Abstract
Background
Bone marrow-derived progenitor cells likely contribute to both endothelial- and smooth muscle cell-dependent healing responses in stent-injured vessel sites. This study aimed to assess mobilization of progenitor cells and vessel healing after zotarolimus-eluting (ZES) and everolimus-eluting (EES) stents.
Methods and results
In 63 patients undergoing coronary stent implantation, we measured circulating CD34 + CD133 + CD45low cells and serum levels of biomarkers relevant to stem cell mobilization. In 31 patients of them, we assessed vessel healing within the stented segment using optical coherence tomography (OCT) imaging. The CD34 + CD133 + CD45low cells increased 68 ± 59% 7 days after bare metal stent (BMS), 10 ± 53% after ZES (P < 0.01 vs BMS), 3 ± 49% after EES (P < 0.001 vs BMS), compared with baseline. Percent change in CD34 + CD133 + CD45low cells was positively correlated with that in stromal cell-derived factor (SDF)-1α (R = 0.29, P = 0.034). Percentage of uncovered struts was higher in the EES group (14.4 ± 17.3%), compared with the BMS (0.7 ± 1.3, P < 0.01) and ZES (0.4 ± 0.5, P < 0.01) groups. The change in CD34 + CD133 + CD45low cells showed positive correlation with OCT-quantified mean neointimal area (R = 0.48, P < 0.01). Finally, circulating mononuclear cells obtained from 5 healthy volunteers were isolated to determine the effect of sirolimus, zotarolimus and everolimus on vascular cell differentiation. The differentiation of mononuclear cells into endothelial-like cells was dose-dependently suppressed by sirolimus, zotarolimus, and everolimus.
Conclusions
Mobilization of progenitor cells was suppressed, and differentiation of mononuclear cells into endothelial-like cells was inhibited, in association with increased number of uncovered stent struts, even after second generation drug-eluting stenting. These data suggest that new approaches are necessary to enhance stent healing.
Keywords: Drug-eluting stent, Vascular injury, Circulating progenitor cell, Re-endothelialization, Optical coherence tomography
1. Introduction
Generational advances in drug-eluting stent (DES) technology have resulted in reduced rates of target lesion revascularization across broad patient and lesion subsets with improved safety with respect to stent thrombosis. However, concerns over incomplete stent healing even with second-generation DES persist because the annual rate of target lesion failure still remains at 2–4% annually, which is similar to the rates observed after implantation of bare metal stent (BMS) or first-generation DES [1]. From a vascular biology perspective, there is consensus that late lumen loss and neointimal thickening (i.e., restenosis) are the biological response to vascular injury characterized by a sequence of endothelial denudation, platelet deposition and inflammatory cell recruitment, smooth muscle cell migration and proliferation, and extracellular matrix deposition. Complete stent coverage and re-endothelialization are commonly viewed as markers of favorable vascular healing [2], [3].
In the course of investigating vascular healing after stent deployment, multiple research laboratories, including our own, discovered that progenitor cells from bone marrow and other tissues serve as a source of both smooth muscle cell and endothelial cell precursors in the healing response [4]. These same progenitor cells play an essential role in angiogenesis [5], [6]. We reported previously that CD34-positive (CD34 +) cells, which include smooth muscle progenitor cells (SMPCs) as well as endothelial progenitor cells (EPCs), are mobilized into the circulation after stenting and are positively correlated with an increased risk of restenosis [7]. Specifically, circulating CD34 + cells increased 7–14 days after BMS deployment and was associated with late lumen loss and restenosis; first-generation sirolimus-eluting stent (SES) suppressed late lumen loss and CD34 + cell mobilization, raising the question of whether neointimal suppression is inexorably linked with impaired re-endothelialization. In the present study, we assessed mobilization of progenitor cells and vessel healing after second generation zotarolimus-eluting (ZES) and everolimus-eluting (EES) stents. In addition, we also investigated in-vitro pharmacological action of the drugs coated on the surface of DESs on the differentiation of progenitor cells into vascular cells.
2. Methods
2.1. Study design
The subjects included 63 patients (46 men and 17 women, aged 69 ± 9 yr) with stable coronary artery disease who underwent elective coronary stent implantation for organic lesions of single coronary artery, using any one of BMS, ZES or EES. Stent selection was based on operators' decision. Consequently, BMS was implanted in 20 patients, ZES in 19 and EES in the remaining 24 patients. All of the patients were receiving dual anti-platelet therapy with 81 mg of aspirin and 75 mg clopidogrel at least until the follow-up coronary angiography was performed. The follow-up coronary angiography was recommended for all patients at 12 months after stent implantation, and was performed earlier if necessary based on clinical indications. In all patients, peripheral blood sample was collected at baseline before stenting and on the day 7 post-stenting. The blood samples were immediately collected into tubes containing ethylene diaminetetraacetate (EDTA) and plain tubes. We measured the number of circulating progenitor cells including EPCs at baseline and on the day 7 post-stenting, using the EDTA blood. We also measured serum level of biomarkers relevant to stem cell mobilization using the plain tube blood. At the time of follow-up coronary angiography, we assessed re-endothelialization and neointimal growth at the site of stent placement using optical coherence tomography (OCT) imaging, in addition to quantitative coronary angiographic analysis (QCA). The local institutional review board in Dokkyo Medical University (Mibu, Tochigi, Japan) approved the study protocol, and written informed consent was obtained from each patient.
