Abstract
The dopaminergic hypothesis of schizophrenia is the main concept explaining the direct reasons of schizophrenia and the effectiveness of current antipsychotics. All antipsychotics present on the market are potent dopamine D2 receptor antagonists or partial agonists. In this work we investigate a series of dopamine D2 receptor antagonists which do not fulfill the criteria of the classical pharmacophore model as they do not possess a protonatable nitrogen atom necessary to interact with the conserved Asp(3.32). Such compounds are interesting, inter alia, due to possible better pharmacokinetic profile when compared to basic, ionizable molecules. By means of homology modeling, molecular docking and molecular dynamics we determined that the compounds investigated interact with Asp(3.32) via their amide nitrogen atom. It was found that the studied compounds stabilize the receptor inactive conformation through the effect on the ionic lock, which is typical for GPCR antagonists. We constructed a CoMFA model for the studied compounds with the following statistics: R2 = 0.95, Q2 = 0.63. The quality of the CoMFA model was confirmed by high value of R2 of the test set, equal 0.96. The CoMFA model indicated two regions where bulky substituents are favored and two regions where bulky substituents are not beneficial. Two red contour regions near carbonyl groups were identified meaning that negative charge would be favored here. Furthermore, the S-oxide group is connected with blue contour region meaning that positive charge is favored in this position. These findings may be applied for further optimization of the studied compound series.
Keywords: Dopamine D2 receptor, Dopamine D2 receptor antagonists, CoMFA, QSAR, Molecular modeling, Non-basic compounds.
Introduction
G protein-coupled receptors (GPCRs) account for approximately 30% of all current drug targets (Overington et al. 2006; Komatsu 2015). The dopamine receptors belong to monoamine GPCRs and they play a significant role in the pathophysiology and treatment of psychosis and movement disorders. In particular, multiple psychomotor signs and symptoms are affected by the activity of dopaminergic neurons and by drugs that selectively interact with neuronal dopamine receptors (Missale et al. 2010). These symptoms include rigidity in Parkinson’s disease, dyskinesia in Huntington’s disease, spontaneous oral dyskinesia in the elderly and hallucinations in Alzheimer’s disease and schizophrenia (Missale et al. 2010).
The causes of schizophrenia and—as a consequence—the mechanism of action of antipsychotic drugs are currently not adequately understood. The dopaminergic hypothesis of schizophrenia is the main concept explaining the direct reasons for schizophrenia and the effectiveness of current antipsychotics. According to this hypothesis the pathomechanism of schizophrenia is attributed to the dysfunction of dopaminergic receptors in the mesolimbic system (positive symptoms) and mesocortical pathway (negative symptoms).
Dopamine D2 receptor antagonists are used as antipsychotics. Starting from the discovery of the antipsychotic activity of chlorpromazine in 1952, all currently known antipsychotics exhibit affinity for dopamine D2-like receptors as an apparently essential aspect of their mechanism of action (Mailman and Murthy 2010), which is in accordance with the dopaminergic hypothesis of schizophrenia. This includes the third generation antipsychotics, aripiprazole, brexpiprazole and cariprazine. The activity of these drugs have been assigned to either D2 receptor partial agonism (dopamine receptor stabilizers) or D2 receptor functional selectivity (Mailman and Murthy 2010). However, a significant percentage of schizophrenia patients do not respond well to the available treatments. There are two other main limitations of current antipsychotics. Firstly, they are considerably efficient against positive symptoms of schizophrenia (e.g., hallucinations, delusions, and thought disorders) whereas they do not address well negative (e.g., anhedonia and social withdrawal) and cognitive symptoms (e.g., memory and attention deficits) of the disease. Secondly, in particular first generation antipsychotics, exert severe side effects, including neurological disorders termed as extrapyramidal symptoms, sexual dysfunctions, and deterioration of cognitive abilities. Second generation antipsychotics often have metabolic effects leading to weight gain. Thus, novel types of potential drugs against schizophrenia are currently under investigation.
All aminergic GPCRs and some other rhodopsin-like GPCRs, e.g., opioid receptors, share the negatively charged and conserved aspartate residue (D3.32) in the transmembrane helix 3 (TM3) which was generally proposed as a key anchor for the basic moieties of aminergic ligands (Shi and Javitch 2002; Surgand et al. 2006; Kooistra et al. 2013). Accordingly, most dopamine D2 receptor orthosteric ligands, including both agonists and antagonists fulfill the criteria of the classical pharmacophore model. The key element of this model is a protonatable nitrogen atom which is able to interact with the conserved Asp(3.32). A positively charged group is also a key element of the pharmacophore model recently constructed for the dopamine D2 receptor antagonists (Ekhteiari Salmas et al. 2016). It was found that common pharmacophore motives for the dopamine D2 receptor antagonists include AADPR, AADRR, AAHPR, AAPRR, ADHRR, ADPRR, AHHPR, AHHRR, AHPRR, and HHPRR (“A”—hydrogen bond acceptor, “D”—hydrogen bond donor, “H”—hydrophobic group, “P”—positively charged group, and “R”—aromatic ring), however the highest correlation coefficients for training set and test set compounds were found as 0.95 and 0.75, respectively at the AADPR.671 model. This work also indicated other top-ranked 3D QSAR hypotheses (AADRR.1398, AAPRR.3900, and ADHRR.2864) and two of them did not contain a positively charged group. This is in accordance with a few reports which have proven that the presence of a basic nitrogen atom enabling formation of the interaction of its protonated form and D3.32 is not indispensable for the dopamine D2 receptor anchoring (Xiao et al. 2014; Kaczor et al. 2016b). Non-basic ligands are also known for some other aminergic GPCRs, e.g., the serotonin 5-HT6 receptor (Ivachtchenko et al. 2010) and for opioid receptors, including κ opioid receptor ligand, salvinorin A and μ opioid receptor ligands, carbonyl derivatives of 1-aryl-2-iminoimidazolidine (Matosiuk et al. 2001, 2002a, b; Sztanke et al. 2005).
