Abstract
Background and Objectives
Short-term fasting differentially alters cytochrome P450 (CYP) mediated drug metabolism. This has been established by using CYP-enzyme selective probe drugs. However, the observed effects of fasting on the pharmacokinetics of these probe drugs may also include the effects of altered plasma protein binding of these drugs. Therefore, we studied the effect of short-term fasting on protein binding of five commonly used probe drugs [caffeine (CYP1A2), metoprolol (CYP2D6), midazolam (CYP3A4), omeprazole (CYP2C19) and S-warfarin (CYP2C9)].
Methods
The free and total plasma concentrations of the five probe drugs were analyzed by LC–MS/MS in samples retrieved in a cross-over study in which nine healthy subjects received an intravenous administration of the cocktail after an overnight fast (control) and after 36 h of fasting.
Results
Short-term fasting increased plasma free fatty acid concentrations from 0.48 mmol/L (control) to 1.29 mmol/L (36 h fasting) (p = 0.012). Short-term fasting did not alter the free fractions of caffeine, metoprolol and omeprazole compared to the control intervention (p > 0.05). Power to detect a difference for midazolam and S-warfarin was low since the majority of free concentrations were below the limit of quantification.
Conclusions
This study demonstrates that short-term fasting does not alter protein binding of the probe drugs caffeine, metoprolol and omeprazole.
Electronic supplementary material
The online version of this article (doi:10.1007/s13318-017-0437-7) contains supplementary material, which is available to authorized users.
Key Points
| Short-term fasting does not alter protein binding of the probe drugs caffeine, metoprolol and omeprazole. |
| Additional research is warranted to study the role of short-term fasting on protein binding of the highly protein bound probe drugs midazolam and S-warfarin. |
| When determining the dose of a probe drug in protein binding studies, it is important to take the degree of protein binding and the sensitivity of the analytical method into account. |
Introduction
Probe drugs are often used to determine the metabolic activity of cytochrome P450 (CYP) enzymes in vivo. When the probe drug is almost exclusively metabolized by the individual CYP enzyme of interest, the pharmacokinetics of the drug reflect the activity of the specific CYP enzyme [1].
Previously, we have shown that short-term fasting differentially altered the pharmacokinetics of five probe drugs administered as a cocktail: caffeine (CYP1A2), metoprolol (CYP2D6), midazolam (CYP3A4), omeprazole (CYP2C19) and S-warfarin (CYP2C9) [1–3]. The probe drugs are selective for the specific CYP enzymes and, as validated by Turpault et al., do not interact with each other [1]. This makes the administration of these drugs as a “cocktail” suitable for in vivo use. Short-term fasting increased systemic caffeine clearance by 17% (p = 0.04) and metoprolol clearance by 13% (p < 0.01) which indicates increased activity of CYP1A2 and CYP2D6, respectively [3]. Furthermore, short-term fasting decreased systemic S-warfarin clearance by 19% (p < 0.01) which indicates decreased CYP2C9 enzyme activity, whereas the pharmacokinetics of omeprazole and midazolam were unaffected [3]. However, these observations were based on total drug concentrations of the probe drugs which includes both protein bound and unbound (free) drugs.
Most drugs are to some extent bound to plasma proteins such as albumin, α1-glycoprotein or β-lipoprotein. The impact of plasma protein binding on the pharmacokinetics of drugs depends on the extraction ratio of the drug and can be described by the well-stirred model (Eqs. 1 and 2):
| 1 |
| 2 |
where CL indicates total clearance, Q H the hepatic blood flow, f u the fraction of unbound drug in plasma, CLint the intrinsic clearance of unbound drug and E H the hepatic extraction ratio [4]. For drugs with a low-extraction ratio (Q H > f u × CLint, E H < 0.3) (caffeine (f u ≅ 0.65) and S-warfarin (f u ≅ 0.01)), clearance (CL) is mainly dependent on f u and CLint (Eq. 1), whereas the three determinants Q H, CLint and f u are important factors for the intermediate-extraction ratio drugs (Q H ~ f u × CLint, E H = 0.3–0.7) metoprolol (E H = 0.67, f u ≅ 0.90), midazolam (E H = 0.31, f u ≅ 0.02) and omeprazole (E H = 0.35, f u ≅ 0.03) [5, 6].
