Abstract
The dorsal striatum is a key node for many neurobiological processes such as motor activity, cognitive functions, and affective processes. The proper functioning of striatal neurons relies critically on metabotropic receptors. Specifically, the main adenosine and endocannabinoid receptors present in the striatum, ie, adenosine A2A receptor (A2AR) and cannabinoid CB1 receptor (CB1R), are of pivotal importance in the control of neuronal excitability. Facilitatory and inhibitory functional interactions between striatal A2AR and CB1R have been reported, and evidence supports that this cross-talk may rely, at least in part, on the formation of A2AR-CB1R heteromeric complexes. However, the specific location and properties of these heteromers have remained largely unknown. Here, by using techniques that allowed a precise visualization of the heteromers in situ in combination with sophisticated genetically modified animal models, together with biochemical and pharmacological approaches, we provide a high-resolution expression map and a detailed functional characterization of A2AR-CB1R heteromers in the dorsal striatum. Specifically, our data unveil that the A2AR-CB1R heteromer (i) is essentially absent from corticostriatal projections and striatonigral neurons, and, instead, is largely present in striatopallidal neurons, (ii) displays a striking G protein-coupled signaling profile, where co-stimulation of both receptors leads to strongly reduced downstream signaling, and (iii) undergoes an unprecedented dysfunction in Huntington’s disease, an archetypal disease that affects striatal neurons. Altogether, our findings may open a new conceptual framework to understand the role of coordinated adenosine-endocannabinoid signaling in the indirect striatal pathway, which may be relevant in motor function and neurodegenerative diseases.
INTRODUCTION
The dorsal striatum is a key node for many neurobiological processes such as motor activity, cognitive functions, and affective processes. The vast majority (~95%) of neurons within the striatum are GABAergic medium spiny neurons (MSNs), which receive glutamatergic inputs primarily from the cortex. MSNs differ in their neurochemical composition and form two major efferent pathways, the direct (striatonigral) pathway and the indirect (striatopallidal) pathway (Kreitzer, 2009). The proper functioning of MSNs relies critically on metabotropic receptor signaling. Many neurotransmitters and neuromodulators such as dopamine, glutamate, endocannabinoids and adenosine control MSN activity and plasticity by engaging their cognate G protein-coupled receptors (GPCRs) (Lovinger, 2010; Girault, 2012). Specifically, the main endocannabinoid and adenosine receptors present in MSNs, ie, cannabinoid type 1 receptor (CB1R) and adenosine subtype 2A receptor (A2AR), are of pivotal importance in the control of neuronal excitability. CB1R is one of the most abundant GPCRs in MSNs (Glass et al, 2000; Castillo et al, 2012). In particular, CB1R is highly expressed in the terminals of both striatonigral and striatopallidal MSNs, where it mediates endocannabinoid-dependent inhibition of GABA release, thus decreasing motor activity (Katona and Freund, 2008; Castillo et al, 2012). CB1R is also expressed in glutamatergic terminals projecting from the cortex onto the striatum, where it controls MSN function by blunting glutamatergic output and mediating the so-called endocannabinoid-dependent long-term depression (Kreitzer, 2009; Castillo et al, 2012). A2AR is also very abundant in the striatum (Schiffmann and Vanderhaeghen, 1993; Schiffmann et al, 2007). Presynaptically, a significant fraction of the corticostriatal projections that expresses CB1R also contains A2AR. These A2AR molecules are mostly located on corticostriatal terminals that form synaptic contacts with striatonigral MSNs (Quiroz et al, 2009; Ferreira et al, 2015). Blockade of presynaptic A2AR counteracts glutamate release and motor output evoked by cortical stimulation (Quiroz et al, 2009; Orru et al, 2011; Tebano et al, 2012). Postsynaptically, A2AR is selectively located on striatopallidal MSNs, which co-express dopamine D2 receptor (D2R) (Schiffmann et al, 2007; Azdad et al, 2009; Tebano et al, 2012). Blockade of postsynaptic A2AR mediates the motor-activating effects of A2AR antagonists, consistent with an inactivation of the indirect pathway (Orru et al, 2011; Tebano et al, 2012).
The high expression of A2AR and CB1R in the striatum, together with the key involvement of both receptors in the control of motor and goal-directed behaviors, have led to a large number of studies on the interactions between them (Ferre et al, 2010; Tebano et al, 2012). Understanding these interactions is of special relevance not only physiologically but also pharmacologically as these receptors are targets of widely consumed psychoactive substances such as caffeine (an A2AR antagonist) and Δ9-tetrahydrocannabinol (a CB1R agonist). Both facilitatory and inhibitory functional interactions between striatal A2AR and CB1R have been demonstrated (Ferre et al, 2010; Tebano et al, 2012; Justinova et al, 2014). The precise molecular mechanisms underlying the cross-talk between these receptors is yet to be fully understood, but some evidence supports that they may rely, at least in part, on the formation of A2AR-CB1R heteromeric complexes (Carriba et al, 2007; Ferre et al, 2010; Tebano et al, 2012; Chiodi et al, 2016). Despite >10 years of research on GPCR heteromers, there continues to be a major gap in our understanding of where exactly heteromers are expressed as well as linking them to precise signal transduction pathways and biological functions. In the case of the A2AR-CB1R heteromer, factors to consider include (i) the additional partners with which A2AR and CB1R could interact differently at presynaptic sites (eg, A1R) (Ciruela et al, 2006) or postsynaptic sites (eg, D2R and mGluR5) (Navarro et al, 2008; Azdad et al, 2009; Cabello et al, 2009; Bonaventura et al, 2014; Bonaventura et al, 2015), (ii) the convergence of adenosine and endocannabinoid actions on various intracellular signaling pathways (Ferre et al, 2010; Tebano et al, 2012), and (iii) the intricate network of molecular processes controlling adenosine and endocannabinoid release (Kreitzer and Malenka, 2005; Lerner et al, 2010).
Previous studies on the A2AR-CB1R heteromer have relied essentially on energy transfer-based assays in cells ectopically expressing A2AR and CB1R, as well as co-immunolocalization and co-immunoprecipitation experiments (Carriba et al, 2007; Navarro et al, 2008; Bonaventura et al, 2014). These approaches, although widely exploited and certainly valuable, possess limitations of spatial resolution (co-immunolocalization), molecular specificity (co-immunoprecipitation), and biological interpretation (energy transfer using protein overexpression) to characterize GPCR heteromers. Hence, here we made use of techniques to allow a precise visualization of the heteromers in situ in combination with sophisticated genetically modified mouse models, together with biochemical and pharmacological approaches, to cogently characterize the anatomy and signaling profile of the A2AR-CB1R heteromer in the dorsal striatum.
MATERIALS AND METHODS
The experimental procedures used in this study are extensively described in Supplementary Materials and Methods. That section provides precise details on animal models (genetic mouse models to study the location of the A2AR-CB1R heteromer, as well as mouse models of Huntington’s disease (HD)), human post mortem brain samples (see also Supplementary Table S1), recombinant adeno-associated viral vectors, HIV TAT peptides designed to disrupt the A2AR-CB1R heteromer, cell culture and transfection procedures, in situ proximity ligation assays (PLA), fluorescence complementation assays, dynamic mass redistribution (DMR) label-free assays, cAMP and Ca2+ concentration assays, western blotting assays, immunomicroscopy procedures, and statistical analyses (see also Supplementary Table S2).