2.2. Measurement of circulating CD34 + CD133 + CD45low cells
We measured circulating CD34 + CD133 + CD45low cells, which include EPCs, using flow cytometry based on a previously described method [7], [8], [9] with minor modifications. In brief, the reagent mixture consisted of a nucleic acid dye (SY-III-8; Molecular Probe), a peridinine chlorophil protein (PerCP)-conjugated anti-CD45 (Becton Dickinson), a fluorescein isothiocyanate (FITC)-conjugated anti-CD34 (Becton Dickinson) and a phycoerythrin (PE)-conjugated anti-CD133 (Miltennyi Biotec). Isotype controls were used as negative controls based on the species and immunoglobulin (Ig) G control antibodies (IgG1 isotype control; Becton Dickinson). Flow cytometric analysis was performed using the FACS Calibur laser flow cytometer (Becton Dickinson) according to the manufacturer's instructions (Supplemental file 1). The absolute number of CD34 + CD133 + CD45low cells per milliliter was calculated based on the cells-to-the white blood cell count. To minimize any methodological variations, each sample was analyzed with two independent experiments, and the mean value was calculated.
2.3. Serum biomarker assays
In this study, we also measured serum levels of angiogenic biomarkers relevant to stem cell mobilization, such as stromal cell-derived factor (SDF)-1α, interleukin (IL)-8, and matrix metalloproteinase (MMP)-9, using enzyme-linked immunosorbent assay (ELISA). We used each commercially available ELISA kit, the Quantikine ELISA kit (R&D Systems) for SDF-1α (Human CXCL12/SDF-1α Immunoassay), IL-8 (Human CXCL8/IL-8 Immunoassay), and MMP-9 (Human MMP-9 Immunoassay). The procedure was performed according to the manufacturer's instructions. Each sample was assayed in duplicate standards and controls; high, medium, and low were included in each run. All results were reported within the linearity of the assay. The colorimetric reactions were read as the value of the optical density directly on the automatic microplate reader set to 450 nm.
2.4. Quantitative coronary angiography analysis
Coronary lesions were assessed by QCA using a computer-based CASS system (Pie Medical Instruments) before and immediately after stent implantation and at the time of follow-up coronary angiography. Lesion length, reference diameter and minimal lumen diameter were measured and late lumen loss (minimal lumen diameter after stenting minus minimal lumen diameter at follow-up angiography) was calculated.
2.5. Optical coherence tomography imaging and analysis
At the time of follow-up coronary angiography, OCT examination was performed using a frequency-domain system (C7-XR FD-OCT Intravascular Imaging System; LightLab Imaging). The cross-sectional neointimal area and the neointimal volume was calculated. In every cross-sectional image, neointimal coverage was assessed for all of the struts, and the percentage of uncovered struts to total struts in all OCT cross-sections was calculated [10]. The OCT analysis was performed by an independent investigator blinded to the study protocol in University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University School of Medicine (Cleveland, OH).
2.6. In-vitro experiment
We investigated in-vitro pharmacological action of the drugs coated on the surface of SES, ZES and EES, i. e., sirolimus, zotarolimus and everolimus, respectively, regarding differentiation of bone marrow-derived progenitor cells into vascular endothelial cells as well as vascular smooth muscle cells, using a previously described method [11] with minor modifications. Briefly, peripheral mononuclear cells were isolated from the peripheral blood of 5 healthy human volunteers (3 men and 2 women, aged 37 ± 8 yr). Mononuclear cells were cultured for 14 days in EGM-2 (Lonza) supplemented with hydrocortisone, bovine brain extract, vascular endothelial growth factor (VEGF) and fetal bovine serum (EPC medium) and also in HuMedia-SG2 (Kurabo) supplemented with platelet derived growth factor-BB and basic fibroblast growth factor (SMPC medium). Sirolimus (Sigma), zotarolimus (Toronto Research Chemicals) and everolimus (Sigma) were added to each culture medium at a concentration of 0.01, 0.1 and 1 nM each.
Immunocytochemistry was used to assay cultured cells for the expression of CD31 and von Willebrand factor (vWF) as endothelial cell markers, and for the expression of α-smooth muscle actin (α-SMA) and smooth muscle myosin heavy chain isoforms, SM1 and SM2, as smooth muscle cell markers, to identify endothelial- as well as smooth muscle-like cells. Primary monoclonal antibodies against human CD31 (JC70A; Dako Cytomation), vWF (F8/86; Dako Cytomation), α-SMA (1A4; Dako Cytomation), SM1 (3F8; Yamasa) and SM2 (1G12; Yamasa) were applied. Non-immune mouse IgG2a and IgG1 (Dako Cytomation) were used as negative controls. To visualize the immunoreactive products, Fast Red Substrate System (Dako Cytomation) was used according to the manufacturer's instructions. To identify endothelial-like cells, incorporation of acetylated LDL labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyamine perchlorate (DiI-Ac-LDL) into the cultured cells was also observed. The cultured cells were incubated with DiI-Ac-LDL (Alfa Aesar), and counterstained with FITC-labeled lectin from Bandeiraea simplicifolia (FITC-BS lectin, Sigma). The cells positive for both DiI-Ac-LDL and FITC-BS lectin were assessed [11]. These in-vitro experiments were also approved in Dokkyo Medical University institutional review board, and written informed consent was obtained from each of 5 participants.
2.7. Statistical analyses
Normality of the distribution of variables was assessed using Kolmogorov-Smirnov test with Lilliefors' correlation. If the distribution of values were non-parametric, the data were transformed into logarithmic values. Data were presented as means ± standard deviation (SD). Differences among the 3 groups were assessed with a chi-square test for categorical variables and with an analysis of variance (ANOVA) followed by post-hoc Scheffe's test for continuous variables. Serial changes in parameters were analyzed using repeated measures ANOVA. Spearman correlation analyses were used to assess the relationship between 2 parameters. P < 0.05 was considered to be significant.