In most clinical studies total plasma drug concentrations are measured to assess pharmacokinetic parameters such as clearance [7]. This assumes that protein binding is relatively constant between and within subjects. However, free fatty acids in plasma, which are increased by fasting, can compete with drugs for protein binding sites, thereby increasing the free fraction (ratio of free per total drug in plasma) of a drug [8]. This can result in altered total clearance rates, which may erroneously be interpreted as altered CYP-mediated clearance. To determine whether the previously observed effects of fasting on the pharmacokinetics of the probe drugs include the effects of altered plasma protein binding instead of altered CYP-enzyme activity, our study aimed to determine the effect of short-term fasting on protein binding by analyzing the free and total plasma concentrations of the five probe drugs used in the cocktail (caffeine, metoprolol, midazolam, omeprazole and S-warfarin).
Materials and Methods
We performed an open-label, randomly assigned crossover intervention study in healthy male subjects. Nine subjects received an intravenous administration of a five probe drug cocktail after (1) an overnight fast (control) and (2) after 36 h of fasting [3]. The drugs were administered sequentially and consisted of 20 mg omeprazole (40 mg powder for solution for infusion, AstraZeneca BV, Zoetermeer, The Netherlands), 0.015 mg/kg midazolam (5 mg/mL, 1 mL ampoules, Roche Nederland BV, Woerden, The Netherlands), 50 mg caffeine (10 mg/mL, 1 mL ampoules, VUMC, Amsterdam, The Netherlands), 5 mg racemic warfarin (5 mg/mL, 3 mL ampoules, RadboudUMC, Nijmegen, The Netherlands) and 20 mg metoprolol (1 mg/mL, 5 mL ampoules, AstraZeneca BV, Zoetermeer, The Netherlands). The administered dosages were based on a previously performed study by Turpault et al. and on the (lower) quantification limits of the analytical method that was validated and used for the analysis of the drugs [1, 2]. Dosages were generally low, and no clinical effects were observed. Per plasma concentration–time curve six samples, drawn at t = 0.2, t = 0.5, t = 1, t = 3, t = 3.5 and t = 5 h after administration of the first probe drug, were used for the measurement of the free and total plasma concentrations.
Plasma was separated by centrifugation and stored at −80 °C until analysis. Furthermore, before administration of the cocktail, samples were drawn for analyses of biochemical parameters such as serum albumin and free fatty acids [3]. Free fatty acid concentrations were determined using a validated spectrophotometric assay [9, 10]. For additional details on the study protocol we refer to our study on the effects of short-term fasting on CYP-mediated drug metabolism [3].
Analytical Method
Total plasma concentrations of the five drugs were simultaneously analyzed using a previously validated analytical LC–MS/MS method [2]. For the analyses of the free drug concentrations, unbound drug was separated from plasma protein bound drug by ultrafiltration: the same plasma samples were thawed at room temperature and kept in a water bath (37 °C) for 30 min after which 250 µL was transferred to an ultrafiltration device (Centrifree® Ultracel, YM-T membrane, Merck Millipore, Bad Schwalbag, Germany) for 30 min centrifugation at 37 °C while running at 2000×g. The ultrafiltrate samples containing the free probe drug were then further processed in a similar manner as the samples for assessment of the total plasma concentrations. For caffeine, midazolam, omeprazole and S-warfarin, recovery after ultrafiltration was 95.1–99.3% at the lower limit of quantification (LLOQ), 93.0–117.4% at a midlevel of quantification (MLQ) and 94.6–106.6% at the upper limit of quantification (ULOQ). This indicates that no specific binding occurred to the ultrafiltration device. Some binding was present for midazolam; recovery was 78.7, 77.7 and 82.5% for the LLOQ, MLQ and ULOQ, respectively. To be able to simultaneously quantify free drug concentration of the five probe drugs in a single sample, measured midazolam concentrations were corrected by a multiplication factor of 1.25. The LLOQ was 50 µg/L for caffeine, 1 µg/L for metoprolol and 2 µg/L for omeprazole [2]. By fourfold dilution of the QC samples, the LLOQ for the high protein bound drugs was semi-quantitatively lowered from 0.5 to 0.125 µg/L for midazolam and from 4 to 1 µg/L for S-warfarin, to be able to measure the very low free concentrations of these drugs.