RESULTS
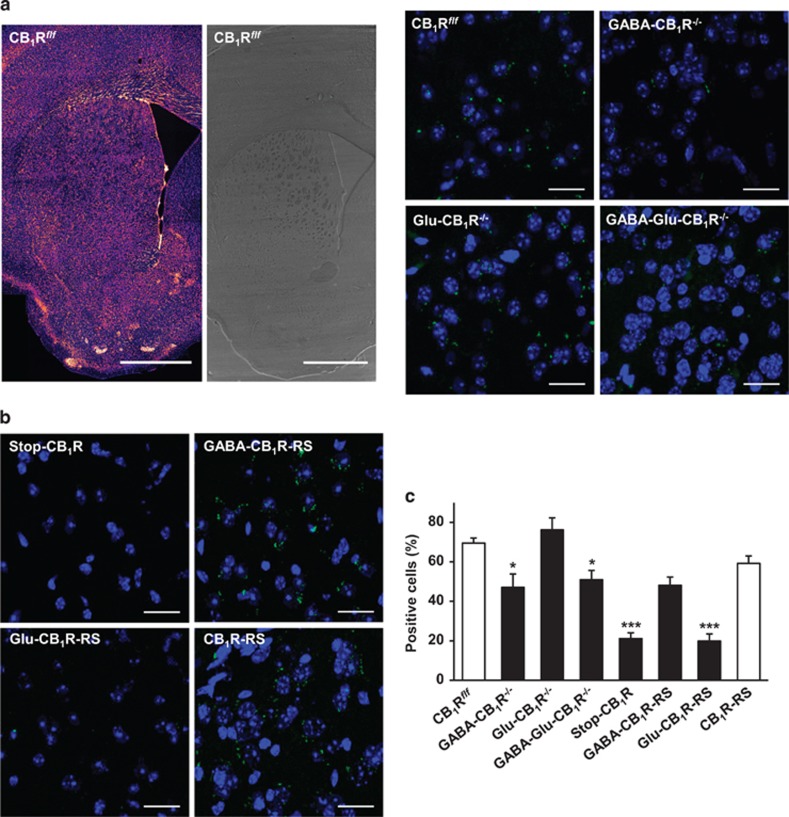
A2AR-CB1R Heteromers are Located on GABAergic Neurons Rather Than Glutamatergic Projections in the Mouse Dorsal Striatum
To clarify the precise location of A2AR-CB1R heteromers in the dorsal striatum we conducted PLA experiments. The PLA assay is a powerful and straightforward technique to detect protein–protein interactions in general, and GPCR oligomers in particular, and to localize these complexes in situ with cell sub-population selectivity, thus allowing an unbiased demonstration and quantification of protein complexes in unmodified cells and tissues (Taura et al, 2015). Importantly, PLA permits assessing close proximity between proteins within an oligomer with high resolution (<40 nm). As PLA relies on the amplification of a small signal, its main limitation is antibody specificity/background noise, which we minimize by adapting refined technical protocols as well as employing multiple genetic mouse models and controls (Taura et al, 2015). Here, we first used conditional mutant mice bearing a genetic deletion of CB1R in forebrain GABAergic neurons (CB1Rfloxed/floxed;Dlx5/6-Cre/+ mice; herein referred to as GABA-CB1R−/− mice) or dorsal telencephalic glutamatergic neurons (CB1Rfloxed/floxed;Nex-Cre/+ mice; herein referred to as Glu-CB1R−/− mice) (Monory et al, 2006). Striatal A2AR-CB1R heteromers were evident almost exclusively as dots in the vicinity of cell nuclei, and showed a remarkable reduction in GABA-CB1R−/− mice (Figure 1a and c). In contrast, no significant differences were observed between Glu-CB1R−/− mice and CB1Rfloxed/floxed;+/+ controls (Figure 1a and c) when data were expressed either as a percentage of cells containing one or more dots relative to total cell nuclei (Figure 1c) or as a total number of dots relative to total cell nuclei (CB1Rfloxed/floxed mice: 2.23±0.16; Glu-CB1R−/− mice: 2.40±0.20; n=3 animals of each genotype). In addition, Glu-CB1R−/− mice did not show any significant reduction in the percentage of A2AR-CB1R heteromer-positive cells relative to total cell nuclei in their motor cortices (CB1Rfloxed/floxed mice: 70.3±2.3; Glu-CB1R−/− mice: 71.4±3.0; n=3 animals of each genotype). Likewise, the expression levels of A2AR-CB1R heteromers displayed by GABA-CB1R−/− mice were not decreased further when the CB1R gene was simultaneously ablated in glutamatergic neurons (CB1Rfloxed/floxed;Dlx5/6-Cre;Nex-Cre mice; herein referred to as GABA-Glu-CB1R−/− mice) (Bellocchio et al, 2010) (Figure 1a and c). Control experiments conducted in the absence of one of the two primary antibodies, as well as in full CB1R−/− mice (Marsicano et al, 2002) and full A2AR−/− mice (Ledent et al, 1997), provided strong support to the specificity of the PLA analyses performed (Supplementary Figure S1a–c). Of note, a different anti-CB1R primary antibody provided a similar A2AR-CB1R heteromer detection (Supplementary Figure S1d). Moreover, the specificity of the primary antibodies used was also demonstrated by immunocytofluorescence studies conducted in HEK-293T cells transfected or not with cDNAs encoding human A2AR or human CB1R (Supplementary Figure S1e).
Figure 1.
A2AR-CB1R heteromers are located on GABAergic neurons rather than glutamatergic projections in the mouse dorsal striatum. (a, b) PLA assays were performed in dorsal-striatum sections from 3–4-month-old mice of different genotypes. A2AR-CB1R heteromers are shown as green dots. Nuclei are colored in blue by DAPI staining. (a) Representative low-magnification image of tissue sections used for PLA assays. Left, DAPI-stained field; right, bright field. Scale bar: 1 mm. Representative pictures from control CB1R-floxed, GABA-CB1R−/−, Glu-CB1R−/−, and GABA-Glu-CB1R−/− mice. Scale bar: 20 μm. (b) Representative pictures from Stop-CB1R, GABA-CB1R-RS mice, Glu-CB1R-RS mice and CB1R-RS mice. Scale bar: 20 μm. (c) Quantification of the number of cells containing one or more dots expressed as the percentage of the total number of cells (blue nuclei). Data are the mean±SEM of counts in 5–14 different fields from three different animals of each type. One-way ANOVA followed by Dunnet post hoc test showed a significant (*p<0.05, ***p<0.001) decrease of heteromer expression compared to control CB1R-floxed mice (a) or to CB1R-RS mice (b). Further details of statistical analyses are given in Supplementary Table S2.
To unequivocally ascribe A2AR-CB1R heteromers to GABAergic neurons we made use of a Cre-mediated, lineage-specific CB1R re-expression/rescue strategy in a CB1R-null background (herein referred to as Stop-CB1R mice) (Ruehle et al, 2013; De Salas-Quiroga et al, 2015). The selective rescue of CB1R expression in forebrain GABAergic neurons (herein referred to as GABA-CB1R-RS mice) was achieved by expressing Cre under the regulatory elements of the Dlx5/6 gene (De Salas-Quiroga et al, 2015). In parallel, we rescued CB1R expression selectively in dorsal telencephalic glutamatergic neurons (herein referred to as Glu-CB1R-RS mice) by using a Nex-Cre mouse line (Ruehle et al, 2013). As a control, an EIIa-Cre-mediated, global CB1R expression-rescue in a CB1R-null background was conducted (herein referred to as CB1R-RS mice) (Ruehle et al, 2013). Remarkably, the expression levels of A2AR-CB1R heteromers were notably restored in GABA-CB1R-RS mice (Figure 1b and c). In contrast, no significant rescue of the heteromer was observed in Glu-CB1R-RS animals when data were expressed either as a percentage of cells containing one or more dots relative to total cell nuclei (Figure 1c) or as a total number of dots relative to total cell nuclei (Stop-CB1R mice: 0.24±0.01; Glu-CB1R-RS mice: 0.28±0.04; n=3 animals of each genotype).
Taken together, these data strongly support that, in the mouse dorsal striatum, A2AR-CB1R heteromers are located on GABAergic neurons rather than glutamatergic projections.
A2AR-CB1R Heteromers are Located on Indirect-Pathway MSNs in the Mouse Dorsal Striatum
The vast majority (~95%) of neurons within the striatum are MSNs (Kreitzer, 2009). These neurons differ in their neurochemical composition and form two major efferent pathways. The direct pathway consists of MSNs expressing markers such as dopamine D1 receptor (D1R) and substance P. It mainly projects to the substantia nigra pars reticulata and the internal segment of the globus pallidus. The indirect pathway is composed of MSNs expressing markers such as D2R and enkephalin. It mainly projects to the external segment of the globus pallidus, which, in turn, projects to the subthalamic nucleus (Kreitzer, 2009). CB1R is located on both direct-pathway and indirect-pathway MSNs, whereas A2AR resides essentially on indirect-pathway MSNs (Schiffmann et al, 2007; Kreitzer, 2009; Castillo et al, 2012). As a consequence, A2AR-CB1R heteromers would conceivably be located on indirect-pathway MSNs. To substantiate this possibility, we first used conditional mutant mice bearing a genetic deletion of CB1R in D1R-expressing neurons (CB1Rfloxed/floxed;Drd1a-Cre/+ mice; herein referred to as D1R-CB1R−/− mice) (Monory et al, 2007). No differences were observed in the expression of A2AR-CB1R heteromers, as assessed by PLA analyses, between D1R-CB1R−/− mice and control mice (Supplementary Figure S2a), thus confirming that the heteromer is not located on direct-pathway MSNs. CB1R is essentially a presynaptic receptor that, in MSNs, resides, mainly on terminals and collaterals (Katona and Freund, 2008; Kreitzer, 2009; Castillo et al, 2012). Hence, we also studied the projection sites of MSNs in CB1Rfloxed/floxed mice. Specifically, we injected stereotactically these CB1Rfloxed/floxed mice with a recombinant adeno-associated viral vector encoding Cre (or EGFP to gain visualization of neuronal projections) into the dorsal striatum (or the motor cortex as control). Cre expression was driven by a CaMKIIα promoter, so it was confined to MSNs (injections into the striatum) or principal neurons (injections into the cortex) (Chiarlone et al, 2014). Cre-mediated excision of the loxP-flanked CB1R gene in dorsal-striatum MSNs of CB1Rfloxed/floxed mice reduced the expression of A2AR-CB1R heteromers in the globus pallidus (Supplementary Figure S2b). In contrast, inactivation of the CB1R gene in the motor cortices of CB1Rfloxed/floxed mice did not affect the expression of A2AR-CB1R heteromers on corticostriatal inputs (Supplementary Figure S2c).
Collectively, these data show that, in the mouse dorsal striatum, A2AR-CB1R heteromers are primarily located on indirect-pathway MSNs.
