Abstract
Objective
To evaluate dolutegravir pharmacokinetics during pregnancy compared to postpartum and in infant washout samples after delivery.
Design
Ongoing, nonrandomized, open-label, parallel-group, multi-center phase-IV prospective study of antiretroviral pharmacokinetics in HIV-infected pregnant women and infants.
Methods
Intensive steady-state 24 hour pharmacokinetic profiles after dolutegravir 50mg once-daily were performed during the second trimester (2T), third trimester (3T) and postpartum (PP). Infant samples were collected after birth. Dolutegravir was measured by validated LC-MS/MS; quantitation limit was 0.005mcg/mL. A two-tailed Wilcoxon signed-rank test (α = 0.10) was employed for paired within-subject comparisons.
Results
Twenty-nine enrolled participants had a median age of 32 years (range 21–42). Pharmacokinetic data were available for 15 (2T), 28 (3T) and 23 (PP) women. Median dolutegravir AUC0-24, Cmax and C24 were 25–51% lower in the 2T and 3T compared to PP. The median cord blood/maternal plasma concentration ratio was 1.25 (n=18). In 21 infants, median elimination half-life was 32.8 hours after in utero exposure. Viral load at delivery was <50 copies/mL for 27/29 women (93%). Twenty-nine infants were HIV-negative. Renal abnormalities noted on ultrasound in two infants were deemed possibly related to dolutegravir.
Conclusions
Dolutegravir exposure is lower in pregnancy compared to postpartum in the same women on once-daily dosing. Median AUC0-24 during pregnancy was similar to, while trough concentrations were lower than, those seen in non-pregnant adults. Trough concentrations in pregnancy were well above dolutegravir EC90 (0.064mcg/mL). Dolutegravir readily crosses the placenta. Infant elimination is prolonged, with half-life over twice that of historical adult controls.
Keywords: dolutegravir, Tivicay ™, integrase inhibitor, HIV infection, pharmacokinetics, perinatal transmission
Introduction
Integrase strand transfer inhibitors (INSTI) are a potent class of antiretrovirals that block the action of integrase, a viral enzyme which inserts the viral genome into the DNA of the host cell. This class is currently recommended as first-line treatment for antiretroviral-naïve adults and children in the U.S. living with HIV[1,2]. While highly effective and well-tolerated antiretroviral treatment options continue to increase, pharmacokinetic and safety data on newer agents for use in pregnant women remain sparse.
Pregnant women living with HIV use antiretrovirals for their own health and to prevent perinatal HIV transmission. Physiologic changes during pregnancy may substantially impact medication exposure[3]. Drugs metabolized by the cytochrome P450 3A (CYP3A) enzyme family, such as protease inhibitors, often show decreased exposure during pregnancy[4–6]. Exposure to other antiretrovirals may be unchanged or even increased during pregnancy[7–13]. Lower antiretroviral exposure increases the risk of inadequate maternal viral suppression and transmission of HIV to the infant, while increased drug exposure may subject mother and child to increased risk of drug toxicities.
Dolutegravir is a second generation INSTI, primarily metabolized by uridine diphosphate (UDP)-glucuronosyl transferase 1A1 (UGT1A1) with minor contributions from CYP3A[14]. Raltegravir, a first-generation INSTI also metabolized by UGT1A1, displayed decreased exposure during pregnancy by 29% and 50% in two prior studies[15,16]. Several case reports described dolutegravir exposure in pregnancy[17–19]. One reported pharmacokinetics over 8 hours in one pregnant woman, and in cord blood from two women at delivery who were taking dolutegravir 50mg twice daily[19]. No studies have described dolutegravir pharmacokinetics with standard dosing (50mg once daily) during pregnancy. The primary objective of this study was to determine dolutegravir 50mg once daily pharmacokinetics in pregnant and postpartum women living with HIV, and infant washout pharmacokinetics after birth.
Methods
Study population and design
IMPAACT P1026s “Pharmacokinetic Properties of Antiretroviral and Related Drugs during Pregnancy and Postpartum” (ClinicalTrials.gov NCT00042289), is an ongoing non-randomized, open-label, parallel-group, multi-center, phase IV prospective study. The study recruited pregnant HIV-infected women receiving dolutegravir 50mg once daily prescribed for clinical care in the U.S. Participants had to be stable on their antiretroviral regimen for two weeks, and intend to continue the same regimen through 6–12 weeks postpartum. Maternal exclusion criteria were multiple gestation, a clinical or laboratory toxicity necessitating a medication change during the study, and the use of specific medications known to interact with dolutegravir.
Each study site received ethical and local institutional review board approval. All subjects gave informed consent prior to study participation. Medications were prescribed by each participant’s clinical care provider. Pharmacokinetic sampling was performed during the second trimester, third trimester, and postpartum, if possible. Collected samples were assayed in real time and results reported to each study participant and her clinician.
Infant enrollment occurred immediately after maternal enrollment with maternal consent, with eligibility confirmed at birth. Infant inclusion criteria were birth weight >1,000g, singleton delivery and maternal enrollment in P1026s. Infant exclusion criteria were a severe congenital malformation or medical condition that would interfere with study participation as deemed by site clinicians and use of specific medications known to interfere with dolutegravir disposition.
Clinical and laboratory monitoring
Each study visit included monitoring of HIV-1 RNA, CD4+ lymphocyte cell count, hematology, and serum biochemistry. The lower limit of detection for HIV-1 RNA assays performed locally ranged from 50–400 copies/mL. All infants received physical examinations after birth and laboratory evaluations were performed if clinically indicated. Adverse events were reported at each study visit and toxicity management was determined by each participant’s clinician.
