Abstract
Nanotechnology is widely used in our day to day life including its use in medicine. Using nanotechnology, it is easy to analyze and manipulate atoms, chemical bonds and molecules present between various compounds. Nanotechnology is used in the dental field as nano dentistry. While choosing the nanoparticle for the use in the field of nano dentistry its chemical, physical, along with the biological aspect of nanostructures are taken into account. Often various atoms or molecules are added to form the functional structure. Nanostructures are used in innovations or diagnosis of dentistry. Some nanoparticles are used for oral disease preventive drugs, prostheses and for teeth implantation. Nanomaterials further deliver oral fluid or drugs, preventing and curing some oral disease (oral cancer) and maintain oral health care up to a high extent. This review summarises the use of various widely used nanoparticle in the field of dentistry.
Keywords: Nanoparticles, Dental filling, Dental implant, Dental polishing
1. Introduction
Teeth are present within the oral cavity and include various parts like dentin, enamel, cementum, pulp, and periodontal ligament. The function of teeth is to cut and crush the food to make it easy for swallowing and digesting.1 Besides that tooth enhances self-confidence and improve the quality of life. Thus the loss of teeth due to disease or decay affect eating pattern, speaking, or laughing to certain extend.2 Thus a lot of effort has given to protect the teeth in the field of dentistry.
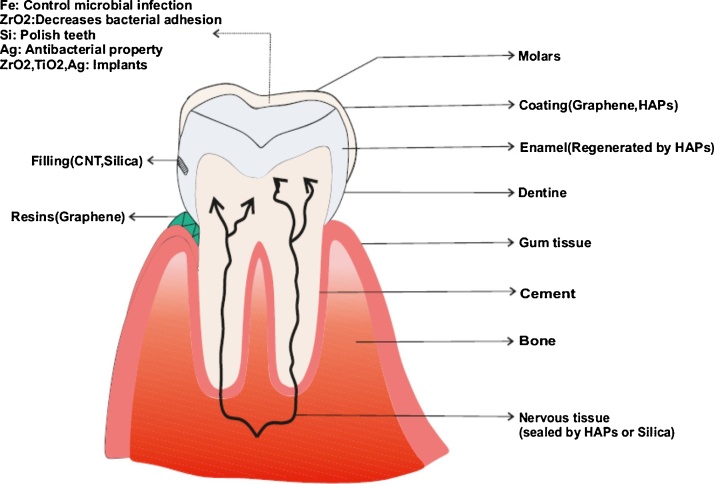
Various compounds are used in the field of dentistry to protect the teeth (Fig. 1). For filling of teeth amalgam having good mechanical properties is used. The amalgam composite is used to seal crowns and bridges.3 The sealing was permanently hardened by a polymerization lamp inserted into the oral cavity. Glass ionomers are used for temporary filling of deciduous teeth. It gives well tolerance capacity and permanently seals crowns and bridges.4 For prosthesis, gold is an ideal material as it is harmless, precision, and rigid.5 Ceramics are used in fixed prosthetics (crowns and bridges).6 Steel is used for framework and clasps in removable prosthetics.7 For bleaching the yellow coloured tooth, “carbamide peroxide” gels8 and hydrogen peroxide derivatives9 are used. Hg replaces heterogeneous and homogeneous transplants. Although these methods are used in the field of dentistry, they have some disadvantages too (Table 1). For example, the main flaw of amalgam is that it contains mercury which is harmful to our body.10 Composite fillings are hypersensitive11 towards cold. Ceramics can sometimes fracture and extremely hard in nature.12 Taking these disadvantages into account nanoparticles are introduced in dentistry.
Fig. 1.
Conventional materials used in dentistry.
Table 1.
Advantages and disadvantages of conventional materials used in dentistry.
| Conventional Materials | Advantages | Disadvantages |
|---|---|---|
| Amalgam | Durable, provide great resistance to surface corrosion, easy to manipulate, needs less time to get placed than other materials; prevention of bacterial leakage, lasts long when placed under controlled conditions, money saving. | Disruption of the tooth tissue, reduced aesthetic qualities, local allergic responses may occur, mercury which is component may have toxic effects. |
| Nickel or cobalt chrome alloys | Good resistance to further decay, long term effectivity, shows no fracture issues in stress conditions, does not decay in the mouth, and needs removal of the minimum tooth, resistance against leakage. | Dark silver metal colored, may irritate sensitivity tooth as it conducts heat and cold, costly. |
| Glass Ionomer | Provide aesthetic beauty, prevents decay by fluoride release, needs removal of the minimum tooth, low tooth sensitivity. | Usage is limited, the material becomes rough as time goes and can increase the accumulation of plaque resulting in periodontal disease. |
| Gold alloy | Good resistance to decay, durable, shows no fracture in stress, resists leakage because of good fitting, needs removal of the minimum tooth. | It is colored yellow, may result in tooth sensitivity, high cost. |
| Ceramics | Provide excellent resistance to further decay, resistant to surface wear, resistant to leakage, very minimal or no tooth sensitivity. | The weak material, which can break under biting forces, not suggested for molar teeth, costly material. |
| Ceramics fused to metals | Resistance to decay, very durable, no sensitivity of tooth, resists leakage. | More tooth needs to be removed than ceramics, higher cost. |
| Resin ionomer | Very good aesthetics, act against decay by releasing fluoride, needs removal of the minimum tooth, well usable for non-biting surfaces, can be used for the primary restoration of teeth, better than glass ionomers, provide resistance against leakage, low rate of dental sensitivity. | Cost is similar to the composite resin, limited use wears faster than composite and amalgam. |
| Composite resin | Strong and durable, tooth colored, break free, preservation of maximum tooth, the low hazard of leakage, do not undergo decay and provide good resistance to the biting forces. | Moderate tooth sensitivity occurs, more costly, shrinks when it hardened which could lead to temperature sensitivity, may leak over time. |
Nanotechnology13, a technology which deals objects of nanometer size and the particles are called as nanoparticles (NPs). Silver is the frequently used NPs, used in various products in different form,14 which is followed by carbon and ion oxides (TiO2).15 The nanoparticle can improve the quality of the products by adding many functional groups to it.16 Thus nano products are widely used in various industrial sectors,15 medicine and in the field of dentistry.17
Hybrid bionanomaterials are used to build electronic and memory devices.18 The dental implant formed of biocompatible materials like hydroxyapatite and titanium embedded into the alveolar bone along with an artificial tooth is available for implantation.19 This implant, mainly, forms a periodontium and that varies from the structure of the original tissue . It may cause dental ankyloses when alveolar bone directly contacts the dental implant.20 Nanoporous anodic alumina (NAA), porous silicon (pSi) and Titania nanotubes (TNTs) are used for development of drug-releasing implants by anodizing silicon, titanium and aluminum electrochemically.21, 22, 23, 24, 25 Nano pores of TNTs, NAA and pSi are developed electrochemically to change the length and shape, the diameter of the pore to develop implants which can release drugs. Drug-releasing action can be studied by accurately making the nanoporous structure which is based on therapeutic requirements such as drug loading, dosages of the drug, the rate of drug release, etc.26 The various nanoparticles used in the field of dentistry are summarised in Fig. 2. This review summarises utilisation of some of the widely used nanomaterial in various field of dentistry.