A2AR-CB1R Heteromers Expressed in the Mouse Dorsal Striatum are Functional
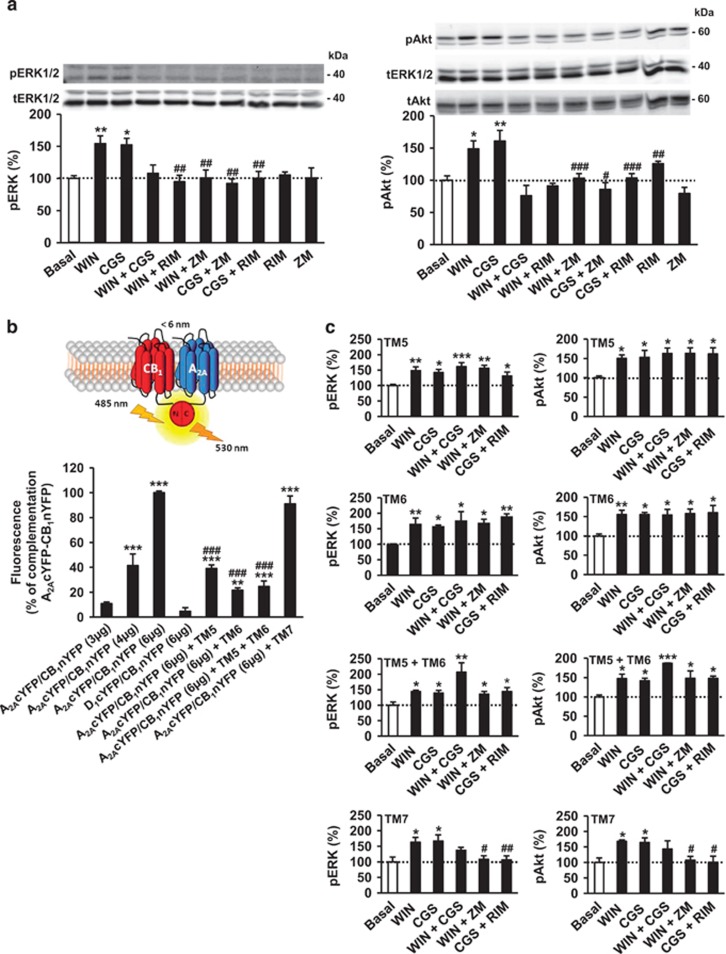
Previous reports have shown the existence of both facilitatory and inhibitory functional interactions between A2AR and CB1R (Ferre et al, 2010; Tebano et al, 2012). To investigate the possible role of the A2AR-CB1R heteromer in these interactions we characterized in detail heteromer functionality in the dorsal striatum. For this purpose we used C57BL/6N-mouse striatal slices and conducted cell signaling experiments on two pathways coupled to A2AR and CB1R: extracellular signal-regulated kinase (ERK) and Akt. The CB1R agonist WIN-55,212-2 or the A2AR agonist CGS21680 increased ERK phosphorylation (activation) in the dorsal striatum, whereas co-incubation with both agonists abrogated ERK phosphorylation, thus demonstrating a negative cross-talk between A2AR and CB1R (Figure 2a). In addition, the CB1R antagonist SR141716 (rimonabant) or the A2AR antagonist ZM241385 prevented the ERK-activating effect of WIN-55,212-2 or CGS21680 (Figure 2a). These data show a cross-antagonism between the two receptors, a phenomenon not uncommon in heteromers. When these cross-pharmacological assays were conducted for Akt phosphorylation (activation), similar negative cross-talk and cross-antagonism processes were observed (Figure 2a). Collectively, these findings demonstrate the existence of inhibitory interactions between A2AR and CB1R in the mouse dorsal striatum.
Figure 2.
A2AR-CB1R heteromers expressed in the mouse dorsal striatum are functional. (a, c) ERK and Akt phosphorylation was determined in striatal slices from 3–4-month-old C57BL/6N mice pre-treated for 4 h with medium (a) or with 4 μM TM5, TM6 or TM7 peptides alone or in combination (c). Slices were then preincubated for 20 min with vehicle, the CB1R antagonist SR141716 (10 μM) or the A2AR antagonist ZM241385 (10 μM) before the addition of vehicle, the CB1R agonist WIN-55,212-2 (1 μM), the A2AR agonist CGS21680 (1 μM) or both, for 10 min. Immunoreactive bands from 3–6 slices from 12 different animals were quantified for each condition. Values represent mean±SEM of percentage of phosphorylation relative to basal levels found in vehicle only-treated slices (100%, dotted line). One-way ANOVA showed a significant (*p<0.05, **p<0.01, ***p<0.001) effect over basal, or of agonist plus antagonist treatment over agonist-only treatment (#p<0.05, ##p<0.01, ###p<0.001). Further details of statistical analyses are given in Supplementary Table S2. In (a), representative western blots are shown at the top of each panel. (b) Schematic representation of the bimolecular fluorescence complementation technique showing that fluorescence only appears after the YFP Venus hemiprotein (cYFP or nYFP) complementation owing to the proximity of the two receptors fused to hemi-YFP Venus proteins (top panel). In the bottom panel, fluorescence at 530 nm was monitored in HEK-293T cells transfected with the indicated amounts of cDNA encoding CB1R-nYFP and A2AR-cYFP (equal amount for each construct) or, as a negative control, transfected with cDNA encoding CB1R-nYFP and the non-interacting D1R-cYFP. Transfected cells were treated for 4 h with medium or with 4 μM TM5, TM6, and/or TM7 peptides before fluorescence reading. Values represent mean±SEM of percentage of fluorescence relative to A2AR-cYFP/CB1R-nYFP maximal complementation (n=4–12 replicates from three independent experiments for each condition). One-way ANOVA showed a significant change in fluorescence over non-transfected cells (**p<0.01, ***p<0.001), or of the peptide-treated over the corresponding non-peptide treated cells (###p<0.001). Further details of statistical analyses are given in Supplementary Table S2.
Next, we sought to substantiate that the aforementioned negative cross-talk and cross-antagonism between A2AR and CB1R rely on A2AR-CB1R heteromers. It is generally believed that agonist binding to the extracellular pocket of GPCRs induces local conformational changes that increase signaling by opening an intracellular cavity via the movement of transmembrane helices (TMs) 5 and 6 for receptor activation, whereas, conversely, inverse agonists decrease the basal, agonist-independent, level of signaling by closing this cavity (Shoichet and Kobilka, 2012; Venkatakrishnan et al, 2013). In fact, the reported crystal structure of the agonist-bound A2AR, compared with the inactive, antagonist-bound A2AR, shows an outward tilt and rotation of the cytoplasmic half of TM6 and a movement of TM5, thus resembling the changes associated with the active-state structure of other class A GPCRs (Xu et al, 2011). Likewise, the crystal structure of the antagonist-bound CB1R has been recently reported, showing a similar opsin-like behavior for this receptor (Hua et al, 2016; Shao et al, 2016). Our aforementioned observation that A2AR-CB1R heteromers display both negative cross-talk and cross-antagonism suggests a negative modulation between both receptors through protein–protein interactions involving the TM5/TM6 interface. Hence, to test this hypothesis, we studied whether synthetic peptides with the sequence of TM5, TM6 or TM7 (as negative control) of CB1R, fused to HIV TAT peptide to allow efficient intracellular delivery and plasma membrane insertion (Schwarze et al, 1999; He et al, 2011), were able to disrupt A2AR-CB1R heteromerization and the observed bidirectional cross-signaling. This approach has been recently used by us and others to disrupt other heteromers (Guitart et al, 2014; Lee et al, 2014; Viñals et al, 2015).
We first characterized the TM interference peptides by the bimolecular fluorescence complementation technique. In this assay, fluorescence only appears after correct folding of two YFP Venus hemiproteins. This occurs when two receptors fused to hemi-YFP Venus proteins (cYFP or nYFP) come within proximity to facilitate YFP Venus folding (Figure 2b, scheme). Fluorescence was detected in HEK-293T cells transfected with different amounts of cDNA encoding CB1R-nYFP and A2AR-cYFP, but not in negative controls in which cells were transfected with cDNA encoding CB1R-nYFP and the non-interacting D1R-cYFP (Figure 2b). The TM-targeted peptides were subsequently tested. We found that treatment of cells expressing CB1R-nYFP and A2AR-cYFP with TM5 or TM6 (but not TM7) peptides disrupted the heteromer structure, as revealed by a loss of fluorescence (Figure 2b). We next studied the effect of the interference peptides on A2AR and CB1R signaling in mouse striatal slices. When the peptides were evaluated in cross-pharmacological assays, we found that pretreatment of brain slices with TM5, TM6 or both (but not TM7) peptides disrupted (i) the ability of the CB1R agonist WIN-55,212-2 and the CB1R antagonist SR141716 to dampen A2AR-evoked actions on ERK and Akt, as well as (ii) the ability of the A2AR agonist CGS21680 and the A2AR antagonist ZM241385 to dampen CB1R-evoked actions on these two signaling pathways (Figure 2c). Of note, when the TM5 and TM6 peptides were used in combination, the increase in ERK and Akt phosphorylation upon receptor co-activation tended to be higher compared with TM5-only or TM6-only incubations (Figure 2c), thus conceivably reflecting that the peptide combination is more efficient than each peptide alone in disrupting the heteromer.
Together, these data provide evidence for the importance of the TM5/TM6 interface in the A2AR-CB1R heteromer, and support that the negative cross-talk and cross-antagonism that occurs between CB1R and A2AR are due to protein–protein interactions and are a specific biochemical characteristic of the A2AR-CB1R heteromer.