Sample collection
Intensive 24-hour pharmacokinetic evaluations occurred at second trimester (20–26 weeks gestation), third trimester (30–38 weeks gestation) and 6–12 weeks following delivery. Requirements prior to pharmacokinetic sampling were self-reported dolutegravir adherence for two weeks and consistent dosing times for the last three days. Dolutegravir was dosed without regard to meals. On sampling days the pre-dose sample was drawn and dolutegravir was administered under observation. Post-dose samples were drawn at 1, 2, 4, 6, 8, 12 and 24 hours. At delivery, cord and maternal blood samples were collected. In infants, four blood samples were collected at 2–10 hours, 18–28 hours, 36–72 hours and 5–9 days after birth.
Dolutegravir concentration assays
Dolutegravir samples were analyzed at the IMPAACT Pharmacology Support Laboratory at the University of Alabama at Birmingham using a sensitive liquid chromatography tandem mass spectrometry assay validated to quantitate total dolutegravir concentrations in human plasma samples[20]. Briefly, this method requires only a 20 μL aliquot of human plasma and has a dynamic range of 0.005–10 mcg/mL. Samples are subjected to a simple acetonitrile protein precipitation containing a dolutegravir stable isotope as an internal standard. The laboratory adheres to Clinical Laboratory Improvement Amendments (CLIA) and performs standardized interlaboratory testing though the AIDS Clinical Trial Group (ACTG) clinical pharmacology quality assurance and quality control program.
Pharmacokinetic analyses
Dolutegravir maximum, minimum, and last plasma concentrations (Cmax, Cmin, C24) along with corresponding time points (Tmax, Tmin) were observed directly. Steady-state area under the concentration versus time curve from time 0 to 24 hours post-dose (AUC0-24) was estimated with the trapezoidal rule. Half-life (t1/2) was calculated as 0.693/λz; λz is the elimination rate constant derived from the terminal slope of the log concentration versus time curve. For participants with pre-dose concentrations below the assay quantification limit (indicating probable non-adherence), single-dose AUC from time 0 to infinity was estimated as AUC0-24 plus the C24 divided by λz. Apparent oral clearance (CL/F) was calculated as dose divided by AUC0-24. Undetectable concentrations were set at half the lower limit of quantification to calculate summary statistics. The 50th percentile AUC0-24 in non-pregnant adults was 53.6mcg*hr/mL and the minimal exposure target was the 10th percentile AUC0-24, 37.5mcg*hr/mL, which was approximated from published pharmacokinetic parameters[14].
Statistical analyses
The target sample size was 25 women with evaluable third trimester pharmacokinetic data, with at least 12 who had evaluable second trimester pharmacokinetic data. Each individual woman’s dolutegravir exposure during pregnancy was determined in real time, compared with the 50th and 10th percentile AUCs estimated for non-pregnant adult historical controls, and reported to each participant’s care provider[14]. A stopping criterion to evaluate the adequacy of drug exposure was predefined as 6/25 women (24%; exact 80% confidence limits: 13%–38%) falling below the target AUC. The goal was to prevent excess accrual to a cohort with known inadequate exposure. The statistical rationale for this early stopping criterion was previously described[4].
Descriptive statistics were calculated for pharmacokinetic parameters during each study period. Ninety percent confidence limits of the geometric mean ratio of dolutegravir pharmacokinetic parameters in the pregnant versus non-pregnant conditions were calculated. If the confidence limits exclude 1.0, the pharmacokinetic exposure parameter is significantly lower (or higher) in one condition than in the other, with 1-sided P value of 0.05 (2-sided P value of 0.10). Pharmacokinetic parameters during the second trimester versus postpartum and during the third trimester versus postpartum were also compared at within-subjects using the Wilcoxon signed-rank test, with P<0.10 considered statistically significant. Nominal variables were compared with Fisher Exact (including all participants) and McNemar (paired data only) tests.
Results
Subject Characteristics
Twenty-nine pregnant women taking dolutegravir 50mg once-daily enrolled in the study. Paired postpartum data were available for twelve of fifteen women who had second trimester visits, and for twenty-two who had third trimester visits. Participant demographic and clinical characteristics are summarized in Table 1. Twenty-nine live born infants had a median (interquartile range; IQR) birth weight of 3090g (1480–4000). Infant birth characteristics are summarized in Table 1. All infants were confirmed eligible at birth; none met the infant exclusion criteria.
Table 1.