Statistical Analysis
Due to the sequential intravenous administration and differences in half-life of the probe drugs, the number of samples to be included in the analysis differed per probe: six samples per plasma concentration–time curve for midazolam, 5 for caffeine and 4 for metoprolol, omeprazole and S-warfarin, respectively. Per sample, the free fraction (ratio of free per total drug in plasma) was calculated for each probe drug.
Most drugs display linear protein binding, whereby the free fraction remains unchanged as drug concentrations increase [11]. In order to test for linearity, the relationship between the free fractions and total drug concentrations were analyzed for each drug and compared between the control and 36 h of fasting intervention using linear regression. In the absence of concentration-dependent protein binding, the differences in the free fractions of the probe drugs between the control and the fasting intervention were assessed: the average free fraction per patient per intervention was calculated and compared using a paired t-tests (normally distributed data) or Wilcoxon signed-ranks test (not normally distributed data). The Shapiro–Wilk test was used to assess the normality of data distribution. A p value ≤ 0.05 was considered significant. Statistical analysis was performed using IBM SPSS Statistics version 23.0. Based on the results of the free fractions of all probe drugs, a post hoc power analysis was performed using nQuery Advisor version 7.0.
Results
Short-term fasting increased plasma free fatty acid concentrations from 0.48 mmol/L (range 0.19–0.73) in the control condition to 1.29 mmol/L (range 0.63–2.57) after short-term fasting (p = 0.012). However, short-term fasting did not alter serum albumin concentrations: the median concentration was 47 g/L (range 44–49) in the control condition and 48 g/L (range 45–51) after short-term fasting (p = 0.256).
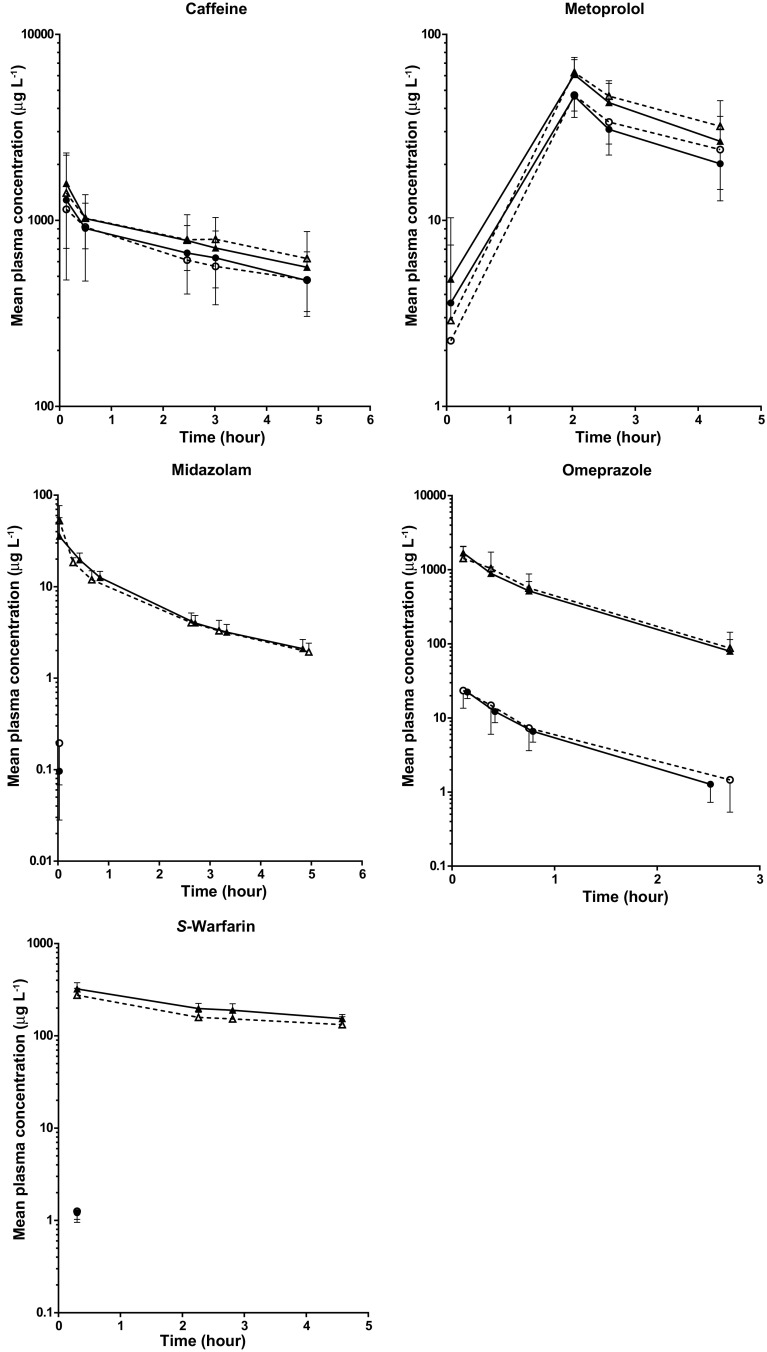
The mean total and unbound (free) plasma concentration–time curves of the five probe drugs are presented in Fig. 1.