The development of non-basic, non-ionizable ligands for the treatment of central nervous system (CNS) diseases may be an important improvement taking into account the pharmacokinetics of a potential drug. The most important barrier for drug permeation is due to many lipid barriers which separate body compartments. The ionization state of the drug is an important factor as all the charged drugs diffuse through lipid environments with difficulty. The charged forms of drugs are aqueous-soluble and relatively lipid-insoluble so they does not pass biological membranes easily. pH and the drug pKa are crucial for determination of the ionization state and will significantly influence drug transport. As most drugs are weak acids and/or bases, knowledge of the dissociation constant can help in understanding the ionic form a compound will take across a range of pH values (Manallack 2008). This is especially crucial in physiological systems where ionization state will influence the rate at which the molecule is able to diffuse across membranes and obstacles such as the blood-brain barrier (BBB) (Manallack 2008). The pKa of a drug affects lipophilicity, solubility, protein binding and permeability which—as a consequence—directly influences pharmacokinetic (PK) features, like absorption, distribution, metabolism, and excretion (ADME) (Manallack 2008).
As it was already mentioned, Xiao et al. (2014) obtained a series of the dopamine D2 receptor antagonists without a protonatable nitrogen atom. Such ligands are worth detailed investigation as they may exhibit a unique pharmacological profile and may turn out better antipsychotics as well as may be developed as drugs with better pharmacokinetic properties. In this context, we studied the interactions of these dopamine D2 receptor antagonists without a protonatable nitrogen atom with the dopamine D2 receptor by means of homology modeling, molecular docking and molecular dynamics and we constructed a CoMFA model for them which can enable further modifications within this series of compounds.
Material and methods
Homology modeling
The homology model of the human dopamine D2 receptor (P14416) in inactive conformation and in complex with an antagonist eticlopride was constructed as previously described (Kaczor et al. 2016a, b, c). The X-ray structure of the dopamine D3 receptor in complex with an antagonist eticlopride (PDB ID: 3PBL) (Chien et al. 2010) was used as a template as it is currently the template with the highest sequence identity and similarity to the dopamine D2 receptor target. Multiple sequence alignment of 50 rhodopsin-like GPCRs was carried out with the GPCR module of MOE Molecular Environment and refined manually, in particular to satisfy disulfide bridges which were not automatically identified by the software. The dopamine D2 receptor model was built without N-terminus (the first 36 residues were removed, the model starts with Tyr37), and without the intracellular loop 3 (ICL3, residues Arg217-Lys362 were removed). Homology modeling was performed using Modeler v.9.10 (Webb and Sali 2014). A hundred homology models of the dopamine D2 receptor in complex with eticlopride were generated, and subsequently assessed by Modeler objective function and discrete optimized protein energy profiles (Shen and Sali 2006). The best model was subjected to quality assessments using the Schrödinger suite of software tool for Ramachandran plots.
Compound preparation
The investigated compounds (the reference ligand chlorprothixene and compounds 1–44 were modeled using the LigPrep protocol from the Schrödinger Suite. In order to sample different protonation states of ligands in physiological pH, Epik module was used. The compounds were further optimized with the Wavefunction Spartan10 software. The procedure involved geometry optimization performed with B3LYP DFT using the 6-31G(d,p) basis set.
Molecular docking
Molecular docking was performed using Glide from the Schrödinger suite of software. The grid file for chlorprothixene docking was generated at default settings, indicating eticlopride as a reference ligand. The complex of the dopamine D2 receptor with chlorprothixene was constructed using the SP (standard precision) protocol of Glide. The complex was further refined with induced-fit docking of Schrödinger suite of software and used for grid generation with default settings indicating chlorprothixene as a reference ligand. Molecular docking of compounds 1–44 was performed using the SP (standard precision) protocol of Glide. 50 poses were generated for each ligands. The selected docking poses were used for CoMFA alignment. PyMol v. 0.99 was used for visualization of results.
Molecular dynamics
Molecular dynamics studies of selected ligand-receptor complexes were performed using Desmond v. 3.0.3.1 (Bowers et al. 2006) as described previously (Kaczor et al. 2014; Jozwiak et al. 2014; Kaczor et al. 2016a, c). The complexes were inserted into POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) membrane, hydrated and ions were added to neutralize protein charges and then to the concentration of 0.15 M NaCl. The complexes were minimized and subjected to MD first in the NVT ensemble for 1 ns and then in NPT ensemble for 20 ns with the restrictions on the protein backbone in each case. The production run was performed in NPT ensemble with no restrictions for 50 ns. Analysis of molecular dynamics simulations was performed with application of Schrödinger suite of software tools.
CoMFA studies
Compounds were divided into the training set (40 compounds) and the test set (4 compounds). For constructing 3D-QSAR models, the compounds should span at least four orders of activity magnitude and be well proportioned in each activity magnitude (Yuan et al. 2014). The activity of the compounds was published elsewhere (Xiao et al. 2014). The AC50 (nM) values were converted into pAC50, which were applied as dependent variables for subsequent 3D-QSAR analyses. The pAC50 of compounds with AC50 above 77 μM was arbitrarily assigned the value of 3.
Molecular alignment is the most sensitive factor which has a significant effect on the 3D-QSAR models (Yuan et al. 2014). In this study, by identification of the binding conformations of the compounds, molecular alignment was obtained through molecular docking. Thus, all the molecules were well aligned in the binding site of the dopamine D2 receptor for developing 3D-QSAR model.
CoMFA model was developed applying the QSAR module in Sybyl v. 2.1. The standard Tripos force field was used for CoMFA analysis with Gasteiger-Hückel point charges and the default sp3 carbon probe with point charge +1.0 (Kaczor et al. 2015). The optimal number of components was designated so that Q2 was maximal and the standard error of prediction was minimal (Kaczor et al. 2015).
PLS analysis was applied to linearly correlate the CoMFA fields to the pAC50 activity values. The cross-validation analysis was performed using the leave-one-out (LOO) method, in which one compound is removed from the data set, and its activity is predicted using the model derived from the rest compounds of the data set (Kaczor et al. 2015). The model resulting in the highest Q2, optimum number of components (ONC), and the lowest standard error of prediction were taken for further analysis. In addition, the statistical significance of the model was described by the standard error of estimate (SEE) and probability value (F value) (Kaczor et al. 2015).
The predictive capability of the 3D-QSAR model was evaluated with the external test set of 4 compounds. The test set molecules were also optimized and aligned in the same manner as described above, and their activities were predicted using the developed model.