Participant Demographics
| N/Total N (%) or Median (Range) | |
|---|---|
| Race/Ethnicity: | |
| Black Non-Hispanic | 21/29 (72.4) |
| Hispanic | 3/29 (10.3) |
| White Non-Hispanic | 3/29 (10.3) |
| Other | 2/29 (6.9) |
|
| |
| Age at Third Trimester Visit (years) | 32 (21 – 42) |
|
| |
| Weight at Second Trimester Visit (kg) | 81.8 (46.8 – 138.5) |
| Weight at Third Trimester Visit (kg) | 84.9 (51.4 – 141.1) |
| Gestational Age at Second Trimester Visit (weeks) | 23.9 (20.0 – 26.4) |
| Gestational Age at Third Trimester Visit (weeks) | 33.7 (30.1 – 37.6) |
| Duration of Dolutegravir Therapy at Third Trimester Visit (weeks) | 19 (3.6 – 195) |
| Weeks after Delivery at Postpartum Visit | 10 (6 – 32) |
|
| |
| Concomitant Antiretroviral Regimens: | |
| Abacavir/Lamivudine | 20/29 (69.0) |
| Emtricitabine/Tenofovir disoproxil fumarate | 5/29 (17.2) |
| Lamivudine/Zidovudine | 1/29 (3.4) |
| Rilpivirine/Atazanavir/Ritonavir | 1/29 (3.4) |
| Emtricitabine/Tenofovir disoproxil fumarate/Zidovudine | 1/29 (3.4) |
| Emtricitabine/Maraviroc | 1/29 (3.4) |
|
| |
| HIV-1 RNA at Second Trimester (copies/mL) | <20 (<20 – <20) |
| < 50 copies/mL | 14/14 (100) |
|
| |
| HIV-1 RNA at Third Trimester (copies/mL) | <20 (<20 – 42) |
| < 50 copies/mL | 28/28 (100) |
|
| |
| HIV-1 RNA at Delivery (copies/mL) | <20 (<20 – 870) |
| < 50 copies/mL | 27/29 (93.1) |
|
| |
| HIV-1 RNA at Postpartum (copies/mL) | <20 (<20 – 36,926) |
| < 50 copies/mL | 14/19 (73.7) |
|
| |
| Infant Gestational Age at Birth (weeks) | 38.9 (34.9 – 42.3) |
| Number of Preterm Infants (< 37 weeks) | 4 (14) |
| Infant Weight at Birth (grams) | 3090 (1480 – 4000) |
| Number of Low Birth Weight Infants (< 2500 grams) | 4 (14) |
| Number of Small for Gestational Age Infants | 5 (17) |
| Number of Large for Gestational Age Infants | 2 (7) |
| Infant Length at Birth (cm) | 50 (40.7 – 71.1) |
|
| |
| Infant Infection Status: | |
| Negative Based on Best Available Data | 24/29 (82.8) |
| Indeterminate | 5/29 (17.2) |
Dolutegravir Pharmacokinetics
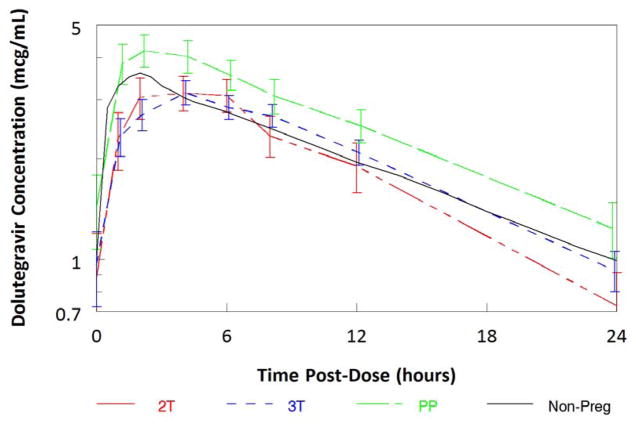
Median (IQR) dolutegravir AUC0-24 in the second trimester, third trimester and postpartum periods were 47.6mcg*hr/mL (33.4–63.7), 49.2mcg*hr/mL (36.4–62.0) and 65mcg*hr/mL (47.8–88.4), respectively. Compared to paired postpartum data, dolutegravir AUC0-24 was 29% lower in the third trimester (n=22, p=0.0003; Table 2, Figure 1) and 37% lower in the second trimester (n=12, p=0.002). The frequency of participants meeting the estimated AUC0-24 10th percentile (37.5mcg*hr/mL) was 9/15 (60%) in the second trimester (p=0.0018 compared to postpartum), 20/28 (71%) in the third trimester (p=0.0057 compared to postpartum) and 23/23 (100%) postpartum. Excluding non-paired data, 7/12 (58%) in the second trimester (p=0.0625 compared to postpartum) and 16/22 (73%) in the third trimester (p=0.0313 compared to postpartum) participants met the estimated 10th percentile AUC0-24.
Table 2.
Maternal Dolutegravir Pharmacokinetic Parameters, Median (IQR)
| Parameter | Second Trimester n = 15 |
Third Trimester n = 28 |
Postpartum n = 22 |
Historical Control1 | Geometric Mean Ratio2 (90% CI) 2T/PP, n=12 |
Geometric Mean Ratio2 (90% CI) 3T/PP, n=22 |
|---|---|---|---|---|---|---|
| AUC0-24 (mcg* hr/mL) | 47.6 (33.4 – 63.7)* | 49.2 (36.4 – 62.0)* | 65.0 (47.8 – 88.4) | 53.6 (27) | 0.63 (0.52–0.75) | 0.71 (0.63–0.81) |
| C0 (mcg/mL) | 0.88 (0.56 – 1.58)* | 0.96 (0.61 – 1.63) | 1.54 (0.98 – 2.59) | - | 0.75 (0.20–2.77) | 1.17 (0.51–2.70) |
| Cmax (mcg/mL) | 3.62 (2.57 – 4.63)* | 3.54 (2.66 – 4.24)* | 4.85 (3.83 – 5.97) | 3.67 (20) | 0.74 (0.61–0.89) | 0.75 (0.64–0.88) |
| Tmax (hr) | 2 (2 – 4) | 3 (2 – 4) | 2 (2 – 4) | 2 to 3 | - | - |
| C24 (mcg/mL) | 0.73 (0.63 – 1.34)* | 0.93 (0.68 – 1.34)* | 1.28 (0.80 – 1.95) | - | 0.49 (0.35–0.68) | 0.66 (0.52–0.84) |
| Cmin (mcg/mL) | 0.69 (0.56 – 1.28)* | 0.81 (0.55 – 1.31)* | 0.97 (0.70 – 2.06) | 1.11 (46) | 0.86 (0.24–3.05) | 1.14 (0.51–2.55) |
| CL/F (L/hr) | 1.05 (0.79 – 1.50)* | 1.02 (0.81 – 1.37)* | 0.77 (0.57 – 1.05) | 1 | 1.60 (1.33–1.93) | 1.40 (1.24–1.59) |
| T1/2 (hr) | 11.0 (8.9 – 13.1)* | 12.2 (10.4 – 15.0)* | 13.5 (10.6 – 18.6) | 14 | 0.74 (0.60–0.91) | 0.91 (0.79–1.05) |
p<0.10 compared to postpartum
Historical data from Tivicay™ (Dolutegravir) package insert, represented as geometric mean (%CV)
Paired comparisons
Figure 1.