Functional A2AR-CB1R Heteromers are Present in Wild-Type and Mutant Huntingtin-Expressing Striatal Neuroblasts
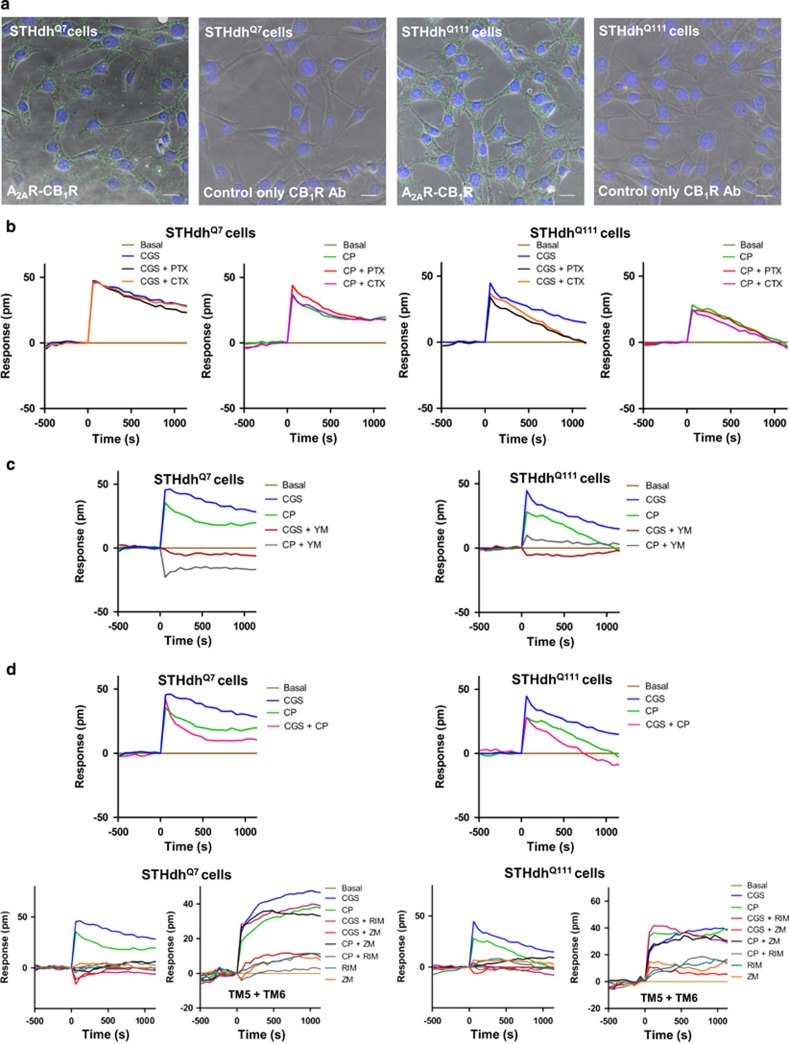
To evaluate the relevance of the A2AR-CB1R heteromer in a pathological setting we selected HD as a model because (i) it is the paradigmatic disease primarily caused by a selective loss of MSNs in the dorsal striatum (Walker, 2007), and (ii) changes in the expression and function of A2AR and CB1R have been shown to occur in the dorsal striatum of patients and animal models of the disease (Glass et al, 2000; Fernandez-Ruiz et al, 2011; Lee and Chern, 2014). We first characterized the heteromer in conditionally immortalized striatal neuroblasts expressing two normal (STHdhQ7) or mutant (STHdhQ111) full-length endogenous huntingtin alleles with 7 or 111 glutamine residues, respectively, which represent a widely accepted cellular model to investigate huntingtin actions. These cells do not exhibit mutant-huntingtin inclusions (Trettel et al, 2000), thus allowing the modeling of changes occurring at early HD stages.
We readily detected PLA-positive A2AR-CB1R heteromers in both STHdhQ7 and STHdhQ111 cells (Figure 3a), indicating that the mere expression of mutant huntingtin does not prevent heteromerization of both receptors. To evaluate the functional characteristics of A2AR-CB1R heteromers, we first measured the global cellular response using DMR label-free assays, which detect changes in light diffraction in the bottom 150 nm of a cell monolayer. In these experiments we had a preference for CP-55,940 over WIN-55,212-2 as the CB1R agonist because the former is less hydrophobic than the latter and so conceivably more accessible to cultured cells. In fact, dose–response experiments conducted in both STHdhQ7 and STHdhQ111 cells showed that CP-55,940 impacted the DMR signal more markedly than WIN-55,212-2 (Supplementary Figure S3a and b). Both the A2AR agonist CGS21680 and the CB1R agonist CP-55,940 induced time-dependent signaling in STHdhQ7 and STHdhQ111 cells (Figure 3b). Of note, A2AR and CB1R-evoked signaling was essentially insensitive to pertussis toxin (PTX) or cholera toxin (CTX) (Figure 3b), thus indicating that these receptors do not significantly couple to Gi or Gs proteins in these cells. This notion was further supported by the observation that, in both STHdhQ7 cells (Supplementary Figure S4a) and STHdhQ111 cells (Supplementary Figure S4b), neither the A2AR agonist nor the CB1R agonist was able to affect basal or forskolin-elevated cAMP concentrations in the absence or presence of PTX or CTX. In line with this apparent lack of ‘classical’ A2AR-Gs/olf and CB1R-Gi coupling, the Gq protein inhibitor YM-254890 was able to abrogate the A2AR and CB1R-evoked changes in DMR (Figure 3c). This non-conventional coupling did appear to be due to heteromer formation as experiments conducted with the TM5 and TM6 peptides on STHdhQ7 and STHdhQ111 cells showed that the peptide combination, presumably by disrupting the heteromer, turned A2AR and CB1R action to their ‘classical’, ‘protomeric’ Gs/olf, and Gi-mediated signaling, respectively (Supplementary Figure S4c). This strongly supports that there is no limitation of Gs/olf or Gi protein availability in these cells, as previously indicated by others’ work (Araki et al, 2006), and that the A2AR-CB1R heteromer couples selectively to Gq. Moreover, and further supporting a Gq-dependent signaling for the heteromer, engagement of A2AR or CB1R increased intracellular free Ca2+ concentration in both STHdhQ7 and STHdhQ111 cells (Supplementary Figure S5).
Figure 3.
A2AR-CB1R heteromers expressed in wild-type STHdhQ7 and mutant huntingtin-expressing STHdhQ111 striatal neuroblasts signal via Gq protein rather than Gi or Gs protein. (a) PLA assays were performed in STHdhQ7 and STHdhQ111 cells. A2AR-CB1R heteromers are shown as green dots. Nuclei are colored in blue by DAPI staining. Controls in the absence of anti-A2AR primary antibody were also performed. Representative pictures are shown. Scale bar: 20 μm. (b) Dynamic mass redistribution (DMR) assays were performed in STHdhQ7 and STHdhQ111 cells pretreated overnight with vehicle, pertussis toxin (PTX; 10 ng/ml) or cholera toxin (CTX; 100 ng/ml), and further treated with vehicle, the A2AR agonist CGS21680 (1 μM) or the CB1R agonist CP-55,940 (1 μM). (c) DMR assays in STHdhQ7 and STHdhQ111 cells preincubated for 30 min with vehicle or the Gq protein inhibitor YM-254890 (1 μM), and then activated with the A2AR agonist CGS21680 (1 μM) or the CB1R agonist CP-55,940 (1 μM). (d) DMR assays showing negative cross-talk (top panels) and cross-antagonism (bottom panels) between A2AR and CB1R signaling. STHdhQ7 and STHdhQ111 cells were not pre-treated (top panels) or pre-treated for 4 h with medium (left bottom panels) or with 4 μM TM5 plus TM6 (right bottom panels) before incubation for 30 min with vehicle, the CB1R antagonist SR141716 (RIM; 1 μM) or the A2AR antagonist ZM241385 (1 μM), and then activated with vehicle, CGS21680 (1 μM) or CP-55,940 (1 μM). (b–d) The resulting shifts of reflected light wavelength (pm) were monitored over time. Each panel is a representative experiment of n=3 different experiments. Each curve is the mean of a representative optical trace experiment carried out in triplicates.
We next investigated whether the heteromer-specific biochemical properties described above could influence Gq-driven signaling. Regarding negative cross-talk, the DMR signal induced by the A2AR agonist CGS21680 alone or the CB1R agonist CP-55,940 alone was attenuated when both agonists were added together to STHdhQ7 or STHdhQ111 cells (Figure 3d, top panels). Regarding cross-antagonism, the DMR signal induced by the CB1R agonist was prevented not only by the CB1R antagonist SR141716 but also by the A2AR antagonist ZM241385, and, similarly, the DMR signal induced by the A2AR agonist CGS21680 was also prevented by either antagonist (Figure 3d, bottom panels). Of note, the combination of the TM5 and TM6 peptides disrupted the cross-antagonism between A2AR and CB1R in STHdhQ7 and STHdhQ111 cells (Figure 3d, bottom panels).
Collectively, these data indicate that co-expression of A2AR and CB1R, likely through the formation of A2AR-CB1R heteromers, facilitates Gq rather than Gs or Gi coupling in wild-type and mutant huntingtin-expressing mouse striatal neuroblasts.