Fig. 1.
Mean (± SD) total and unbound (free) plasma concentration–time curves of the five probe drugs. The dashed line with open triangles represents the mean total plasma concentration–time curve after the control intervention. The solid line with closed triangles represents the mean total plasma concentration–time curve after the short-term fasting intervention. The dashed line with open circles represents the mean unbound plasma concentration–time curve after the control intervention. The solid line with open circles represents the mean unbound plasma concentration–time curve after the short-term fasting intervention
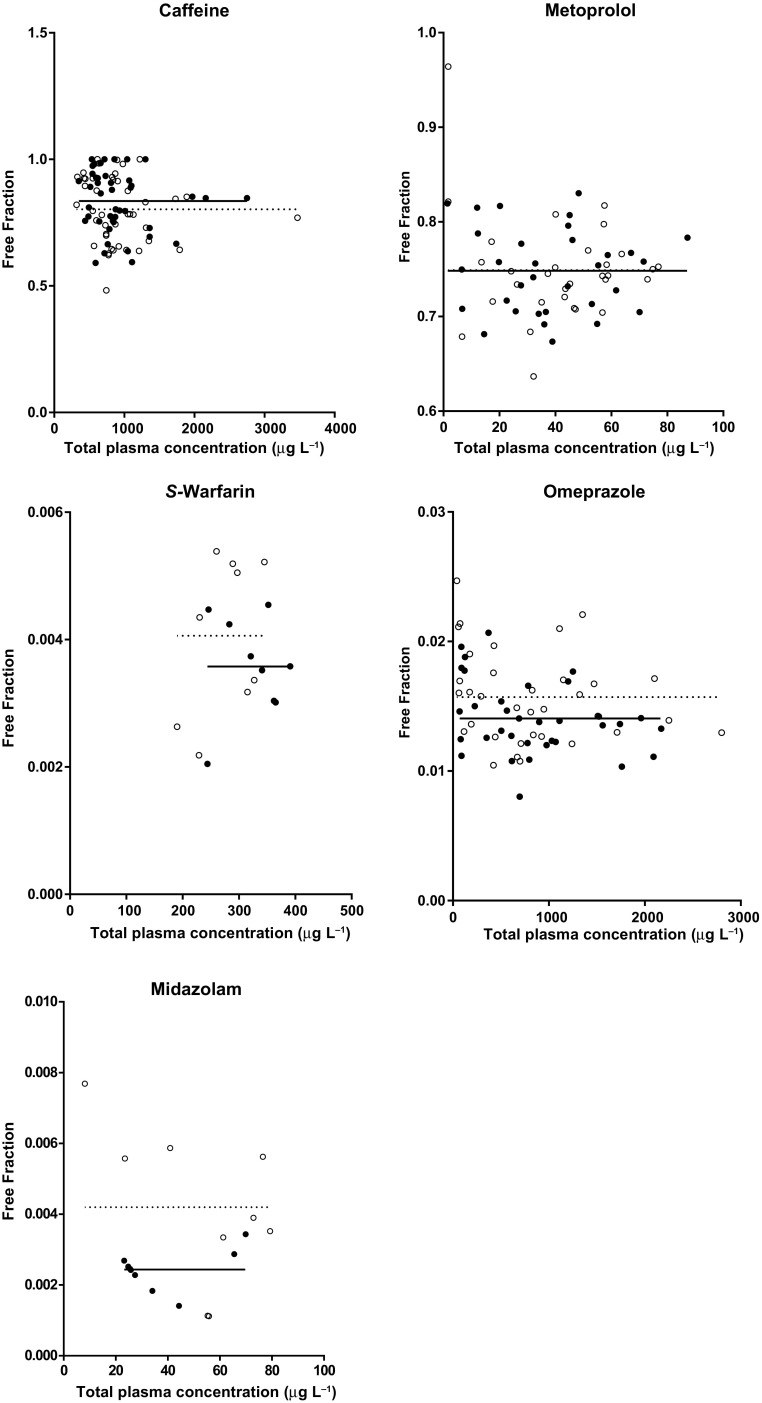
All five probe drugs demonstrated a linear relationship (no concentration-dependency) between protein binding and the total plasma concentration (Fig. 2): free fractions are evenly distributed and the slopes for both the control and the short-term fasting intervention did not significantly differ from zero (data not shown).
Fig. 2.
Distribution of the free fractions (ratio of free per total drug in plasma) versus the total plasma concentrations of the five probe drugs. The open circles represent the free fractions analyzed after the control intervention and the closed circles represent the short-term fasting intervention. The solid (short-term fasting) and dashed (control) lines represent the Y-intercepts for the best fits
Short-term fasting did not significantly alter the free fractions of the five probe drugs compared to the control intervention (Table 1). This may also be appreciated in Fig. 1S (supplementary data). For S-warfarin and midazolam only one sample per plasma concentration–time curve [drawn at the time at which the maximum concentration was observed (t max)] could be included in the analysis since the free plasma concentrations of the other samples were all below the LLOQ (BLOQ) (Table 1, Fig. 1). Within the t max samples, 55.6% of the free plasma