Fig. 2.
The different approach of nanoparticle used in dentistry.
1.1. NP used in dentistry
Various applications of NPs that are used in the field of dentistry are shown in Fig. 3. The chemical and physical property based on the metals or the compounds used to prepare the NPs (Table 2). We are summarising the pure NPs as well as the composite NPs used in the field of dentistry. Bio-composite materials like tiny calcium phosphate crystallites having similarity towards hydroxyapatite showing biological characteristics of forming a chemical bond interface with bone. Hydroxyapatite is a natural nanocomposite of the body, formed of collagen. Typically they are used for implants coatings27 having biocompatibility and better wear resistance and for preparation of bone graft.15 Recently, ceramics are modified organically and its fillers are used in dentistry to enhance dispersion and enhanced biocompatibility and for high toughness.15
Fig. 3.
Different nanoparticles used in various application of dentistry.
Table 2.
Properties of nanoparticles.
| Name of NP | Compressive strength, Malleability, ductility. | Physical property | Chemical property |
|---|---|---|---|
| Carbon nanotubes | Carbon nanotubes can offer tensile strength because of the hexagonal arrangement yet it has the malleability of rubber, high tensile ductility (8–13%), good mechanical strength. | Surface area is large, ultra-light weight, heat stability, high strength, lower density. | Heat transmission efficiency Strong bond between carbons atoms make this material quite stable; the carbon atoms in nanotubes are arranged in hexagonal rings. |
| Graphene | Graphene is transparent, flexible (high malleability and ductility) and very stable. | The unique structure gives rise to a high planar surface area, superior mechanical strength, electronic properties are remarkable and alluring optical characteristics. | Single, thick carbon sheets of honeycomb lattice orientation having two-dimensional (2D) origin make up the grapheme structure. Due to the structure, graphene has acquired a number of unique and exceptional characters. |
| Hydroxy apatite (HAp) | Greater surface area, hexagonal structure. | It is a calcium phosphate. It is quite stable when compared to other calcium phosphates. | |
| Zirconia | The ductile, soft and malleable matter which provide great resistance to corrosion. | Zirconium nanoparticles are lighter and less susceptible to embrittlement by hydrogen. | Available in the form of nanodots, Nano fluids nanocrystals with the white surface area. Provide great resistance to corrosion by acids, alkalis, salt water and other agents. |
| Silica | Compressive strength–1600 MPa with minimal ductility and significant hardness. | Two types based on their structure – P-type and S-type. P-type is characterized by numerous Nano pores whereas S-type is having smaller surface area. P-type nano silica is having comparatively higher UV reflectivity. | Chemical composition- |
| Silica- 46.83% | |||
| Oxygen- 53.33% Molar mass- 59.96 g/mol | |||
| Density- 2.4 g/cm3 | |||
| Titania | Having a compressive strength of about 3675 MPa with null ductility, quite hard and an elasticity limit of 367.5 MPa. | Found as nanocrystals or nano drops having large surface area, exhibit magnetic properties. | Chemical composition |
| Titania- 59.93% | |||
| Oxygen- 40.55% | |||
| Insoluble in water and organic solvent | |||
| Silver | Silver nanoparticles are having high ductility and malleability. They are also good conductors. | Small size, the surface area is large, having exceptional optical, electrical and thermal conductivity. | Chemically very stable having catalytic activity, unique surface chemistry which helps them better act as an antimicrobial agent. |
2. Carbon based nano-materials
2.1. Carbon nanotubes
Carbon nanotubes (CNTs) have unique electrical as well as mechanical properties. Strength and flexibility of CNTs are because of C22C covalent bond and hexagonal orientation. CNTs also have thermal and electrical conductivity (semi conductivity).28 Because of it's excellent mechanical and electrical properties such as heat stability, heat transmission efficiency, high strength and lower density, it is used as a candidate for teeth filling and various applications (Fig. 4, Fig. 5). CNTs needles are used for bringing active agents to live cells (Kanzius RF Therapy).15
Fig. 4.
Different approaches of nanoparticles applied in dentistry.
Fig. 5.
Dental application of nanoparticles.
2.2. Graphene
Graphene, an allotrope of carbon having sp2 hybridized carbon atoms has one-atom thick densely packed planar sheets structure arranged in honeycomb crystal lattice. This makes graphene as the thinnest material forming a uniform crystal lattice without any vacancies or structural dislocations. This property yielded graphene new physical properties.15 Conducting electrons of graphene, mostly behave like electrons or neutrons which move like the speed of light.29 Thus graphene is used in photovoltaics,30 bio-devices,31 ultra-capacitors32 for diagnosis and detection of disease and building of anti-bacterial surfaces.33 Thus graphene is used to treat various bacterial biofilm.34
Oral biofilms are important for dental caries development (Streptococcus mutants – 10 etiological agents in dental caries formation) and several periodontal diseases. Implants can replace absent teeth.35 Biofilms are important due to its implant failure nature. Graphene/zinc oxide nanocomposite (GZNC) has the potential to the biofilm caused by Streptococcus mutans.36 Acrylic teeth coated with graphene (Fig. 4) are used due to its cost effectiveness, fracture resistant and low-density property is suitable for implantation.36 Anti-biofilm assays show biofilm reduction in presence of GZNC.36 PCL/graphene was also used for porous scaffolds formation.37
3. Hydroxy apatite (HAp)
Hydroxyl apatite NPs has been used widely in medicine and dentistry. It's similar composition with teeth and bone make it a biocompatible substance for the physiological process. This is the main composition of mineralized tissues of the human body (Ca10 (PO4)6·2(OH)). It is a natural calcium phosphate ceramic, predominant in 97% enamel. The hardest tissue of our body is tooth enamel which has HAp nano crystals as the building blocks. Teeth are acellular in nature, thus it cannot be logically repaired like a bone. Thus regenerating the enamel surface is a significant challenge.38
The recently developed interest in nanotechnology is providing new insights about the application of nano-hydroxyapatite in dentistry. The nano sized HAp particles can easily integrate into the dental tubules. The function of the tubule is to seal the opening and thus prevents the nerves to expose towards external stimuli. Thus, HAp helps to reduce dental hypersensitivity.39 HAp NPs is having greater surface area, as a result of which they can bind strongly with proteins as well as with bacterial and plaque fragments. Their high biological activity and reactivity enable them to bind to the dentin apatite and tooth enamel.40 Hydroxyl apatite nanoparticles can fit well with the very small cavities present in the enamel originated by acidic erosion.41 The HAp NPs are adsorbed robustly to the enamel of the teeth41 and thus retard auxiliary erosive demineralization.41 Various toothpaste, mouth-rinsing solutions integrate these nanocrystals to repair the enamel surfaces. The biomimetic function of hydroxyapatite is to protect the teeth by making a film of artificial enamel around the tooth (Fig. 4). The granular hydroxyapatite is employed in dental clinical rehearsal to reform periodontal shortcomings.42
Biocompatibility studies indicated that hydroxyapatite has the ability to bind to bone and do not lead to any inflammatory response, either local or systemic nor toxic phenomena. Some scientists have shown that the hydroxyapatite, as like tricalcium phosphate, do not go through any resorption.42 Due to its chemical property and ability to form crystals with inorganic components of teeth, it can build chemical bonds and make sure the immediate integration of titanium implants to the teeth and the tissues around it. Several studies have put forward the role of hydroxyapatite in facilitating the method of step integration.42
4. Iron oxide
Iron compound (FeOx) nanoparticle has an important role in biology and medicinal field.43 Magnetite and Maghemite, the two common forms of iron oxide nanoparticles are most popular in biomedical science due to its biocompatibility and non-toxic property to humans.44 Furthermore, iron oxide is well decomposable and thus helpful in favor of in vivo applications. Mostly used NPs in medical science are nanoparticles based on Super-paramagnetic iron oxide.43
Bacterial biofilms on dental implants are difficult to eliminate by antibiotics as a result of their protective layer of exopolymers that insert the microorganisms in a matrix, which is impenetrable for immune-cells and for many antibiotics. The nanoparticulate substance is presently used to control microbial infections. Iron-oxide nanoparticles are widely utilized to eradicate biofilms on dental implants45 (Fig. 4, Fig. 5).