Maternal median (± standard error of the median) dolutegravir concentrations versus time post-dose.
Dolutegravir Cmax was 26% lower in the second trimester and 25% lower in the third trimester compared to paired postpartum data (p=0.0098, p=0.0025). Dolutegravir C24 was 51% lower in the second trimester and 34% lower in the third trimester compared to paired postpartum data (p=0.0039, p=0.0062). Pre-dose concentrations below the quantitation limit were seen in two participants at the postpartum visit, suggesting recent non-adherence.
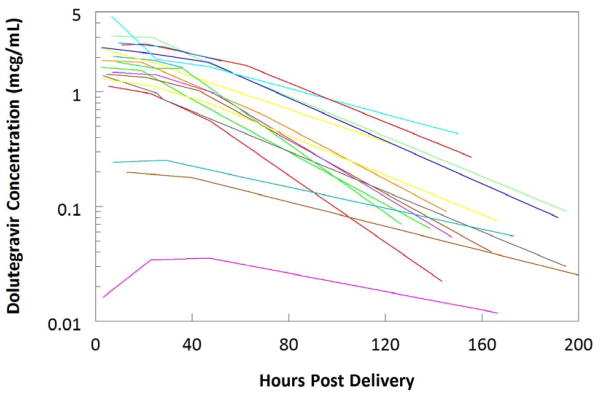
Median (IQR) concentrations of dolutegravir in cord blood and maternal plasma at delivery were 1.67mcg/mL (1.17–2.00) and 1.24mcg/mL (0.57–1.68), respectively. The ratio of cord blood to maternal plasma was 1.25 (1.07–1.40). In infants after birth, median dolutegravir Cmax was 1.85mcg/mL (1.42–2.48) at a median 6.9 hours (3.5–9.2). Concentrations through 9 days of life for all infants are shown in Table 3 and Figure 2. At the final washout sample (between 5–9 days of life), all samples still had measurable dolutegravir (>0.005mcg/mL). One of sixteen infants providing washout pharmacokinetic data was breast fed but dolutegravir concentration in breast milk was not assayed. Plasma dolutegravir concentrations in this infant were 4.57mcg/mL, 1.94mcg/mL, 1.66mcg/mL, and 0.44mcg/mL at 7, 25, 46, and 150 hours after delivery. Excluding the breast fed infant in whom half-life cannot be reliably calculated, the median (IQR) dolutegravir half-life was 32.8 hours (25.9–35.9).
Table 3.
Dolutegravir Exposure at Delivery and in Infants, Median (IQR)
| At Delivery, n=23 | Median (IQR) | n |
|---|---|---|
| Cord Blood (mcg/mL) | 1.67 (1.17 – 2.00) | 19 |
| Maternal Plasma (mcg/mL) | 1.24 (0.57 – 1.68) | 23 |
| Cord Blood/Maternal Plasma Ratio | 1.25 (1.07 – 1.40) | 18 |
| Infant Washout Samples after Delivery, n=22 | ||
| Cmax (mcg/mL) | 1.64 (1.31 – 2.38) | 18 |
| T1/2 (hr)* | 32.8 (25.9 – 35.9) | 16 |
| Concentration (2 – 10 hours, mcg/mL) | 1.73 (1.33 – 2.41) | 17 |
| Concentration (18 – 28 hours, mcg/mL) | 1.53 (1.04 – 1.91) | 18 |
| Concentration (36 – 72 hours, mcg/mL) | 1.00 (0.66 – 1.65) | 17 |
| Concentration (5 – 9 days, mcg/mL) | 0.06 (0.04 – 0.14) | 17 |
excludes the breast-fed infant
Figure 2.
Individual infant dolutegravir concentrations versus time post-delivery.
Maternal and Infant Outcomes
Eight women reported grade 3 adverse events. These included low hemoglobin (n=3), pre-eclampsia (n=2), and pre-term labor, nausea/vomiting, cesarean wound infection/fever, blurry vision/headache, low albumin, and proteinuria (n=1 for each). In the third trimester, 28/28 (100%) of maternal participants had an HIV-1 RNA ≤50 copies/mL. At delivery, 27/29 (93%) of maternal participants had an HIV-1 RNA ≤50 copies/mL and one participant had a viral load over 400 copies/mL (Table 1). The two women with detectable viral loads at delivery had dolutegravir exposure at the third trimester approximately equal to the median exposure for the group at that time point. They had been receiving dolutegravir for 22 weeks and 42 weeks. One woman had decreased but still detectable HIV-1 RNA at the postpartum visit, and the other woman was undetectable at the postpartum visit. At postpartum pharmacokinetic sampling, 14/19 (74%) maternal participants had an HIV-1 RNA ≤50 copies/mL.
