematic reviews, calculated cost savings, and wrote the first draft of the manuscript; J.P.H. and F.X. carried out statistical analysis of the HPTN 067/ADAPT data; K.R.A. HPTN 067 behavioural scientist; K.B. HPTN 067 leadership and operations center representative; A.C., L.G.B., T.H.H., S.M. HPTN 067 sites PIs; RMG HPTN 067 protocol chair; D.D., J.P.H., D.D., K.R.A., K.B., T.H.H., S.M., R.M.G., M.C.B. edited the manuscript; all authors saw and approved the final version of the manuscript.
Sources of funding: This work was supported by the HIV Prevention Trials Network (HPTN) Modelling Centre, which is funded by the US National Institutes of Health (grant number UM1 AI068617) through the HPTN Statistical and Data Management Center. The HPTN 067 trial was supported by the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health and the National Institute on Drug Abuse through the US National Institutes of Health (Award Numbers UM1 AI068619, UM1 AI068613).
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention, the National Institutes of Health, or the United States Department of Health and Human Services.
Conflicts of interest
K.R.A. has an education grant from Gilead Sciences through the University of Connecticut. Other authors have no conflicts of interest to declare.
Supplementary Material
References
- 1.Spinner CD, Boesecke C, Zink A, Jessen H, Stellbrink HJ, Rockstroh JK, et al. HIV pre-exposure prophylaxis (PrEP): a review of current knowledge of oral systemic HIV PrEP in humans. Infection 2016; 44:151–158. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS, The Henry J Kaiser Family Foundation. Donor government funding for low- and middle-income countries in 2016. 2017. [Google Scholar]
- 3.Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med 2013; 10:e1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cambiano V, Miners A, Phillips A. What do we know about the cost–effectiveness of HIV preexposure prophylaxis, and is it affordable?. Curr Opin HIV AIDS 2016; 11:56–66. [DOI] [PubMed] [Google Scholar]
- 5.Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 6.Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One 2012; 7:e33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kibengo FM, Ruzagira E, Katende D, Bwanika AN, Bahemuka U, Haberer JE, et al. Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected Ugandan volunteers living in HIV-serodiscordant relationships: a randomized, clinical trial. PLoS One 2013; 8:e74314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekker LG, Roux S, Sebastien E, Yola N, Amico KR, Hughes JP, et al. Daily and nondaily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. Lancet HIV 2017; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Lerma JG, Paxton L, Kilmarx PH, Heneine W. Oral pre-exposure prophylaxis for HIV prevention. Trends Pharmacol Sci 2010; 31:74–81. [DOI] [PubMed] [Google Scholar]
- 10.Division of AIDS (DAIDS), US National Institute of Allergy and Infectious Diseases (NIAID), US National Institutes of Mental Health (NIMH), US National Institites of Health (NIH). HPTN 067 the ADAPT study: a phase II, randomized, open-label, pharmacokinetic and behavioral study of the use of intermittent oral emtricitabine/tenofovir disoproxil fumarate pre-exposure prophylaxis (PrEP). Protocol version 5.0. 2013. [Google Scholar]
- 11.Holtz TH, Chitwarakorn A, Curlin ME, Hughes J, Amico KR, Hendrix C, et al. HPTN 067/ADAPT study: a comparison of daily and nondaily pre-exposure prophylaxis dosing in Thai men who have sex with men, Bangkok, Thailand. J Int AIDS Soc 2015; 18:25–26. [Google Scholar]
- 12.Mannheimer S, Hirsch-Moverman Y, Loquere A, Franks J, Hughes J, Ou S-S, et al. HPTN 067/ADAPT study: a comparison of daily and intermittent preexposure prophylaxis dosing for HIV prevention in men who have sex with men and transgender women in New York city. J Int AIDS Soc 2015; 18:24–25. [Google Scholar]
- 13.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong KJ, Desai S, Desai M, Nardone A, van Hoek AJ, Gill ON. Cost and cost-effectiveness of an HIV pre-exposure prophylaxis (PrEP) programme for high-risk men who have sex with men in England: results of a static decision analytical model. Lancet 2015; 386:S16. [Google Scholar]
- 15.Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy 2010; 6:51–59. [Google Scholar]
- 16.World Bank. World Bank country and lending groups. 2017. [Google Scholar]
- 17.Lorente N, Fugon L, Carrieri MP, Andreo C, Le Gall JM, Cook E, et al. Acceptability of an ‘on-demand’ preexposure HIV prophylaxis trial among men who have sex with men living in France. AIDS Care 2012; 24:468–477. [DOI] [PubMed] [Google Scholar]
- 18.Parsons JT, Rendina HJ, Grov C, Ventuneac A, Mustanski B. Accuracy of highly sexually active gay and bisexual men's predictions of their daily likelihood of anal sex and its relevance for intermittent event-driven HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2015; 68:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volk JE, Liu A, Vittinghoff E, Irvin R, Kroboth E, Krakower D, et al. Sexual frequency and planning among at-risk men who have sex with men in the United States: implications for event-based intermittent pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2012; 61:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Griensven F, Thienkrua W, Sukwicha W, Wimonsate W, Chaikummao S, Varangrat A, et al. Sex frequency and sex planning among men who have sex with men in Bangkok, Thailand: implications for pre- and post-exposure prophylaxis against HIV infection. J Int AIDS Soc 2010; 13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark D, Amico KR, Wallace M, Roux S, Grant R, Wood R, et al. Acceptability of oral intermittent pre-exposure prophylaxis as a biomedical HIV prevention strategy: results from the South African ADAPT (HPTN 067) Preparatory Study. J Int AIDS Soc 2012; 15:141. [Google Scholar]