5. Zirconia
The use Zirconia (Zirconium dioxide, ZrO2) has considerable significance in dental science.46 It has similar metallic properties and color like tooth.47 Zirconia is a chemical oxide 48 which is insoluble in water. Thus, it reduces the bacterial adhesion and has low cytotoxicity.49, 50 This makes ZrO2 as a widespread biomaterial for dental implants.51 Zirconia implants encompass glorious resistance against corrosion and carry, as well as sensible biocompatibility.52 Moreover, high fracture resistance can be acquired by ZrO2 because of energy retention property throughout the conversion of polygonally shaped molecules into monoclinic ones.53 Zirconia NPs is a bio inert material, the encapsulation by animal tissue is weak and also the unleash of remains virtually unnoticeable. Additionally, Zirconia is osseo conductive,54 thereby it facilitates bone formation. Besides ZrO2 NPs various nanocomposites are also used in various application of dentistry (Fig. 4, Fig. 5).
Nano zirconia-alumina materials combine the physical and chemical properties of ceramic material.55, 56 In these NPs, low percentage of tetragonal ZrO2 particles is in an aluminum oxide matrix. Thus, the toughness and longevity which are the principal interest in the dentistry are retained.55, 56 Alumina/zirconia nano composites are new implant materials which show better efficacy as compared to the ceramic materials.57 Zirconia oxide nanoparticles are found to have anti-biofilm activity against certain bacteria (such as Enterococcus faecalis) and therefore they can be effectively used as a polishing agent in dental practices.58
6. Silica
Silica-based NPs have a significant role in nanotechnology, due to its size, surface area, biocompatibility, low toxicity, low density and adsorption capacity.59 SiNPs are used in various filed of medical science60 to diagnose, control diseases and correcting genetic disorders. Thus Silica NPs used in biomedical research, such as drug delivery,61 enzyme supporters62 and biosensors.63
In the field of dentistry silica NPs used as dental filler. Variation in dimension and outline of packing particles affect the possessions of the materials.64 Thus various dental filler products developed to improve their mechanical properties.65 Tooth polishing is a conventional practice, which uses silica particles. Silica particles are used in polishing for their biocompatibility and low cost. Some cariogenic bacteria that lives inside the oral cavity causes damage of the tooth enamel66 by producing acids that demineralize the enamel, thereby causing caries. Polishing of teeth surfaces is often done67, 68, 69 to protect the enamel surfaces. Studies have shown a considerable lower roughness of the polished surface when silica nanoparticles were used for polishing.70 Thus, polishing prevents dental caries, which acts as a primary defense mechanism against the cariogenic bacteria.
Modified silica nanoparticles are used to treat dental hypersensitivity (Fig. 4, Fig. 5). Enamel loss exposes dentinal tubules, thereby increasing the risk of dental hypersensitivity. Over the years, a number of desensitizing agents are commercially available that aim at occluding dentinal tubules. But none of them are consistent and reliable. Recently, a number of dental care products have been developed for the occlusion of exposed dentinal tubules.71, 72 Unfortunately, the products can penetrate only up to a small depth into the dentinal tubules which may not combat the daily adverse conditions.73, 74. Therefore, it's necessary to develop biomaterials that can penetrate deeper inside the dentinal tubules without irritating the pulp, rapid in action, and provide lasting tubule occlusion in dentin.75 Mesoporous silica possesses well-defined structures, high surface areas, tunable pore sizes, hydrocarbon sorption efficacy, and high thermal/hydrothermal stability; as such, mesoporous silicas have been widely researched over the past two decades.76, 77, 78
7. Titania (TiO2)
TiO2 nanoparticles are mostly used in medical and in the field of dentistry.15 Insertion of implants gives rise to the allergic reaction by inducing antigen/antibody type 1 and type IV complex reaction. However, adhesion of microbes on Titania implants has a strong effect on teeth healing process and show the long-term effect on implants. Roughness or chemical decomposition on implants can form plaque inside the oral cavity. Titanium plates were placed in bacterial suspension for around 1 h for bacterial colony count, adhesion angle measurements represent no significant difference on surface modification. Adherent bacteria number reduces significantly on stable Titania (titanium/zirconia nanoparticles) rather than polished titanium. So physical modification (silica/titania nanoparticle coating) significantly decreases microbial adhesion for result promotion.79 Bone reacts to physically modified titanium implants for the formation of inorganic nano porous substances and intensively used to form drug-releasing implants (Fig. 4, Fig. 5). Surface modification improves hardness and strength of dental RBCs and affects linkage and dispersion of TiO2 present inside a resin matrix.80
8. Silver
Silver compounds are known to exhibit antibacterial activity.81 With the advancement of nanotechnology more silver nanoparticles are synthesized (AgNPs).82, 83 AgNPs are widely used in water purification, toothpaste, washing machines, shampoo, kitchen utilities, fabrics, nursing bottles, and humidifiers.84 AgNPs85 have also been studied for use in several areas of dentistry which includes endodontics,86 dental restorative material,87 dental prosthetics,88 dental implants.89 Incorporation of AgNPs decreases microbial colonization over dental parts and increases oral health.90 As the nanoparticles possess small size having the larger surface area, they show the antimicrobial effect at very low level.91, 92 Because of its minute size, AgNPs can able to penetrate easily the bacterial cell membrane resulting in rapid bactericidal activity.93 Silver can interfere with DNA and proteins by interacting with —SH groups, and also alters the base pairing, DNA unwinding, cell wall synthesis and respiratory processes,94 resulting in bacterial death Various NPs used in the field of dentistry and its positive and negative impacts are summerised in (Table 3).
