Abstract
Neuropeptide S (NPS) is an important anxiolytic substance of the brain. However, the signaling pathways downstream of NPS receptor (NPSR) activation, underlying the behavioral effect of NPS, remain largely unknown. Here, we show that bilateral microinfusion of NPS (0.2 nmol/0.5 μl) into the medial amygdala (MeA) of male adult Wistar rats reduced anxiety-related behavior on both the elevated plus-maze and the open field. Moreover, as shown in amygdala tissue micropunches intracerebroventricular infusion of NPS (1 nmol/5 μl) (1) evoked phosphorylation and synthesis of CaMKIIα in relation to reference protein β-tubulin representing Ca2+ influx, and (2) induced phosphorylation of mitogen-activated protein kinase ERK1/2. The NPS-induced anxiolysis was prevented by local inhibition of phospholipase C signaling using U73122 (0.5 nmol/0.5 μl) in the MeA, indicating the behavioral relevance of this pathway. Conversely, local pharmacological blockade of adenylyl cyclase signaling using 2’,5’-dideoxyadenosine (12.5 nmol/0.5 μl) failed to inhibit the anxiolytic effect of NPS infused into the MeA. Hence, NPS promotes acute anxiolysis within the MeA dependent on NPSR-mediated phospholipase C signaling. Taken together, our study extends the knowledge about the intracellular signaling mechanisms underlying the potent anxiolytic profile of NPS.
Introduction
Anxiety disorders have a lifetime prevalence of 28%, and despite substantial research novel therapeutics are still required (Kessler et al, 2005). Neuropeptide S (NPS) represents a potential candidate because of its strong anxiolytic activity following intracerebroventricular (ICV) infusion (Xu et al, 2004; Leonard et al, 2008; Rizzi et al, 2008; Vitale et al, 2008; Wegener et al, 2011; Slattery et al, 2015; Zoicas et al, 2016) in both rats and mice and local infusion into lateral and basolateral amygdala in mice (Jungling et al, 2008; Chauveau et al, 2012). An anxiolytic effect was also described after intranasal application of NPS (Ionescu et al, 2012; Lukas and Neumann, 2012; Dine et al, 2015) in both species. Moreover, NPS has been shown to potently reverse cued fear expression and to promote cued fear extinction in mice and rats (Jungling et al, 2008; Zoicas et al, 2016), also in those selectively bred for extremes in anxiety-related behavior (Slattery et al, 2015), and in a psychopathological mouse model of extinction-deficient 129S1/SvImJ mice (Sartori et al, 2016).
The pericoerulear region and the parabrachial nucleus harbor a cluster of predominantly glutamatergic NPS-immunoreactive neurons in rats (Xu et al, 2007). Moreover, scattered NPS precursor mRNA signals have been detected in the amygdala and the dorsomedial hypothalamus (Xu et al, 2007). In contrast to the 20-amino acid peptide, the NPS receptor (NPSR) has a wider distribution in the brain. The NPSR is mainly expressed in regions of the limbic system (Xu et al, 2007). The in vitro studies in NPSR-transfected human embryonic kidney 293T (HEK293T) cells and Chinese hamster ovary cells revealed increased cyclic adenosine monophosphate (cAMP) concentration by adenylyl cyclase (AC) activation and a rise in intracellular Ca2+ levels due to phospholipase C (PLC) stimulation, suggesting that the NPSR is coupled to both Gs and Gq proteins (Reinscheid and Xu, 2005; Camarda et al, 2009; Erdmann et al, 2015; Liao et al, 2016), respectively. More detailed, Pape and colleagues (Erdmann et al, 2015) demonstrated that NPS-evoked Ca2+ influx is marked by a biphasic time course in NPSR-transfected hippocampal mouse neurons with the fast component being maintained in an inositol triphosphate- and a ryanodine receptor-dependent manner and the slow component depending on store-operated Ca2+ entry from the extracellular space. However, the underlying mechanism(s) mediating the anxiolytic activity following NPSR activation is still unknown.
The in situ hybridization studies in mice revealed NPSR mRNA expression in the medial nucleus of the amygdala (MeA) as well as the lateral and basolateral amygdala nuclei (Clark et al, 2011), whereas NPSR mRNA expression in rats is restricted to the MeA and intercalated nucleus of the amygdala (Xu et al, 2007). As the amygdala regulates fear and anxiety-related behavior (Tovote et al, 2015), we analyzed, whether (1) NPS promotes anxiolysis within the MeA, (2) central NPS infusion activates Ca2+-dependent signaling pathways in the amygdala in vivo, and (3) acute NPS-induced anxiolysis is mediated in a PLC- and/or AC-dependent manner. These findings will increase our understanding of the involvement of NPS in emotion regulation and in the pathophysiology of anxiety disorders.
Materials and methods
Animals
Male Wistar rats (230–250 g, Charles River Laboratories, Germany) were housed in groups of 3–4 under standard laboratory conditions (food and water ad libitum, 12 : 12 h light/dark cycle, lights on at 0600 h, 21–23 °C, 55% humidity). Rats were allowed at least 1 week of habituation before they were used for surgical procedures. All experiments were performed between 0800 and 1100 h in accordance with the Guide for the Care and Use of Laboratory Animals by the NIH, and the ARRIVE guidelines (Kilkenny et al, 2010), and were approved by the government of the Oberpfalz.