3. Results
3.1. Circulating CD34 + CD133 + CD45low cells and serum biomarkers after stenting
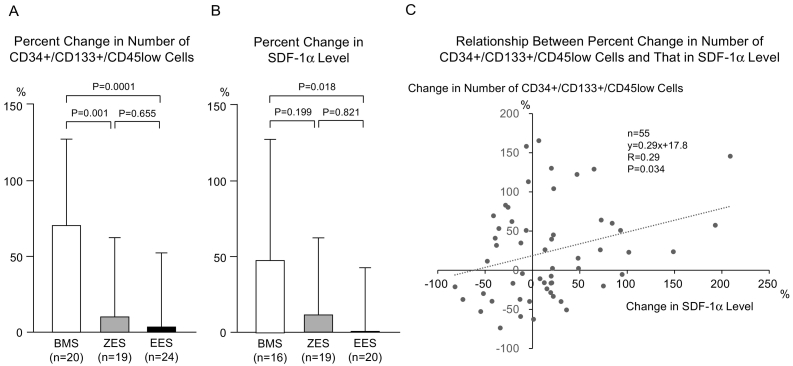
Baseline characteristics and QCA results of all 63 patients were compared among the 3 stent groups (BMS, ZES and EES). There were no significant differences in the baseline characteristics among the 3 groups. Baseline reference vessel diameter, acute gain, and late loss were less in the EES compared to the BMS group (Supplemental file 2). Baseline circulating CD34 + CD133 + CD45ow cell numbers were similar (P = 0.165) among BMS (331 ± 170/ml), ZES (326 ± 185/ml), and EES (422 ± 208/ml) treated patients. The number of CD34 + CD133 + CD45low cells increased significantly 7 days post-stenting in the BMS group (to 512 ± 252/ml, P < 0.001), but did not in the ZES (to 326 ± 186/ml) or EES (to 443 ± 319/ml) groups. Percent change in the number of CD34 + CD133 + CD45low cells was significantly reduced in both ZES (10 ± 53%, P < 0.01 vs. BMS) and EES (3 ± 49%, P < 0.001 vs. BMS) treated patients compared with BMS (68 ± 59%) (Fig. 1A).
Fig. 1.
Circulating CD34 + CD133 + CD45low cells and SDF-1α level. (A) Percent change in the number of CD34 + CD133 + CD45low cells at the day 7 from the baseline value was less in the ZES group and was still less in the EES group, compared with the BMS group. (B) Percent change in the SDF-1α level was less in the EES group, compared with the BMS group. The level in the ZES group did not show significant difference from that in BMS group as well as that in EES group. (C) In overall patients, the percent change in number of CD34 + CD133 + CD45low cells was positively correlated with that in SDF-1α level.
BMS indicates bare metal stent; ZES, zotarolimus-eluting stent; EES, everolimus-eluting stent; SDF, stromal cell-derived factor.
Since quantification of progenitor cell number requires flow cytometric analysis, which may be impractical for routine clinical use, we also investigated whether serum-based biomarkers implicated in progenitor cell mobilization, including SDF-1α, IL-8, and MMP-9, might serve as useful surrogate markers. Baseline serum levels of SDF-1α were similar in BMS (1847 ± 822 pg/ml), ZES (1597 ± 874 pg/ml), EES (2174 ± 967 pg/ml) treated patients. Although SDF-1α level on day 7 post-stenting was similar among the 3 groups (2266 ± 768, 1444 ± 512 and 2035 ± 882 pg/ml, respectively), percent change in SDF-1α level was reduced significantly in the EES compared to BMS group (0 ± 41 vs. 47 ± 79%, P = 0.018). The change in SDF-1α serum level in the ZES group was 12 ± 49% and not significantly different from the EES group (Fig. 1B). Among BMS, ZES, and EES groups, baseline levels of IL-8 (227 ± 188, 205 ± 81 and 207 ± 67 pg/ml, respectively) and MMP-9 (3127 ± 1560, 3212 ± 1765 and 3644 ± 2286 pg/ml, respectively) were similar. Seven days post-stenting, there were no significant differences in the serum levels of IL-8 (BMS 188 ± 70, ZES 190 ± 77, and EES 218 ± 92 pg/ml) and MMP-9 (BMS 2702 ± 1360, ZES 3187 ± 1893 and EES 4160 ± 3414 pg/ml), or in the percent change in serum IL-8 (BMS –12 ± 32, ZES 1 ± 41, EES 8 ± 38%) and MMP-9 (BMS –5 ± 39, ZES 4 ± 46, EES 23 ± 57%) levels. The percent change in serum SDF-1α level correlated positively with the percent change in CD34 + CD133 + CD45low cell number (R = 0.29, P < 0.05) (Fig. 1C). There were no significant correlations between the percent change in serum levels of IL-8 (R = 0.03) or MMP-9 (R = 0.01) and percent change in CD34 + CD133 + CD45low cell number.
3.2. Neointima formation and circulating CD34 + CD133 + CD45low cells
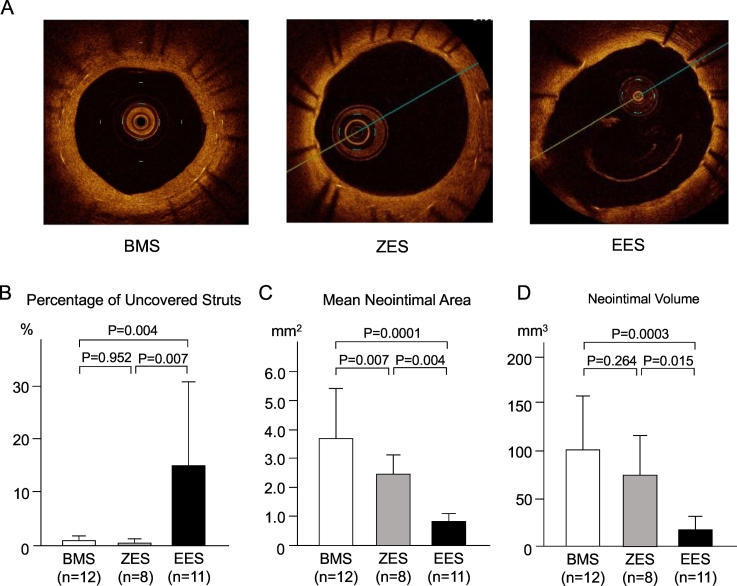
We performed intravascular imaging with OCT at the time of follow-up coronary angiography in 31 (BMS n = 12, ZES n = 8 and EES n = 11) out of 63 patients. By study design, OCT was limited to patients without angiographic restenosis (i.e., < 50% diameter stenosis), because OCT was not performed in patients with > 50% stenosis due to likely need for balloon pre-dilation to obtain technically adequate images. Single strut analysis was performed and the number and percentage of uncovered struts quantified. Mean neointinal area (an average of all measured cross-sectional neointimal area) and neointimal volume were also assessed. Representative OCT findings in BMS, ZES and EES treated patients are shown in Fig. 2A. The percentage of uncovered struts was higher in EES (14.4 ± 17.3%, P < 0.01 vs. BMS) compared to BMS (0.7 ± 1.3%) and ZES (0.4 ± 0.5%, P < 0.01 vs. EES) groups (Fig. 2B). Mean neointimal area was 3.8 ± 1.6, 2.4 ± 0.7 and 0.8 ± 0.4 mm2, respectively, in the BMS, ZES and EES groups. Neointimal area was significantly reduced in EES compared with both BMS (P < 0.001) and ZES (P < 0.01) (Fig. 2C). Neointimal volume was also significantly lower in EES (21 ± 16 mm3) compared to BMS (101 ± 61 mm3, P < 0.001 vs BMS) and ZES (77 ± 47 mm3, P = 0.015 vs. EES) (Fig. 2D).