Table 3.
Nanoparticles used in dentistry.
| Name of the material | Purpose of use | Advantage of this material | Toxicity/side effect known | Reference |
|---|---|---|---|---|
| Carbon nanotubes | Teeth filling, a coating of the teeth surface. | Large surface area, bring active agents to live cells, adheres easily to the tooth surfaces and to the surfaces of dentin and cementum. | The structure, size, surface, and purity have a significant effect on CNTs reactivity. Nanotubes can induce inflammatory and fibrotic reactions under some conditions by crossing membrane barriers. | 98, 99, 100, 101, 102 |
| Graphene | Teeth coating, suitable for implantation, biofilm reduction | Cost effectiveness, fracture resistant, low density form a uniform crystal lattice, treat bacterial biofilm | The toxicity depends on the shape, size, and oxidative state of graphene. Post synthesis processing steps can incorporate metallic impurities which may elicit variable toxicity responses | 103, 104, 105, 106, 107, 108 |
| Hydroxy apatite (HAp) | reduce dental hypersensitivity, also act as cavity filler, retard auxiliary demineralization, repairment of enamel surfaces | The nano sized HAp particles can easily integrate into the dental tubules. Similar composition with teeth and bone, biocompatible, adsorbed to the enamel of the teeth, protect the teeth by making a film of artificial enamel around the tooth, reform periodontal shortcomings. | They can bind with proteins and produce protein-particle complexes which eventually get killed by macrophages in tissues. These particles traveled to and dispersed into lungs, spleen, and liver by blood. The inflammatory response, signaling pathway, and oxidative stress can be affected by the toxicity of nanoparticles. | 38,39,109, 110, 111, 112 |
| Zirconia | Reduces bacterial adhesion to the tooth surface, provide protection against dental carries, effective polishing agent. | Similar mechanical properties and color like a tooth, have low cytotoxicity, sensible biocompatibility, and high fracture resistance. | Zirconium oxide nanomaterials may have some short- and long-term risks such as a significant DNA damage in human T-cells, and induce apoptosis, inhibition of cell proliferation in human mesothelioma and rodent fibroblast cell lines are reported after exposure to zirconium. It also found to induce cellular oxidative stress causing cell mortality. Studies have indicated that these NPs can stop the cell cycle as well as they can cross the various physiological barriers resulting in adverse effects. | 113, 114, 115, 116, 117, 118 |
| Silica | Dental filling agent, tooth polishing, prevents dental caries, an antibacterial agent, to treat dental hypersensitivity. | Biocompatible, have a low toxic effect, low density and a significant adsorption ability and most importantly they are cost effective. The reduced roughness of teeth surface when used as the polishing agent. | Toxic effects depend on the route of entry and physio-chemical characters. Recent studies showed that silica nanoparticles can also induce silicosis as well as lung cancer as like crystalline particles. SiNPs induce cytotoxicity. Furthermore, it can also induce oxidative stress; mediate apoptosis which is dependent on the size and dose. Some studies have also reported that SiNPs also has several genotoxic (DNA damage, regulation of genes involves in apoptosis and autophagy) as well as immunotoxic effects. | 119, 120, 121, 122, 123, 124 |
| Titania | Mainly dental implants | Long term effect on dental implants, surface modification provided more advantages like less bacterial adhesion, improved hardness. | NPs provide more toxicity than Fine Particles. It goes to the body through inhalation. Workers in TiO2 production factories have cancers (revealed in epidemiologic studies). TiO2 NPs can build up inside the brain by crossing blood-brain barrier in the region of cortex and hippocampus. TiO2 exposure results in microglia activation, ROS (reactive oxygen species) production, signaling pathways activation having a role in cell death and inflammation. | 125, 126, 127, 128, 129, 130, 131 |
| Silver | Antimicrobial agent, dental restorative material, dental prosthetics, dental implants | Known to decrease bacterial colonization and increases oral health, can easily penetrate bacterial membrane due to its smaller size. It is biocompatible having low toxicity and long-term antibacterial activity. | AgNPs induce toxicity. Chronic exposure to silver can have argyria. Toxic effects of AgNPs are due to ROS production. Both silver ions and AgNPs contribute to the toxicity. Ag NPs involved in oxidative stress production and genotoxicity, lysosomal AcP activation, actin disruption, hemocytes phagocytosis stimulation and inhibition of Na-K-ATPase. | 132,133 |
Summerisation of NPs used in dentistry.
Although Ag acts as a potent antimicrobial agent, it also has many advantages, such as its biocompatibility, low toxicity,85 low bacterial resistance95 and long-term antibacterial activity.96 The disadvantages of using AgNPs is that it changes dental colour.97
9. Conclusion
Nanotechnology is going to be an essential part of the clinical dental practice. Nanomaterials are used in toothpaste and other rinsing solutions for better oral healthcare services which will become less stressful for the dental surgeons. New nano strategies cope up to address the dental problems. Nanomaterials used in the dental filling, polishing of the enamel surface to prevent caries, also used as implant materials that are more effective than the conventional materials. Some of the nanoparticles act as antimicrobial agent thus prevent bacterial growth. Nanodentistry attracts patients towards dentistry, since, it will be cost effective, time-saving and prevent the patient from mental trauma. Development of modified nanomaterials is surely going to help to solve the dental problems. Nanotechnology’s effect for treatment of oral disease is limited, which rapidly progress the investigations ensuring developments that are possible in near future.
Conflict of interest
None.
Acknowledgements
SS and SM are thankful to MHRD for the fellowship they received for their doctoral work.
References
- 1.Tortora G.J., Derrickson B.H. John Wiley & Sons; 2008. Principles of anatomy and physiology. [Google Scholar]
- 2.TO IG. Digestive system.
