Abstract
Phenolics, as the main bioactive compounds in tea, have been suggested to have potential in the prevention of various human diseases. However, little is known about phenolics and their bioactivity in Zhangping Narcissue tea cake which is considered the most special kind of oolong tea. To unveil its bioactivity, three phenolic-enriched extracts were obtained from Zhangping Narcissue tea cake using ethyl acetate, n-butanol, and water. Their main chemical compositions and in vitro bioactivity were analyzed by high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography-mass spectrometry (UPLC-MS). The ethyl acetate fraction (ZEF) consisted of higher content of phenolics, flavonoids, procyanidins, and catechin monomers (including epigallocatechin gallate (EGCG), epicatechin gallate (ECG), and gallocatechin gallate (GCG)) than n-butanol fraction (ZBF) and water fraction (ZWF). ZEF exhibited the strongest antioxidant capacity in vitro due to its abundant bioactive compounds. This was validated by Pearson correlation and hierarchical clustering analyses. ZEF also showed a remarkable inhibition on the growth, migration, and invasion of 4T1 murine breast cancer cells.
Keywords: Oolong tea, Antioxidant activity, Metastasis, Cell cycle
1. Introduction
Tea (Camellia sinensis) was originally used as a medicine in the treatment of various illnesses in ancient China and now it is one of the most popular drinks in many countries worldwide. Many beneficial effects of tea may be due to the abundant phenolic compounds in tea leaves (Yang et al., 2009). Phenolics in tea leaves mainly include catechins, proanthocyanidins, anthocyanins, gallotannins, ellagitannins, flavonol glycosides, etc. (Tanaka et al., 2009). As is known, different processing of tea alters the composition of phenolic compounds in tea products, and this leads to differences in their bioactivity.
Oolong tea undergoes a distinct process named shaking and is classified as semi-fermented tea. It retains relatively high levels of catechins and includes some oligomers of catechins such as theaflavin and theasinensins. Recently, more attention has been paid to oolong tea due to its unique flavor and health benefits. It has been found that oolong tea had remarkable health benefits such as anti-obesity effect (Yang et al., 2015), antioxidant effect (Chen H et al., 2009), and antitumor effect (Matsumoto et al., 1996). Among various oolong teas, Zhangping Narcissus tea cake is considered as the most special one because of its pressure processing which leads to a distinct shape and “mysterious” scent. Zhangping Narcissus tea cake is made from the tea plant variety called Fujian Narcissus and its unique processing includes setting, sun fixation, fresh leaf airing, leaf laying, fixation, rolling, shaping, and hot-air drying. This tea is the most characteristic tea with its distinct shape, special floral scent, and mellow taste compared to other teas. However, to the best of our knowledge, little information is known on the phenolic compounds in this tea cake and their potential bioactivity and anticancer activity.
The 4T1 murine breast cancer cell is a transplantable breast tumor cell line that is highly tumorigenic and invasive. It spontaneously metastasizes from the primary site to multiple distant sites, which is widely applied in human breast cancer study. Thus, taking this cell as a research model is suitable for evaluating the effects of oolong tea extracts on human breast cancer in vitro.
In the present study, three phenolic-enriched extracts were obtained from Zhangping Narcissus tea cake using three solvents with various polarities to compare their chemical constituents and antioxidant activity. In addition, inhibitory effects of the extracts on growth and migration of 4T1 tumor cells were comparatively studied, and this may help assess, in a fundamental way, the medical value of Zhangping Narcissus tea cake in the food and pharmacological industry.
2. Materials and methods
2.1. Materials and reagents
Zhangping Narcissus tea cake was obtained from Baolong Tea Company (Fujian Province, China). Folin-Ciocalteu’s phenol reagent, 3-(2-pyridyl)-5,6-diphenrl-1,2,4-triazine-4',4-disulfonic acid sodium salt (Ferrozine), 2,2-diphenyl-1-pikryl-hydrazyl (DPPH), 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), 2,4,6-tripyridyl-S-triazine (TPTZ), epigallocatechin gallate (EGCG), gallic acid (GA), butylated hydroxytoluene (BHT), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), rutin, vanillin, ninhydrin, and the standards for high-performance liquid chromatography (HPLC) analysis were purchased from Sigma-Aldrich, Germany. RPMI-1640 medium, fetal bovine serum (FBS), and trypsin were purchased from Gibco, USA. The cell cycle detection kit was obtained from KeyGen Biotech, China. All the other relevant reagents were purchased from Sinopharm Chemical Reagent Co., Ltd., China.
2.2. Cell lines
4T1 mouse mammary carcinoma cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 medium with 10% (0.1 g/ml) FBS. Cells were maintained in a humidified atmosphere with 5% (v/v) CO2 at 37 °C.
2.3. Preparations of phenolic-enriched extracts from Zhangping Narcissus tea cake
Zhangping Narcissus tea cake (100 g) was mixed with 1 L acetone (70%, v/v) at room temperature for 1 h and the extraction was repeated three times. The total extracted solution was concentrated to 200 ml by a vacuum evaporator at 50 °C. The concentrated solution was then fractionated with 200 ml chloroform, ethyl acetate, and n-butanol three times, respectively. The ethyl acetate, n-butanol, and water fractions were concentrated by the vacuum evaporator and dried with a freeze drier, and the fractions were named ZEF, ZBF, and ZWF, respectively (Fig. S1).