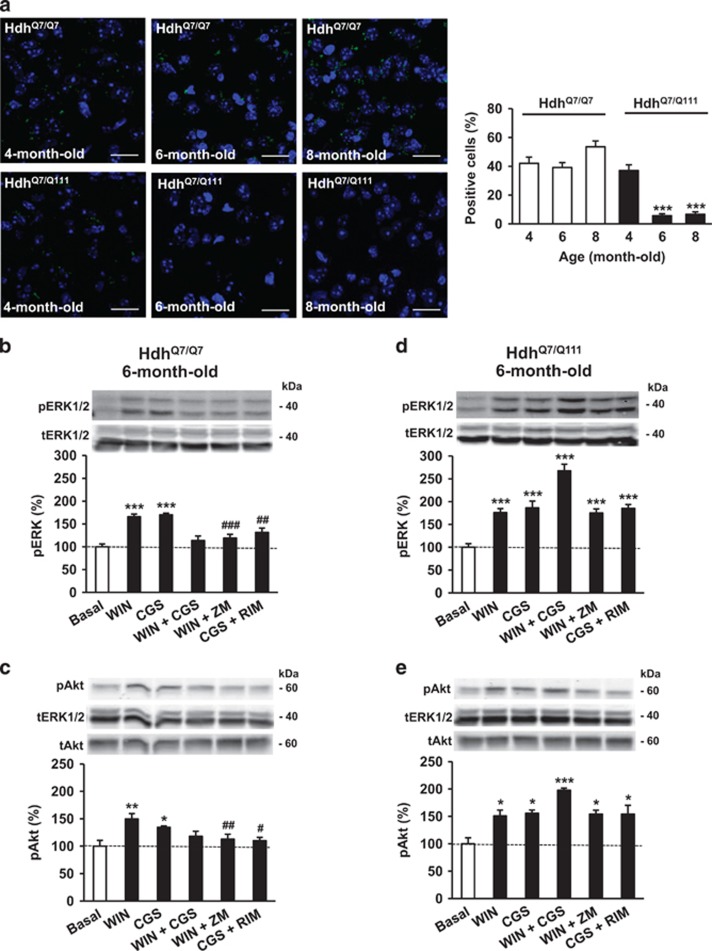
Functional A2AR-CB1R Heteromers are Expressed in HD Mice at Early but not Advanced Disease Stages
To study the role of A2AR-CB1R heteromers in HD in vivo we analyzed their expression and function in a widely accepted model of HD, heterozygous mutant knock-in HdhQ7/Q111 mice, that express in heterozygosity a mutant full-length huntingtin allele with 111 glutamine residues, and wild-type HdhQ7/Q7 mice, that express two wild-type full-length huntingtin alleles with 7 glutamine residues. At an early stage of the disease (4 months of age), mutant HdhQ7/Q111 mice displayed A2AR-CB1R heteromers in the dorsal striatum at similar levels as wild-type HdhQ7/Q7 mice (Figure 4a). However, at more advanced stages (6 and 8 months of age), the expression of A2AR-CB1R heteromers was almost completely lost in mutant HdhQ7/Q111 mice but not wild-type HdhQ7/Q7 mice (Figure 4a). Of note, total striatal A2AR and CB1R expression, as determined by western blot (Supplementary Figure S6a) and immunofluorescence microscopy (Supplementary Figure S6b), was largely preserved in 6-month-old mutant HdhQ7/Q111 mice compared with age-matched wild-type HdhQ7/Q7 mice. Hence, irrespective of the small differences found between the western blot and immunofluorescence data, which can be conceivably due to the intrinsic characteristics of the two techniques, these findings suggest that the massive loss of A2AR-CB1R heteromers found in HdhQ7/Q111 mice is mostly heteromer-selective and not primarily due to a mere reduction of total A2AR and CB1R molecules. In agreement with this notion, and as a further proof of the selective loss, the expression of another CB1R heteromer previously reported in indirect-pathway MSNs, namely CB1R-D2R (Navarro et al, 2008; Bonaventura et al, 2014), was not reduced in 6-month-old mutant HdhQ7/Q111 mice compared with their wild-type controls (Supplementary Figure S6c). Moreover, a remarkable loss of A2AR-CB1R heteromers was also observed in advanced stages of mouse models of HD transgenic for human mutant huntingtin exon 1, specifically R6/1 mice (Supplementary Figure S7a) and R6/2 mice (Supplementary Figure S7b). Again, the expression of CB1R-D2R heteromers, used as a control, did not decrease in advanced-stage R6/1 or R6/2 mice compared with age-matched wild-type animals (Supplementary Figure S7c).
Figure 4.
Functional A2AR-CB1R heteromers are expressed in HdhQ7/Q111 HD mice at early but not advanced disease stages. (a) PLA assays were performed in dorsal-striatum sections from wild-type HdhQ7/Q7 mice and mutant huntingtin-expressing knock-in HdhQ7/Q111 mice. A2AR-CB1R heteromers are shown as green dots in mice at 4, 6, and 8 months of age. Nuclei are colored in blue by DAPI staining. Representative pictures are shown. Scale bar: 20 μm. Quantification of the number of cells containing one or more dots expressed as the percentage of the total number of cells (blue nuclei). Data are the mean±SEM of counts in 11–26 different fields from five different animals of each type. One-way ANOVA followed by Bonferroni post hoc test showed showed a significant (***p<0.001) decrease of heteromer expression in HdhQ7/Q111compared with the respective age-matched HdhQ7/Q7 mice. (b–e) ERK phosphorylation (b, d) and Akt phosphorylation (c, e) were determined in striatal slices from 6 month-old wild-type HdhQ7/Q7 mice (b, c) and mutant huntingtin-expressing knock-in HdhQ7/Q111 mice (d, e). Slices were preincubated for 20 min with vehicle, the CB1R antagonist SR141716 (RIM; 1 μM) or the A2AR antagonist ZM241385 (1 μM) before the addition of vehicle or the CB1R agonist WIN-55,212-2 (1 μM), the A2AR agonist CGS21680 (1 μM), or both, for 10 min. Immunoreactive bands from 4–6 slices of 5–6 different animals were quantified for each condition. Values represent mean±SEM of percentage of phosphorylation relative to basal levels found in vehicle only-treated slices (100%, dotted line). Representative western blots are shown at the top of each panel. One-way ANOVA showed a significant effect over basal (*p<0.05, **p<0.01, ***p<0.001), or of the antagonist plus agonist treatment over the agonist-only treatment (#p<0.05, ##p<0.01, ###p<0.001). Further details of statistical analyses are given in Supplementary Table S2.
CB1R is highly abundant in most MSNs (Katona and Freund, 2008; Castillo et al, 2012), but it has been reported that the downregulation of CB1R mRNA expression in R6 transgenic mice is striatum subregion-selective, occurring preferentially in the dorsolateral than the dorsomedial striatum (Denovan-Wright and Robertson, 2000; McCaw et al, 2004). Hence, we analyzed the expression of total A2AR and CB1R immunoreactivity, as well as that of the A2AR-CB1R heteromer, in the dorsolateral vs the dorsomedial striatum of wild-type HdhQ7/Q7 and mutant HdhQ7/Q111 mice at 6 months of age. We found no significant differences between the two dorsal-striatum compartments in total A2AR immunoreactivity in either HdhQ7/Q7 mice (relative values: dorsolateral: 100±5.7; A2AR, dorsomedial: 101.8±5.7; n=3 animals) or HdhQ7/Q111 mice (relative values: dorsolateral: 100±5.2; A2AR, dorsomedial: 114.8±7.8; n=3 animals). There was a moderate preference of total CB1R protein expression for the dorsolateral striatum in HdhQ7/Q7 mice (relative values: dorsolateral: 100±3.8; dorsomedial: 83.1±2.5; n=3 animals; p=0.032), as well as a non-significant trend in HdhQ7/Q111 mice (relative values: dorsolateral: 100±2.9; dorsomedial: 85.8±2.7; n=3 animals). Regarding the A2AR-CB1R heteromer, we found no significant differences between the two dorsal-striatum compartments in the percentage of heteromer-positive cells relative to total cell nuclei in either HdhQ7/Q7 mice (dorsolateral: 45.0±4.9; dorsomedial: 44.0±3.8; n=3 animals) or HdhQ7/Q111 mice (dorsolateral: 10.4±2.3; dorsomedial: 7.5±1.4; n=4 animals). Overall, these data show that the A2AR-CB1R heteromer has a rather similar expression pattern in the mouse dorsolateral and dorsomedial striatum.
To study the function of the A2AR-CB1R heteromer in HD mice, we performed cross-signaling experiments in striatal slices from 6-month-old HdhQ7/Q7 and HdhQ7/Q111 mice. Consistently with the aforementioned data on both cell and slice cultures from control C57BL/6N mice, dual agonist treatment with WIN-55,212-2 and CGS21680 depressed phospho-ERK or phospho-Akt signal compared with single-agonist stimulation in wild-type HdhQ7/Q7 mice, thus showing a negative cross-talk (Figure 4b and c). In addition, the action of both agonists was blocked when the slices were preincubated with the partner receptor antagonists, SR141716 or ZM241385, thus showing cross-antagonism (Figure 4b and c). Interestingly, in HdhQ7/Q111 mice this negative cross-talk and cross-antagonism signature was not detected (Figure 4d and e), in line with the PLA data showing that the A2AR-CB1R heteromer is indeed not expressed in 6-month-old HdhQ7/Q111 mice. Of note, and also in line with the data shown above, this loss of cross-signaling did not appear to be simply due to the loss of surface expression of functional receptors, as the extent of single agonist-evoked ERK and Akt stimulation was roughly equivalent in both HdhQ7/Q111 and HdhQ7/Q7 mice (Figure 4b–e).