Surgical Procedures
Rats were injected subcutaneously with the analgesic Buprenorphin (Bayer, 0.05 mg Buprenovet/kg) and the antibiotic Baytril (Baxter, 10 mg Enrofloxacin/kg) 30 min before surgery. All stereotaxic procedures were performed under isoflurane anesthesia and semisterile conditions. For ICV infusions, a 12 mm long 21-G guide cannula was stereotaxically placed 2 mm above the lateral ventricle (AP: −1.0 mm, ML: −1.6 mm, DV: −2.0 mm; (Paxinos and Watson, 1998). For bilateral intra-MeA infusions, 12 mm long 23-G guide cannulas were implanted 2 mm above the left and right MeA (AP: −2.3 mm, ML: ±3.2, DV: −7.5 mm). The implants were fixed to two stainless steel screws using dental cement. Rats were housed singly after surgery, allowed to recover for at least 5 days and handled daily to habituate them to the infusion procedure and to minimize nonspecific stress responses at the day of experiment. Guide cannulas were closed using dummy cannulas that were cleaned daily during the handling procedure.
Experimental Design
Drugs and drug infusions
For central infusions, NPS (Bachem) was diluted in sterile Ringer’s solution (vehicle (Veh); B. BRAUN Melsungen AG). NPS was infused ICV at 1 nmol/5 μl, or locally into the MeA at 0.2 nmol/0.5 μl.
For local inhibition of either AC or PLC before NPS infusion, the AC inhibitor 2’,5’-dideoxydenosine (DDA) or the PLC blocker U73122 were infused into the MeA. The infused doses of DDA (12.5 nmol/0.5 μl, EnzoLifeScience) and U73122 (0.5 nmol/0.5 μl, EnzoLifeScience) were selected on the basis of previous in vitro studies and their half-maximal inhibitory dose (Ogawa et al, 1995; Feisst et al, 2005). As both DDA and U73122 stocks were diluted in dimethyl sulfoxide (DMSO, Merck), Veh1 was composed of 20% DMSO and 80% sterile Ringer’s solution. Veh2 (NPS control) was composed of sterile Ringer’s solution.
The infusion systems (ICV: 14.7 mm, 25 G; local: 14 mm, 27 G) were connected to a 10-μl Hamilton syringe via PE20 tubing. After infusion at a rate of 1 μl/min, the infusion system was kept in place for 10 s to allow local substance diffusion. None of the drug-infused rats showed any signs of tremor, convulsions, or wet-dog shaking in their home cage.
Experiment 1: Effect of bilateral infusion of NPS into the MeA on anxiety-related behavior
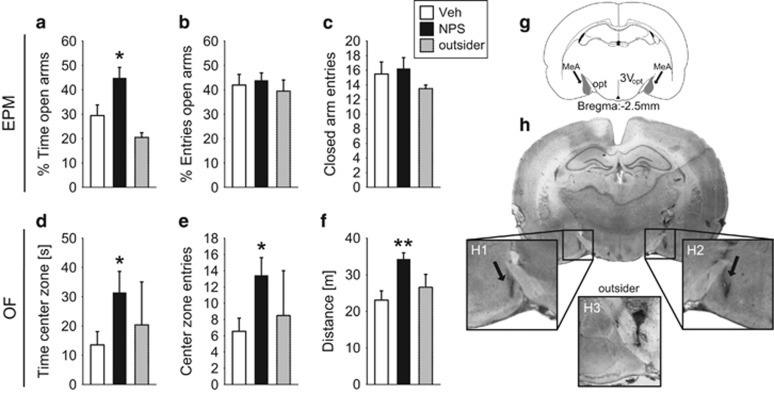
To examine the local effect of NPS within the MeA on anxiety-related behavior, rats were bilaterally infused with either NPS (0.2 nmol) or Veh, and were tested on the elevated plus-maze (EPM; Supplementary Information 1) 15 min thereafter. After 2 days, the same rats were tested in the open field (OF; Supplementary Information 1) 15 min after they received a randomized infusion of either NPS or Veh. Behavioral effects of NPS infusion outside the MeA (outsider, n=2) are displayed in Figure 1.
Figure 1.
Anxiolytic effect of NPS infusion (0.2 nmol/0.5 μl) bilaterally into the MeA of male rats as assessed on the EPM (a–c) and the OF (d–f). During the 5 min of testing on the EPM, (a) the percentage of time spent on the EPM, (b) the percentage of entries into the open arms, and (c) number of closed arm entries were monitored. During the 5 min of testing in the OF, (d) the time spent in the OF, (e) the entries into the center zone, and (f) traveled distance were analyzed. Data represent mean+SEM, *p<0.05, **p<0.01 vs Veh; group sizes: n=7–9; except outsider n=2. (g) Schematic drawing of the MeA in gray (opt: optic tract, 3V: third ventricle, Paxinos and Watson, 1998) and (h) representative microphotographs of bilateral MeA infusion sites in a coronal brain slice (H1 and H2). H3 represents an infusion site considered as outsider.