2.4. Chemical compound analysis
Total phenolic content was determined using the Folin-Ciocalteu method of Meda et al. (2005). Total flavonoid content was determined using the method of Kim et al. (2011). The content of procyanidin was measured according to the method of Garcia-Parrilla et al. (1997). The sugar content was determined by the method of Morris (1948) with glucose as standard. The protein content was determined by the method of Bradford (1976) using bovine serum albumin (BSA) as the standard. The amino acid content was measured using Nihydrin Colorimetry (Sun et al., 2006) with a standard of theanine. Caffeine and tea catechins, including GA, theaflavin (TF1), (+)-catechin (C), (+)-catechin gallate (CG), (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), (−)-epigallocatechin gallate (EGCG), (+)-gallocatechin (GC), and (+)-gallocatechin gallate (GCG), were measured based on the HPLC method of Liang et al. (2007), in a Shimadzu SCL-10A HPLC system (Shimadzu Corporation, Tokyo, Japan). Ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) analysis was performed using ACQUITY UPLC system (Waters, Milford, MA, USA) coupled to a Quattro Premier XE mass spectrometer equipped with an electrospray interface, according to the method of Monbaliu et al. (2010).
2.5. Determination of antioxidant activity
The DPPH scavenging activity, ABTS scavenging capacity, and ferric-reducing antioxidant power (FRAP) assays were used to evaluate the antioxidant activity of the extracts. The DPPH assay was determined by the method of Mohsen and Ammar (2009). The ABTS assay was carried out according to the method of Cai et al. (2004). The FRAP assay was performed according to the method of Benzie and Strain (1999). EGCG and BHT were used as the positive controls in the three assays.
2.6. Cell viability assay
The effects of ZEF and ZBF on the viability of 4T1 cells were measured by MTT assay. Briefly, 4T1 cells were seeded in 96-well plates by 1×105 cells per well with treatments of different sample concentrations (0, 50, 100, 150, and 200 μg/ml) for 24 and 48 h. After adding 20 μl MTT to each well with 4 h incubation, 100 μl dimethyl sulfoxide (DMSO) was added and the 96-well plate was agitated for 5 min. The absorbance was measured at 570 nm.
2.7. Cell cycle analysis assay
After treatment with ZEF, ZBF, and EGCG at the concentrations of 0, 50, 100, 150, and 200 μg/ml for 24 h, 4T1 cells were collected for further cell cycle analysis. After phosphate buffered saline (PBS) washing, cells were fixed in cold 70% (v/v) ethanol and put in −20 °C fridge overnight. Then the cells were washed with PBS before staining (100 μg/ml of RNase A, 25 μg/ml of propidium iodide (PI)), and incubated at 4 °C for 30 min. Cell cycle analysis was measured by the flow cytometer (Lai et al., 2012).
2.8. Wound healing assay
4T1 cells (5×105 cells/ml, 1 ml/well) were put into 6-well plates and incubated until 90% confluent. Cells were starved for 24 h and the confluent monolayers were wounded by scratching lines with a 200-μl pipette tip. The cells were washed with PBS, and observed and photographed under the microscope. Cells were treated with RPMI-1640 medium containing 1% (v/v) FBS with ZEF, ZBF, and EGCG, at concentrations of 0, 50, 100, 150, and 200 μg/ml. Images of the wound surfaces were recorded after 24 and 48 h (Yuan et al., 2013).
2.9. Cell migration assay
Cell migration assay was performed using chambers (24-well, 8-μm pore size, Corning, NY, USA) according to the method of Chen Y et al. (2009) with some modifications. A volume of 600 μl RPMI-1640 medium containing 20% (v/v) FBS was used as attractant in the lower chamber. A volume of 100 μl RPMI-1640 medium containing 5×105 cells was added to the upper compartment of the insert and was allowed to migrate through the pores. Cells on the lower side of the insert filter were fixed by 4% (0.04 g/ml) paraformaldehyde for 10 min and then stained with 0.1% (1 g/L) crystal violet in 0.5% (v/v) ethanol for 15 min. Numbers of cells on the underside of the filter from five randomly selected microscopic views were counted and photographed.
2.10. Statistical analysis
All the experiments were carried out in triplicate. Data were expressed as mean±standard deviation (SD) and evaluated by analysis of variance (ANOVA) using SPSS (Version 16.0). P<0.05 was considered statistically significant. Heatmap and hierarchical cluster were generated in Rstudio (Version 3.2.0).
3. Results
3.1. Bioactive compounds in tea extracts
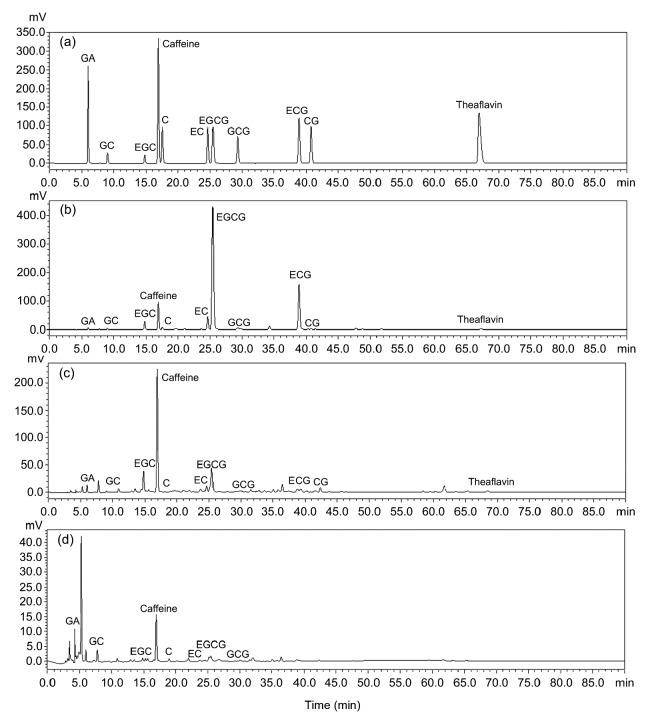
Acetone (70%) was used to get the crude extracts in the first step since much literature mentioned that it was a suitable solvent for phenolic compound extraction (Chavan et al., 2001; Kuźma et al., 2014). Then the crude extraction was extracted with chloroform to remove caffeine. The chemical compositions of the three extracts are shown in Table 1. ZEF, which was extracted by ethyl acetate, had the highest amount of phenolics (678.8 mg/g), flavonoids (529.6 mg/g), and proteins (32.9 mg/g). ZBF, extracted by n-butanol, also had many phenolics (373.9 mg/g) and flavonoids (264.6 mg/g), and contained the highest amount of procyanidins (453.3 mg/g) and sugars (131.5 mg/g). ZWF, obtained by water extraction, contained the lowest amount of compounds. HPLC analysis was used to compare the amount of different catechin monomers and caffeine among these three extracts. As shown in Fig. 1 and Table 2, the result of HPLC was similar with that exhibited in Table 1. ZEF contained the highest catechin monomer content, especially the content of EGCG ((495.7±1.7) mg/g), which was about 8 times higher than that of ZBF ((57.4±0.5) mg/g) and 4125 times higher than that of ZWF ((0.1±0.0) mg/g). ZBF had the highest amount of caffeine ((63.6±0.6) mg/g), while ECG, CG, or TF1 was not detected in ZWF.