Results and discussion
Homology modeling
Homology model of the human dopamine D2 receptor (P14416) in inactive conformation and in complex with an antagonist eticlopride was built using homology modeling with Modeler 9.10 (Webb and Sali 2014) and X-ray structure of the dopamine D3 receptor in complex with eticlopride (PDB ID: 3PBL) (Chien et al. 2010) as a template as previously described (Kaczor et al. 2016a, b, c). The sequence identity between the template and the target was 79% and the sequence similarity was 90%. The stereochemical quality of the obtained homology model of dopamine D2 receptor is confirmed by the respective Ramachandran plot (Fig. 1). High sequence identity and similarity of the template and the target determine the high quality of the homology model, the credibility of resulting docking poses and the obtained CoMFA model. Moreover, this homology model of the human dopamine D2 receptor was used for structure-based virtual screening (Kaczor et al. 2016b). In that study, from 21 compounds investigated in vitro we identified ten dopamine D2 receptor ligands (47.6% success rate, among them the dopamine D2 receptor antagonists as designed) possessing additional affinity to other receptors tested, in particular to 5-HT2A receptors. The affinity (Ki) of the identified compounds ranged from 58 nM to about 24 µM. Importantly, we found one dopamine D2 receptor antagonist that did not have a protonatable nitrogen atom, which is a key structural element of the classical D2 pharmacophore model necessary to interact with the conserved Asp(3.32) which is rather unusual in structure-based virtual screening. This compound had over 20-fold binding selectivity for D2 receptor compared to D3 receptor and was also selective over other receptors tested. These findings further confirm the quality of the constructed dopamine D2 receptor homology model.
Fig. 1.
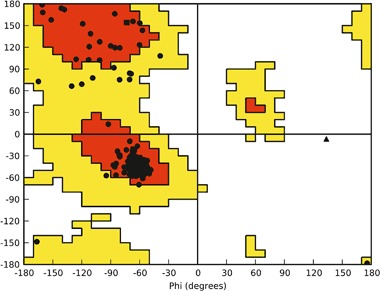
The Ramachandran plot for the D2 receptor homology model (Kaczor et al. 2016b)
Homology model of dopamine D2 receptor in inactive conformation was also built by Malo et al. (2012), Yap et al. (2012), and Duan et al. (2015) using β2 adrenergic receptor as a template. Sukalovic et al. (2013) used D3 receptor as a template while Kołaczkowski et al. (2013) compared models built on both templates.
The studied compounds
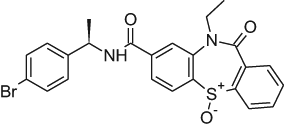
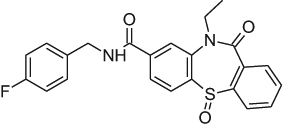
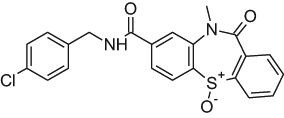
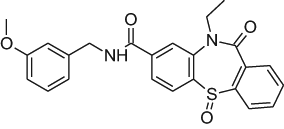
The studied dopamine D2 receptor antagonists 1–44 accompanied by their activity (experimental and predicted) are presented in Table 1.
Table 1.
The investigated dopamine D2 receptor antagonists with the experimental and predicted pAC50 values
| Compound number | 2D structure | pAC50 Exp | pAC50 Pred | Residual |
|---|---|---|---|---|
| Training set | ||||
| 1 |
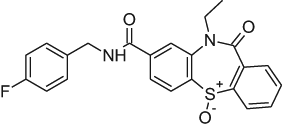
|
7.25 | 6.66 | 0.59 |
| 3 |
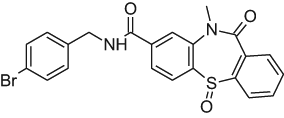
|
7.15 | 6.78 | 0.37 |
| 5 |
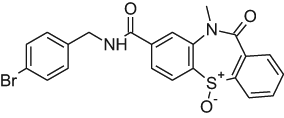
|
7.05 | 6.71 | 0.34 |
| 6 |
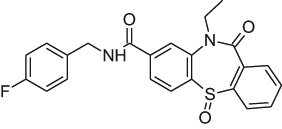
|
7.05 | 6.84 | 0.21 |
| 7 |
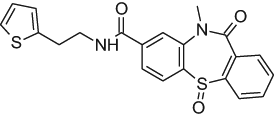
|
7.05 | 7.04 | 0.01 |
| 8 |
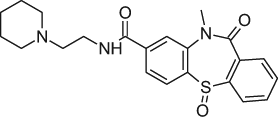
|
6.95 | 6.78 | 0.17 |
| 9 |