Fig. 2.
OCT-based neointima formation and circulating CD34 + CD133 + CD45low cells. (A) Typical OCT findings indicate that neointima formation and neointimal coverage over the stent struts are adequate in a BMS lesion, a little inferior in a ZES lesion and poor in an EES lesion. (B) Percentage of uncovered struts was higher in the EES group, compared with the BMS group and ZES group. (C) Mean neointimal area was less in EES group, compared with the BMS group and ZES group. The value in the EES group was still less than that in the ZES group. (D) Neointimal volume was less in the EES group, compared with the BMS group and ZES group.
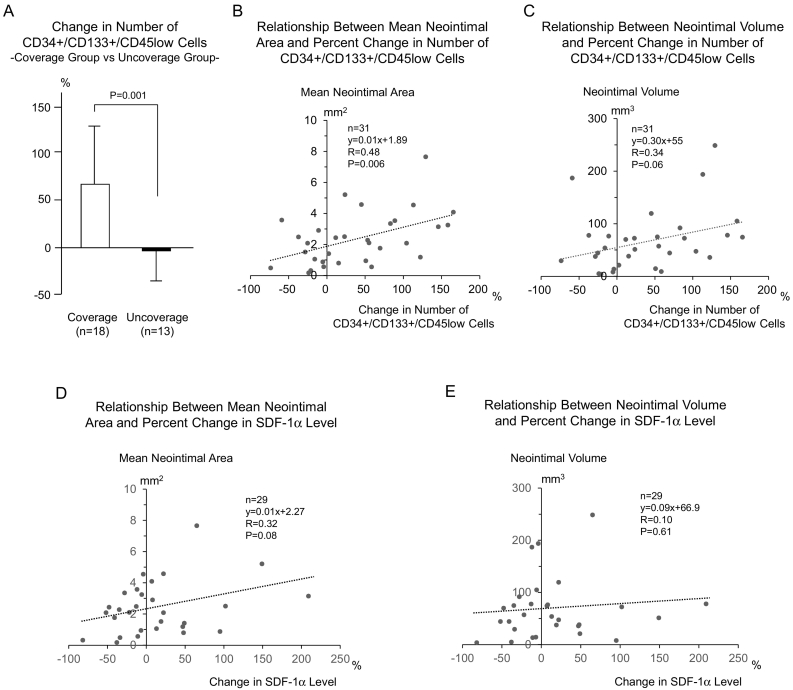
Next the 31 patients providing OCT findings were divided into 2 groups independent of stent type according to the percentage of uncovered struts. < 1% uncovered struts were defined as “covered” (covered group: n = 18; BMS n = 10, ZES n = 7, EES n = 1) and greater than or equal to 1.0% defined as “uncovered” (uncovered group: n = 13; BMS n = 2, ZES n = 1, EES n = 10). The percent change in CD34 + CD133 + CD45low cell number on day 7 was significantly reduced in the uncovered compared to the covered group (uncovered − 3 ± 38% vs. covered 70 ± 65, P < 0.01) (Fig. 3A). The percent change in serum SDF-1α level was similar in the covered and uncovered groups (9 ± 56 vs. 19 ± 69%, P = 0.69).
Fig. 3.
Neointima formation vs circulating CD34 + CD133 + CD45low cells and SDF-1α level. (A) If the patients were divided into 2 groups, i.e., coverage group (percent uncovered struts < 1.0%) or uncoverage group (1.0%≤), independently of stent type, percent changes in the number of CD34 + CD133 + CD45low cells were greater in the coverage group, compared with the uncoverage group. (B) Percent change in the number of CD34 + CD133 + CD45low cells showed significant positive correlation with the mean neointimal area. (C) Percent change in the number of CD34 + CD133 + CD45low cells showed a trend of positive correlation with the neointimal volume. (D) Percent change in SDF-1α level showed a trend of positive correlation with the mean neointimal area. (E) Percent change in SDF-1α level was not correlated with the neointimal volume.
The percent change CD34 + CD133 + CD45low cell number showed significant positive correlation with mean neointimal area (R = 0.48, P < 0.01) (Fig. 3B) and a trend toward positive correlation with neointimal volume (R = 0.34, P = 0.06) (Fig. 3C). The percent change in serum SDF-1α level also correlated positively with the mean neointimal area (R = 0.32, P = 0.08) (Fig. 3D).