Table 1.
Content of catechin monomers from ZEF, ZBF, and ZWF
| Sample | Main chemical composition (mg/g) |
|||||
| Phenolics | Flavonoids | Procyanidins | Sugars | Proteins | Amino acids | |
| ZEF | 678.8±20.2a | 529.6±15.6a | 419.3±8.0b | 26.4±0.8c | 32.9±0.5a | 2.9±0.1b |
| ZBF | 373.9±19.2b | 264.6±22.3b | 453.3±8.8a | 131.5±3.9a | 24.5±0.9b | 4.6±0.1a |
| ZWF | 73.0±8.2c | ND | 22.6±0.6c | 78.3±3.3b | 8.4±0.7c | 4.9±0.5a |
ZEF, ZBF, and ZWF are extracts from Zhangping Narcissus tea cake by ethyl acetate, n-butanol, and water, respectively. All the main chemical compositions are calculated based on the weight of extracts. ND means not detected. a–c Different letters mean significant difference (P<0.05). Data are expressed as mean±SD (n=3)
Fig. 1.
HPLC chromatograms of catechin monomers and theaflavin in ZEF, ZBF, and ZWF
(a) Standard mixture; (b–d) ZEF (b), ZBF (c), and ZWF (d) are extracts from Zhangping Narcissus tea cake by ethyl acetate, n-butanol, and water, respectively
Table 2.
Main chemical compositions of ZEF, ZEB, and ZWF
| Sample | Catechin monomer (mg/g) |
|||||
| GA | GC | EGC | Caffeine | C | EC | |
| ZEF | 1.3±0.0 | 14.2±0.5 | 136.3±1.7 | 30.7±0.3 | 4.6±0.1 | 55.8±0.9 |
| ZBF | 3.9±0.4 | 18.0±0.3 | 188.0±1.0 | 63.6±0.6 | 0.2±0.0 | 13.1±0.1 |
| ZWF | 2.4±0.6 | 6.1±0.2 | 5.1±0.1 | 0.2±0.0 | 0.3±0.0 | 2.4±0.5 |
|
| ||||||
|
| ||||||
| Sample | Catechin monomer (mg/g) | |||||
|
| ||||||
| EGCG | GCG | ECG | CG | TF1 | ||
|
| ||||||
| ZEF | 495.7±1.7 | 9.5±0.8 | 124.0±1.0 | 1.4±0.1 | 1.3±0.0 | |
| ZBF | 57.4±0.5 | 1.2±0.0 | 5.5±0.1 | 0.4±0.0 | 0.3±0.0 | |
| ZWF | 0.1±0.0 | 0.5±0.0 | ND | ND | ND | |
ZEF, ZBF, and ZWF are extracts from Zhangping Narcissus tea cake by ethyl acetate, n-butanol, and water, respectively. The catechin monomer content is calculated based on the weight of extracts. ND means not detected. Data are expressed as mean±SD (n=3)
Furthermore, UPLC-MS assay was used to analyze other monomers that ZEF and ZBF contained (Figs. S2 and S3). The results in Table 3 reveal that (−)-epigallocatechin 3-O-(4''-O-methyl) gallate and theaflavin were detected in ZEF, while ZBF turned out to have nine monomers such as quinic acid, myricetin-3-O-galactose, and quercetin-3-O-galactose-rutinoside.
Table 3.
Identification of the compounds of ZEF and ZBF on single-dimension UPLC-MS
| Sample | Retention time (min) | [M−H]− (m/z) | Identification |
| ZEF | 15.08 | 471 | (−)-Epigallocatechin 3-O-(4''-O-methyl) gallate |
| 20.73 | 563 | Theaflavin | |
| ZBF | 6.74 | 190 | Quinic acid |
| 14.31 | 479 | Myricetin-3-O-galactose | |
| 14.80 | 771 | Quercetin-3-O-galactose-rutinoside | |
| 14.97 | 593 | Kaempferol-3-O-p-coumaroyl-glucose | |
| 15.09 | 771 | Quercetin-3-O-glucose-rutinoside | |
| 16.14 | 755 | Kaempferol-3-O-galactose-rutinoside | |
| 19.58 | 1049 | Quercetin-3-O-glucose-rhamnose-(p-coumaroyl-arabinose)-hexoside | |
| 20.45 | 1033 | Galloylated prodelphinidin | |
| 20.73 | 563 | Theaflavin |
3.2. Antioxidant activity of the tea extracts
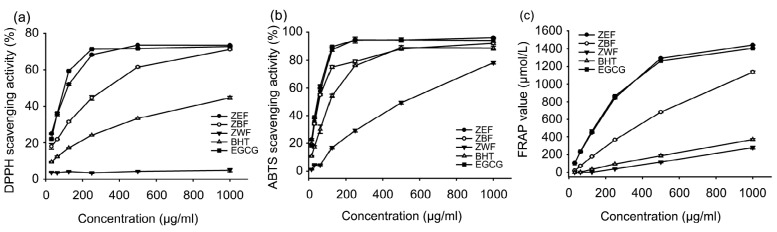
Due to the complex nature of phytochemicals, the antioxidant activity of the extracts was evaluated by three different assays. DPPH scavenging activity has been widely used to test the free radical scavenging ability of various natural products (Wang et al., 2011). Scavenging capacity of ABTS is more reactive than that of DPPH radicals and involves an H atom transfer process (Kaviarasan et al., 2007). FARP shows the antioxidant activity of any substance in the reaction medium as reducing ability (Shi et al., 2009). The DPPH scavenging abilities of ZEF, ZBF, and ZWF are shown in Fig. 2a. BHT and EGCG were used as the positive controls. ZEF, ZBF, and two controls (BHT and EGCG) showed effective scavenging capability on DPPH radicals in a dose-dependent way. In the tested concentration range, the DPPH scavenging ability was in the order of EGCG>ZEF>ZBF>BHT, with their IC50 (half maximal inhibitory concentration) values of 93.3, 112.7, 310.5, and 971.9 μg/ml, respectively (Table 4).