- 3.Spencer A. Dental amalgam and mercury in dentistry. Austral Dent J. 2000;45:224–234. doi: 10.1111/j.1834-7819.2000.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson A.D., Kent B. The glass-ionomer cement, a new translucent dental filling material. J Chem Technol Biotechnol. 1971;21 313-313. [Google Scholar]
- 5.Carr A.B., Brunski J.B., Hurley E. Effects of fabrication, finishing, and polishing procedures on preload in prostheses using conventional 'gold' and plastic cylinders. Int J Oral Maxillofac Implants. 1996:11. [PubMed] [Google Scholar]
- 6.Kelly J.R. Ceramics in restorative and prosthetic dentistry. Annu Rev Mater Sci. 1997;27:443–468. [Google Scholar]
- 7.Riley M.A., Walmsley A.D., Harris I.R. Magnets in prosthetic dentistry. J Prosthetic Dent. 2001;86:137–142. doi: 10.1067/mpr.2001.115533. [DOI] [PubMed] [Google Scholar]
- 8.Gökay O., Müjdeci A., Algin E. In vitro peroxide penetration into the pulp chamber from newer bleaching products. Int Endod J. 2005;38:516–520. doi: 10.1111/j.1365-2591.2005.00979.x. [DOI] [PubMed] [Google Scholar]
- 9.Tredwin C., Naik S., Lewis N., Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: review of adverse effects and safety issues. Br Dent J. 2006;200:371. doi: 10.1038/sj.bdj.4813423. [DOI] [PubMed] [Google Scholar]
- 10.Hörsted-Bindslev P. Amalgam toxicity—environmental and occupational hazards. J Dent. 2004;32:359–365. doi: 10.1016/j.jdent.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Axéll T. Hypersensitivity of the oral mucosa: clinics and pathology. Acta Odontol Scand. 2001;59:315–319. doi: 10.1080/000163501750541192. [DOI] [PubMed] [Google Scholar]
- 12.Hondrum S.O. A review of the strength properties of dental ceramics. J Prosthetic Dent. 1992;67:859–865. doi: 10.1016/0022-3913(92)90602-7. [DOI] [PubMed] [Google Scholar]
- 13.Eshed M., Lellouche J., Banin E., Gedanken A. MgF 2 nanoparticle-coated teeth inhibit Streptococcus mutans biofilm formation on a tooth model. J Mater Chem. B. 2013;1:3985–3991. doi: 10.1039/c3tb20598c. [DOI] [PubMed] [Google Scholar]
- 14.Xiu Z.-M., Ma J., Alvarez P.J. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol. 2011;45:9003–9008. doi: 10.1021/es201918f. [DOI] [PubMed] [Google Scholar]
- 15.McINTYRE RA Common nano-materials and their use in real world applications. Sci Prog. 2012;95:1–22. doi: 10.3184/003685012X13294715456431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moszner N., Klapdohr S. Nanotechnology for dental composites. IJNT. 2004;1:130–156. [Google Scholar]
- 17.Khurshid Z., Zafar M., Qasim S., Shahab S., Naseem M., AbuReqaiba A. Advances in nanotechnology for restorative dentistry. Materials. 2015;8:717–731. doi: 10.3390/ma8020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan H., Park S.H., Finkelstein G., Reif J.H., LaBean T.H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science. 2003;301:1882–1884. doi: 10.1126/science.1089389. [DOI] [PubMed] [Google Scholar]
- 19.H. Fukuda, Dental implant: Google Patents; 1992.
- 20.Oh T.-J., Yoon J., Misch C.E., Wang H.-L. The causes of early implant bone loss: myth or science? J Periodontol. 2002;73:322–333. doi: 10.1902/jop.2002.73.3.322. [DOI] [PubMed] [Google Scholar]
- 21.Pacholski C., Sartor M., Sailor M.J., Cunin F., Miskelly G.M. Biosensing using porous silicon double-layer interferometers: reflective interferometric fourier transform spectroscopy. J Am Chem Soc. 2005;127:11636–11645. doi: 10.1021/ja0511671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacholski C., Yu C., Miskelly G.M., Godin D., Sailor M.J. Reflective interferometric fourier transform spectroscopy: a self-compensating label-free immunosensor using double-layers of porous SiO2. J Am Chem Soc. 2006;128:4250–4252. doi: 10.1021/ja056702b. [DOI] [PubMed] [Google Scholar]
- 23.Paulose M., Shankar K., Yoriya S. Anodic growth of highly ordered TiO2 nanotube arrays to 134 μm in length. J Phys Chem B. 2006;110:16179–16184. doi: 10.1021/jp064020k. [DOI] [PubMed] [Google Scholar]
- 24.Lee W.-J., Alhoshan M., Smyrl W.H. Titanium dioxide nanotube arrays fabricated by anodizing processes electrochemical properties. J Electrochem Soc. 2006;153:B499–B505. [Google Scholar]
- 25.de la Escosura-Muñiz A., Merkoçi A. Nanochannels preparation and application in biosensing. ACS Nano. 2012;6:7556–7583. doi: 10.1021/nn301368z. [DOI] [PubMed] [Google Scholar]
- 26.Santos A., Aw M.S., Bariana M., Kumeria T., Wang Y., Losic D. Drug-releasing implants: current progress, challenges and perspectives. J Mater Chem B. 2014;2:6157–6182. doi: 10.1039/c4tb00548a. [DOI] [PubMed] [Google Scholar]
- 27.Ma J., Wong H., Kong L., Peng K.-W. Biomimetic processing of nanocrystallite bioactive apatite coating on titanium. Nanotechnology. 2003;14:619. [Google Scholar]
- 28.Ajayan P.M., Zhou O.Z. CNT: Springer; 2001. Applications of carbon nanotubes; pp. 391–425. [Google Scholar]
- 29.Pincock P.G. 2017. Graphene, a Definition, Its Properties, and Practical Uses. [Google Scholar]
- 30.Bonaccorso F., Sun Z., Hasan T., Ferrari A. Graphene photonics and optoelectronics. Nat Photon. 2010;4:611–622. [Google Scholar]
- 31.Xu M., Fujita D., Hanagata N. Perspectives and challenges of emerging single-molecule DNA sequencing technologies. Small. 2009;5:2638–2649. doi: 10.1002/smll.200900976. [DOI] [PubMed] [Google Scholar]
- 32.Stoller M.D., Park S., Zhu Y., An J., Ruoff R.S. Graphene-based ultracapacitors. Nano Lett. 2008;8:3498–3502. doi: 10.1021/nl802558y. [DOI] [PubMed] [Google Scholar]
- 33.Hu W., Peng C., Luo W. Graphene-based antibacterial paper. ACS Nano. 2010;4:4317–4323. doi: 10.1021/nn101097v. [DOI] [PubMed] [Google Scholar]
- 34.Carpio I.E.M., Santos C.M., Wei X., Rodrigues D.F. Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale. 2012;4:4746–4756. doi: 10.1039/c2nr30774j. [DOI] [PubMed] [Google Scholar]
- 35.Misch C.E. Elsevier Health Sciences; 2014. Dental Implant Prosthetics-E-Book. [Google Scholar]
- 36.Kulshrestha S., Khan S., Meena R., Singh B.R., Khan A.U. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling. 2014;30:1281–1294. doi: 10.1080/08927014.2014.983093. [DOI] [PubMed] [Google Scholar]
- 37.Bartolo P., Kruth J.-P., Silva J. Biomedical production of implants by additive electro-chemical and physical processes. Cirp Ann Manuf Technol. 2012;61:635–655. [Google Scholar]