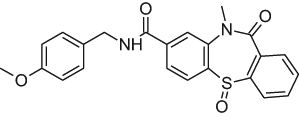
|
6.95 | 6.91 | 0.04 |
| 10 |
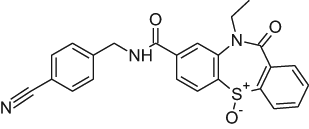
|
6.95 | 7.06 | −0.11 |
| 11 |
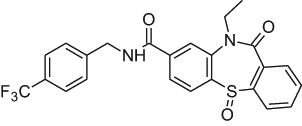
|
6.85 | 7.10 | −0.25 |
| 12 |
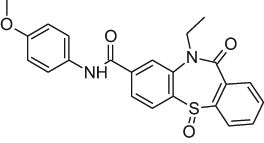
|
6.85 | 6.71 | 0.14 |
| 14 |
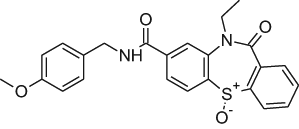
|
6.85 | 6.93 | −0.08 |
| 15 |
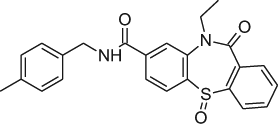
|
6.75 | 6.57 | 0.18 |
| 16 |
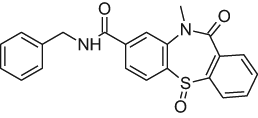
|
6.65 | 6.49 | 0.16 |
| 17 |
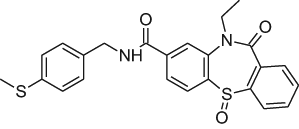
|
6.65 | 6.72 | −0.07 |
| 18 |
|
6.65 | 6.73 | −0.08 |
| 19 |
|
6.65 | 6.79 | −0.14 |
| 20 |
|
6.55 | 6.81 | −0.26 |
| 21 |
|
6.55 | 6.30 | 0.25 |
| 22 |
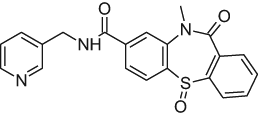
|
6.55 | 6.45 | 0.1 |
| 23 |
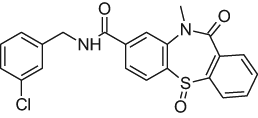
|
6.55 | 6.60 | −0.05 |
| 24 |
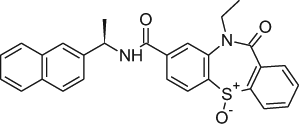
|
6.45 | 6.34 | 0.11 |
| 25 |

|
6.45 | 6.65 | −0.2 |
| 26 |
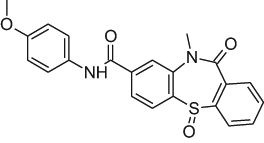
|
6.45 | 6.20 | 0.25 |
| 27 |
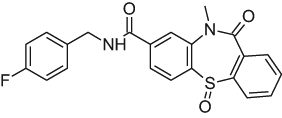
|
6.45 | 6.76 | −0.31 |
| 28 |
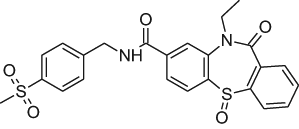
|
6.45 | 6.67 | −0.22 |
| 29 |
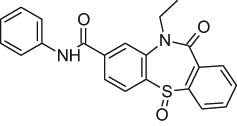
|
6.45 | 6.49 | −0.04 |
| 30 |
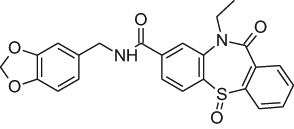
|
6.45 | 6.46 | −0.01 |
| 31 |
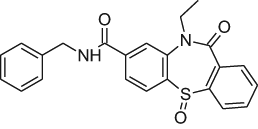
|
6.35 | 6.46 | −0.11 |
| 32 |
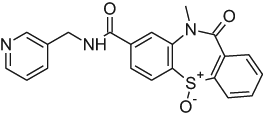
|
6.35 | 6.60 | −0.25 |
| 33 |
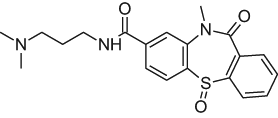
|
6.25 | 6.31 | −0.06 |
| 34 |
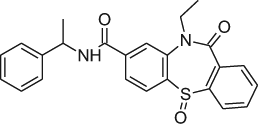
|
6.25 | 6.35 | −0.1 |
| 35 |
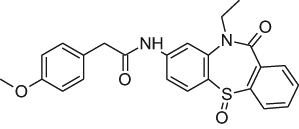
|
6.15 | 6.04 | 0.11 |
| 37 |
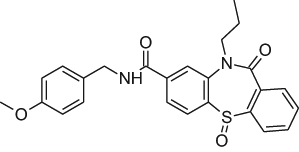
|
6.15 | 6.26 | −0.11 |
| 38 |
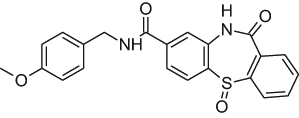
|
6.05 | 6.04 | 0.01 |
| 39 |
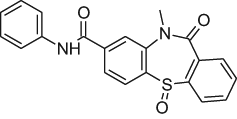
|
5.80 | 6.01 | −0.21 |
| 40 |
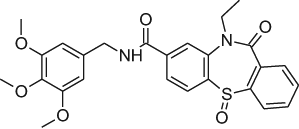
|
5.75 | 6.00 | −0.25 |
| 41 |
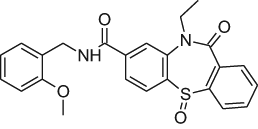
|
5.35 | 5.43 | −0.08 |
| 42 |
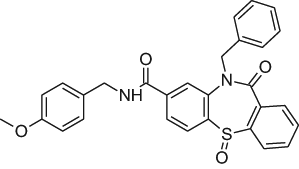
|
5.05 | 5.14 | −0.09 |
| 43 |

|
3.00 | 3.02 | −0.02 |
| 44 |
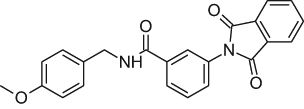
|
3.00 | 2.93 | 0.07 |
| Test set | ||||
| 2 |
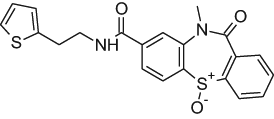
|
7.15 | 6.92 | 0.23 |
| 4 |
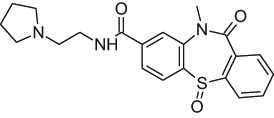
|
7.05 | 6.75 | 0.3 |
| 13 |
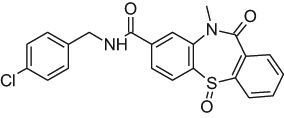
|
6.85 | 6.76 | 0.09 |
| 36 |
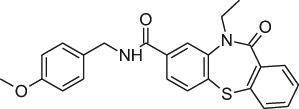
|
6.15 | 6.24 | −0.09 |
Molecular docking
A reference ligand, chlorprothixene, was docked to the homology model of dopamine D2 receptor using the standard precision (SP) protocol of Glide from Schrödinger suite of software. The selected docking poses were refined using induced-fit docking approach of Schrödinger suite of software. The final docking pose was identified by visual inspection among the poses where the protonatable nitrogen atom of the ligands interacted with the conserved Asp(3.32) of the receptors. The docking pose of chlorprothixene is shown in Fig. 2. It can be seen that a key interaction for this ligand is an electrostatic interaction between the protonatable nitrogen atom of the ligand and Asp(3.32). Moreover, Trp(6.48), Phe(6.61), and His(6.55) were also found to be important for binding of the ligand.
Fig. 2.