Fig. 2.
Antioxidant activity of ZEF, ZBF, ZWF, BHT, and EGCG and inhibition of 4T1 cell growth by ZEF, ZBF, and EGCG
(a) DPPH scavenging activity; (b) ABTS scavenging capacity; (c) FRAP. ZEF, ZBF, and ZWF are extracted from Zhangping Narcissus tea cake by ethyl acetate, n-butanol, and water, respectively. BHT and EGCG are the positive controls. Error bars indicate the standard deviations (n=3)
Table 4.
Effective concentrations for the antioxidant activity of three extracts and two controls
| Group | DPPH assay (IC50, μg/ml) | ABTS assay (IC50, μg/ml) | FRAP assay (μg/ml) |
| ZEF | 112.7 | 43.3 | 1292.3 |
| ZBF | 310.5 | 60.0 | 683.7 |
| ZWF | >1000.0 | 468.9 | 116.7 |
| BHT | 971.9 | 115.1 | 187.3 |
| EGCG | 93.3 | 42.4 | 1262.8 |
ZEF, ZBF, and ZWF are extracted from Zhangping Narcissus tea cake by ethyl acetate, n-butanol, and water, respectively. FRAP value was calculated at 500 μg/ml
The ABTS scavenging capacity of the three extracts and two controls are shown in Fig. 2b. In this assay, ZEF exhibited the strongest ABTS scavenging capacity with an IC50 value of 43.3 μg/ml, followed by ZBF (IC50 value of 60.0 μg/ml), BHT (IC50 value of 115.1 μg/ml), and ZWF (IC50 value of 468.9 μg/ml). EGCG turned out to have a similar ABTS scavenging capacity as that of ZEF, with IC50 value of 42.4 μg/ml (Table 4).
As presented in Fig. 2c and Table 4, when treated at 500 μg/ml, ZEF possessed the highest FRAP value of 1292.3 μg/ml, followed by EGCG (1262.8 μg/ml), ZBF (683.7 μg/ml), BHT (187.3 μg/ml), and ZWF (116.7 μg/ml). The result was dose-dependent on ferric-reducing power which was increased in the order of ZEF>EGCG>ZBF>BHT>ZWF.
3.3. Correlation between the antioxidant activity and the amount of main bioactive compounds in the tea extracts
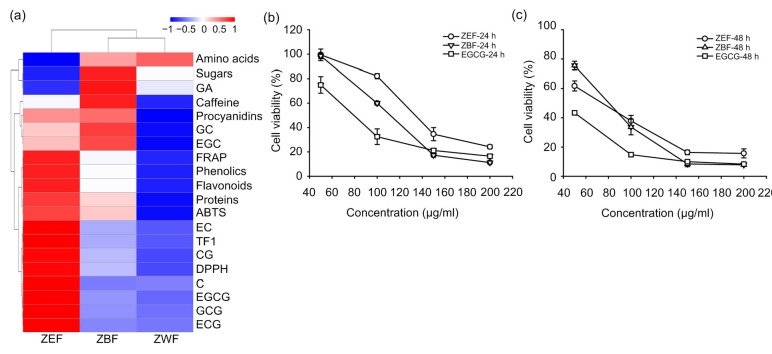
To unveil the correlation between the antioxidant activity and the chemical composition of the tea extracts from the tea cake, Pearson correlations were calculated between the main chemical compounds and antioxidant activity of the phenolic-enriched extracts (Table 5). The amounts of phenolics, flavonoids, procyanidins, and proteins of the extracts were significantly (P<0.01) associated with their DPPH scavenging ability, ABTS scavenging ability, and FRAP. To further validate the correlation, hierarchical clustering of chemical composition and antioxidant activity of the extracts was generated in Rstudio. As can be seen in Fig 3a, ZEF contained higher bioactive compounds and possessed better antioxidant capabilities than ZBF and ZWF. The amounts of phenolics, flavonoids, proteins, TF1, and some catechin monomers (such as EC, EGCG, GCG, ECG, C, and CG) of the extracts were clustered into one group with the three antioxidant assays, which indicated that these main bioactive compounds were highly correlated with the antioxidant activity of the tea extracts. However, the amounts of amino acids, sugars, and GA were not so related with the antioxidant capacities of tea extracts in this study. These results were consistent with our Pearson analysis results.
Table 5.