Together, these data demonstrate that a selective loss of functional A2AR-CB1R heteromers accompanies disease progression in mouse models of HD.
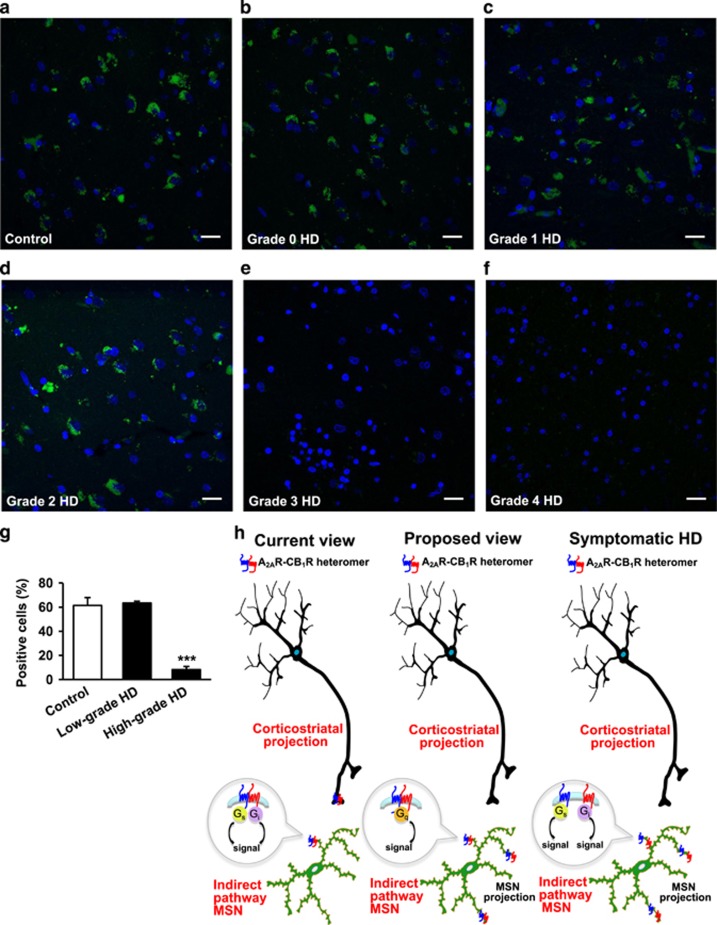
A2AR-CB1R Heteromers are Lost in the Caudate-Putamen of High-Grade HD Patients
We next investigated whether the aforementioned changes in A2AR-CB1R heteromer expression found in HD mouse models are also evident in HD. Thus, we used the PLA technique to analyze human caudate-putamen post mortem samples from control subjects and HD patients at different grades. A2AR-CB1R heteromers were readily evident in the caudate-putamen of control individuals, with a high fraction (~65%) of total cells expressing heteromers (Figure 5a and g, and Supplementary Table S1). These complexes were also detected at those normal levels in asymptomatic huntingtin gene-mutation carriers (HD grade 0) and early symptomatic HD patients (HD grades 1–2) (Figure 5b–d and g, and Supplementary Table S1). In contrast, A2AR-CB1R heteromers were strongly reduced in caudate-putamen samples from high-grade, advanced HD patients (HD grades 3–4), with only ~10% of total cells containing PLA-positive dots (Figure 5e–g, and Supplementary Table S1). PLA labeling was quite uniform in the caudate-putamen sections analyzed, and thus no perceptible differences in A2AR-CB1R heteromer expression were detected between those two nuclei within each subject (Supplementary Figure S8a and b). In addition, the demographic characteristics of the samples used indicated that the control, low-grade HD and high-grade HD subject populations were rather homogeneous (Supplementary Table S1), thus supporting that the differences found in A2AR-CB1R heteromer expression were not due to those confounding factors. Taken together, these data support that the human brain expresses A2AR-CB1R heteromers, and suggest that these complexes might serve specific functions that are impaired at late stages of HD progression.
Figure 5.
A2AR-CB1R heteromers are lost in the caudate-putamen of high-grade HD patients. PLA assays were performed in caudate-putamen sections of post mortem samples from control subjects (a) and HD patients at different grades (b–f). A2AR-CB1R heteromers are shown as green dots. Nuclei are colored in blue by DAPI staining. Representative pictures are shown. Scale bar: 20 μm. (g) Quantification of the number of cells containing one or more dots expressed as the percentage of the total number of cells (blue nuclei). Data are the mean±SEM of counts in 21–43 different fields from five control subjects, five low-grade HD patients (1 grade 0, 2 grade 1, plus 2 grade 2) and five high-grade HD patients (2 grade 3, plus 3 grade 4). The characteristics of these human samples are shown in Supplementary Table S1. One-way ANOVA followed by Dunnet post hoc test showed a significant (***p<0.001) decrease of heteromer expression compared to control subjects. Further details of statistical analyses are given in Supplementary Table S2. (h) Scheme depicting the proposed location and G protein-coupling of the A2AR-CB1R heteromer in the dorsal striatum. It is currently believed (left) that the A2AR-CB1R heteromer is located on corticostriatal projections as well as on the somatodendritic compartment of indirect-pathway MSNs. Each protomer would maintain its canonical G protein coupling (Gs for A2AR, and Gi for CB1R). In this study we propose (middle) that the A2AR-CB1R heteromer is located mostly on indirect-pathway MSNs, not only on their somatodendritic compartment but also likely on their terminals. According to our data, the A2AR-CB1R heteromer would facilitate Gq rather than Gs or Gi coupling. In symptomatic HD (right), the A2AR-CB1R heteromer would be disrupted into its constituting protomers.
DISCUSSION
Despite the progress made toward identifying and understanding GPCR heteromers, their promise as precision drug targets has yet to be fully realized due to the lack of detailed expression maps and functional profiles. A first important conclusion of our study refers to the precise location of the A2AR-CB1R heteromer in the mouse dorsal striatum. The current view in the field supports that a major site of A2AR and CB1R colocalization is the corticostriatal-neuron terminal, at which the two receptors could physically interact to form A2AR-CB1R heteromers (Figure 5h). These presynaptic heteromers have been suggested to provide a frame to explain, at least in part, the negative pharmacological interactions between A2AR and CB1R that occur in the corticostriatal pathway (Ferre et al, 2010; Tebano et al, 2012; Ferreira et al, 2015; Chiodi et al, 2016). However, those previous studies on A2AR-CB1R heteromers, although elegant and carefully conducted, lacked state-of-the-art genetic controls and heteromer-detecting techniques. Thus, to evaluate the possible existence of A2AR-CB1R heteromers in corticostriatal neurons, we have made use of three potent genetic models, namely (i) mice lacking CB1R selectively in cortical glutamatergic neurons, (ii) CB1R-deficient mice in which CB1R expression is selectively rescued in cortical glutamatergic neurons, and (iii) CB1R-floxed mice in which CB1R is selectively excised in corticostriatal neurons. Systematic PLA assays conducted in these mouse models strikingly showed that the expression of the A2AR-CB1R heteromer in corticostriatal projections to the dorsal striatum is negligible (Figure 1c). This finding supports that the inhibitory cross-talk processes between A2AR and CB1R reported to date in corticostriatal terminals do not rely primarily on physical interactions between the two receptors at the plasma membrane, but on other potential factors such us an opposite Gs/Gi protein-dependent downstream signaling converging on glutamate release at the presynapse, which, in turn, would conceivably lead to an opposite modulation of the mGluR5/phospholipase C-β/diacylglycerol lipase-α (DAGLα)/2-arachidonoylglycerol (2-AG) retrograde-signaling machinery at the postsynapse (Uchigashima et al, 2007; Katona and Freund, 2008). In any case, this observed absence of presynaptic A2AR-CB1R heteromers does certainly not preclude that A2AR and CB1R could interact with other partners at corticostriatal terminals to form GPCR complexes, for example, the A1R-A2AR heteromer (Ciruela et al, 2006; Quiroz et al, 2009).