Infants were born at a median of 38.9 weeks of gestation (range: 34.9–42.3). Four infants were pre-term (< 37 weeks gestation), five were small for gestational age, and four were low birth weight (< 2500 grams), with one of those four being very low birth weight (< 1500 grams). All were HIV-negative based on best available data, with twenty-four infants confirmed uninfected and 5 indeterminate due to incomplete testing.
Clinical abnormalities were noted at or shortly after birth in seven infants. The clinical findings in three of these infants were considered to be normal variants-one each with congenital filum terminale fibrolipoma, two vessel umbilical cord without other abnormalities, and post-axial polydactyly (supernumerary digit). These findings were considered unrelated to dolutegravir exposure. Total anomalous pulmonary venous return was diagnosed in one infant. This infant’s mother began dolutegravir treatment during week 16 of gestation and the cardiac abnormality was judged unrelated to dolutegravir exposure. One infant was noted to have increased jitteriness and chin tremors which resolved over the first months of life; these neurologic findings were considered unrelated to dolutegravir exposure. Renal abnormalities diagnosed by ultrasound in two infants were deemed possibly related to dolutegravir exposure. One infant, whose mother began dolutegravir treatment during week 12 of gestation, had an isolated renal cyst in the left kidney. Another infant, whose mother began dolutegravir treatment during week 11 of gestation, had a multicystic dysplastic right kidney.
Other adverse events were reported in five infants. Two infants had transient low blood glucose shortly after birth. The infant with the multicystic dysplastic right kidney was also diagnosed with cystic fibrosis and experienced numerous adverse events over the first months of life, including anemia, MRSA bacteremia, enterovirus bronchiolitis, malnutrition, pleural effusion, pneumonia and respiratory failure. One infant had neonatal abstinence syndrome following maternal opiate use during pregnancy and respiratory failure. A fifth infant was diagnosed with sickle cell trait on routine newborn screening.
Discussion
Increasing use of INSTIs as first-line treatment regimens among adults living with HIV will lead to more pregnancies occurring in women on dolutegravir-containing regimens. This study is the first to analyze the pharmacokinetics of dolutegravir 50mg once-daily dosing in a large population of pregnant women. Paired data demonstrated a 29% decrease in dolutegravir AUC0-24 in the third trimester compared to postpartum. Second trimester AUC0-24 was also lower than postpartum. Both second and third trimester Cmax and C24 were significantly lower than paired postpartum data by 25–51%. The general risk of decreased antiretroviral exposure is failure to suppress viral replication. This study designated sub-therapeutic exposure less than the 10th percentile dolutegravir AUC0-24 (37.5mcg*hr/mL) of non-pregnant adults. Significantly fewer participants met this minimum threshold during pregnancy (60% in second trimester, 71% in third trimester) compared to postpartum (100% postpartum). Although all women had HIV-1 RNA less than 50 copies/mL at the time of the second and third trimester pharmacokinetic sampling, two women had detectable viral loads at delivery. One of these women had a lower but still detectable viral load postpartum, while the other woman had an undetectable viral load postpartum.
Even though exposure was lower during pregnancy compared to postpartum, median AUC during pregnancy in this study (47–49mcg*hr/mL) were similar to the reported values in non-pregnant adults (53.6mcg*hr/mL)[14]. Also, postpartum AUC and maximum concentrations in this study were higher than reported values in non-pregnant adults; the significant differences seen between pregnancy and postpartum were in part due to the higher than expected dolutegravir exposures postpartum. This increased exposure postpartum has been noted with other antiretrovirals[15,21–22]. The postpartum visits were a median of 10 weeks after delivery. This time frame post-delivery may not represent “non-pregnant adults”, as physiologic changes that could affect pharmacokinetics of medications may happen abruptly or slowly postpartum and can variably rebound in the opposite direction of pregnancy changes. For example, cardiac output takes 6–12 weeks to return to non-pregnant levels, and kidneys usually take 2–3 months (sometimes up to 4 months) to return to normal, non-pregnant function[23].
Trough concentrations in this study during pregnancy (0.73 and 0.93mcg/mL) were somewhat lower than those reported in non-pregnant adults (1.11mcg/mL). However, even these lower values are still approximately 11–14 times higher than the reported dolutegravir in vitro protein-adjusted 90% effective concentrations (EC90) of 0.064mcg/mL[24]. Notably, the Phase I and Phase II studies of dolutegravir found similar high efficacy rates with doses ranging from 10mg to 50mg[24,25]. Geometric mean trough concentrations with 10mg once-daily dosing for 10 – 14 days are approximately 0.2–0.3 mcg/mL[24,25]. The troughs seen in this study during pregnancy were still about 4 times higher than those with 10mg daily doses that have shown similar efficacy to the 50mg dose in prior clinical studies. During pregnancy, none of the women in this study had dolutegravir concentrations below the quantitative limit of the assay, 0.005mcg/mL, and no infants acquired HIV infection from their mothers.