- 38.Ferreira J., Pires P.T., Almeida C., Jerónimo S., Melo P. Avaliação da Eficácia do nanoXIM CarePaste na Oclusão dos Túbulos Dentinários/Evaluation of the Efficacy of nanoXIM CarePaste in Dentinal Tubule Occlusion. Int Poster J Dent Oral Med-XXIII Congresso. 2014 OMD. [Google Scholar]
- 39.Khetawat S., Lodha S. Nanotechnology (nanohydroxyapatite crystals): recent advancement in treatment of dentinal hypersensitivity. J Interdiscipl Med Dent Sci. 2015;3:181. [Google Scholar]
- 40.Besinis A., van Noort R., Martin N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent Mater J. 2012;28:1012–1023. doi: 10.1016/j.dental.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Li L., Pan H., Tao J. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–4084. [Google Scholar]
- 42.Pepla E., Besharat L.K., Palaia G., Tenore G., Migliau G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol. 2014;5:108. [PMC free article] [PubMed] [Google Scholar]
- 43.Motte L. What are the current advances regarding iron oxide nanoparticles for nanomedicine. J Bioanal Biomed. 2012:4. [Google Scholar]
- 44.Laurent S., Forge D., Port M. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 45.Sathyanarayanan M.B., Balachandranath R., Genji Srinivasulu Y., Kannaiyan S.K., Subbiahdoss G. The effect of gold and iron-oxide nanoparticles on biofilm-forming pathogens. ISRN Microbiol. 2013;2013 doi: 10.1155/2013/272086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denry I., Kelly J.R. State of the art of zirconia for dental applications. Dent Mater J. 2008;24:299–307. doi: 10.1016/j.dental.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Piconi C., Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1–25. doi: 10.1016/s0142-9612(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 48.Zarone F., Russo S., Sorrentino R. From porcelain-fused-to-metal to zirconia: clinical and experimental considerations. Dent Mater J. 2011;27:83–96. doi: 10.1016/j.dental.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 49.Lughi V., Sergo V. Low temperature degradation-aging-of zirconia: a critical review of the relevant aspects in dentistry. Dent Mater. 2010;26:807–820. doi: 10.1016/j.dental.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Dion I., Bordenave L., Lefebvre F. Physico-chemistry and cytotoxicity of ceramics. J Mater Sci Mater Med. 1994;5:18–24. doi: 10.1023/a:1018520630500. [DOI] [PubMed] [Google Scholar]
- 51.Ramesh T., Gangaiah M., Harish P., Krishnakumar U., Nandakishore B. Zirconia ceramics as a dental biomaterial–an over view. Trends Biomater Artif Organs. 2012:26. [Google Scholar]
- 52.Rocchietta I., Fontana F., Addis A., Schupbach P., Simion M. Surface-modified zirconia implants: tissue response in rabbits. Clin Oral Implants Res. 2009;20:844–850. doi: 10.1111/j.1600-0501.2009.01727.x. [DOI] [PubMed] [Google Scholar]
- 53.Akagawa Y., Hosokawa R., Sato Y., Kamayama K. Comparison between freestanding and tooth-connected partially stabilized zirconia implants after two years’ function in monkeys: a clinical and histologic study. J Prosthet Dent. 1998;80:551–558. doi: 10.1016/s0022-3913(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 54.Josset Y., Oum'Hamed Z., Zarrinpour A., Lorenzato M., Adnet J.-J., Laurent-Maquin D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J Biomed Mater Res. 1999;47:481–493. doi: 10.1002/(sici)1097-4636(19991215)47:4<481::aid-jbm4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 55.Deville S., Chevalier J., Fantozzi G. Low-temperature ageing of zirconia-toughened alumina ceramics and its implication in biomedical implants. J Eur Ceram Soc. 2003;23:2975–2982. [Google Scholar]
- 56.Pecharroman C., Bartolomé J.F., Requena J. Percolative mechanism of aging in zirconia-containing ceramics for medical applications. J Eur Ceram Soc. 2003;15:507–511. [Google Scholar]
- 57.Benzaid R., Chevalier J., Saâdaoui M. Fracture toughness, strength and slow crack growth in a ceria stabilized zirconia–alumina nanocomposite for medical applications. Biomaterials. 2008;29:3636–3641. doi: 10.1016/j.biomaterials.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Guerreiro-Tanomaru J.M., Trindade-Junior A., Cesar Costa B. Effect of zirconium oxide and zinc oxide nanoparticles on physicochemical properties and antibiofilm activity of a calcium silicate-based material. Scientific World J. 2014:2014. doi: 10.1155/2014/975213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halas N.J. Nanoscience under glass: the versatile chemistry of silica nanostructures. ACS Nano. 2008;2:179–183. doi: 10.1021/nn800052e. [DOI] [PubMed] [Google Scholar]
- 60.Bitar A., Ahmad N.M., Fessi H., Elaissari A. Silica-based nanoparticles for biomedical applications. Drug Discov Today. 2012;17:1147–1154. doi: 10.1016/j.drudis.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Vivero-Escoto J.L., Slowing I.I., Trewyn B.G., Lin V.S.Y. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 62.Coll C., Mondragón L., Martínez-Máñez R. Enzyme-mediated controlled release systems by anchoring peptide sequences on mesoporous silica supports. Angew Chem Int Ed. 2011;50:2138–2140. doi: 10.1002/anie.201004133. [DOI] [PubMed] [Google Scholar]
- 63.Tallury P., Payton K., Santra S. 2008. Silica-based multimodal/multifunctional nanoparticles for bioimaging and biosensing applications. [DOI] [PubMed] [Google Scholar]
- 64.Chen M.-H. Update on dental nanocomposites. J Dent Res. 2010;89:549–560. doi: 10.1177/0022034510363765. [DOI] [PubMed] [Google Scholar]
- 65.Mitra S.B., Wu D., Holmes B.N. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003;134:1382–1390. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 66.Loesche W.J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roulet J., Roulet-Mehrens T. The surface roughness of restorative materials and dental tissues after polishing with prophylaxis and polishing pastes. J Periodontol. 1982;53:257–266. doi: 10.1902/jop.1982.53.4.257. [DOI] [PubMed] [Google Scholar]
- 68.Güorgan S., Bolay S., Alacam R. In vitro adherence of bacteria to bleached or unbleached enamel surfaces. J Oral Rehab. 1997;24:624–627. doi: 10.1046/j.1365-2842.1997.00534.x. [DOI] [PubMed] [Google Scholar]
- 69.Banerjee A., Watson T. Dentine caries excavation: a review of current clinical techniques. Br Dent J. 2000;188:476. doi: 10.1038/sj.bdj.4800515. [DOI] [PubMed] [Google Scholar]