Pearson correlation analysis between main chemical compositions and antioxidant activity of ZEF, ZBF, and ZWF
| Main chemical composition | DPPH scavenging ability |
ABTS scavenging ability |
FRAP value |
|||
| Pearson correlation | P value | Pearson correlation | P value | Pearson correlation | P value | |
| Total phenolic content | 0.80** | 0.00 | 0.64** | 0.00 | 0.94** | 0.00 |
| Total flavonoid content | 0.80** | 0.00 | 0.64** | 0.00 | 0.94** | 0.00 |
| Procyanidin content | 0.80** | 0.00 | 0.67** | 0.00 | 0.90** | 0.00 |
| Sugar content | 0.27 | 0.27 | 0.44 | 0.07 | 0.34 | 0.17 |
| Protein content | 0.75** | 0.00 | 0.69** | 0.00 | 0.90** | 0.00 |
| Amino acid content | 0.24 | 0.34 | 0.50* | 0.04 | 0.40 | 0.10 |
P<0.05,
P<0.01
3.4. Inhibitory effects of extracts on the growth of 4T1 cells
Inhibitory effects of ZEF and ZBF on the growth of 4T1 cells were further investigated due to the abundance of chemical compounds and outstanding antioxidant ability. As shown in Figs. 3b and 3c, different concentrations of ZEF and ZBF exhibited a time-and dose-dependent inhibition on 4T1 cell growth compared to the control group. The IC50 value of ZEF was 136.5 μg/ml by 24-h treatment and it went to 67.6 μg/ml after 48-h treatment. Similar results were observed on the IC50 values of ZBF and EGCG at the 24th and 48th hour, which changed from 111.3 to 75.3 μg/ml and from 79.4 to 40.9 μg/ml, respectively.
Fig. 3.
Hierarchical clustering of chemical constitution and antioxidant activity in ZEF, ZBF, and ZWF (a) and inhibition of growth by ZEF, ZBF, and EGCG in 4T1 cells for 24 h (b) and 48 h (c)
The values of DPPH and ABTS were used as 1/IC50 value. 4T1 cells were treated with 50, 100, 150, and 200 μg/ml ZEF, ZBF, and EGCG for 24 and 48 h. Cell viability was monitored by MTT assay. Data are expressed as mean±SD (n=3)
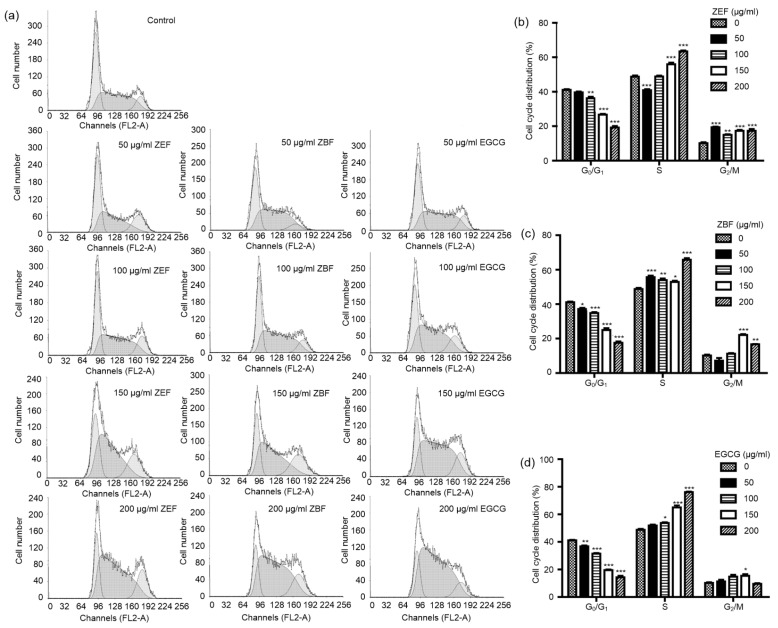
After incubation with different concentrations of ZEF and ZBF (ranging from 50 to 200 μg/ml) for 24 h, cell cycle distributions were analyzed by the flowcytometry method (FCM). As shown in Fig. 4, with the concentrations of ZEF, ZBF, and EGCG increased, the percentage of cells in G0/G1 phase significantly (P<0.05) declined, while the percentages of cells in S phase and G2/M phase moderately increased. The percentages of cells in G2/M phase treated by ZEF and ZBF increased much more than that of EGCG.
Fig. 4.
ZEF, ZBF, and EGCG caused the accumulation of the 4T1 cells in the S and G2/M phases
4T1 cells were treated with 50, 100, 150 and 200 μg/ml ZEF, ZBF, and EGCG for 24 h. The cells were washed, fixed, stained with propidiumiodide, and analyzed for DNA content by flow cytometry. (a) One representative result is shown. (b–d) Percentages of 4T1 cells in different phases of cell cycle after ZEF (b), ZBF (c), and EGCG (d) treatments. * P<0.05, ** P<0.01, *** P<0.001 as compared with control (0 μg/ml)
3.5. Effects of extracts on the migration and invasion of 4T1 cells
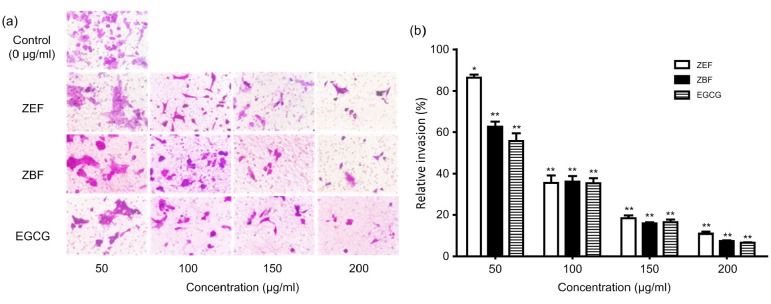
We used wound healing assay and Transwell assay to evaluate the effect of tea extracts on the migration of 4T1 cells in vitro. Phenolic-enriched extracts from the tea cake significantly inhibited the migration of 4T1 cells both after 24-h and 48-h treatments based on the results of the wound healing assay in Fig. 5. These two extracts exhibited the same outstanding inhibitory abilities on 4T1 cells migration as EGCG. After the treatments of the Zhangping Narcissus tea cake extracts and EGCG at the concentrations of 50, 100, 150, and 200 μg/ml for 24 h, the number of invasion cells reduced significantly (P<0.05) compared to the control group (Fig. 6). The relative invasion ratio of 4T1 cells treated by ZEF, ZBF, and EGCG was 86.2%, 62.7%, and 55.7%, respectively, at the concentration of 50 μg/ml. Furthermore, the results also suggested that the phenolic-enriched extracts from the tea cake and EGCG have effective inhibition on migration and invasion of 4T1 cells in a dose-dependent manner (Fig. 6b).