Another widely accepted site at which striatal A2AR-CB1R heteromers are believed to reside is the somatodendritic compartment of MSNs, the main target of corticostriatal inputs (Carriba et al, 2007; Schiffmann et al, 2007; Ferre et al, 2010) (Figure 5h). Here, by using (i) mice lacking CB1R selectively in GABAergic neurons, (ii) CB1R-deficient mice in which CB1R expression is selectively rescued in GABAergic neurons, (iii) mice lacking CB1R selectively in D1R-expressing MSNs, and (iv) CB1R-floxed mice in which CB1R is selectively excised in MSNs, we cogently demonstrated that the A2AR-CB1R heteromer is indeed present in indirect-pathway MSNs (Figure 1 and Supplementary Figure S2). It is well established that CB1R is largely a presynaptic receptor that is highly abundant in the resident collaterals and long-range projections of MSNs (Uchigashima et al, 2007; Katona and Freund, 2008). Our data support that A2AR-CB1R heteromers are not solely expressed in the somatodendritic compartment of indirect-pathway MSNs, but, most likely, also at terminals of these neurons (Figure 5h). Nonetheless, the higher PLA signal found in GABA-CB1R−/− and GABA-Glu-CB1R−/−mice compared with full CB1R−/− mice (Figure 1c and Supplementary Figure S1c) suggests that, in the dorsal striatum, A2AR-CB1R heteromers may also be located on non-GABAergic, non-glutamatergic cells/terminals such as cholinergic interneurons, dopaminergic projections, or astrocytes. We are also aware that understanding the precise role of A2AR-CB1R complexes in indirect-pathway MSNs is an extremely complex issue. This complexity is due, in part, to the possibility that A2AR and CB1R can interact with other receptors in indirect-pathway MSNs. For example, A2AR is highly co-expressed with both D2R and mGluR5, which colocalizes with DAGLα at the perisynaptic border of dendritic spines of MSNs (Uchigashima et al, 2007; Katona and Freund, 2008). The activation of mGluR5 by glutamate spillover derived from corticostriatal overactivity, which leads to DAGLα-mediated 2-AG generation, can be tuned by D2R in MSN dendritic spines (Kreitzer and Malenka, 2005; Yin and Lovinger, 2006). In addition, A2AR antagonists potentiate 2-AG release and long-term depression in indirect-pathway MSNs (Lerner et al, 2010). Whether these intricate interactions between A2AR, D2R and mGluR5 rely, at least in part, on putative A2AR-D2R-mGluR5 heteromers (Cabello et al, 2009) has still to be defined. To complicate the situation further, postsynaptic A2AR and D2R might form other higher-order heteromeric complexes, including a proposed A2AR-CB1R-D2R heteromer (Navarro et al, 2010; Bonaventura et al, 2014). This functional conundrum notwithstanding, the present study provides a cogent understanding of the anatomical distribution of the A2AR-CB1R heteromer, or the complexes containing the heteromer, in the corticostriatal circuit.
Our data also support that the selective coupling to Gq protein, rather than to Gs or Gi proteins, is a biochemical hallmark of the A2AR-CB1R heteromer in striatal cells (Figure 5h). A G protein switch has in fact been suggested to occur in several GPCR heteromerization processes. For example, a change from the archetypical Gs-coupled D1R (either as monomer or as D1R-D1R homomers) to non-canonical Gi-coupled D1R-HT3R heteromer has been observed (Ferrada et al, 2009). In addition, formation of the CB1R-5-HT2AR heteromer may lead to a switch in G protein coupling for 5-HT2AR from Gq to Gi protein (Viñals et al, 2015). Thus, it is possible that in a striatopallidal MSN, there is a coexistence of A2AR and CB1R (as both monomers and A2AR-A2AR and CB1R-CB1R homomers), which are widely believed to couple to Gs/olf and Gi proteins, respectively, together with A2AR-CB1R heteromers, which could couple non-canonically to Gq protein. How these processes of GPCR protein–protein interaction and subsequent G protein ‘shuffling’ affect corticostriatal circuitry is as yet unknown. It is conceivable that the arrangement of the aforementioned heteromers from A2AR and CB1R protomers in striatopallidal MSNs, by recruiting activatory Gq proteins, would be a way to fuel the indirect pathway and therefore blunt motor activity. However, such a functional outcome is difficult to predict as, according to the currently accepted models of basal ganglia function, motor activation relies on the simultaneous and coordinated activation of the direct and indirect striatal pathways (Nelson and Kreitzer, 2014). In any case, our data support the existence of different pools of A2AR and CB1R with different G protein coupling in corticostriatal projections, striatopallidal MSNs and striatonigral MSNs, thus providing adenosinergic and cannabinergic cross-signaling with an extreme degree of complexity.
To study whether the A2AR-CB1R heteromer is affected in a pathological setting we selected HD as the archetypal neurodegenerative disease that primarily affects MSNs in a selective manner. A significant number of studies have dealt with CB1R expression and function in HD. In particular, a downregulation of CB1R expression has been documented in the caudate-putamen of HD patients and the dorsal striatum of some HD animal models, which seems to reflect the characteristic damage pattern of MSNs (Glass et al, 2000; Fernandez-Ruiz et al, 2011). In addition, we (Blazquez et al, 2011) and others (Mievis et al, 2011b) have demonstrated a neuroprotective role of CB1R in transgenic mouse models of HD. Likewise, administration of the cannabinoid agonist THC to HD mice prevented disease progression as assessed by behavioral, neuropathological, and molecular markers (Blazquez et al, 2011). In sum, it is currently believed that CB1R may be neuroprotective in HD. Regarding A2AR, its expression has been shown to decrease in striatopallidal MSNs from the caudate-putamen of HD patients and the dorsal striatum of some HD animal models (Glass et al, 2000; Lee and Chern, 2014). However, the precise role of A2AR in HD progression is not obvious yet, as conflicting results have been reported. Thus, administration of the A2AR agonist CGS21680 to HD mice prevented neuropathological deficits and improved motor alterations, although it had no effect on body weight or lifespan (Chou et al, 2005). Likewise, the dual-function compound T1–11, which simultaneously activates A2AR and blocks adenosine transport, improved motor coordination deficits, reduced striatal huntingtin aggregates, and normalized proteasomal activity (Huang et al, 2011). Genetic ablation of A2AR in HD mice worsened motor performance, decreased animal survival, and reduced striatal enkephalin expression (Mievis et al, 2011a), and also reversed working memory deficits (Li et al, 2015). However, and in striking contrast, administration of the A2AR antagonist SCH58261 exerted beneficial effects in HD mice by attenuating anxiety-like responses and sensitivity to excitotoxins, although it had no effect on motor coordination (Domenici et al, 2007). Because of these (at least apparently) contradictory data coming from various A2AR gain-of-function and loss-of-function approaches, it is conceivable that A2AR can mediate different (even opposing) molecular and physiopathological mechanisms depending on its cellular location and, hence, its extent of heteromerization. It has been proposed that a selective functional impairment of A2AR located on striatopallidal MSNs occurs at pre-symptomatic stages of HD, whereas presynaptic A2AR function is not affected (Orru et al, 2011). Of note, CB1R is also lost in MSNs but not in corticostriatal projections along HD progression (Chiodi et al, 2012; Chiarlone et al, 2014). This suggests that the corticostriatal pool of non-heteromerizing A2AR and CB1R would be the main target of adenosinergic and cannabinergic drugs aimed at relieving the symptoms of HD at late stages, whereas the MSN pool of A2AR-CB1R heteromers could be an additional target of those drugs at early disease stages. As A2AR-CB1R heteromers are lost in the caudate-putamen of high-grade HD patients, the heteromer’s specific functions would be impaired at advanced stages of HD progression. Thus, the fine negative cross-talk between adenosine and endocannabinoids would conceivably disappear in advanced HD, and one might speculate that the Gq specific signaling would be lost as well at those late disease stages (Figure 5h). The A2AR-CB1R heteromer is singular in both its specific localization on indirect-pathway MSNs and its biochemical characteristics owing to its coupling to non-canonical Gq-mediated signaling. Together, our findings may open a new conceptual framework to understand the role of coordinated adenosine-cannabinoid function in the indirect striatal pathway, which may be relevant in motor function and neural diseases.
FUNDING AND DISCLOSURE
This work was supported by grants from the Spanish Ministry of Economy and Competitiveness (MINECO/FEDER; grant SAF2015-64945-R to MG; grant SAF-2014-54840-R to EIC and VC; grant SAF2015-65034-R to PG; grant SAF2015-67474-R to SG; grants SAF2014-55700-P and PCIN-2013-019-C03-03 to FC); Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED; grant PI2013/05 to MG, PJM and EIC); Comunidad de Madrid (grant S2010/BMD-2308 to MG); Generalitat de Catalunya (grant 2014-SGR-1236 to EIC); ‘La Marató de TV3’ Foundation (grant 20140610 to EIC; grant 20152031 to FC); Agentschap voor Innovatie door Wetenschap en Technologie (grant SBO-140028 to FC); BBSRC DTP studentship (to PJM and LB); EPSRC (grant EP/M006379/1 to LAH); Deutsche Forschungsgemeinschaft (DFG; grant MO 1920/1-1 to KM; grant CRC-TRR 58 to BL); Institute of Health Carlos III from the Spanish Ministry of Economy and Competitiveness (grant PIE14/00034 to FC; grant PI10/00172 and funding from FEDER grants to MJC and JP); The Basque Government (grant IT764-13 to PG); University of the Basque Country UPV/EHU (grant UFI11/41 to PG); Red de Trastornos Adictivos-Institute of Health Carlos III (grant RD12/0028/0004 to PG). AC is supported by the Spanish Ministry of Economy and Competitiveness (Juan de la Cierva Program). MM is supported by the Spanish Ministry of Education, Culture and Sport (FPU Program). The authors declare no conflict of interest.