- 70.Gaikwad R., Sokolov I. Silica nanoparticles to polish tooth surfaces for caries prevention. J Dent Res. 2008;87:980–983. doi: 10.1177/154405910808701007. [DOI] [PubMed] [Google Scholar]
- 71.Petrou I., Heu R., Stranick M. A breakthrough therapy for dentin hypersensitivity: how dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teeth. J Clin Dent. 2009;20:23. [PubMed] [Google Scholar]
- 72.Golpayegani M.V., Sohrabi A., Biria M., Ansari G. Remineralization effect of topical NovaMin versus sodium fluoride (1.1%) on caries-like lesions in permanent teeth. J Clin Dent (Tehran, Iran) 2012;9:68. [PMC free article] [PubMed] [Google Scholar]
- 73.Orchardson R., Gillam D.G. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137:990–998. doi: 10.14219/jada.archive.2006.0321. [DOI] [PubMed] [Google Scholar]
- 74.Moritz A., Schoop U., Goharkhay K. Long-term effects of CO2 laser irradiation on treatment of hypersensitive dental necks: results of an in vivo study. J Clin Laser Med Surg. 1998;16:211–215. doi: 10.1089/clm.1998.16.211. [DOI] [PubMed] [Google Scholar]
- 75.Addy M., Edgar W.M., Embery G. Thieme; 2000. Tooth wear and sensitivity: Clinical advances in restorative dentistry. [Google Scholar]
- 76.Wu S.-H., Mou C.-Y., Lin H.-P. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013;42:3862–3875. doi: 10.1039/c3cs35405a. [DOI] [PubMed] [Google Scholar]
- 77.Yeh Y.-Q., Lin H.-P., Tang C.-Y., Mou C.-Y. Mesoporous silica SBA-15 sheet with perpendicular nanochannels. J Colloid Interface Sci. 2011;362:354–366. doi: 10.1016/j.jcis.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 78.Chiang Y.-C., Lin H.-P., Chang H.-H. A mesoporous silica biomaterial for dental biomimetic crystallization. ACS Nano. 2014;8:12502–12513. doi: 10.1021/nn5053487. [DOI] [PubMed] [Google Scholar]
- 79.Größner-Schreiber B., Griepentrog M., Haustein I. Plaque formation on surface modified dental implants. Clin Implant Dent Relat Res. 2001;12:543–551. doi: 10.1034/j.1600-0501.2001.120601.x. [DOI] [PubMed] [Google Scholar]
- 80.Xia Y., Zhang F., Xie H., Gu N. Nanoparticle-reinforced resin-based dental composites. J Dent. 2008;36:450–455. doi: 10.1016/j.jdent.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 81.García-Contreras R., Argueta-Figueroa L., Mejía-Rubalcava C. Perspectives for the use of silver nanoparticles in dental practice. Int Dent J. 2011;61:297–301. doi: 10.1111/j.1875-595X.2011.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morones J.R., Elechiguerra J.L., Camacho A. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 83.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Maynard A.D. Nanotechnology, Woodrow Wilson International Center for Scholars. 2006. A research strategy for addressing risk. [Google Scholar]
- 85.Slenters T.V., Hauser-Gerspach I., Daniels A.U., Fromm K.M. Silver coordination compounds as light-stable, nano-structured and anti-bacterial coatings for dental implant and restorative materials. J Mater Chem. 2008;18:5359–5362. [Google Scholar]
- 86.Samiei M., Aghazadeh M., Lotfi M., Shakoei S., Aghazadeh Z., Pakdel S.M.V. Antimicrobial efficacy of mineral trioxide aggregate with and without silver nanoparticles. Iran Endod J. 2013;8:166. [PMC free article] [PubMed] [Google Scholar]
- 87.Jia H., Hou W., Wei L., Xu B., Liu X. The structures and antibacterial properties of nano-SiO 2 supported silver/zinc–silver materials. Dent Mater J. 2008;24:244–249. doi: 10.1016/j.dental.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 88.Nam K.-Y. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. J Adv Prosthodont. 2011;3:20–24. doi: 10.4047/jap.2011.3.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheikh F.A., Barakat N.A., Kanjwal M.A. Electrospun titanium dioxide nanofibers containing hydroxyapatite and silver nanoparticles as future implant materials. J Mater Sci Mater Med. 2010;21:2551–2559. doi: 10.1007/s10856-010-4102-9. [DOI] [PubMed] [Google Scholar]
- 90.Corrêa J.M., Mori M., Sanches H.L., ADd Cruz, Poiate E., Poiate I.A.V.P. Silver nanoparticles in dental biomaterials. Int J Biomater. 2015:2015. doi: 10.1155/2015/485275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng L., Weir M.D., Xu H.H. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng L., Zhang K., Melo M.A., Weir M., Zhou X., Xu H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park H.-J., Park S., Roh J. Biofilm-inactivating activity of silver nanoparticles: a comparison with silver ions. Ind Eng Chem Res. 2013;19:614–619. [Google Scholar]
- 94.Lansdown A.B. Vol. 33. Karger Publishers; 2006. Silver in health care: antimicrobial effects and safety in use; pp. 17–34. (Biofunctional textiles and the skin). [DOI] [PubMed] [Google Scholar]
- 95.Percival S., Bowler P., Russell D. Bacterial resistance to silver in wound care. J Hosp Infect. 2005;60:1–7. doi: 10.1016/j.jhin.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 96.Damm C., Münstedt H., Rösch A. Long-term antimicrobial polyamide 6/silver-nanocomposites. J Mater Sci. 2007;42:6067–6073. [Google Scholar]
- 97.Sadhasivam S., Shanmugam P., Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B. 2010;81:358–362. doi: 10.1016/j.colsurfb.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 98.Akasaka T., Nakata K., Uo M., Watari F. Modification of the dentin surface by using carbon nanotubes. Bio-med Mater Eng. 2009;19:179–185. doi: 10.3233/BME-2009-0578. [DOI] [PubMed] [Google Scholar]
- 99.Sharifi S., Behzadi S., Laurent S., Forrest M.L., Stroeve P., Mahmoudi M. Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y., Zhao Y., Sun B., Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2012;46:702–713. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- 101.Kou W., Akasaka T., Watari F., Sjögren G. An in vitro evaluation of the biological effects of carbon nanotube-coated dental zirconia. ISRN Dent. 2013:2013. doi: 10.1155/2013/296727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolosnjaj J., Szwarc H., Moussa F. Bio-Applications of Nanoparticles. Springer; 2007. Toxicity studies of carbon nanotubes; pp. 181–204. [Google Scholar]