Dolutegravir metabolism is primarily dictated by UGT1A1 activity with minor CYP3A contributions[14]. UGT1A1 enzymes can be found in the liver, lungs, intestine mucosa, brain, uterus and placenta[26]. Progesterone-induced UGT1A1 expression and the resulting net increase in metabolism, along with increased CYP 3A activity, may explain decreased dolutegravir concentrations during pregnancy[27][28]. This study found significantly shorter half-lives and decreased trough dolutegravir concentrations during pregnancy compared to postpartum, consistent with significantly increased clearance. However, half-lives are difficult to accurately estimate in a multiple-dose study with sampling in the elimination phase over less than two half-lives. Dolutegravir is highly bound to plasma proteins[14] but may be displaced by increased hormone binding to these proteins in pregnancy or may have higher unbound concentrations due to lower albumin concentrations in pregnancy. The fraction of unbound dolutegravir mediates the therapeutic action of this antiretroviral, but unbound concentrations were not measured in this study. Though this study observed lower total concentrations in pregnancy compared to postpartum, the therapeutic free fraction of dolutegravir may be increased, stable or decreased during pregnancy.
This study determined the washout kinetics of dolutegravir transferred in utero across the placenta in these infants born to mothers receiving dolutegravir during pregnancy. The median ratio of the dolutegravir concentration in cord blood/maternal plasma at delivery was 1.25, suggesting high placental transfer of dolutegravir consistent with another small study[29]. Elimination of dolutegravir in these newborns was prolonged, with a median elimination half-life in 16 infants of 33 hours compared to a median maternal postpartum elimination half-life of 13.5 hours. These findings are consistent with a case report of dolutegravir pharmacokinetics in a pregnant woman that described a four-fold increase in elimination half-life in the infant compared to the mother[17].
In neonates, although UGT1A1 is found in equal concentrations to adults, its activity is low at birth and only reaches adult levels at 3.8 months[30]. Furthermore, UGT1A1 metabolism in adults and neonates is highly variable. Raltegravir, another INSTI metabolized by UGT1A1, had highly variable and extremely prolonged elimination in nineteen infants[31]. A common indicator of UGT1A1 activity is bilirubin. For instance, infants with UGT1A1 polymorphisms that decrease enzyme activity are at higher risk of hyperbilirubinemia which can lead to kernicterus[32]. This study did not measure bilirubin in infants so the correlation between dolutegravir elimination and UGT1A1 activity cannot be assessed this way. Metabolism of dolutegravir by CYP3A may not play a major role in extended dolutegravir elimination half-lives in infants. Neonatal CYP3A7 activity is high at birth and slowly decreases as adult CYP3A4 activity increases; CYP3A5 remains stable throughout development. This may result in stable CYP3A metabolism in infants compared to adults[33].
Although clinical abnormalities at birth were noted in seven infants, these findings were thought to be unrelated to dolutegravir exposure in five infants. Findings in three infants were considered normal variants. The mother of the infant with total anomalous pulmonary venous return did not start dolutegravir until week 16 of gestation, after cardiac development was complete, and so the cardiac anomaly was judged unrelated to fetal dolutegravir exposure. Renal abnormalities were found on ultrasound in two infants whose mothers began dolutegravir at the end of the first trimester, and these were the only birth abnormalities considered possibly related to dolutegravir exposure. The infant with the multicystic dysplastic kidney was also diagnosed with cystic fibrosis and had several other adverse events thought to be unrelated to dolutegravir exposure because they were consequences of cystic fibrosis. All of the other adverse events were judged not related to in utero dolutegravir exposure because they were either common newborn events (hypoglycemia), associated with maternal opiate use during pregnancy (neonatal abstinence syndrome) or was a genetic trait (sickle cell trait). Although our sample size is small for assessing adverse fetal effects from in utero drug exposure, our data are consistent with the lack of adverse fetal effects seen in preclinical reproductive toxicology studies of dolutegravir[14]. Our data are also consistent with the 83 prospective cases of dolutegravir exposure during pregnancy reported so far to the Antiretroviral Pregnancy Registry, which includes two reports of birth defects in 41 infants with first trimester dolutegravir exposure and one report of a birth defect in 42 infants with second trimester exposure[34].
One limitation of this study is that opportunistic recruitment of women already taking dolutegravir generally means that these women are responding to and not experiencing adverse effects from this regimen. This selection bias may overestimate positive outcomes and underestimate adverse events. This study was also limited to subjects taking dolutegravir 50mg once-daily, indicated for treatment-naïve or INSTI-naïve patients. Dolutegravir 50mg is taken twice-daily for patients with specific INSTI-associated resistance mutations or use of a potent inducer of UGT1A1 or CYP3A metabolism. Once-daily and twice-daily dolutegravir have a non-linear dose-concentration relationship among non-pregnant adults[14]. Therefore, these findings cannot be applied to patients on a twice-daily dolutegravir regimen. Additionally, dolutegravir was taken regardless of food to align with FDA label recommendations. However, low fat meals increase dolutegravir AUC0-24 by 33%, moderate fat meals increase AUC0-24 by 41%, and high fat meals increase AUC0-24 by 66%[35]. Therefore, dolutegravir dosing in relation to meals may have added greater variability to the pharmacokinetic data and statistical comparisons in this study.
Study limitations in our infant washout analysis included wide sampling windows, sparse pharmacokinetic time points and lack of correlation with bilirubin concentrations. Also, these results cannot be extrapolated to breastfeeding populations. Prior raltegravir studies identified a lag in elimination with raltegravir concentrations first increasing prior to decreasing in newly born infants[31]. Our sampling windows were too wide to capture this effect, so it is not known if dolutegravir also follows this pattern.