Acknowledgments
We are very grateful to Cristina Blázquez, Manel Bosch, Elena García-Taboada, Ana Gómez, Bernadette Mohr, María P Muñoz, and Alicia Poplawski for their expert technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Araki KY, Fujimura S, MacDonald ME, Bhide PG (2006). Characterization of mouse striatal precursor cell lines expressing functional dopamine receptors. Dev Neurosci 28: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azdad K, Gall D, Woods AS, Ledent C, Ferre S, Schiffmann SN (2009). Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology 34: 972–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafenetre P, Cannich A, Cota D, Puente N, Grandes P et al (2010). Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 13: 281–283. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Chiarlone A, Sagredo O, Aguado T, Pazos MR, Resel E et al (2011). Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain 134: 119–136. [DOI] [PubMed] [Google Scholar]
- Bonaventura J, Navarro G, Casado-Anguera V, Azdad K, Rea W, Moreno E et al (2015). Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer. Proc Natl Acad Sci USA 112: E3609–E3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura J, Rico AJ, Moreno E, Sierra S, Sanchez M, Luquin N et al (2014). L-DOPA-treatment in primates disrupts the expression of A2A adenosine-CB1 cannabinoid-D2 dopamine receptor heteromers in the caudate nucleus. Neuropharmacology 79: 90–100. [DOI] [PubMed] [Google Scholar]
- Cabello N, Gandia J, Bertarelli DC, Watanabe M, Lluis C, Franco R et al (2009). Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J Neurochem 109: 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A et al (2007). Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 32: 2249–2259. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron 76: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M et al (2006). Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci 26: 2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarlone A, Bellocchio L, Blazquez C, Resel E, Soria-Gomez E, Cannich A et al (2014). A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc Natl Acad Sci USA 111: 8257–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi V, Ferrante A, Ferraro L, Potenza RL, Armida M, Beggiato S et al (2016). Striatal adenosine-cannabinoid receptor interactions in rats overexpressing adenosine A2 receptors. J Neurochem 136: 907–917. [DOI] [PubMed] [Google Scholar]
- Chiodi V, Uchigashima M, Beggiato S, Ferrante A, Armida M, Martire A et al (2012). Unbalance of CB1 receptors expressed in GABAergic and glutamatergic neurons in a transgenic mouse model of Huntington’s disease. Neurobiol Dis 45: 983–991. [DOI] [PubMed] [Google Scholar]
- Chou SY, Lee YC, Chen HM, Chiang MC, Lai HL, Chang HH et al (2005). CGS21680 attenuates symptoms of Huntington’s disease in a transgenic mouse model. J Neurochem 93: 310–320. [DOI] [PubMed] [Google Scholar]
- De Salas-Quiroga A, Diaz-Alonso J, Garcia-Rincon D, Remmers F, Vega D, Gomez-Canas M et al (2015). Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc Natl Acad Sci USA 112: 13693–13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denovan-Wright EM, Robertson HA (2000). Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington’s disease mice. Neuroscience 98: 705–713. [DOI] [PubMed] [Google Scholar]
- Domenici MR, Scattoni ML, Martire A, Lastoria G, Potenza RL, Borioni A et al (2007). Behavioral and electrophysiological effects of the adenosine A2A receptor antagonist SCH 58261 in R6/2 Huntington’s disease mice. Neurobiol Dis 28: 197–205. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Moreno-Martet M, Rodriguez-Cueto C, Palomo-Garo C, Gomez-Canas M, Valdeolivas S et al (2011). Prospects for cannabinoid therapies in basal ganglia disorders. Br J Pharmacol 163: 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrada C, Moreno E, Casado V, Bongers G, Cortes A, Mallol J et al (2009). Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br J Pharmacol 157: 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Lluis C, Justinova Z, Quiroz C, Orru M, Navarro G et al (2010). Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br J Pharmacol 160: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SG, Goncalves FQ, Marques JM, Tome AR, Rodrigues RJ, Nunes-Correia I et al (2015). Presynaptic adenosine A2A receptors dampen cannabinoid CB1 receptor-mediated inhibition of corticostriatal glutamatergic transmission. Br J Pharmacol 172: 1074–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA (2012). Integrating neurotransmission in striatal medium spiny neurons. Adv Exp Med Biol 970: 407–429. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL (2000). The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABAA receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 97: 505–519. [DOI] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sanchez-Soto M et al (2014). Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol Pharmacol 86: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB et al (2011). Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron 69: 120–131. [DOI] [PubMed] [Google Scholar]
- Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y et al (2016). Crystal structure of the human cannabinoid receptor CB1. Cell 167: 750–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NK, Lin JH, Lin JT, Lin CI, Liu EM, Lin CJ et al (2011). A new drug design targeting the adenosinergic system for Huntington’s disease. PLoS One 6: e20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Redhi GH, Goldberg SR, Ferre S (2014). Differential effects of presynaptic versus postsynaptic adenosine A2A receptor blockade on delta-9-tetrahydrocannabinol (THC) self-administration in squirrel monkeys. J Neurosci 34: 6480–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Freund TF (2008). Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14: 923–930. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC (2009). Physiology and pharmacology of striatal neurons. Annu Rev Neurosci 32: 127–147. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC (2005). Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci 25: 10537–10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ et al (1997). Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388: 674–678. [DOI] [PubMed] [Google Scholar]
- Lee CF, Chern Y (2014). Adenosine receptors and Huntington’s disease. Int Rev Neurobiol 119: 195–232. [DOI] [PubMed] [Google Scholar]
- Lee LT, Ng SY, Chu JY, Sekar R, Harikumar KG, Miller LJ et al (2014). Transmembrane peptides as unique tools to demonstrate the in vivo action of a cross-class GPCR heterocomplex. FASEB J 28: 2632–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Horne EA, Stella N, Kreitzer AC (2010). Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J Neurosci 30: 2160–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Silva HB, Real J, Wang YM, Rial D, Li P et al (2015). Inactivation of adenosine A2A receptors reverses working memory deficits at early stages of Huntington’s disease models. Neurobiol Dis 79: 70–80. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (2010). Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG et al (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530–534. [DOI] [PubMed] [Google Scholar]
- McCaw EA, Hu H, Gomez GT, Hebb AL, Kelly ME, Denovan-Wright EM (2004). Structure, expression and regulation of the cannabinoid receptor gene (CB1) in Huntington’s disease transgenic mice. Eur J Biochem 271: 4909–4920. [DOI] [PubMed] [Google Scholar]
- Mievis S, Blum D, Ledent C (2011. a). A2A receptor knockout worsens survival and motor behaviour in a transgenic mouse model of Huntington’s disease. Neurobiol Dis 41: 570–576. [DOI] [PubMed] [Google Scholar]
- Mievis S, Blum D, Ledent C (2011. b). Worsening of Huntington disease phenotype in CB1 receptor knockout mice. Neurobiol Dis 42: 524–529. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G et al (2007). Genetic dissection of behavioural and autonomic effects of delta-9-tetrahydrocannabinol in mice. PLoS Biol 5: e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R et al (2006). The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51: 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Carriba P, Gandia J, Ciruela F, Casado V, Cortes A et al (2008). Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. ScientificWorldJournal 8: 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Ferre S, Cordomi A, Moreno E, Mallol J, Casado V et al (2010). Interactions between intracellular domains as key determinants of the quaternary structure and function of receptor heteromers. J Biol Chem 285: 27346–27359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Kreitzer AC (2014). Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci 37: 117–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Zanoveli JM, Quiroz C, Nguyen HP, Guitart X, Ferre S (2011). Functional changes in postsynaptic adenosine A2A receptors during early stages of a rat model of Huntington disease. Exp Neurol 232: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz C, Lujan R, Uchigashima M, Simoes AP, Lerner TN, Borycz J et al (2009). Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. ScientificWorldJournal 9: 1321–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehle S, Remmers F, Romo-Parra H, Massa F, Wickert M, Wortge S et al (2013). Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J Neurosci 33: 10264–10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S (2007). Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 83: 277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ (1993). Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci 13: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF (1999). In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285: 1569–1572. [DOI] [PubMed] [Google Scholar]
- Shao Z, Yin J, Chapman K, Grzemska M, Clark L, Wang J et al (2016). High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Shoichet BK, Kobilka BK (2012). Structure-based drug screening for G-protein-coupled receptors. Trends Pharmacol Sci 33: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura J, Fernandez-Duenas V, Ciruela F (2015). Visualizing G protein-coupled receptor-receptor interactions in brain using proximity ligation in situ assay. Curr Protoc Cell Biol 67: 17 17 11–16. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Popoli P (2012). Adenosine A2A-cannabinoid CB1 receptor interaction: an integrative mechanism in striatal glutamatergic neurotransmission. Brain Res 1476: 108–118. [DOI] [PubMed] [Google Scholar]
- Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F et al (2000). Dominant phenotypes produced by the HD mutation in STHdhQ111 striatal cells. Hum Mol Genet 9: 2799–2809. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M (2007). Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci 27: 3663–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM (2013). Molecular signatures of G-protein-coupled receptors. Nature 494: 185–194. [DOI] [PubMed] [Google Scholar]
- Viñals X, Moreno E, Lanfumey L, Cordomi A, Pastor A, de La Torre R et al (2015). Cognitive impairment induced by delta-9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol 13: e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FO (2007). Huntington’s disease. Lancet 369: 218–228. [DOI] [PubMed] [Google Scholar]
- Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG et al (2011). Structure of an agonist-bound human A2A adenosine receptor. Science 332: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM (2006). Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci USA 103: 8251–8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.