Experiment 2: Effects of ICV NPS on CaMKIIα and ERK1/2 in the amygdala
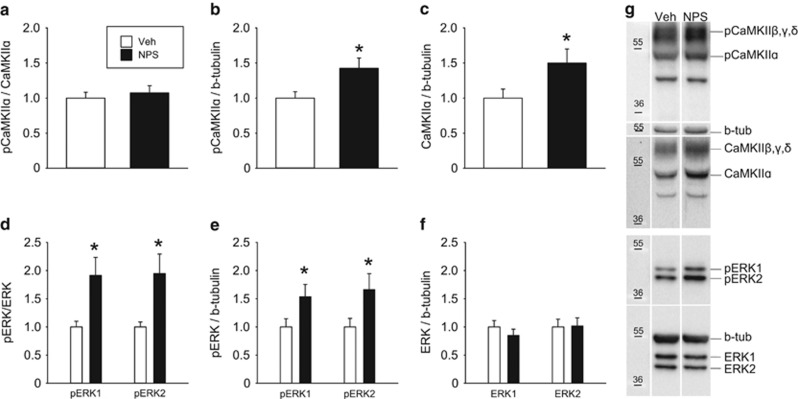
In order to assess NPS-induced activation of intracellular signaling pathways, rats were infused ICV with either NPS (1 nmol/5 μl) or Veh (5 μl). Animals were killed by rapid decapitation 15 min after drug infusion, brains were removed, and amygdala tissue from both hemispheres was harvested using a 2 mm-wide micropuncher accordant to the brain atlas. Relative levels of phospho-Ca2+/calmodulin-dependent kinase IIα (pCaMKIIα) and pERK1/2 in relation to respective total protein and the reference protein β-tubulin were analyzed by western blot analysis (Supplementary Information 2).
Experiment 3: Effects of simultaneous blockade of both PLC and AC on NPS-induced anxiolysis
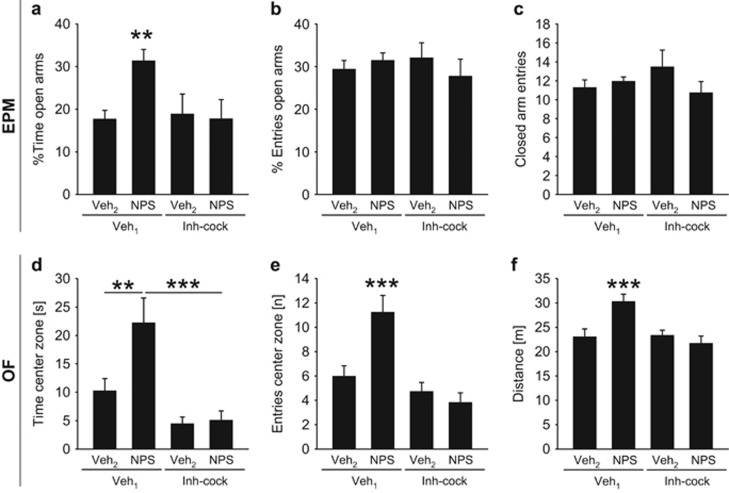
In order to reveal the relevance of NPSR-induced cAMP formation and rise in intracellular Ca2+ levels on NPS-induced anxiolysis within the MeA, PLC and AC were inhibited simultaneously by infusion of an inhibitor-cocktail (Inh-cock) composed of U73122 (0.5 nmol) and DDA (12.5 nmol). Consequently, four groups of conscious rats received the following two subsequent intra-MeA infusions of Veh1/Veh2, Veh1/NPS, Inh-cock/Veh2, or Inh-cock/NPS with a 20 min interval between infusions. Anxiety-related behavior was assessed on the EPM and OF (Supplementary Information 1), respectively, 15 min after the last infusion.
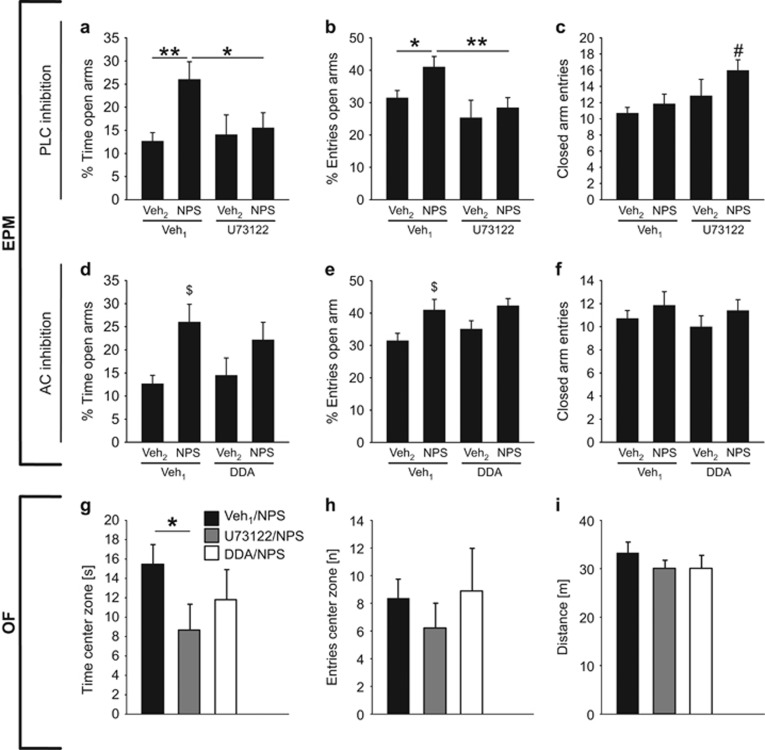
Experiment 4: Effects of specific blockade of either PLC or AC on NPSR-mediated anxiolysis in the MeA
To assess whether the anxiolytic activity of NPS is mediated by either NPS-evoked PLC or AC activation, or via both membrane-bound proteins within the MeA, we separately infused U73122 (0.5 nmol), DDA (12.5 nmol), or Veh1 20 min before infusion of either NPS (0.2 nmol) or Veh2 into the MeA. Anxiety-related behavior was assessed on the EPM (Supplementary Information 1) 15 min after the last infusion. After 2 days, the same rats were tested in the OF (Supplementary Information 1) following the same treatment, ie, they received a randomized preinfusion of Veh1, U73122, or DDA followed by an infusion of NPS 20 min later. They were tested on the OF 15 min after the last infusion.