To conclude, exposure in pregnancy is decreased compared to postpartum, but standard once daily dosing during pregnancy provides exposure well above that needed to suppress viral replication. Women in this study experienced few adverse events and 93% had <50 copies/mL of HIV-RNA at delivery. Dolutegravir crosses the placenta easily, resulting in infant concentrations comparable to maternal plasma concentrations. Low UGT1A1 activity at birth may account for the greater than two-fold increase in dolutegravir elimination half-life in infants as compared to postpartum women. Of the clinical abnormalities observed at birth, only two infants with renal abnormalities, exposed to dolutegravir at the end of the first trimester, were considered possibly related to dolutegravir exposure. Additional data are needed on the use of dolutegravir in pregnant women to assess infant safety or to assess twice daily dosing. In summary, dolutegravir exposure is decreased in pregnancy compared to postpartum, but remains at therapeutic concentrations during pregnancy with standard once daily dosing.
Acknowledgments
We thank the study participants and their families.
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH).
All authors substantially contributed to interpretation of the data, critically revised the manuscript for important intellectual content, approved the final version submitted, and agree to be accountable for the accuracy and integrity of the work. B.M.B, J.W., E.V.C., A.S., E.B., E.S., N.C., S.B., and M.M. made substantial contributions to the conception and design of the work. A.S., E.B., S.L.B., S.B, and M.M. substantially contributed to acquisition of data. N.M., B.M.B, J.W., E.V.C., and E.P.A. substantially contributed to data analysis.
Appendix
In addition to the authors, members of the IMPAACT P1026s protocol team include Francesca Aweeka, Michael Basar, Emily Barr, Mark Byroads, Nantasak Chotivanich, Lisa M. Frenkel, Kathy George, Elizabeth Hawkins, Kathleen Adriane Hernandez, Amy Jennings, Rita Patel and Pra-ornsuda Sukrakanchana. We thank the participating staff and sites: Texas Children’s Hospital (Mary Paul, MD; Chivon McMullen-Jackson, RN, BSN; Norma Cooper, MA, BSN, ACRN); Lurie Children’s Hospital of Chicago (Donna McGregor, APN; Patricia Garcia, MD; Sarah Sutton, MD); University of Miami Pediatric Perinatal HIV/AIDS (Charles D. Mitchell, MD; Grace A. Alvarez A, M, MPH; Ernesto Ruiz Valdes, MD); Jacobi Medical Center (Michelle Giannone, MD; Laveena Kondagari, MD; Raphaelle Auguste, RN); University of Colorado Denver (Jenna Wallace, MSW, CCRP; Torri D. Metz, MD, MS; Kay Kinzie, MSN, FNP); Rush University Cook County Hospital Chicago (Julie Schmidt, MD; Maureen McNichols, RN, MSN, CCRC; Mariam Aziz, MD); Johns Hopkins University (Allison Agwu, MD, ScM, FAAP, FIDSA; Aleisha Collinson-Streng, RN, ACRN; Thuy Anderson, RN, BSN); David Geffen School of Medicine at UCLA (Jaime G. Deville, MD; Carla Janzen, MD, PhD; Michele F. Carter, BS, RN); St Jude Children’s Research Hospital (Luis M. Gomez, MD, MScE; Nina K. Sublette, PhD, APRN; Katherine M. Knapp, MD); University of Puerto Rico (Irma L. Febo MD; Carmen D. Zorrilla MD; Ruth Santos RN, MPH).
Footnotes
Conflicts of Interest and Sources of Funding:
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. No author conflicts were declared.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Section accessed February 26, 2017]. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 2.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. [Accessed February 26, 2017];Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 3.Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33:328–343. doi: 10.2165/00003088-199733050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 5.Acosta EP, Bardeguez A, Zorrilla CD, Van Dyke R, Hughes MD, Huang S, et al. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004;48:430–436. doi: 10.1128/AAC.48.2.430-436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stek A, Best BM, Wang J, Capparelli EV, Burchett SK, Kreitchmann R, et al. Pharmacokinetics of once versus twice daily darunavir in pregnant HIV-infected women. J Acquir Immune Defic Syndr. 2015;70:33–41. doi: 10.1097/QAI.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulligan N, Schalkwijk S, Best BM, Colbers A, Wang J, Capparelli EV, et al. Etravirine pharmacokinetics in HIV-infected pregnant women. Front Pharmacol. 2016;7:1–10. doi: 10.3389/fphar.2016.00239. Article 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moodley J, Moodley D, Pillay K, Coovadia H, Saba J, van Leeuwen R, et al. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis. 1998;178:1327–1333. doi: 10.1086/314431. [DOI] [PubMed] [Google Scholar]
- 9.Best BM, Mirochnick M, Capparelli EV, Stek A, Burchett SK, Holland DT, et al. Impact of pregnancy on abacavir pharmacokinetics. AIDS. 2006;20:553–560. doi: 10.1097/01.aids.0000210609.52836.d1. [DOI] [PubMed] [Google Scholar]
- 10.Colbers AP, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, et al. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS. 2012;27:739–748. doi: 10.1097/QAD.0b013e32835c208b. [DOI] [PubMed] [Google Scholar]
- 11.Best BM, Burchett SK, Li H, Stek A, Hu C, Wang J, et al. Pharmacokinetics of tenofovir during pregnancy and postpartum. HIV Med. 2015;16:502–11. doi: 10.1111/hiv.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stek AM, Best BM, Luo W, Capparelli E, Burchett S, Hu C, et al. Antepartum and postpartum pharmacokinetics of emtricitabine. HIV Med. 2012;13:226–35. doi: 10.1111/j.1468-1293.2011.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capparelli EV, Aweeka F, Hitti J, Stek A, Hu C, Burchett S, et al. Chronic administration of nevirapine during pregnancy: impact on pharmacokinetics. HIV Med. 2008;9:214–220. doi: 10.1111/j.1468-1293.2008.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tivicay [package insert] [Accessed February 26, 2017];ViiV Healthcare. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf.