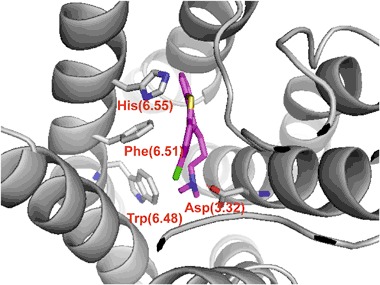
The reference ligand, chlorprothixene docked into the orthosteric site of dopamine D2 receptor homology model. Key interaction of the protonatable nitrogen atom of the ligand with the conserved Asp(3.32) (presented as sticks) is shown as red dashed lines. Other important residues, Trp(6.48), Phe(6.51) and His(6.55) also shown as sticks. Transmembrane helices colored in gray. Hydrogen atoms not shown for clarity (color figure online)
The studied ligands 1–44 (Table 1) were docked to the orthosteric site of the dopamine D2 receptor homology model using chlorprothixene-based grid file and Glide of Schrödinger suite of software. The final poses of selected compounds are presented in Fig. 3. In the case of all ligands the key interaction is the hydrogen bond between the amide nitrogen atom of the ligand and the conserved Asp(3.32). Thus, instead of lack of an important pharmacophoric feature, the studied ligands are able to maintain the main contact with the receptor, typical for dopamine D2 receptor orthosteric ligands. A similar binding mode was proposed earlier for already mentioned dopamine D2 receptor antagonist without a protonatable nitrogen atom identified in virtual screening (Kaczor et al. 2016b).
Fig. 3.
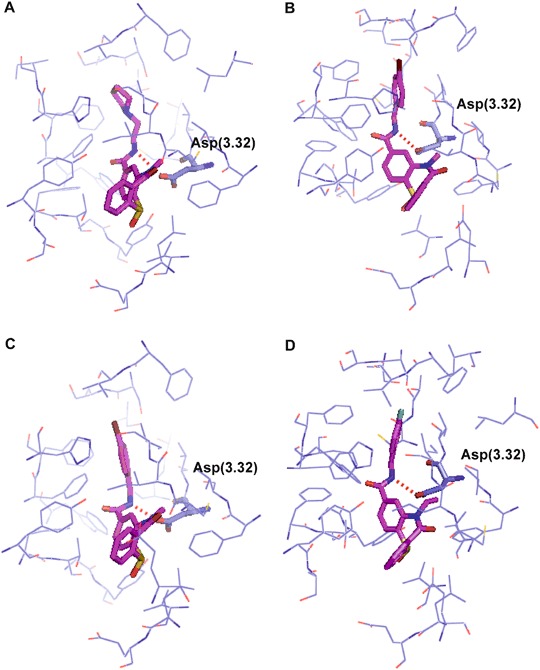
Compounds 2 (a), 3 (b), 5 (c), and 6 (d) docked to the orthosteric site of the dopamine D2 receptor homology model. Ligands shown as stick with carbon atoms colored in magenta. Protein shown in wire representation with carbon atoms colored in purple. Conserved Asp(3.32) shown as sticks. Hydrogen bonds presented as red dashed lines. Hydrogen atoms not shown for clarity (color figure online)
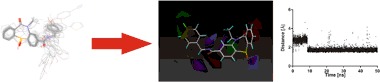
Molecular dynamics
The ligand-receptor complexes were subjected to molecular dynamics with Desmond in order to check their stability. The potential energy changes (Fig. 4) confirm that the simulations were well-equilibrated. The ligand RMSD for selected ligand-receptor complexes is presented in Fig. 5. It can be concluded that the studied ligands are stable in the orthosteric pocket of the dopamine D2 receptor which is confirmed by ligand RMSD value below 2.5 Å.
Fig. 4.
Changes of potential energy during 50 ns molecular dynamics simulation for dopamine D2 receptor in complex with 2 (a), 3 (b), 5 (c), and 6 (d)
Fig. 5.
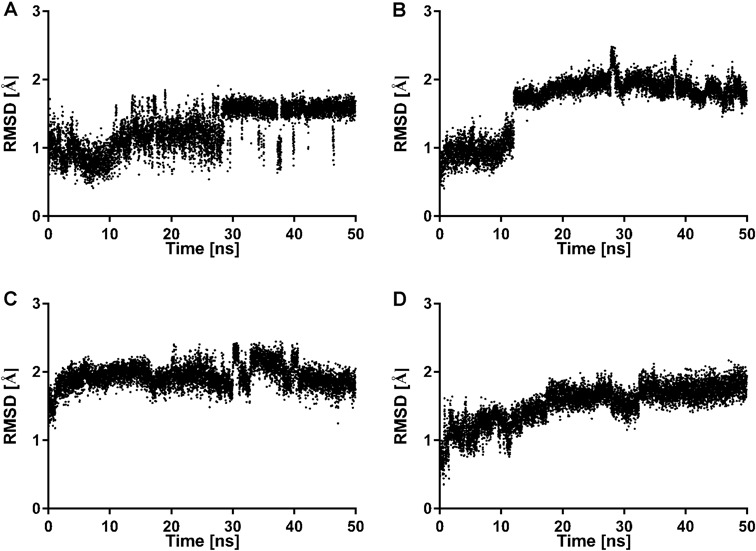
Changes of ligand RMSD during 50 ns molecular dynamics simulation for dopamine D2 receptor in complex with 2 (a), 3 (b), 5 (c), and 6 (d)
Furthermore, the effect of the studied ligands on the stabilization of the dopamine D2 receptor inactive conformation was investigated and compared with the simulations of the apo form of the receptor (Fig. 6). In the inactive state of family A GPCRs there is a strong intramolecular interaction between Arg(3.50) of the conserved (D/E)RY motif in TM3 and residues Glu(6.30) in TM6 (Trzaskowski et al. 2012). Thus, the changes in distance between these ionic lock residues during 50 ns simulations were analyzed. In case of the dopamine D2 receptor apo form the distance between Arg(3.50) and Glu(6.30) is stable around 3 Å (Fig. 6a). However, for compound 3 the distance between Arg(3.50) and Glu(6.30) is about 3 Å for the first 10 ns of simulations and then is stabilized below 2 Å (Fig. 6b). This effect is typical for GPCR antagonists and was earlier reported for the dopamine D2 bivalent antagonists (Kaczor et al. 2016a).
Fig. 6.