Verification of Cannula Placements
After the behavioral experiments, placement of cannulas was verified on 40-μm Nissl-stained coronal cryosections. Only animals with correctly positioned cannulas were included for statistical analyses.
Statistical Analyses
Two-tailed t-test or nonparametric Mann–Whitney U-test was used in order to analyze differences in the state of anxiety following single infusion of either Veh or NPS, and to analyze NPS-induced phosphorylation and synthesis of CaMKII and ERK1/2. To examine the effect of PLC and/or AC inhibition on NPS-induced anxiolysis, two-way analysis of variance has been used (ANOVA, factors first infusion × second infusion). Any overall statistical differences, which were set at p<0.05, were further analyzed using Tukey’s post hoc test. Data are expressed as group mean+SEM. Statistical analyses were performed using SigmaPlot 13 (Systat).
Results
Experiment 1: Effect of Bilateral Infusion of NPS into the MeA on Anxiety-Related Behavior
Bilateral infusion of NPS into the MeA significantly reduced anxiety-related behavior of male rats on both the EPM and OF compared with Veh infusion (Figure 1). NPS-treated rats spent a higher percentage of time on the open arms (t15=−2.46, p=0.027) without effects on the percentage of entries into the open arms (t15=−0.37, p=0.72) and on the number of closed arm entries (t15=−0.32, p=0.75). Notably, the anxiolytic effect of NPS in the MeA was confirmed in the OF test 2 days later, as NPS increased the time the animals spent in the center zone (U=11.0, p=0.034) and almost double the number of center zone entries (t14=−2.58, p=0.022), when compared with Veh-treated rats. Moreover, NPS increased the distance rats traveled in the OF (t14=−3.44, p=0.004) indicative of a hyperlocomotor effect.
Experiment 2: Effects of ICV NPS on CaMKIIα and ERK1/2 in the Amygdala
Western blot analysis of amygdala tissue micropunches revealed increased amounts of both pCaMKIIα (t20=−2.48, p=0.022 Figure 2b) and total CaMKIIα (t20=−2.10, p=0.049, Figure 2c) relative to β-tubulin following ICV infusion of NPS. The phospho-CaMKIIα levels compared with total CaMKIIα were not altered (U=57.00, p=0.84, Figure 2a).
Figure 2.
Effects of ICV NPS (1 nmol/5 μl) on relative amount of phosphorylated CaMKIIα (pCaMKIIα) compared with (a) total CaMKIIα and (b) β-tubulin in the cytosolic fraction of amygdala tissue micropunches of male rats. NPS evoked synthesis of (c) CaMKIIα in relation to β-tubulin. Relative amount of phosphorylated ERK1/2 (pERK1/2) compared with (d) total ERK1/2 and (e) β-tubulin without affecting (f) total ERK1/2 levels relative to β-tubulin following NPS infusion. Data represent mean+SEM, *p<0.05 versus Veh; group sizes: n=9–12. (g) Representative western blots of (p)CaMKIIα, (p)ERK1/2, and β-tubulin.
Moreover, NPS increased cytoplasmic pERK1/2 levels. This applied to both the pERK1/2/ERK1/2 (pERK1/ERK1: U=23.00, p=0.027; pERK2/ERK2: U=21.00, p=0.018, Figure 2d) and the pERK1/2/β-tubulin ratios (pERK1/β-tubulin: t18=−2.12, p=0.048; pERK2/β-tubulin: t18=−2.18, p=0.043; Figure 2e). Total ERK1/2 levels were not altered by ICV NPS (ERK1/β-tubulin: t19=0.92, p=0.37; ERK2/β-tubulin: t18=−0.11, p=0.91, Figure 2f).
Experiment 3: Effects of Simultaneous Blockade of both PLC and AC on NPS-Induced Anxiolysis
Preinfusion of an inhibitor cocktail consisting of both the PLC blocker U73122 and the AC inhibitor DDA bilaterally into the MeA prevented the anxiolytic effect of subsequent locally infused NPS, substantiating that PLC and/or AC mediate the anxiolytic effect (Figure 3). On the EPM, we found an interaction between the first and second infusion on the percentage of time the rats spent on the open arms (F(1, 46)=4.23, p=0.046), whereas the percentage of entries into the open arms was not altered (F(1, 46)=1.13, p=0.29). The post hoc analysis revealed that NPS increased the percentage of time on the open arms in Veh1-pretreated (p=0.003) rats but not in rats pretreated with U73122/DDA (p=0.004). In Veh2-infused rats, U73122/DDA preinfusion did not influence anxiety on the EPM. Local NPS did not affect locomotor activity, as measured by the number of closed arm entries (F(1, 46)=2.86, p=0.098) in both Veh1- and U73122/DDA-pretreated rats.
Figure 3.