3.3. Effect of zotarolimus and everolimus on peripheral blood mononuclear cell differentiation
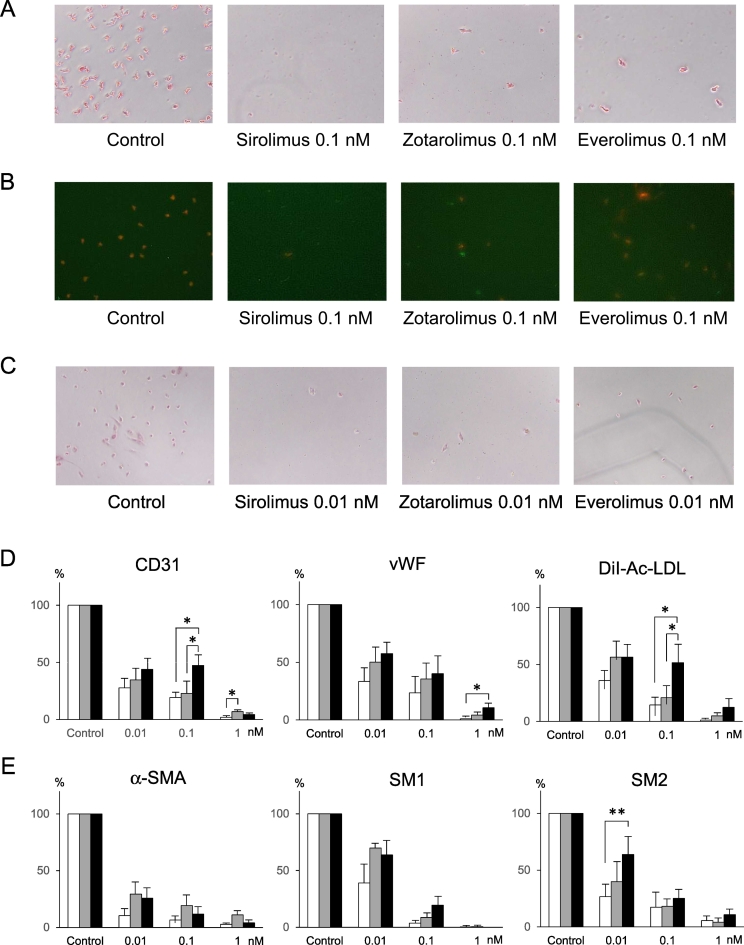
Next, we assessed the effect of everolimus (EES -limus analogue) and zotarolimus (ZES) compared to sirolimus (SES) on progenitor cell differentiation into endothelial and smooth muscle-like lineages. Endothelial cell differentiation was determined using CD31 and vWF markers, smooth muscle cell differentiation with α-SMA, SM1 and SM2. After 14 days of cell culture in media that promote EPC or SMPC lineage differentiation, round mononuclear cells differentiated into spindle- and stellate-shaped cells, respectively. Immunohistochemistry showed that almost all cells cultured in the EPC differentiating medium were positive for CD31 and vWF. Sirolimus, zotarolimus and everolimus significantly reduced the number of cells positive both for CD31 (Fig. 4A, D) and for vWF (Fig. 4D) in a concentration-dependent manner. The number of cells double-positive for both DiI-Ac-LDL and FITC-BS lectin, i.e., DiI-Ac-LDL incorporation, were also reduced by sirolimus, zotarolimus and everolimus in a concentration-dependent manner (Fig. 4B, D). After culturing cells for 14 days in SMPC medium, nearly all of the cells were positive for α-SMA, SM1 and SM2. Sirolimus, zotarolimus and everolimus significantly reduced the number of α-SMA positive cells (Fig. 4C, E) and the number of SM1 and SM2 positive cells in a concentration-dependent manner (Fig. 4E).
Fig. 4.
Effect of sirolimus, zotarolimus and everolimus on differentiation of progenitor cells into endothelilal- and smooth muscle-like cells. (A) Sirolimus, zotarolimus and everolimus (0.1 nM each) reduced differentiation into CD31-positive endothelial-like cells, compared with control. The reduction was a little weaker in zotarolimus and everolimus, compared with sirolimus. (B) Compared with control, sirolimus, zotarolimus and everolimus (0.1 nM each) reduced differentiation into DiI-Ac-LDL/FITC-BS lectin-double positive cells, shown as orange-colored cells, in which red fluorescence (DiI) and green fluorescence (FITC) were merged. The reduction was a little weaker in zotarolimus and everolimus, compared with sirolimus. (C) Sirolimus, zotarolimus and everolimus (0.01 nM each) reduced differentiation into α-SMA-positive endothelial-like cells, compared with control. (D) Sirolimus, zotarolimus and everolimus significantly inhibited differentiation into endothelial-like cells, concentration-dependently. The inhibition seemed a little less in the everolimus, compared with sirolimus and zotarolimus. (E) Sirolimus, zotarolimus and everolimus significantly inhibited differentiation into smooth muscle-like cells, concentration-dependently. The inhibition seemed a little less in the everolimus, compared with sirolimus.
vWF indicates von Willebrand factor; α-SMA, α-smooth muscle actin.
 sirolimus
sirolimus  zotarolimus
zotarolimus  everolimus
everolimus
*P < 0.05, **P < 0.01.
4. Discussion
In this study, we demonstrated that second generation DES suppressed the mobilization of CD34 + CD133 + CD45low cells, inhibited neointimal formation, and, in the case of EES, were associated with increased number of uncovered stent struts compared to BMS. We reported previously that first generation SES stent suppressed late lumen loss and CD34 + cell mobilization. The results of this study strongly suggest that neointimal suppression utilizing DES with -limus derivatives is biologically linked with impaired wound healing responses (i.e., re-endothelialization and incomplete stent strut coverage) as a consequence of reduced mobilization of stem cell progenitors.
4.1. Mobilization of EPCs and wound healing at stent-induced site of injury
The biological response to stent-induced vascular injury is characterized by a cascade of cellular events, including endothelial denudation, platelet deposition, leukocyte recruitment and accumulation, smooth muscle cell proliferation and migration, and the deposition of extracellular matrix proteins [4]. Utilizing animal models of neointimal formation coupled with bone marrow transplantation, multiple laboratories have reported that bone marrow-derived hematopoietic cells, including EPCs and SMPCs, contribute to both endothelial and smooth muscle cell components of the developing neointima [12]. Human studies of transplanted female donor hearts into male recipients confirm that hematopoietic cells contribute to neointimal formation in allograft vasculopathy [13].