- 15.Watts DH, Stek A, Best BM, Wang J, Capparelli EV, Cressey TR, et al. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr. 2014;67:375–81. doi: 10.1097/QAI.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blonk MI, Colbers AP, Hidalgo-Tenorio C, Kabeya K, Weizsacker K, Haberl AE, et al. Raltegravir in HIV-1-infected pregnant women: pharmacokinetics, safety, and efficacy. Clin Infect Dis. 2015;61:809–16. doi: 10.1093/cid/civ366. [DOI] [PubMed] [Google Scholar]
- 17.Pain JB, Lê MP, Caseris M, Amiel C, Lassel L, Charpentier C, et al. Pharmacokinetics of dolutegravir in a premature neonate after HIV treatment intensification during pregnancy. Antimicrob Agents Chemother. 2015;59:3660–3662. doi: 10.1128/AAC.00173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinnetti C, Tintoni M, Ammassari A, Tamburrini E, Bernardi S, Liuzzi G, et al. Successful prevention of HIV mother-to-child transmission with dolutegravir-based combination antiretroviral therapy in a vertically infected pregnant woman with multiclass highly drug-resistant HIV-1. AIDS. 2015;29:2534–2537. doi: 10.1097/QAD.0000000000000888. [DOI] [PubMed] [Google Scholar]
- 19.Lewis JM, Railton E, Riordan A, Khoo S, Chaponda M. Early experience of dolutegravir pharmacokinetics in pregnancy: high maternal levels and significant foetal exposure with twice-daily dosing. AIDS. 2016;30:1313–1315. doi: 10.1097/QAD.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennetto-Hood C, Talbot G, Savina P, Acosta EP. A sensitive HPLC–MS/MS method for the determination of dolutegravir in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;945–6:225–32. doi: 10.1016/j.jchromb.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best BM, Stek AM, Mirochnick M, Hu C, Li H, Burchett SK, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54:381–8. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stek A, Best BM, Wang J, Capparelli EV, Burchett SK, Kreitchmann R, et al. Pharmacokinetics of once versus twice daily darunavir in pregnant HIV-infected women. J Acquir Immune Defic Syndr. 2015;70:33–41. doi: 10.1097/QAI.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackburn ST. Maternal, fetal, and neonatal physiology: A clinical perspective. 4E. St. Louis: Saunders; 2013. [Google Scholar]
- 24.Min S, Sloan L, DeJesus E, Hawkins T, McCurdy L, Song I, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25:1737–1745. doi: 10.1097/QAD.0b013e32834a1dd9. [DOI] [PubMed] [Google Scholar]
- 25.van Lunzen J, Maggiolo F, Arribas JR, Rakhmanova A, Yeni P, Young B, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12:111–8. doi: 10.1016/S1473-3099(11)70290-0. [DOI] [PubMed] [Google Scholar]
- 26.Guillemette C. Pharmacogenomics of human UDP-glucuronosyltransferase enzymes. Pharmacogenomics J. 2003;3:136–158. doi: 10.1038/sj.tpj.6500171. [DOI] [PubMed] [Google Scholar]
- 27.Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38:62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papageorgiou I, Grepper S, Unadkat JD. Induction of hepatic CYP3A enzymes by pregnancy-related hormones: studies in human hepatocytes and hepatic cell lines. Drug Metab Dispos. 2013;41:281–90. doi: 10.1124/dmd.112.049015. [DOI] [PubMed] [Google Scholar]
- 29.Rimawi BH, Johnson E, Rajakumar A, Tao S, Jiang Y, Gillespie S, et al. Pharmacokinetics and placental transfer of elvitegravir, dolutegravir, and other antiretrovirals during pregnancy. Antimicrob Agents Chemother. 2017;61:e02213–16. doi: 10.1128/AAC.02213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyagi SJ, Collier AC. The development of UDP-glucuronosyltransferases 1A1 and 1A6 in the pediatric liver. Drug Metab Dispos. 2011;39:912–9. doi: 10.1124/dmd.110.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke DF, Acosta EP, Rizk ML, Bryson YJ, Spector SA, Mofenson LM, et al. Raltegravir pharmacokinetics in neonates following maternal dosing. J Acquir Immune Defic Syndr. 2014;67:310–5. doi: 10.1097/QAI.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allegaert K, Vanhaesebrouck S, Verbesselt R, van den Anker JN. In vivo glucuronidation activity of drugs in neonates: extensive interindividual variability despite their young age. Ther Drug Monit. 2009;31:411–5. doi: 10.1097/FTD.0b013e3181a8cc0a. [DOI] [PubMed] [Google Scholar]
- 33.Yokoi T. Essentials for starting a pediatric clinical study (1): Pharmacokinetics in children. J Toxicol Sci. 2009;34(Suppl 2):SP307–12. doi: 10.2131/jts.34.sp307. [DOI] [PubMed] [Google Scholar]
- 34.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International. Interim Report for 1 January 1989 through 31 July 2016. Wilmington, NC: Registry Coordinating Center; 2016. Available from URL: www.APRegistry.com. [Google Scholar]
- 35.Song I, Borland J, Chen S, Patel P, Wajima T, Peppercorn A, et al. Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother. 2012;56:1627–9. doi: 10.1128/AAC.05739-11. [DOI] [PMC free article] [PubMed] [Google Scholar]