- 22.Juusola JL, Brandeau ML, Owens DK, Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med 2012; 156:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horberg M, Raymond B. Financial policy issues for HIV preexposure prophylaxis: cost and access to insurance. Am J Prev Med 2013; 44:S125–128. [DOI] [PubMed] [Google Scholar]
- 24.Ouellet E, Durand M, Guertin JR, LeLorier J, Tremblay CL. Cost effectiveness of ’on demand’ HIV pre-exposure prophylaxis for noninjection drug-using men who have sex with men in Canada. Can J Infect Dis Med Microbiol 2015; 26:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider K, Gray RT, Wilson DP. A cost-effectiveness analysis of HIV preexposure prophylaxis for men who have sex with men in Australia. Clin Infect Dis 2014; 58:1027–1034. [DOI] [PubMed] [Google Scholar]
- 26.Mabileau G, Schwarzinger M, Flores J, Patrat C, Luton D, Epelboin S, et al. HIV-serodiscordant couples desiring a child: ’treatment as prevention,’ preexposure prophylaxis, or medically assisted procreation?. Am J Obstet Gynecol 2015; 213:341.e1–341.e12. [DOI] [PubMed] [Google Scholar]
- 27.Gomez GB, Borquez A, Caceres CF, Segura ER, Grant RM, Garnett GP, et al. The potential impact of pre-exposure prophylaxis for HIV prevention among men who have sex with men and transwomen in Lima, Peru: a mathematical modelling study. PLoS Med 2012; 9:e1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pretorius C, Stover J, Bollinger L, Bacaër N, Williams B. Evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS One 2010; 5:e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cremin I, Alsallaq R, Dybul M, Piot P, Garnett G, Hallett TB. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS 2013; 27:447–458. [DOI] [PubMed] [Google Scholar]
- 30.Stover J, Hallett TB, Wu Z, Warren M, Gopalappa C, Pretorius C, et al. How can we get close to zero? The potential contribution of biomedical prevention and the investment framework towards an effective response to HIV. PLoS One 2014; 9:e111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walensky RP, Jacobsen MM, Bekker LG, Parker RA, Wood R, Resch SC, et al. Potential clinical and economic value of long-acting preexposure prophylaxis for South African women at high-risk for HIV infection. J Infect Dis 2016; 213:1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of oral pre-exposure prophylaxis in a portfolio of prevention programs for injection drug users in mixed HIV epidemics. PLoS One 2014; 9:e86584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols BE, Baltussen R, van Dijk JH, Thuma PE, Nouwen JL, Boucher CA, et al. Cost-effectiveness of PrEP in HIV/AIDS control in Zambia: a stochastic league approach. J Acquir Immune Defic Syndr 2014; 66:221–228. [DOI] [PubMed] [Google Scholar]
- 34.Nichols BE, Boucher CA, van Dijk JH, Thuma PE, Nouwen JL, Baltussen R, et al. Cost-effectiveness of pre-exposure prophylaxis (PrEP) in preventing HIV-1 infections in rural Zambia: a modeling study. PLoS One 2013; 8:e59549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiragu M, Kofi H, Wanjala S, Otsio L, Resch SC, Hecht R, et al. Prospective cost of implementing oral HIV preexposure prophylaxis among MSM and young women at risk of HIV in Kenya [abstract number A-641-0439-07017]. 20th International AIDS Conference. Melbourne, Australia; 2014. [Google Scholar]
- 36.Mitchell KM, Lépine A, Terris-Prestholt F, Torpey K, Khamofu H, Folayan MO, et al. Modelling the impact and cost-effectiveness of combination prevention amongst HIV serodiscordant couples in Nigeria. AIDS 2015; 29:2035–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying R, Sharma M, Heffron R, Celum CL, Baeten JM, Katabira E, et al. Cost-effectiveness of pre-exposure prophylaxis targeted to high-risk serodiscordant couples as a bridge to sustained ART use in Kampala, Uganda. J Int AIDS Soc 2015; 18:20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying R, Heffron R, Celum C, Baeten J, Katabira E, Bulya N, et al. Cost-effectiveness of preexposure prophylaxis for high-risk HIV-discordant couples [abstract number 1106]. CROI. Seattle, US; 2015. [Google Scholar]
- 39.Clinton Health Access Initiative. 2016 antiretroviral (ARV) CHAI reference price list. 2016. [Google Scholar]
- 40.World Health Organization. Global price reporting mechanism. Geneva: World Health Organization; 2015. [Google Scholar]
- 41.Curran K, Mugo NR, Kurth A, Ngure K, Heffron R, Donnell D, et al. Daily short message service surveys to measure sexual behavior and pre-exposure prophylaxis use among Kenyan men and women. AIDS Behav 2013; 17:2977–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Elst EM, Mbogua J, Operario D, Mutua G, Kuo C, Mugo P, et al. High acceptability of HIV pre-exposure prophylaxis but challenges in adherence and use: qualitative insights from a phase I trial of intermittent and daily PrEP in at-risk populations in Kenya. AIDS Behav 2013; 17:2162–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimitrov DT, Masse BR, Donnell D. PrEP adherence patterns strongly affect individual HIV risk and observed efficacy in randomized clinical trials. J Acquir Immune Defic Syndr 2016; 72:444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimitrov DT, Mitchell KM, Hughes JP, Donnell D, Bekker L-G, Grant R, et al. Modeled effectiveness of nondaily PrEP based on sex coverage data from HPTN 067/ADAPT [abstract number 1055]. CROI. Boston, US; 2016. [Google Scholar]
- 45.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landovitz RJ. Preexposure prophylaxis for HIV prevention: what we know and what we still need to know for implementation. Top Antivir Med 2015; 23:85–90. [PMC free article] [PubMed] [Google Scholar]
- 47.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016; 214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