concentrations of midazolam and 16.7% of the free plasma concentrations of S-warfarin were still below LLOQ, whereas all total plasma concentrations within these samples could well be quantified. In order to calculate the free fractions (ratio between the free and total plasma concentrations), these BLOQ data (55.6% for midazolam and 16.7% for S-warfarin) were set at 50% of the LLOQ.
Table 1.
Free fractions and percentages of BLOQ samples of the five probe drugs
| Free fractions | Control, median (range) | Fasting, median (range) | Difference (%) | p value | BLOQ samples | |||
|---|---|---|---|---|---|---|---|---|
| Total plasma concentrations | Unbound (free) plasma concentrations | |||||||
| Control (%) | Fasting (%) | Control (%) | Fasting (%) | |||||
| Caffeine | 0.85 (0.65–1.00) | 0.86 (0.66–1.00) | + 0.57 | 0.214 | 0.00 | 0.00 | 0.00 | 0.00 |
| Metoprolol | 0.80 (0.72–0.83) | 0.77 (0.72–0.82) | − 3.5 | 0.767 | 13.9 | 11.1 | 13.9 | 11.1 |
| S-Warfarin | 0.0043 (0.0022–0.0054) | 0.0036 (0.0020–0.0045) | − 16.2 | 0.110 | 0.00 | 0.00 | 80.6 | 77.8 |
| Omeprazol | 0.0141 (0.012–0.019) | 0.0138 (0.010–0.017) | − 2.1 | 0.374 | 2.78 | 0.00 | 11.1 | 13.9 |
| Midazolam | 0.0039 (0.0011–0.0077) | 0.0025 (0.0014–0.0034) | − 36 | 0.260 | 0.00 | 0.00 | 88.9 | 96.3 |
BLOQ below lower limit of quantification
Based on the results of the free fractions of all probe drugs, a post hoc power analysis was performed. Using a paired t test with a 0.05 two-sided significance level, a sample size of nine will have ≥ 98% power to detect a 15% difference in mean free fraction of caffeine, metoprolol and omeprazole between the control and short-term fasting interventions. However, for S-warfarin, a sample size of nine subjects will have 36% power to detect a 15% difference and, for midazolam, a similar sample size will only have 7% power to detect a 15% difference in free fraction between the control and the short-term fasting intervention.