Effects of preinfusion of an inhibitor-cocktail (Inh-cock) consisting of both U73122 (PLC inhibitor, 0.5 nmol) and DDA (AC inhibitor, 12.5 nmol) on the anxiolytic effect of NPS bilaterally infused into the MeA (0.2 nmol/0.5 μl) on the EPM (a–c) and OF (d–f). (a) Percentage of time spent on the EPM, (b) percentage of entries performed into the open arms, (c) closed arm entries, and (d) time spent in the OF, (e) entries performed into the center zone, and (f) distance traveled. As Veh1, 0.5 μl of Ringer’s solution supplemented with DMSO, and as Veh2, 0.5 μl of Ringer’s solution were used. Data represent mean+SEM, **p<0.01, ***p<0.001 versus all other groups (a, e, f) or as indicated; group sizes: n=13–15, except Inh-cock/Veh2: n=6–8.
In the OF, the anxiolytic effect of intra-MeA NPS was similarly prevented by preinfusion of U73122/DDA, as assessed by the time the rats spent in the center zone (F(1, 50)=3.36, p=0.073, first infusion: F(1, 50)=13.72, p<0.001, second infusion: F(1, 50)=4.12, p=0.048) and the number of center zone entries (F(1, 50)=7.99, p=0.007). Moreover, statistical analysis revealed increased locomotion (F(1, 50)=8.28, p=0.006), an effect caused by NPS in Veh1-preinfused rats (p<0.001). In the OF, preinfusion of the inhibitor cocktail also blocked NPS-induced hyperlocomotion (p<0.001).
Experiment 4: Effects of Specific Blockade of Either PLC or AC on NPSR-Mediated Anxiolysis in the MeA
To assess whether the anxiolytic activity of NPS is mediated via the NPSR-evoked activation of either PLC or AC or via both pathways within the MeA, we next preinfused U73122 (PLC inhibitor), DDA (AC inhibitor), or Veh1 bilaterally into the MeA before local NPS or Veh2 infusion. Selective inhibition of PLC signaling by preinfusion of U73122 prevented NPS-induced anxiolysis, whereas specific blockade of the AC by DDA preinfusion failed to inhibit the anxiolytic properties of NPS (Figure 4).
Figure 4.
Effects of preinfusion of either U73122 (PLC inhibitor, 0.5 nmol), DDA (AC inhibitor, 12.5 nmol), or Veh1 (0.5 μl of Ringer’s solution supplemented with DMSO) on the anxiolytic effect of NPS bilaterally infused into the MeA (0.2 nmol/0.5 μl) on the EPM (a–f) and the OF (g–i). (a, d) Percentage of time spent on the EPM, (b, e) percentage of entries performed into the open arms, and (c, f) closed arm entries (group sizes: n=13–15, except Inh-cock/Veh2: n=6–8). As Veh2, 0.5 μl of Ringer’s solution were used. (g) Time spent in the center zone, (h) center zone entries, and (i) traveled distance in the OF (group sizes: n=5–8). All data represent mean+SEM. *P<0.05, **p<0.01 as indicated, #p<0.05 vs Veh1/NPS, $p<0.05 vs Veh1/Veh2.
In more detail, specific inhibition of the PLC signaling affected the percentage of time spent on the open arms (first infusion: F(1, 44)=1.75, p=0.19; second infusion: F(1, 44)=4.72, p=0.036; interaction: F(1, 44)=3.01, p=0.09) and the percentage of open arm entries (first infusion: F(1, 44)=7.47, p=0.009; second infusion: F(1, 44)=3.42, p=0.072; interaction: F(1, 44)=0.87, p=0.36) on the EPM. The post hoc analysis revealed that NPS-infused rats spent more time on the open arms (% time; p=0.003 vs Veh1/Veh2 rats) when preinfused with Veh1. This anxiolytic effect of NPS was prevented in the presence of U73122 (p=0.024 vs Veh1/NPS). Similarly, preinfusion of U73122 reduced the percentage of entries into the open arms compared with Veh1 (p=0.009 vs Veh1/NPS). In addition, U73122 exerted a slight but significant stimulatory effect on locomotor activity (p=0.015 vs Veh1/NPS), as U73122/NPS animals showed an increased number of closed arm entries.
Separate inhibition of the AC signaling by preinfusion of DDA revealed an effect for the second infusion on the percentage of time spent on the open arms (first infusion: F(1, 44)=0.09, p=0.76; second infusion: F(1, 44)=9.32, p=0.004; interaction: F(1, 44)=0.68, p=0.42) and on the percentage of open arm entries (first infusion: F(1, 44)=0.76, p=0.39; second infusion: F(1, 44)=8.77, p=0.005; interaction: F(1, 44)=0.16, p=0.69). The post hoc analysis indicated that NPS almost doubled the percentage of time the animals spent on the open arms (p=0.004) and increased the percentage of open arm entries (p=0.005) in comparison with Veh2-infused rats. More importantly, preinfusion of DDA failed to block this anxiolytic effect of NPS (percentage of time spent on open arms: p=0.41 vs Veh1/NPS; percentage of open arm entries: p=0.73), suggesting that the NPS-induced anxiolysis is independent of AC signaling. None of the drug-infused rats of this experiment showed any signs of hyperlocomotion as the number of closed arm entries was not altered (F(1,,45)=0.016, p=0.90).
In addition, the effect of either Veh1, U73122, or DDA before NPS infusion was assessed 2 days later in the OF. One-way ANOVA did not reveal any significant changes with respect to time spent in the center (F2, 20=2.03, p=0.16), entries into the center (ANOVA on ranks: H=2.15, p=0.34), or traveled distance (F2, 20=0.49, p=0.62). However, when performing separate statistics our previous findings could be confirmed. In detail, specific inhibition of the PLC signaling by U73122 decreased the time spent in the center zone compared with Veh1/NPS-treated animals (U=11.0, p=0.028), but inhibition of AC signaling by DDA failed to block NPS-induced anxiolysis (t11=1.03, p=0.32). None of the drug-infused rats showed any difference in center zone entries (Veh1/NPS vs U73122/NPS: U=15.5, p=0.08; Veh1/NPS vs DDA/NPS: U=17.5, p=0.72) or locomotor activity, as the traveled distance was not altered (Veh1/NPS vs U73122/NPS: t14=0.92, p=0.37; Veh1/NPS vs DDA/NPS: t11=−0.10, p=0.93).