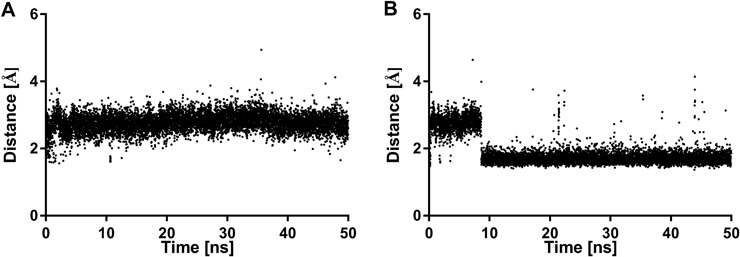
The changes in the ionic lock distance between Arg(3.50) and Glu(6.30) during 50 ns simulations: a dopamine D2 receptor apo form; b dopamine D2 receptor in complex with compound 3
Molecular alignment
The quality of 3D-QSAR models depends on the molecular alignment because the compound activities strongly correlate with different substitutions on a specific point in the same compound series (Yuan et al. 2014; Kaczor et al. 2015). The common substructure has been extensively applied as a base for molecular alignment (Verma et al. 2010; Damale et al. 2014). However, better results can be obtained when the 3D-QSAR models could be built and verified on the active conformations of training and test set compounds, in particular when similar ligands occupy different binding poses in the binding site (Urniaż and Jóźwiak 2013). The molecular alignment of the compounds from the training and test sets is shown in Fig. 7. The alignment using docking conformations will facilitate understanding the contour maps of the models in a structure-based manner (Yuan et al. 2014; Kaczor et al. 2015).
Fig. 7.
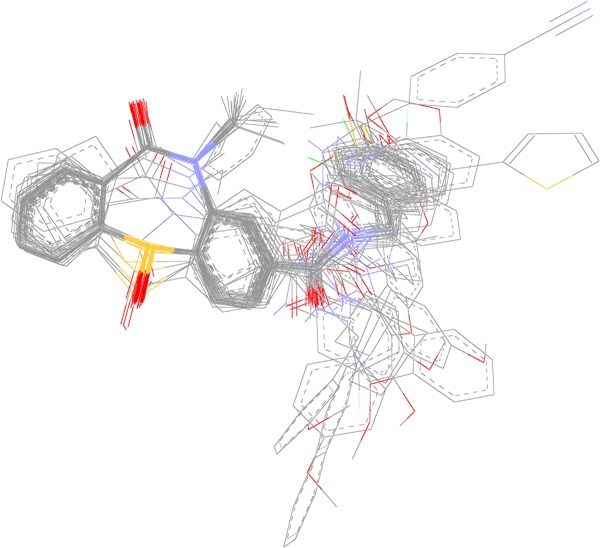
Alignment of 44 compounds in the training and test sets based on the molecular docking results to dopamine D2 receptor orthosteric binding site. Non-polar hydrogen atoms omitted for clarity
CoMFA statistics
The 3D-QSAR CoMFA model was built using Sybyl-X v. 2.1. The CoMFA model gave a cross-validated coefficient Q2 of 0.63 with an optimal number of components of 4, R2 of 0.95 and F value of 174.133. The standard error of estimate was 0.207. These statistical parameters indicate that the CoMFA model is statistically significant. A model for which R2 is above 0.9 and Q2 is above 0.4 is usually considered to be predictive (Wang et al. 2009). The respective field contribution parameters were 62.9% for the electrostatic field and 27.1% for the steric field descriptor. Experimental and predicted AC50 values are presented in Table 1. It can be seen that they are not deviated significantly from each other (not more than 0.3 logarithmic unit for the majority of compounds, with the exception of compound 1 from which was slightly under-predicted). Figure 8 shows a very good correlation between the experimental and computed IC50 values for the training set.
Fig. 8.
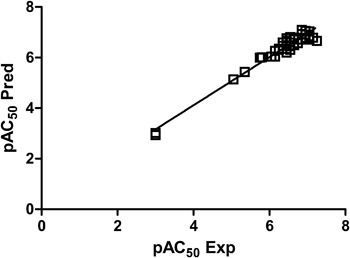
The experimental versus predicted pAC50 values for the training set
Validation of CoMFA model
The obtained CoMFA model was validated by the external test set of four compounds (10% of the number of training set compounds). The R2 of test set was 0.96 so it was close to R2 of the training set which confirms the good predictability of the CoMFA model (Fig. 9) (Golbraikh and Tropsha 2002). Thus, the activities of all the test set compounds were correctly predicted.
Fig. 9.
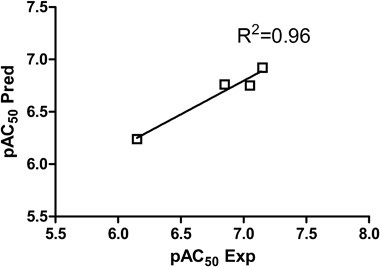
The experimental versus predicted pAC50 values for the test set
Contour map
Figure 10 shows the steric and electrostatic contour maps gained via CoMFA modeling. Steric contour maps gave the information about the spatial volume of substituted groups on different positions. There were two green contour regions located in the active site, meaning that bulky groups were favored. There is a green region near the nitrogen atom of the tricyclic dihydrodibenzo[b,f][1,4]thiazepine system meaning that some substituents may be beneficial in this position. Indeed, the methyl group in this position leads to compounds with better potency than compounds bearing this position not alkylated. However, ethyl, propyl and benzyl moiety lead to decreased potency with increasing substituent size. Another position for a bulky group was identified in the para position of the benzyl group found as primary amide substituent in several compounds. The nature of the substituent in this position does not have an effect on the compound potency. Both the electronic and steric nature of substituents is tolerated. Meta substitution is less favored and ortho substitution is not tolerated (two yellow regions in the contour map). Two red contour regions near carbonyl groups indicate that more negative charge is favored here. As negative charge is also connected with H-bonding acceptors this would most probably also indicate H-bonding acceptors to be favored. Furthermore, the S-oxide group is connected with blue contour region meaning that more positive (or less negative) charge and, correspondingly, H-bond donors are favored and negative charge /H-bond acceptors are disfavored.
Fig. 10.