Fig. 5.
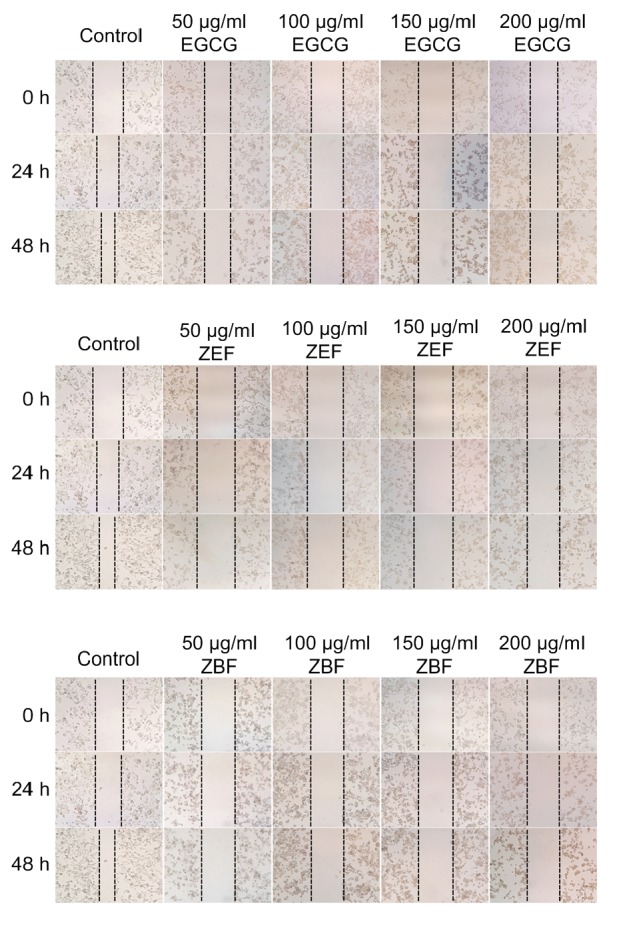
Effects of ZEF, ZBF, and EGCG on 4T1 cell migration by wound healing assay after 24 and 48 h incubation at 50, 100, 150, and 200 μg/ml (×100 magnification)
Fig. 6.
Effects of ZEF, ZBF, and EGCG on 4T1 cell migration and invasion
4T1 cells were treated with 0, 50, 100, 150, and 200 μg/ml ZEF, ZBF and EGCG for 24 h. (a) Invasion images were taken with microscopy (×400 magnification). (b) Relative invasion ratio was calculated by counting three different images of migration cell numbers. * P<0.05, ** P<0.01 as compared with control (0 μg/ml). Data are expressed as mean±SD (n=3)
4. Discussion
Phenolics, as the main bioactive compounds in tea, are supposed to be beneficial for human health. Fermentation, a key step in the process of tea making, determines the distinct flavor and presentation of teas by directly altering chemical composition. As is known, phenolics (mainly catechins) are susceptible to fermentation and of being easily oxidized. About 30% catechins and 20% proanthocyanidins were oxidized in the manufacture of oolong tea from fresh tea shoots, and 20% of total flavonoids were decomposed in a follow-up drying process (Dou et al., 2007). Zhangping Narcissus tea cake is classified into oolong tea because of its slight fermentation, and it is compressed to a cake shape. However, little is known about the alteration of phenolics in this tea cake or their potential bioactivity despite it being so special. In the present study, three phenolic-enriched extracts named ZEF, ZBF, and ZWF were obtained from Zhangping Narcissus tea cake using ethyl acetate, n-butanol, and water, respectively. It was found that ZEF and ZBF possessed higher amounts of phenolics, flavonoids, and procyanidins than did ZWF. Moreover, ZEF had the highest amount of EGCG while ZBF had the highest amount of EGC based on HPLC analysis. Clearly, ethyl acetate is a good option for effective extraction of phenolic extracts (Singh et al., 2007). According to UPLC-MS results, ZEF contained some catechin monomers, theaflavin, and methylated catechin derivatives while ZBF was shown to have some catechin dimers and flavonoid glycosides such as kaemperol, myricetin, and quercetin, which is consistent with some previous research (Meda et al., 2005; Dou et al., 2007).
Much evidence suggests that oxidative stress is associated with a wide range of diseases (Finkel and Holbrook, 2000). Therefore, the antioxidant activity of bioactive compounds could be very important for human health. In this study, the antioxidant ability of three phenolic-enriched extracts was evaluated by three different assays including DPPH, ABTS, and FRAP. All the results based on the three different assays suggested that ZEF was the most potent phenolic-enriched extract, followed by ZBF and ZWF. The results of Pearson correlation analysis and hierarchical clustering indicated that the main bioactive compounds (such as phenolics, flavonoids, procyanidins, proteins, TF1, and catechin monomers) were highly correlated with the antioxidant activity of the tea extracts. Phenolics are the most abundant phytochemicals in many kinds of fruits, vegetables, and tea and were considered to be strongly associated with beneficial effects in many metabolic diseases, which suggested that they could reduce low-density lipoprotein (LDL) oxidation, decrease blood pressure, regulate inflammation, and inhibit cancer cell proliferation (Milenkovic et al., 2013). Procyanidins are a type of polyphenolic secondary metabolites that can be either oligomeric or polymeric and are widely distributed in different plants (Fraser et al., 2012). Moreover, protein contents of the extracts were found to have significant associations with their antioxidant activity, especially with FRAP. Some studies also mentioned that protein content was somehow related to the antioxidant capacity (Huang et al., 2010). However, total sugar and amino acid content of the extracts were not so related with the antioxidant capacity of tea extracts in this study.