Discussion
The present study describes the MeA as an important brain target of NPS to induce anxiolytic effects in male rats and NPSR-evoked PLC signaling as the essential signaling cascade underlying this local anxiolytic effect. More specifically, the PLC inhibitor U73122 prevented NPS-induced anxiolysis in NPS-sensitive amygdalar neurons.
The MeA pointed out as an important relay station and regulator of emotional responses including anxiety-related behaviors (LeDoux, 2007; Keshavarzi et al, 2014). It is critically involved in processing of predator odor-induced defensive reactions, ie, freezing and risk assessment, a defensive behavior related to panic attacks (Herdade et al, 2006). The MeA receives dense synaptic input from various brain regions involved in sensory processing and neuroendocrine and autonomic regulation including the accessory olfactory bulb, the pericoerulear region, and the parabrachial nucleus (Moore and Bloom, 1979; Saper and Loewy, 1980; Keshavarzi et al, 2014), whereas the latter two brain regions harbor a cluster of predominantly glutamatergic NPS-immunoreactive neurons (Xu et al, 2007). The NPSR is differentially expressed in amygdala subnuclei in rats and mice (Xu et al, 2007; Clark et al, 2011). However, both species express the NPSR in the MeA, but not the central nucleus of the amygdala (CeA), the major output nucleus of the amygdala (Tovote et al, 2015) regulating, ie, pericoerulear NPS neurons by monosynaptic, GABAergic projections in mice (Jungling et al, 2015). How MeA neurons stimulated by NPS regulate the activity of CeA neurons and its output signaling remains elusive.
Our data demonstrating that NPS infusion into the MeA reduced the anxiety-related behavior in both the EPM and OF of male rats confirm the robust anxiolytic profile of NPS described following ICV infusion (Xu et al, 2004; Leonard et al, 2008; Rizzi et al, 2008; Vitale et al, 2008; Wegener et al, 2011; Slattery et al, 2015; Zoicas et al, 2016) and intranasal application (Ionescu et al, 2012; Lukas and Neumann, 2012; Dine et al, 2015) in both rats and mice, and infusion into the lateral and basolateral amygdala in mice (Jungling et al, 2008). Specifically, intra-MeA infusion of NPS increased the percentage of time the animals spent on the open arms of the EPM and in the exposed center zone of the OF indicating reduced anxiety levels.
The in vitro activation of the NPSR has been described to increase intracellular Ca2+ levels and to phosphorylate ERK1/2 in NPSR-transfected HEK293T cells (Reinscheid et al, 2005). Furthermore, NPS-induced mobilization of intracellular Ca2+ has been reported in NPSR-transfected hippocampal mouse neurons in vitro, an effect that was strictly dependent on PLC activity (Erdmann et al, 2015). As activation of intracellular signaling cascades is cell-type specific and depends on the cellular microenvironment, the NPS-induced increase in intracellular Ca2+ levels and activation of ERK1/2 found in vitro may not necessarily reflect intracellular signaling upon NPS binding to its receptor within the brain. Our in vivo results are the first to show NPSR-induced activation of intracellular signaling cascades within the brain. Specifically, quantification of pCaMKIIα as a biological marker of NPSR-mediated Ca2+ influx after ICV NPS revealed increased relative quantities of pCaMKIIα reflecting NPS-induced rise in intracellular Ca2+ levels. Our results also demonstrate NPS-induced activation of the MAPK pathway in amygdala punches as reflected by increased pERK1/2 levels in relation to both total ERK1/2 and β-tubulin. Thus, NPSR-evoked phosphorylation and synthesis of CaMKIIα and the phosphorylation of ERK1/2 provide important confirmation of intracellular actions of NPS in the amygdala. However, our results are based on amygdala micropunches. Therefore, it remains elusive whether the described NPSR-mediated pathway activation is restricted to NPSR-positive neurons. Thus, future studies should address signal amplification at cellular level by fluorescent labeling of neurons using NPSR promoter-based expression of reporter proteins.