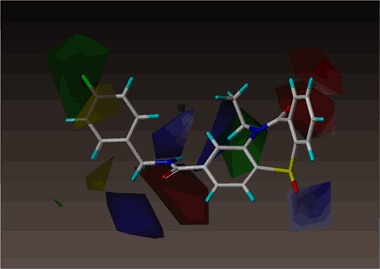
CoMFA steric and electrostatic contour fields. Fields drawn with 90/10 proportion of favorable and unfavorable interactions. The most active compound 1 shown
Conclusion
We built a statistically valid CoMFA model for the dopamine D2 receptor antagonists without a protonatable nitrogen atom. The model was based on molecular alignment derived from molecular docking to the orthosteric site of the dopamine D2 receptor homology model. In spite of having no protonatable nitrogen atom which is a key element of the classical pharmacophore model, the studied compounds are able to interact with the conserved Asp(3.32) thanks to their amide nitrogen atom. The constructed CoMFA model was characterized with the following statistics: R2 = 0.95, Q2 = 0.63. The quality of the CoMFA model was confirmed by high value of R2 of the test set, equal 0.96. The CoMFA model allowed to identify two regions where bulky substituents are favored and two regions where bulky substituents are not favorable Two red contour regions near carbonyl groups were identified meaning that negative charge would be favored here while the S-oxide group is connected with blue contour region meaning that positive charge is favored in this position. These findings may be applied for further optimization of the studied compound series. Moreover, the studied compounds stabilize the receptor inactive conformation through the effect on the ionic lock which is typical for GPCR antagonists.
Acknowledgements
The paper was developed using the equipment purchased within the project “The equipment of innovative laboratories doing research on new medicines used in the therapy of civilization and neoplastic diseases” within the Operational Program Development of Eastern Poland 2007–2013, Priority Axis I modern Economy, operations I.3 Innovation promotion. Part of the calculations was performed under a computational grant by Interdisciplinary Center for Mathematical and Computational Modeling (ICM), Warsaw, Poland, grant number G30-18 and under resources and licenses by CSC, Finland.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Bowers KJ, Chow E, Xu H, Dror RO, Eastwood MP, Gregersen MP, Klepeis JL, Kolossvary I, Moraes MA, Sacerdoti FD, Salmon JK, Shan Y, Shaw DE (2006) Scalable algorithms for molecular dynamics simulations on commodity clusters. In: Proceedings of the ACM/IEEE Conference on Supercomputing (SC06). Tampa, Florida
- Chien EYT, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, Stevens RC. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330:1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damale MG, Harke SN, Kalam Khan FA, Shinde DB, Sangshetti JN. Recent advances in multidimensional QSAR (4D-6D): a critical review. Mini Rev Med Chem. 2014;14:35–55. doi: 10.2174/13895575113136660104. [DOI] [PubMed] [Google Scholar]
- Duan X, Zhang M, Zhang X, Wang F, Lei M. Molecular modeling and docking study on dopamine D2-like and serotonin 5-HT2A receptors. J Mol Graph Model. 2015;57:143–155. doi: 10.1016/j.jmgm.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Ekhteiari Salmas R, Stein M, Yurtsever M, Seeman P, Erol I, Mestanoglu M, Durdagi S (2016) The signaling pathway of dopamine D2 receptor (D2R) activation using normal mode analysis (NMA) and the construction of universal pharmacophore models for D2R inhibitors. J Biomol Struct Dyn. 10.1080/07391102.2016.1206487 [DOI] [PubMed]
- Golbraikh A, Tropsha A. Beware ofq2! J Mol Graph Model. 2002;20:269–276. doi: 10.1016/S1093-3263(01)00123-1. [DOI] [PubMed] [Google Scholar]
- Ivachtchenko AV, Dmitriev DE, Golovina ES, Dubrovskaya ES, Kadieva MG, Koryakova AG, Kysil VM, Mitkin OD, Tkachenko SE, Okun IM, Vorobiov AA. Synthesis of cycloalkane-annelated 3-phenylsulfonyl-pyrazolo[1,5-a]pyrimidines and their evaluation as 5-HT6 receptor antagonists. Bioorg Med Chem Lett. 2010;20:2133–2136. doi: 10.1016/j.bmcl.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Jozwiak K, Targowska-Duda KM, Kaczor AA, Kozak J, Ligeza A, Szacon E, Wrobel TM, Budzynska B, Biala G, Fornal E, Poso A, Wainer IW, Matosiuk D. Synthesis, in vitro and in vivo studies, and molecular modeling of N-alkylated dextromethorphan derivatives as non-competitive inhibitors of α3β4 nicotinic acetylcholine receptor. Bioorg Med Chem. 2014;22:6846–6856. doi: 10.1016/j.bmc.2014.10.036. [DOI] [PubMed] [Google Scholar]
- Kaczor AA, Jörg M, Capuano M (2016a) Modeling dopamine D2 receptor dimer and its interaction with homobivalent antagonists: homology modeling, docking and molecular dynamics. J Mol Model. 10.1007/s00894-016-3065-2 [DOI] [PMC free article] [PubMed]
- Kaczor AA, Karczmarzyk Z, Fruziński A, Pihlaja K, Sinkkonen J, Wiinämaki K, Kronbach C, Unverferth K, Poso A, Matosiuk D. Structural studies, homology modeling and molecular docking of novel non-competitive antagonists of GluK1/GluK2 receptors. Bioorg Med Chem. 2014;22:787–795. doi: 10.1016/j.bmc.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Kaczor AA, Silva AG, Loza MI, Kolb P, Castro M, Poso A. Structure-based virtual screening for dopamine D2 receptor ligands as potential antipsychotics. ChemMedChem. 2016;11:718–729. doi: 10.1002/cmdc.201500599. [DOI] [PubMed] [Google Scholar]
- Kaczor AA, Targowska-Duda KM, Budzyńska B, Biała G, Silva AG, Castro M. In vitro, molecular modeling and behavioral studies of 3-{[4-(5-methoxy-1H-indol-3-yl)-1,2,3,6-tetrahydropyridin-1-yl]methyl}-1,2-dihydroquinolin-2-one (D2AAK1) as a potential antipsychotic. Neurochem Int. 2016;96:84–99. doi: 10.1016/j.neuint.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Kaczor AA, Targowska-Duda KM, Patel JZ, Laitinen T, Parkkari T, Adams Y, Nevalainen TJ, Poso A. Comparative molecular field analysis and molecular dynamics studies of α/β hydrolase domain containing 6 (ABHD6) inhibitors. J Mol Model. 2015;21:2789. doi: 10.1007/s00894-015-2789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołaczkowski M, Bucki A, Feder M, Pawłowski M. Ligand-optimized homology models of D1 and D2 dopamine receptors: application for virtual screening. J Chem Inf Model. 2013;53:638–648. doi: 10.1021/ci300413h. [DOI] [PubMed] [Google Scholar]