ZEF exhibited a similar strong antioxidant ability to EGCG in this study, which may indicate some synergistic interactions of different bioactive compounds in the tea extracts. ZEF contained EGCG and other kinds of phenolics and flavonoids and exhibited similar bioactivity to EGCG in our study, which may indicate that other types of phenolics and flavonoids that have been reported to exhibit weaker antioxidant activity than EGCG may have synergistic interactions and then lead to strong antioxidant capacities. EGCG and other bioactive compounds have been reported to have synergistic interaction in cancer and other disease therapy (Du et al., 2013; Chen et al., 2014; Liu et al., 2016), which may support our hypothesis.
Moreover, phenolic-enriched extracts from Zhangping Narcissus tea cake showed strong inhibition on 4T1 murine breast cancer cells. After treatment for 24 and 48 h, the growth of 4T1 cells was significantly inhibited in a dose-dependent manner, but there was no significant difference (P>0.05) on inhibition of 4T1 growth between ZEF and ZBF both in 24 h (P=0.64) and 48 h (P=0.93) treatments. There was also no significant (P>0.05) difference between ZEF, ZBF, and EGCG, which suggested that ZEF and ZBF exhibited excellent growth inhibitory effect on 4T1 cells in vitro. Cell cycle results showed that the percentages of 4T1 cells in S phase and G2/M phase were increased after treatment for 24 h while the percentage of G0/G1 phase was decreased, which meant that 4T1 cells were arrested in S phase and G2/M phase from DNA synthesis. The inhibition of 4T1 growth might be due to the impact that phenolic-enriched extracts have on cell cycles. Migration and invasion ability of 4T1 cells was reduced by the treatment of the extracts, which illustrated that the extracts not only inhibited the growth of 4T1 cells, but also suppressed metastatic capacity of 4T1 cells. Phenolic-enriched extracts from Zhangping Narcissus tea cake exhibited similarly excellent antioxidant abilities and inhibition on growth and metastatic capacity on 4T1 murine breast cancer cells to that of EGCG. The phenolic-enriched extracts not only contained some catechin monomers, but also catechin dimers and polymers, which suggested that phenolic compounds such as catechin dimers and polymers may also have outstanding bioactivity.
However, our study had some limitations that are important to acknowledge. Bioavailability of the bioactive compounds from plant extracts in mice or human study is a concern in many studies. However, here, we focused on the in vitro effects of the Zhangping Narcissus tea cake extracts on the 4T1 murine breast cancer cells in this study and we used different concentrations (0, 50, 100, 150, and 200 μg/ml, respectively) of the tea cake extracts in cell viability assay, cell cycle assay, wound healing assay, and cell migration assay. The results indicated a time-and dose-dependent inhibition on 4T1 cell growth and effective inhibitions on migration and invasion of 4T1 cells. However, all these experiments were carried out in vitro systems. We can continue the in vivo anti-tumor study of Zhangping Narcissus tea cake extracts and the efficient concentrations of the tea extracts in mice models, which can be detected due to the bioavailability of bioactive compounds from tea extracts. Some studies have already reported the bioavailability of some important compounds in tea extracts such as EGCG, which showed that the plasma bioavailability of EGCG after intragastric administration in a mouse model was about 26.5% (Lambert et al., 2003). These previous studies may help us to design in vivo experiments more reasonably.
5. Conclusions
Based on the results obtained from this study, we suggest that the phenolic-enriched extracts from Zhangping Narcissus tea cake exhibited strong antioxidant capacity in vitro due to its main bioactive compounds of phenolics, flavonoids, etc., and remarkable inhibition on the growth, migration, and invasion of 4T1 murine breast cancer cells. Thus, the phenolic-enriched extracts from Zhangping Narcissus tea cake are supposed to have a potential application in food and pharmaceutical industry as an antioxidant and anticancer drug in future.