As in vitro studies demonstrated that NPSR can couple to intracellular Ca2+ as well as cAMP pathways, indicating interaction with both Gq and Gs types of G proteins (Reinscheid et al, 2005), we aimed to reveal the specific involvement of PLC and/or AC signaling in NPS-induced anxiolysis. As an initial approach, we infused a cocktail composed of both the aminosteroidic PLC inhibitor U73122 and the noncompetitive AC inhibitor DDA before NPS infusion into the MeA. NPS failed to induce anxiolysis in rats pretreated with an inhibitor cocktail composed of U73122 and DDA, indicating that either both or one of these intracellular pathways in the MeA are critically involved in NPS-induced behavioral changes. Next, each of the inhibitors was separately applied before local infusion of NPS. Although NPS has a relatively equal potency to Ca2+ mobilization and cAMP synthesis in vitro (Reinscheid et al, 2005), in rats separate preinfusion of U73122 prevented the anxiolytic effect of locally infused NPS in both the EPM and OF, suggesting that activation of PLC is crucial to orchestrate acute NPS-induced anxiolysis. In contrast, separate preinfusion of DDA failed to block NPS-induced anxiolysis, suggesting that the behavioral effect is independent of AC activation. Recently, a truncated version of the NPS peptide, called compound 4, has been demonstrated to evoke anxiolysis and to induce memory-promoting effects with reduced levels of locomotor stimulation following ICV infusion in mice (Clark et al, 2017). In HEK293T cells transfected with NPSR107I, compound 4 demonstrated a strong level of bias for the Ca2+ mobilization pathway over the cAMP pathway (Clark et al, 2017), in line with our results demonstrating that a block of PLC signaling in the presence of NPS prevents its anxiolytic profile. Although NPS induces cAMP accumulation in HEK293T cells in vitro (Reinscheid et al, 2005), a cAMP-independent mechanism seems to be involved in the anxiolytic effect of NPS in the MeA. Our observation that U73122 and DDA, when infused alone, did not significantly alter anxiety-like behavior compared with control excluded the possibility that the inhibitors changed baseline anxiety.
Based on their chemical structure, both U73122 and DDA are cell membrane permeable. Thus, inhibitor infusion 20 min before local NPS treatment should be sufficient to locally block the respective effector proteins. The different doses of U73122 and DDA used need some considerations: U73122 is reported to reliably inhibit PLC (MacMillan and McCarron, 2010), and usually used at a concentration of 10 μM in vitro (Erdmann et al, 2015). As the binding domain of PLC isoforms is highly conserved (Rhee et al, 1989; Rhee and Bae, 1997), U73122 should inhibit all isoforms of PLC (Carvou et al, 2007). Moreover, U73122 reduces Ca2+ release, when evoked by direct activation of inositol-1,4,5-triphosphate receptors or ryanodine receptors independent of Gq or PLC activity (MacMillan and McCarron, 2010). Here, we used a dose of 0.5 nmol of U73122 for local infusion into each MeA.
In mammals, nine isoforms of AC are expressed catalyzing the production of the second messenger cAMP (Hanoune and Defer, 2001). A Ca2+/Calmodulin-dependent mechanism was demonstrated to activate the AC isoforms 1, 3, and 8 (MacNeil et al, 1985; Halls and Cooper, 2011). Hence, this demonstrates that intracellular pathways do not necessarily act in a linear manner, but can be integrated to bring about the appropriate physiological and behavioral responses. However, whether NPS-evoked cAMP accumulation depends on local rise in intracellular Ca2+ levels needs further investigation. For example, the analysis of NPSR/G protein interactions using fluorescence resonance energy transfer might demonstrate whether NPSR stimulation recruits both Gq and Gs proteins or whether cAMP accumulation is maintained by a Ca2+-dependent activation of AC. Herein, we used DDA to block the membrane-bound AC. The half-maximal inhibitory concentration of DDA is described with 250 μM in vitro (Holgate et al, 1980; Ogawa et al, 1995). Therefore, we infused DDA at a 25-fold higher concentration in order to exert a potentially similar effective inhibition compared with U73122. The total amount of U73122 (0.5 nmol) and DDA (12.5 nmol) applied was selected following diffusion-based calculations in order to maintain at least half-maximal inhibition of the effector proteins within the MeA.
In summary, our in vivo data identified another brain region in rats, the MeA, where NPS reliably exerts anxiolytic properties, and demonstrated that this behavioral effect is strictly dependent upon local NPSR-mediated PLC activation. We could further show that central NPS infusion induced phosphorylation and synthesis of CaMKIIα in amygdala punches, thereby promoting the activation of the MAPK pathway as another NPSR-mediated intracellular signaling cascade. Our findings contribute to a broader knowledge about the molecular, cellular, and behavioral consequences of NPSR activation in the context of its profound anxiolytic profile that may lead to the development of specific NPSR agonists activating specific intracellular pathways involved in anxiolysis.
Funding and disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Rodrigue Maloumby, Nora Jahnen, and Benedikt Nerb for expert technical assistance. We thank Professor Dr Rainer Landgraf and Dr Ben Jurek for critically reading the manuscript.