- Komatsu H. Novel therapeutic GPCRs for psychiatric disorders. Int J Mol Sci. 2015;16:14109–14121. doi: 10.3390/ijms160614109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra AJ, Kuhne S, de Esch IJP, Leurs R, de Graaf C. A structural chemogenomics analysis of aminergic GPCRs: lessons for histamine receptor ligand design. Br J Pharmacol. 2013;170:101–126. doi: 10.1111/bph.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16:488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo M, Brive L, Luthman K, Svensson P. Investigation of D2 receptor-agonist interactions using a combination of pharmacophore and receptor homology modeling. ChemMedChem. 2012;7:471–482. doi: 10.1002/cmdc.201100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manallack DT. The pK(a) distribution of drugs: application to drug discovery. Perspect Med Chem. 2008;1:25–38. [PMC free article] [PubMed] [Google Scholar]
- Matosiuk D, Fidecka S, Antkiewicz-Michaluk L, Dybała I, Kozioł AE. Synthesis and pharmacological activity of new carbonyl derivatives of 1-aryl-2-iminoimidazolidine. Part 1. Synthesis and pharmacological activity of chain derivatives of 1-aryl-2-iminoimidazolidine containing urea moiety. Eur J Med Chem. 2001;36:783–797. doi: 10.1016/S0223-5234(01)01267-3. [DOI] [PubMed] [Google Scholar]
- Matosiuk D, Fidecka S, Antkiewicz-Michaluk L, Lipkowski J, Dybala I, Koziol AE. Synthesis and pharmacological activity of new carbonyl derivatives of 1-aryl-2-iminoimidazolidine: part 2. Synthesis and pharmacological activity of 1,6-diaryl-5,7(1H)dioxo-2,3-dihydroimidazo[1,2-a][1,3,5]triazines. Eur J Med Chem. 2002;37:761–772. doi: 10.1016/S0223-5234(02)01408-3. [DOI] [PubMed] [Google Scholar]
- Matosiuk D, Fidecka S, Antkiewicz-Michaluk L, Dybala I, Koziol AE. Synthesis and pharmacological activity of new carbonyl derivatives of 1-aryl-2-iminoimidazolidine. Part 3. Synthesis and pharmacological activity of 1-aryl-5,6(1H)dioxo-2,3-dihydroimidazo[1,2-a]imidazoles. Eur J Med Chem. 2002;37:845–853. doi: 10.1016/S0223-5234(02)01407-1. [DOI] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Collo G, Spano P. The neurobiology of dopamine receptors: evolution from the dual concept to heterodimer complexes. J Recept Signal Transduct Res. 2010;30:347–354. doi: 10.3109/10799893.2010.506192. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci Publ Protein Soc. 2006;15:2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Javitch JA. The binding site of aminergic G protein-coupled receptors: the transmembrane segments and second extracellular loop. Annu Rev Pharmacol Toxicol. 2002;42:437–467. doi: 10.1146/annurev.pharmtox.42.091101.144224. [DOI] [PubMed] [Google Scholar]
- Sukalovic V, Soskic V, Sencanski M, Andric D, Kostic-Rajacic S. Determination of key receptor-ligand interactions of dopaminergic arylpiperazines and the dopamine D2 receptor homology model. J Mol Model. 2013;19:1751–1762. doi: 10.1007/s00894-012-1731-6. [DOI] [PubMed] [Google Scholar]
- Surgand JS, Rodrigo J, Kellenberger E, Rognan D. A chemogenomic analysis of the transmembrane binding cavity of human G-protein-coupled receptors. Proteins. 2006;62:509–538. doi: 10.1002/prot.20768. [DOI] [PubMed] [Google Scholar]
- Sztanke K, Fidecka S, Kedzierska E, Karczmarzyk Z, Pihlaja K, Matosiuk D. Antinociceptive activity of new imidazolidine carbonyl derivatives. Part 4. Synthesis and pharmacological activity of 8-aryl-3,4-dioxo-2H,8H-6,7-dihydroimidazo[2,1-c] [1,2,4]triazines. Eur J Med Chem. 2005;40:127–134. doi: 10.1016/j.ejmech.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs - theoretical and experimental studies. Curr Med Chem. 2012;19:1090–1109. doi: 10.2174/092986712799320556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urniaż RD, Jóźwiak K. X-ray crystallographic structures as a source of ligand alignment in 3D-QSAR. J Chem Inf Model. 2013;53:1406–1414. doi: 10.1021/ci400004e. [DOI] [PubMed] [Google Scholar]
- Verma J, Khedkar VM, Coutinho EC. 3D-QSAR in drug design—a review. Curr Top Med Chem. 2010;10:95–115. doi: 10.2174/156802610790232260. [DOI] [PubMed] [Google Scholar]
- Wang P, Wen Y, Han GZ, Sidhu PK, Zhu BT. Characterization of the oestrogenic activity of non-aromatic steroids: are there male-specific endogenous oestrogen receptor modulators? Br J Pharmacol. 2009;158:1796–1807. doi: 10.1111/j.1476-5381.2009.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, Sali A (2014) Comparative protein structure modeling using MODELLER. Curr Protoc Bioinforma Ed Board Andreas Baxevanis Al 47:5.6.1–5.6.32. 10.1002/0471250953.bi0506s47 [DOI] [PubMed]
- Xiao J, Free RB, Barnaeva E, Conroy JL, Doyle T, Miller B, Bryant-Genevier M, Taylor MK, Hu X, Dulcey AE, Southall N, Ferrer M, Titus S, Zheng W, Sibley DR, Marugan JJ. Discovery, optimization, and characterization of novel D2 dopamine receptor selective antagonists. J Med Chem. 2014;57:3450–3463. doi: 10.1021/jm500126s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap BK, Buckle MJC, Doughty SW. Homology modeling of the human 5-HT1A, 5-HT 2A, D1, and D2 receptors: model refinement with molecular dynamics simulations and docking evaluation. J Mol Model. 2012;18:3639–3655. doi: 10.1007/s00894-012-1368-5. [DOI] [PubMed] [Google Scholar]
- Yuan H, Zhuang J, Hu S, Li H, Xu J, Hu Y, Xiong X, Chen Y, Lu T. Molecular modeling of exquisitely selective c-Met inhibitors through 3D-QSAR and molecular dynamics simulations. J Chem Inf Model. 2014;54:2544–2554. doi: 10.1021/ci500268s. [DOI] [PubMed] [Google Scholar]