Discussion
The study demonstrates that short-term fasting did not significantly alter protein binding of the probe drugs caffeine, metoprolol and omeprazole. This supports our previous findings that short-term fasting alters the pharmacokinetics of caffeine and metoprolol by affecting CYP-mediated drug metabolism [3, 12].
Short-term fasting did not alter protein binding of the low extraction ratio drug and CYP1A2 probe caffeine which means that the previously found increased systemic clearance (+ 17%) is due to altered CYP1A2-mediated drug metabolism. As an intermediate-extraction ratio drug, the pharmacokinetics of metoprolol after intravenous infusion depends on hepatic blood flow, protein binding and intrinsic clearance (Eq. 1). We have now demonstrated that the previously found increased systemic clearance (+ 13%) is not due to altered (decreased) protein binding. Furthermore, it is unlikely that short-term fasting would have increased hepatic blood flow to explain the observed effect, since the opposite has been described in literature [13, 14]. Therefore, short-term fasting increased CYP2D6-mediated drug metabolism, considering the fact that metoprolol is a probe for CYP2D6.
Short-term fasting did not significantly alter protein binding of the highly protein bound (> 95%) probes midazolam and S-warfarin. However, these results are underpowered and no conclusions on the effect of short-term fasting on protein binding of both drugs can be drawn. The lack of power could be explained by the high percentage of free plasma concentration samples below the lower limit of quantification (BLOQ) (Table 1). The free concentrations of both midazolam and S-warfarin in the samples were very low, and only the samples drawn at the tmax of each plasma concentration–time curve could be included in the analysis. The t max samples still included a number of free plasma concentrations that were below the LLOQ (55.6% for midazolam and 16.7% for S-warfarin) which were set at 50% of the LLOQ. This may hamper detection of small differences in BLOQ free plasma concentrations between both interventions. Although this may seem to be unimportant, small differences in BLOQ free plasma concentrations can still cause relatively large changes in the already small fractions of unbound midazolam and S-warfarin (Eq. 1). For S-warfarin, the previously found decrease in systemic S-warfarin clearance is in line with the expression of hepatic mRNA of the corresponding CYP enzyme in rats, which indicates an effect of fasting on CYP2C9-mediated metabolism [12]. However, an effect of fasting on protein binding instead or in addition to altered CYP2C9-mediated metabolism cannot be excluded. Additional research on the effect of short-term fasting on protein binding of S-warfarin may be warranted because of conflicting findings described in literature. Some studies suggest that free fatty acids, which were significantly increased by fasting, compete with drugs thereby increasing the free fraction [8].In contrast, Vorum et al. have shown that free fatty acids can increase warfarin binding affinity [15]. The latter may result in a relatively large decrease in free fraction, which could explain decreased total clearance (Eq. 1). Omeprazole is also highly protein bound (≈ 97%). In contrast to S-warfarin and midazolam, omeprazole free concentrations were all above the LLOQ and it was clearly demonstrated that short-term fasting did not alter plasma protein binding. However, this result cannot be extrapolated to S-warfarin as serum albumin has different binding sites for warfarin (site I) and omeprazole (site IIa) [16]. To overcome the potential confounding effects of protein binding when studying intrinsic clearance (CLint) using probe drugs, the use of low protein bound probe drugs might be recommended. However, for CYP2C9, 2C19 and CYP3A4 there are no good alternative probes which have low protein binding or comply with other factors (e.g. selectivity for the enzyme involved, lack of interaction with other probe drugs when administered as cocktail) that are also important for a probe drug and should not be ignored. In the absence of a suitable alternative, administration of a higher dose of the high protein bound probe drug and/or the use of a more sensitive analytical method, which is able to detect changes in the very low free plasma concentrations of these drugs, could be considered.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank M. Pistorius and N. Frens for their specific contribution to the laboratory analysis of this study.
Compliance with Ethical Standards
Funding
This study did not receive funding.