Author contributions
TG and IDN designed the study based on literature searches; TG performed the experiments including the statistical analysis, and wrote the first draft of the manuscript. Both authors contributed to and have approved the final manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Camarda V, Rizzi A, Ruzza C, Zucchini S, Marzola G, Marzola E et al (2009). In vitro and in vivo pharmacological characterization of the neuropeptide s receptor antagonist [D-Cys(tBu)5]neuropeptide S. J Pharm Exp Ther 328: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvou N, Norden AGW, Unwin RJ, Cockcroft S (2007). Signalling through phospholipase C interferes with clathrin-mediated endocytosis. Cell Signal 19: 42–51. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Lange MD, Jungling K, Lesting J, Seidenbecher T, Pape HC (2012). Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala. Neuropsychopharmacology 37: 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SD, Duangdao DM, Schulz S, Zhang L, Liu X, Xu YL et al (2011). Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J Comp Neurol 519: 1867–1893. [DOI] [PubMed] [Google Scholar]
- Clark SD, Kenakin TP, Gertz S, Hassler C, Gay EA, Langston TL et al (2017). Identification of the first biased NPS receptor agonist that retains anxiolytic and memory promoting effects with reduced levels of locomotor stimulation. Neuropharmacology 118: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dine J, Ionescu IA, Avrabos C, Yen YC, Holsboer F, Landgraf R et al (2015). Intranasally applied neuropeptide S shifts a high-anxiety electrophysiological endophenotype in the ventral hippocampus towards a "normal"-anxiety one. PLoS ONE 10: e0120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann F, Kugler S, Blaesse P, Lange MD, Skryabin BV, Pape HC et al (2015). Neuronal expression of the human neuropeptide S receptor NPSR1 identifies NPS-induced calcium signaling pathways. PLoS ONE 10: e0117319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feisst C, Albert D, Steinhilber D, Werz O (2005). The aminosteroid phospholipase C antagonist U-73122 (1-[6-[[17-beta-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5- dione) potently inhibits human 5-lipoxygenase in vivo and in vitro. Mol Pharmacol 67: 1751–1757. [DOI] [PubMed] [Google Scholar]
- Halls ML, Cooper DM (2011). Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol 3: a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J, Defer N (2001). Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41: 145–174. [DOI] [PubMed] [Google Scholar]
- Herdade KC, Strauss CV, Zangrossi Junior H, Viana MB (2006). Effects of medial amygdala inactivation on a panic-related behavior. Behav Brain Res 172: 316–323. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Lewis RA, Austen KF (1980). Role of adenylate cyclase in immunologic release of mediators from rat mast cells: agonist and antagonist effects of purine- and ribose-modified adenosine analogs. Proc Natl Acad Sci USA 77: 6800–6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu IA, Dine J, Yen YC, Buell DR, Herrmann L, Holsboer F et al (2012). Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology 37: 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungling K, Lange MD, Szkudlarek HJ, Lesting J, Erdmann FS, Doengi M et al (2015). Increased GABAergic efficacy of central amygdala projections to neuropeptide S neurons in the brainstem during fear memory retrieval. Neuropsychopharmacology 40: 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD et al (2008). Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59: 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi S, Sullivan RK, Ianno DJ, Sah P (2014). Functional properties and projections of neurons in the medial amygdala. J Neurosci 34: 8699–8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Brandenburg N, Lane M, Roy-Byrne P, Stang PD, Stein DJ et al (2005). Rethinking the duration requirement for generalized anxiety disorder: evidence from the National Comorbidity Survey Replication. Psychol Med 35: 1073–1082. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2007). The amygdala. Curr Biol 17: R868–R874. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ et al (2008). Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology 197: 601–611. [DOI] [PubMed] [Google Scholar]
- Liao Y, Lu B, Ma Q, Wu G, Lai X, Zang J et al (2016). Human neuropeptide S receptor is activated via a galphaq protein-biased signaling cascade by a human neuropeptide S analog lacking the C-terminal 10 residues. J Biol Chem 291: 7505–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Neumann ID (2012). Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: social versus non-social effects. Neuropharmacology 62: 398–405. [DOI] [PubMed] [Google Scholar]
- MacMillan D, McCarron JG (2010). The phospholipase C inhibitor U-73122 inhibits Ca2+release from the intracellular sarcoplasmic reticulum Ca2+store by inhibiting Ca2+pumps in smooth muscle. Br J Pharmacol 160: 1295–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil S, Lakey T, Tomlinson S (1985). Calmodulin regulation of adenylate cyclase activity. Cell Calcium 6: 213–216. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE (1979). Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci 2: 113–168. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Tashima M, Takeda Y, Sawai H, Toi T, Sawada H et al (1995). Erythroid differentiation and growth inhibition of K562 cells by 2',5'-dideoxyadenosine: synergism with interferon-alpha. Leuk Res 19: 749–755. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego. [Google Scholar]
- Reinscheid RK, Xu YL (2005). Neuropeptide S and its receptor: a newly deorphanized G protein-coupled receptor system. Neuroscientist 11: 532–538. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R et al (2005). Pharmacological characterization of human and murine neuropeptide S receptor variants. J Pharm Exp Ther 315: 1338–1345. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS (1997). Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem 272: 15045–15048. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Suh PG, Ryu SH, Lee SY (1989). Studies of inositol phospholipid-specific phospholipase C. Science 244: 546–550. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S et al (2008). Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br J Pharmacol 154: 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD (1980). Efferent connections of the parabrachial nucleus in the rat. Brain Res 197: 291–317. [DOI] [PubMed] [Google Scholar]
- Sartori SB, Maurer V, Murphy C, Schmuckermair C, Muigg P, Neumann ID et al (2016). Combined neuropeptide S and D-cycloserine augmentation prevents the return of fear in extinction-impaired rodents: advantage of dual versus single drug approaches. Int J Neuropsychopharmacol 19: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Naik RR, Grund T, Yen YC, Sartori SB, Fuchsl A et al (2015). Selective breeding for high anxiety introduces a synonymous SNP that increases neuropeptide S receptor activity. J Neurosci 35: 4599–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A (2015). Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16: 317–331. [DOI] [PubMed] [Google Scholar]
- Vitale G, Filaferro M, Ruggieri V, Pennella S, Frigeri C, Rizzi A et al (2008). Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides 29: 2286–2291. [DOI] [PubMed] [Google Scholar]
- Wegener G, Finger BC, Elfving B, Keller K, Liebenberg N, Fischer CW et al (2011). Neuropeptide S alters anxiety, but not depression-like behaviour in Flinders Sensitive Line rats: a genetic animal model of depression. Int J Neuropsychopharmacol 15: 375–387. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK (2007). Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol 500: 84–102. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH et al (2004). Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43: 487–497. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Menon R, Neumann ID (2016). Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology 108: 284–291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.