Circulating EPCs are measured clinically using flow cytometry and their number has been associated with cardiovascular disease or its risk factors [14], [15]. After coronary stent implantation, EPCs mobilize from bone marrow and migrate to sites of stent-induced vascular injury, contributing in part to re-endothelialization and ultimately stent strut coverage [16], [17], [18]. We reported previously that CD34 + cells, which include SMPCs as well as EPCs, are mobilized into the circulation after stenting and are positively correlated with an increased risk of restenosis [7]. Specifically, circulating CD34 + cells increased 7–14 days after BMS deployment and was associated with late lumen loss and restenosis; first generation SES suppressed late lumen loss and CD34 + cell mobilization. In the present study utilizing second generation DESs, we observed that the number of circulating CD34 + CD133 + CD45low cells increased on the day 7 after BMS implantation and was suppressed significantly by ZES and EES. In the present study, we used additional flow cytometric markers to identify EPCs as CD34 + CD133 + CD45low cells. EPCs are characterized by the co-expression of stem cell markers CD34 and/or CD133, and endothelial cell markers such as VEGF receptor-1, VEGF receptor-2/kinase insert domain receptor (KDR), CD31, vascular endothelial-cadherin, von Willebrand factor (vWF) or E-selectin [19]. Although CD34 + KDR + cells, CD34 +/CD133 +/KDR + cells or others as well as CD34 + CD133 + CD45low cells have been used as EPCs [9], [10], [18], [20], [21], the accurate characterization of EPCs by flow cytometry alone is limited by the fact that some of the markers used in phenotyping these cells are expressed in non-EPC lineages. Recently, the EPC colony-forming assay, developed to delineate circulating EPC differentiation [14]. Two types of EPC colony forming units are observed. One termed small EPC colony-forming units representing more primitive state with high proliferative activity and the other large with more differentiated vasculogenic properties [22].
One novel aspect of our study is the correlation of early circulating progenitor cell mobilization and stent healing using OCT, the highest resolution intravascular imaging modality. We observed that the change in CD34 + CD133 + CD45low cell number showed significant positive correlation with OCT-quantified mean neointimal area, consistent with the paradigm that bone marrow precursors giving rise to smooth muscle lineage cells contribute to neointimal formation. However, the percentage of uncovered struts by OCT was highest in the second generation EES group, which also suppressed CD34 + CD133 + CD45low cell number to the greatest extent, suggesting that bone marrow precursors favorably influence stent strut coverage and vessel wall healing. Since BMS and second generation DES strut thickness in the present study were similar, our results suggest that everolimus potently suppressed EPC mobilization and neointimal growth at the expense of strut coverage.
4.2. SDF-1α as a surrogate marker for mobilization of progenitor cells
Although the change in CD34 + CD133 + CD45low cell number was predictive of in-stent late lumen loss, quantification of progenitor cell number requires flow cytometric analysis, which is impractical for routine clinical use. We investigated whether serum levels of biomarkers, potentially related to angiogenesis or stem cell mobilization, such as SDF-1α [23], [24], [25], IL-8 [26], [27], and MMP-9 [28], [29], [30], might serve as useful surrogate biomarkers. As a result, levels of SDF-1α, but not IL-8 or MMP-9 correlated with the number of CD34 + CD133 + CD45low cells. SDF-1α, also known as CXCL12, is a cytokine involved in homing, mobilization, and differentiation of circulating progenitor cells in response to tissue ischemia [23], [24]. Binding of SDF-1α to its receptor CXCR4 activates several signaling pathways leading to a variety of biological responses, including secretion of VEGF, which induces angiogenesis and enhances endothelial regeneration [25]. Our results suggest that serum SDF-1α level would be a potential surrogate marker for mobilization of progenitor cells after coronary stent implantation.
4.3. Effects of specific–limus analogues on progenitor cell differentiation
We assessed the effect of everolimus (EES) and zotarolimus (ZES) compared to sirolimus (SES) on progenitor cell differentiation into endothelial and smooth muscle-like lineages. Endothelial cell differentiation was determined using CD31 and vWF markers, smooth muscle cell differentiation with α-SMA, SM1 and SM2. To verify endothelial cell differentiation, DiI-Ac-LDL incorporation was also assessed. Everolimus, zotarolimus, and sirolimus all inhibited mononuclear cell differentiation into endothelial cell and smooth muscle cell-like lineages, indicating a drug class effect on progenitor cell differentiation.
4.4. Potential limitations
The present study has several potential limitations. First, small sample size might be prone to type I error. Second, selection of BMS, ZES, and EES was not randomized. Stent selection was determined by the implanting interventional cardiologist so we cannot rule out confounding factors. Clinical and lesion characteristics, however, were similar among the 3 stent groups. Third, a limited set of flow cytometric markers were utilized for the identification of EPCs as CD34 + CD133 + CD45low cells. Fourth, OCT was performed on a subset of patients. Those with severe in-stent restenosis were excluded due to technical limitations of optimal image acquisition without balloon pre-dilation. Stent strut coverage was therefore assessed in patients without clinical and angiographic restenosis. Finally, in our OCT analysis, we could not account for data clustering, which several lines of evidence recently indicate as a major issue [31].
4.5. Conclusion/clinical implication
Second generation DESs represent a therapeutic advantage over first generation DESs. Randomized clinical trials showed superiority of second generation DESs with respect to efficacy (i.e., target lesion revascularization) and safety (myocardial infarction and stent thrombosis) [32], [33], [34]. Despite superiority in clinical trial settings, these second-generation DESs are associated with persistent late TLF with a 2–4% annual incidence, which is similar to rates observed with BMS and first-generation DESs [1]. Indeed, we have recently demonstrated using OCT and coronary angioscopy that second generation DESs are still associated with incomplete stent strut coverage and imaging features of inflammatory neoatheroma [35].