Conflict of interest
The authors, L.A. Lammers, R. Achterbergh, J.A. Romijn and R.A.A. Mathôt, have no conflict of interest to declare.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research committee. Furthermore, the study was conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13318-017-0437-7) contains supplementary material, which is available to authorized users.
References
- 1.Turpault S, Brian W, Van Horn R, Santoni A, Poitiers F, Donazzolo Y, et al. Pharmacokinetic assessment of a five-probe cocktail for CYPs 1A2, 2C9, 2C19, 2D6 and 3A. Br J Clin Pharmacol. 2009;68(6):928–935. doi: 10.1111/j.1365-2125.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lammers LA, Achterbergh R, Pistorius MC, Bijleveld Y, de Vries EM, Boelen A, et al. Quantitative method for simultaneous analysis of a 5-probe cocktail for cytochrome P450 enzymes. Ther Drug Monit. 2016;38(6):761–768. doi: 10.1097/FTD.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 3.Lammers LA, Achterbergh R, van Schaik RH, Romijn JA, Mathot RA. Effect of short-term fasting on systemic cytochrome P450-mediated drug metabolism in healthy subjects: a randomized, controlled, crossover study using a cocktail approach. Clin Pharmacokinet. 2017 doi: 10.1007/s40262-017-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benet LZ, Hoener BA. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115–121. doi: 10.1067/mcp.2002.121829. [DOI] [PubMed] [Google Scholar]
- 5.Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147–1161. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
- 6.Micromedex Solutions—Drug Monographs [database on the Internet]. Truven Health Analytics Inc. 2014. https://www.micromedexsolutions.com. Accessed March 2014.
- 7.Butler JM, Begg EJ. Free drug metabolic clearance in elderly people. Clin Pharmacokinet. 2008;47(5):297–321. doi: 10.2165/00003088-200847050-00002. [DOI] [PubMed] [Google Scholar]
- 8.Tesseromatis C, Alevizou A. The role of the protein-binding on the mode of drug action as well the interactions with other drugs. Eur J Drug Metab Pharmacokinet. 2008;33(4):225–230. doi: 10.1007/BF03190876. [DOI] [PubMed] [Google Scholar]
- 9.Duncombe WG. The colorimetric micro-determination of non-esterified fatty acids in plasma. Clin Chim Acta. 1964;9:122–125. doi: 10.1016/0009-8981(64)90004-X. [DOI] [PubMed] [Google Scholar]
- 10.Krebs M, Stingl H, Nowotny P, Weghuber D, Bischof M, Waldhausl W, et al. Prevention of in vitro lipolysis by tetrahydrolipstatin. Clin Chem. 2000;46(7):950–954. [PubMed] [Google Scholar]
- 11.Gonzalez D, Schmidt S, Derendorf H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin Microbiol Rev. 2013;26(2):274–288. doi: 10.1128/CMR.00092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammers LA, Achterbergh R, de Vries EM, van Nierop FS, Klumpen HJ, Soeters MR, et al. Short-term fasting alters cytochrome P450-mediated drug metabolism in humans. Drug Metab Dispos. 2015;43(6):819–828. doi: 10.1124/dmd.114.062299. [DOI] [PubMed] [Google Scholar]
- 13.Lomax MA, Baird GD. Blood flow and nutrient exchange across the liver and gut of the dairy cow. Effects of lactation and fasting. Br J Nutr. 1983;49(3):481–496. doi: 10.1079/BJN19830057. [DOI] [PubMed] [Google Scholar]
- 14.Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16(48):6046–6057. doi: 10.3748/wjg.v16.i48.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vorum H, Honore B. Influence of fatty acids on the binding of warfarin and phenprocoumon to human serum albumin with relation to anticoagulant therapy. J Pharm Pharmacol. 1996;48(8):870–875. doi: 10.1111/j.2042-7158.1996.tb03990.x. [DOI] [PubMed] [Google Scholar]
- 16.Pawar SK, Punith R, Naik RS, Seetharamappa J. Spectroscopic and molecular modeling approaches to investigate the binding of proton pump inhibitors to human serum albumin. J Biomol Struct Dyn. 2016 doi: 10.1080/07391102.2016.1251337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.