- 103.Yin P.T., Shah S., Chhowalla M., Lee K.-B. Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem Rev. 2015;115:2483–2531. doi: 10.1021/cr500537t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Q., Mahmood N., Zhu J., Hou Y., Sun S. Graphene and its composites with nanoparticles for electrochemical energy applications. Nano Today. 2014;9:668–683. [Google Scholar]
- 105.Sava S., Tonea A., Stanca B., Alb C., Sarosi C., Dudea D. Studia Universitatis Babes-Bolyai; Chemia: 2015. The study of new composites with graphene used in dentistry; p. 60. [Google Scholar]
- 106.Sarosi C., Biris A.R., Antoniac A. The nanofiller effect on properties of experimental graphene dental nanocomposites. J Adhesion Sci Technol. 2016;30:1779–1794. [Google Scholar]
- 107.Sava S., Moldovan M., Sarosi C., Mesaros A., Dudea D., Alb C. Effects of graphene addition on the mechanical properties of composites for dental restoration. Materiale Plastice. 2015;52:90–92. [Google Scholar]
- 108.He J., Zhu X., Qi Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl Mater Interfaces. 2015;7:5605–5611. doi: 10.1021/acsami.5b01069. [DOI] [PubMed] [Google Scholar]
- 109.Besinis A., van Noort R., Martin N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent Mater. 2012;28:1012–1023. doi: 10.1016/j.dental.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 110.Li L., Pan H., Tao J. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–4084. [Google Scholar]
- 111.Pepla E., Besharat L.K., Palaia G., Tenore G., Migliau G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Annali di stomatologia. 2014;5:108. [PMC free article] [PubMed] [Google Scholar]
- 112.Wang J., Wang L., Fan Y. Adverse biological effect of TiO2 and hydroxyapatite nanoparticles used in bone repair and replacement. Int J Mol Sci. 2016;17:798. doi: 10.3390/ijms17060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Asadpour E., Sadeghnia H.R., Ghorbani A., Sedaghat M., Boroushaki M.T. Oxidative stress-mediated cytotoxicity of zirconia nanoparticles on PC12 and N2a cells. J Nanoparticle Res. 2016;18:14. [Google Scholar]
- 114.Arefian Z., Pishbin F., Negahdary M., Ajdary M. Potential toxic effects of Zirconia Oxide nanoparticles on liver and kidney factors. Biom Res. 2015:26. [Google Scholar]
- 115.Asadpour E., Sadeghnia H.R., Ghorbani A., Boroushaki M.T. Effect of zirconium dioxide nanoparticles on glutathione peroxidase enzyme in PC12 and N2a cell lines. Iran J Pharm Res: IJPR. 2014;13:1141. [PMC free article] [PubMed] [Google Scholar]
- 116.Caicedo M., Jacobs J.J., Reddy A., Hallab N.J. Analysis of metal ion-induced DNA damage, apoptosis, and necrosis in human (Jurkat) T-cells demonstrates Ni2+ and V3+ are more toxic than other metals: Al3+, Be2+, Co2+, Cr3+, Cu2+, Fe3+, Mo5+, Nb5+, Zr2+ J Biomed Mater Res Part A. 2008;86:905–913. doi: 10.1002/jbm.a.31789. [DOI] [PubMed] [Google Scholar]
- 117.Wang M.L., Tuli R., Manner P.A., Sharkey P.F., Hall D.J., Tuan R.S. Direct and indirect induction of apoptosis in human mesenchymal stem cells in response to titanium particles. J Orthopaedic Res. 2003;21:697–707. doi: 10.1016/S0736-0266(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 118.Brunner T.J., Wick P., Manser P. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006;40:4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- 119.Murugadoss S., Lison D., Godderis L. Toxicology of silica nanoparticles: an update. Arc Toxicol. 2017:1–44. doi: 10.1007/s00204-017-1993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahmad J., Ahamed M., Akhtar M.J. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol Appl Pharmacol. 2012;259:160–168. doi: 10.1016/j.taap.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 121.Ahamed M. Silica nanoparticles-induced cytotoxicity, oxidative stress and apoptosis in cultured A431 and A549 cells. Hum Exp Toxicol. 2013;32:186–195. doi: 10.1177/0960327112459206. [DOI] [PubMed] [Google Scholar]
- 122.Bitar A., Ahmad N.M., Fessi H., Elaissari A. Silica-based nanoparticles for biomedical applications. Drug Discov Today. 2012;17:1147–1154. doi: 10.1016/j.drudis.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 123.Choi J., Zheng Q., Katz H.E., Guilarte T.R. Silica-based nanoparticle uptake and cellular response by primary microglia. Environ Health Perspect. 2010;118:589. doi: 10.1289/ehp.0901534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chu Z., Huang Y., Li L., Tao Q., Li Q. Physiological pathway of human cell damage induced by genotoxic crystalline silica nanoparticles. Biomaterials. 2012;33:7540–7546. doi: 10.1016/j.biomaterials.2012.06.073. [DOI] [PubMed] [Google Scholar]
- 125.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Particle Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee K., Trochimowicz H., Reinhardt C. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol Appl Pharmacol. 1985;79:179–192. doi: 10.1016/0041-008x(85)90339-4. [DOI] [PubMed] [Google Scholar]
- 127.Maynard A.D., Kuempel E.D. Airborne nanostructured particles and occupational health. J Nanoparticle Res. 2005;7:587–614. [Google Scholar]
- 128.Fabian E., Landsiedel R., Ma-Hock L., Wiench K., Wohlleben W., Van Ravenzwaay B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol. 2008;82:151–157. doi: 10.1007/s00204-007-0253-y. [DOI] [PubMed] [Google Scholar]
- 129.Hext P.M., Tomenson J.A., Thompson P. Titanium dioxide: inhalation toxicology and epidemiology. Ann Occup Hygiene. 2005;49:461–472. doi: 10.1093/annhyg/mei012. [DOI] [PubMed] [Google Scholar]
- 130.Baan R., Straif K., Grosse Y., Secretan B., El Ghissassi F., Cogliano V. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006;7:295. doi: 10.1016/s1470-2045(06)70651-9. [DOI] [PubMed] [Google Scholar]
- 131.Boffetta P., Soutar A., Cherrie J.W. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control. 2004;15:697–706. doi: 10.1023/B:CACO.0000036188.23970.22. [DOI] [PubMed] [Google Scholar]
- 132.Katsumiti A., Gilliland D., Arostegui I., Cajaraville M.P. Mechanisms of toxicity of ag nanoparticles in comparison to bulk and ionic ag on mussel hemocytes and gill cells. PloS One. 2015;10:e0129039. doi: 10.1371/journal.pone.0129039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beer C., Foldbjerg R., Hayashi Y., Sutherland D.S., Autrup H. Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicol Lett. 2012;208:286–292. doi: 10.1016/j.toxlet.2011.11.002. [DOI] [PubMed] [Google Scholar]