In the present study, second generation DESs suppressed the mobilization of CD34 + CD133 + CD45low cells, inhibited the differentiation of mononuclear cells into endothelial-like cells, and were associated with increased number of uncovered stent struts, i.e., incomplete stent healing, behaving similarly to our prior observations with first generation DESs. The incomplete stent healing might cause relapse of inflammatory response at late phase after stent implantation, possibly leading to neoatherosclerosis, which we observe more frequently in DESs compared with BMS, although the present study did not focus on this phenomenon. Importantly, we believe that it is reasonable to speculate that DES with bioabsorbable polymers utilizing similar -limus analogues as well as bioresorbable vascular scaffolds eluting -limus analogues will likely be associated with similar effects on progenitor cell mobilization given the observed drug class effect. These data suggest that new approaches are necessary to enhance stent healing.
Although evidence-based efficacy and safety of second generation DESs have been established, post-PCI strategies under the acute phase measurement of cellular response or relating biomarkers, and late phase observation of stent healing using imaging modalities would be also promising as a precision medicine.
Funding source
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (22590794) and by a grant of the Vehicle Racing Commemorative Foundation, Tokyo, Japan (661).
Disclosure
Daniel Simon receives honoraria as course director for Medtronic Vascular.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2017.12.003.
Appendix A. Supplementary data
Supplemental file 1 Detection of circulating CD34 + CD133 + CD45low cells by flow cytomeric analysis. Each measurement consisted of 106 events of all white blood cells. After gating on the CD45-low area, the absolute numbers of CD34 + cells were quantified. To quantify the amount of CD34 + CD133 + CD45low cells, the mononuclear cell fraction was gated and analyzed for the expression of CD34 and CD45 cells. Only the CD34 + CD45low cells were finally investigated for the count of CD133 + cells.
Supplemental file 2 Baseline characteristics in patients treated with bare metal stent, zotarolimus-eluting stent or everolimus-eluting stent
References
- 1.Kereiakes D.J. The TWENTE trial in perspective: stents and stent trials in evolution. JAMA Cardiol. 2017;2:235–237. doi: 10.1001/jamacardio.2016.5208. [DOI] [PubMed] [Google Scholar]
- 2.Inoue T., Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ. J. 2009;73:615–621. doi: 10.1253/circj.cj-09-0059. [DOI] [PubMed] [Google Scholar]
- 3.Inoue T., Croce K., Morooka T., Sakuma M., Node K., Simon D.I. Vascular inflammation and repair: implication for reendothelialization, restenosis, and stent thrombosis. J. Am. Coll. Cardiol. Intv. 2011;4:1057–1066. doi: 10.1016/j.jcin.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa M.A., Simon D.I. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Murohara T., Ikeda H., Duan J., Shintani S., Sasaki K., Eguchi H., Onituka I., Matsui K., Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue T., Sata M., Hikichi Y., Sohma R., Fukuda D., Uchida T., Shimizu M., Komoda H., Node K. Mobilization of CD34-positive bone marrow-derived stem cells after coronary stent implantation: impact on restenosis. Circulation. 2007;115:553–561. doi: 10.1161/CIRCULATIONAHA.106.621714. [DOI] [PubMed] [Google Scholar]
- 8.Umemura T., Soga J., Hidaka T., Takemoto H., Nakamura S., Jitsuki D., Nishioka K., Goto C., Yoshizumi M., Chayama K., Higashi Y. Aging and hypertension are independent risk factors for reduced number of circulating endothelial progenitor cells. Am. J. Hypertens. 2008;21:1203–1209. doi: 10.1038/ajh.2008.278. [DOI] [PubMed] [Google Scholar]
- 9.Arao K., Yasu T., Ohmura N., Tsukamoto Y., Murata M., Kubo N., Umemoto T., Ikeda N., Ako J., Ishikawa S., Kawakami M., Momomura S. Circulating CD34 +/CD133 + progenitor cells in patients with stable angina pectoris undergoing percutaneous coronary interventions. Circ. J. 2010;74:1929–1935. doi: 10.1253/circj.cj-09-0917. [DOI] [PubMed] [Google Scholar]
- 10.Kim B.K., Hong M.K., Shin D.H., Kim J.S., Ko Y.G., Choi D., Jang Y. Optical coherence tomography analysis of strut coverage in biolimus- and sirolimus-eluting stents: 3-month and 12 month serial follow-up. Int. J. Cardiol. 2013;168:4617–4623. doi: 10.1016/j.ijcard.2013.07.160. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda D., Sata M., Tanaka K., Nagai R. Potent inhibitory effect of sirolimus on circulating vascular progenitor cells. Circulation. 2005;111:926–931. doi: 10.1161/01.CIR.0000155612.47040.17. [DOI] [PubMed] [Google Scholar]
- 12.Sata M., Saiura A., Kunisato A., Tojo A., Okada S., Tokuhisa T., Hirai H., Makuuchi M., Hirata Y., Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat. Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 13.Simper D., Wang S., Deb A., Holmes D., McGregor C., Frantz R., Kushwaha S.S., Caplice N.M. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation. 2003;108:143–149. doi: 10.1161/01.CIR.0000081703.34526.5D. [DOI] [PubMed] [Google Scholar]
- 14.Hill J.M., Zalos G., Halcox J.P., Schenke W.H., Waclawiw M.A., Quyyumi A.A., Finkel T. Circulating endothelial progenitor cells, vascular function and cardiovascular risk. N. Engl. J. Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 15.Werner N., Kosiol S., Schiegl T., Ahlers P., Walenta K., Link A., Böhm M., Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 16.Kong D., Melo L.G., Gnecchi M., Zhang L., Mostoslavsky G., Liew C.C., Pratt R.E., Dzau V.J. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation. 2004;110:2039–2046. doi: 10.1161/01.CIR.0000143161.01901.BD. [DOI] [PubMed] [Google Scholar]