List of electronic supplementary materials
Flow chart of phenolic-enriched extracts from Zhangping Narcissus tea cake
UPLC-MS chromatograms of ZBF
UPLC-MS chromatograms of ZEF
Footnotes
Project supported by the Science and Technology Department of Guangdong Province, China (No. 2016B090918118)
Electronic supplementary materials: The online version of this article (https://doi.org/10.1631/jzus.B1700162) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Le YING, De-dong KONG, Yuan-yuan GAO, Feng YAN, Yue-fei WANG, and Ping XU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/S0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 2.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Cai Y, Luo Q, Sun M, et al. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavan U, Shahidi F, Naczk M. Extraction of condensed tannins from beach pea (Lathyrus maritimus L.) as affected by different solvents. Food Chem. 2001;75(4):509–512. doi: 10.1016/S0308-8146(01)00234-5. [DOI] [Google Scholar]
- 5.Chen H, Qu Z, Fu L, et al. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J Food Sci. 2009;74(6):C469–C474. doi: 10.1111/j.1750-3841.2009.01231.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Ye HL, Zhang G, et al. Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells. PLoS ONE. 2014;9(1):e85771. doi: 10.1371/journal.pone.0085771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Lu B, Yang Q, et al. Combined integrin phosphoproteomic analyses and small interfering RNA-based functional screening identify key regulators for cancer cell adhesion and migration. Cancer Res. 2009;69(8):3713–3720. doi: 10.1158/0008-5472.CAN-08-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dou J, Lee VSY, Jason TC, et al. Identification and comparison of phenolic compounds in the preparation of oolong tea manufactured by semifermentation and drying processes. J Agric Food Chem. 2007;55(18):7462–7468. doi: 10.1021/jf0718603. [DOI] [PubMed] [Google Scholar]
- 9.Du GJ, Wang CZ, Qi LW, et al. The synergistic apoptotic interaction of panaxadiol and epigallocatechin gallate in human colorectal cancer cells. Phytother Res. 2013;27(2):272–277. doi: 10.1002/ptr.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 11.Fraser K, Harrison SJ, Lane GA, et al. HPLC-MS/MS profiling of proanthocyanidins in teas: a comparative study. J Food Compost Anal. 2012;26(1-2):43–51. doi: 10.1016/j.jfca.2012.01.004. [DOI] [Google Scholar]
- 12.Garcia-Parrilla MC, Heredia FJ, Troncoso AM, et al. Spectrophotometric determination of total procyanidins in wine vinegars. Talanta. 1997;44(1):119–123. doi: 10.1016/S0039-9140(96)02012-7. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Deng Q, Xie B, et al. Purification and characterization of an antioxidant protein from ginkgo biloba seeds. Food Res Int. 2010;43(1):86–94. doi: 10.1016/j.foodres.2009.08.015. [DOI] [Google Scholar]
- 14.Kaviarasan S, Naik GH, Gangabhagirathi R, et al. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 2007;103(1):31–37. doi: 10.1016/j.foodchem.2006.05.064. [DOI] [Google Scholar]
- 15.Kim IS, Yang MR, Lee OH, et al. Antioxidant activities of hot water extracts from various spices. Int J Mol Sci. 2011;12(12):4120–4131. doi: 10.3390/ijms12064120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuźma P, Drużyńska B, Obiedzinski M. Optimization of extraction conditions of some polyphenolic compounds from parsley leaves (Petroselinum crispum) Acta Sci Pol Technol Aliment. 2014;13(2):145–154. doi: 10.17306/J.AFS.2014.2.4. [DOI] [PubMed] [Google Scholar]
- 17.Lai L, Fu Q, Liu Y, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharm Sin. 2012;33(4):523–530. doi: 10.1038/aps.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert JD, Lee MJ, Lu H, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133(12):4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 19.Liang H, Liang Y, Dong J, et al. Decaffeination of fresh green tea leaf (Camellia sinensis) by hot water treatment. Food Chem. 2007;101(4):1451–1456. doi: 10.1016/j.foodchem.2006.03.054. [DOI] [Google Scholar]
- 20.Liu Z, Luo Z, Jia C, et al. Synergistic effects of Potentilla fruticosa L. leaves combined with green tea polyphenols in a variety of oxidation systems. J Food Sci. 2016;81(5):C1091–C1101. doi: 10.1111/1750-3841.13292. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto N, Kohri T, Okushio K, et al. Inhibitory effects of tea catechins, black tea extract and oolong tea extract on hepatocarcinogenesis in rat. Jpn J Cancer Res. 1996;87(10):1034–1038. doi: 10.1111/j.1349-7006.1996.tb03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meda A, Lamien CE, Romito M, et al. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91(3):571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 23.Milenkovic D, Jude B, Morand C. MiRNA as molecular target of polyphenols underlying their biological effects. Free Radical Biol Med. 2013;64:40–51. doi: 10.1016/j.freeradbiomed.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 24.Mohsen SM, Ammar ASM. Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chem. 2009;112(3):595–598. doi: 10.1016/j.foodchem.2008.06.014. [DOI] [Google Scholar]
- 25.Monbaliu S, Wu A, Zhang D, et al. Multimycotoxin UPLC-MS/MS for tea, herbal infusions and the derived drinkable products. J Agric Food Chem. 2010;58(24):12664–12671. doi: 10.1021/jf1033043. [DOI] [PubMed] [Google Scholar]
- 26.Morris DL. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science. 1948;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- 27.Shi J, Gong J, Liu J, et al. Antioxidant capacity of extract from edible flowers of prunus mume in China and its active components. LWT Food Sci Technol. 2009;42(2):477–482. doi: 10.1016/j.lwt.2008.09.008. [DOI] [Google Scholar]
- 28.Singh R, Singh S, Kumar S, et al. Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn. Food Chem Toxicol. 2007;45(7):1216–1223. doi: 10.1016/j.fct.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Sun SW, Lin YC, Weng YM, et al. Efficiency improvements on ninhydrin method for amino acid quantification. J Food Compost Anal. 2006;19(2-3):112–117. doi: 10.1016/j.jfca.2005.04.006. [DOI] [Google Scholar]
- 30.Tanaka T, Matsuo Y, Kouno I. Chemistry of secondary polyphenols produced during processing of tea and selected foods. Int J Mol Sci. 2009;11(1):14–40. doi: 10.3390/ijms11010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Bian X, Park J, et al. Physicochemical properties, in vitro antioxidant activities and inhibitory potential against α-glucosidase of polysaccharides from Ampelopsis grossedentata leaves and stems. Molecules. 2011;16(12):7762–7772. doi: 10.3390/molecules16097762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CS, Lambert JD, Sang S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch Toxicol. 2009;83(1):11–21. doi: 10.1007/s00204-008-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Qiao L, Zhang X, et al. Effect of methylated tea catechins from Chinese oolong tea on the proliferation and differentiation of 3T3-L1 preadipocyte. Fitoterapia. 2015;104:45–49. doi: 10.1016/j.fitote.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Yuan X, Yu L, Li J, et al. ATF3 suppresses metastasis of bladder cancer by regulating gelsolin-mediated remodeling of the actin cytoskeleton. Cancer Res. 2013;73(12):3625–3637. doi: 10.1158/0008-5472.CAN-12-3879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart of phenolic-enriched extracts from Zhangping Narcissus tea cake
UPLC-MS chromatograms of ZBF
UPLC-MS chromatograms of ZEF