- 17.Werner N., Priller J., Laufs U., Endres M., Böhm M., Dirnagl U., Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler. Thromb. Vasc. Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 18.Gao M., Yao Q., Liu Y., Sun F., Ma Y., Sun G. Association between mobilization of circulating endothelial progenitor cells and time or degree of injury from angioplasty in patients with exertional angina: a prospective study. Exp. Ther. Med. 2015;10:809–815. doi: 10.3892/etm.2015.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padfield G.J., Newby D.E., Mills N.L. Understanding the role of endothelial progenitor cells in percutaneous coronary intervention. J. Am. Coll. Cardiol. 2010;55:1553–1565. doi: 10.1016/j.jacc.2009.10.070. [DOI] [PubMed] [Google Scholar]
- 20.Vasa M., Fichtlscherer S., Aicher A., Adler K., Urbich C., Martin H., Zeiher A.M., Dimmeler S. Number and migratory activity of circulating endothelila progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001;89:1e–7e. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 21.Grisar J., Aletaha D., Steiner C.W., Kapral T., Steiner S., Seidinger D., Weigel G., Schwarzinger I., Wolozcszuk W., Steiger G., Smolen J.S. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111:204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 22.Masuda H., Alev C., Akimaru H., Ito R., Shizuno T., Kobori M., Horii M., Ishihara T., Isobe K., Isozaki M., Itoh J., Itoh Y., Okada K., McIntyre B.A., Kato S., Asahara T. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circ. Res. 2011;109:20–37. doi: 10.1161/CIRCRESAHA.110.231837. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi J., Kusano K.F., Masuo O., Kawamoto A., Silver M., Murasawa S., Bosch-Marce M., Masuda H., Losordo D.W., Isner J.M., Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 24.De Falco E., Porcelli D., Torella A.R., Straino S., Iachininoto M.G., Orlandi A., Truffa S., Biglioli P., Napolitano M., Capogrossi M.C., Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 25.Liang Z., Brooks J., Willard M., Liang K., Yoon Y., Kang S., Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem. Biophys. Res. Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin D., Galisteo R., Gutkind J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009;284:6038–6042. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li A., Varney M.L., Valasek J., Godfrey M., Dave B.J., Singh R.K. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 28.Starckx S., Van den Steen P.E., Wuyts A., Van Damme J., Opdenakker G. Neutrophil geratinase B and chemokines in leukocytosis and stem cell mobilization. Leuk. Lymphoma. 2002;43:233–241. doi: 10.1080/10428190290005982. [DOI] [PubMed] [Google Scholar]
- 29.Pelus L.M., Bian H., King A.G., Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GROβ/CXCL2 and GROβT/CXCL2Δ4. Blood. 2004;103:110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 30.Inoue T., Taguchi I., Abe S., Toyoda S., Nakajima K., Sakuma M., Node K. Activation of matrix metalloproteinase-9 is associated with mobilization of bone marrow-derived cells after coronary stent implantation. Int. J. Cardiol. 2011;152:332–336. doi: 10.1016/j.ijcard.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 31.Davlouros P.A., Mavronasiou E., Xanthopoulou I., Karantalis V., Tsigkas G., Hahalis G., Alexopoulos D. An optical coherence tomography study of two new generation stents with biodegradable polymer carrier, eluting paclitaxel vs. biolimus-A9. Int. J. Cardiol. 2012;157:341–346. doi: 10.1016/j.ijcard.2010.12.072. [DOI] [PubMed] [Google Scholar]
- 32.Kandzari D.E., Mauri L., Popma J.J., Turco M.A., Gurbel P.A., Fitzgerald P.J., Leon M.B. Late-term clinical outcomes with zotarolimus- and sirolimus-eluting stents: 5-year follow-up of the ENDEAVOR III (a randomized controlled trial of the Medtronic Endeavor drug [ABT-578] eluting coronary stent system versus the Cypher sirolimus-eluting coronary stent system in de novo native coronary artery lesions) J. Am. Coll. Cardiol. Intv. 2011;4:543–550. doi: 10.1016/j.jcin.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Gada H., Kirtane A.J., Newman W., Sanz M., Hermiller J.B., Mahaffey K.W., Cutlip D.E., Sudhir K., Hou L., Koo K., Stone G.W. 5-Year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions) J. Am. Coll. Cardiol. Intv. 2013;6:1263–1266. doi: 10.1016/j.jcin.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Smits P.C., Vlachojannis G.J., McFadden E.P., Royaards K.J., Wassing J., Joesoef K.S., van Mieghem C., van de Ent M. Final 5-year follow-up of a randomized controlled trial of Everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice: the COMPARE trial (a trial of everolimus-eluting stents and paclitaxel stents for coronary revascularization in daily practice) J. Am. Coll. Cardiol. Intv. 2015;8:1157–1165. doi: 10.1016/j.jcin.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Masawa T., Abe S., Toyoda S., Sakuma M., Nasuno T., Kageyama M., Tokura M., Koizumi S., Taguchi I., Inoue T. Comparison of the performance of zotarolimus- and everolimus-eluting stents by optical coherence tomography and coronary angioscopy. Heart Vessel. 2016;31:1230–1238. doi: 10.1007/s00380-015-0728-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental file 1 Detection of circulating CD34 + CD133 + CD45low cells by flow cytomeric analysis. Each measurement consisted of 106 events of all white blood cells. After gating on the CD45-low area, the absolute numbers of CD34 + cells were quantified. To quantify the amount of CD34 + CD133 + CD45low cells, the mononuclear cell fraction was gated and analyzed for the expression of CD34 and CD45 cells. Only the CD34 + CD45low cells were finally investigated for the count of CD133 + cells.
Supplemental file 2 Baseline characteristics in patients treated with bare metal stent, zotarolimus-eluting stent or everolimus-eluting stent