Abstract
Insufficient hydrogen sulfide (H2S) has been implicated in Type 2 diabetic mellitus (T2DM) and hyperhomocysteinemia (HHcy)-related cardiovascular complications. We investigated the role of H2S in T2DM and HHcy-induced endothelial dysfunction in small mesenteric artery (SMA) of db/db mice fed a high methionine (HM) diet. HM diet (8 weeks) induced HHcy in both T2DM db/db mice and non-diabetic db/+ mice (total plasma Hcy: 48.4 and 31.3 µM, respectively), and aggravated the impaired endothelium-derived hyperpolarization factor (EDHF)-induced endothelium-dependent relaxation to acetylcholine (ACh), determined by the presence of eNOS inhibitor N(ω)-nitro-L-arginine methyl ester (L-NAME) and prostacyclin (PGI2) inhibitor indomethacin (INDO), in SMA from db/db mice but not that from db/+ mice. A non-selective Ca2+-active potassium channel (KCa) opener NS309 rescued T2DM/HHcy-impaired EDHF-mediated vascular relaxation to ACh. EDHF-induced relaxation to ACh was inhibited by a non-selective KCa blocker TEA and intermediate-conductance KCa blocker (IKCa) Tram-34, but not by small-conductance KCa (SKCa) blocker Apamin. HHcy potentiated the reduction of free sulfide, H2S and cystathionine γ-lyase protein, which converts L-cysteine to H2S, in SMA of db/db mice. Importantly, a stable H2S donor DATS diminished the enhanced O2- production in SMAs and lung endothelial cells of T2DM/HHcy mice. Antioxidant PEG-SOD and DATS improved T2DM/HHcy impaired relaxation to ACh. Moreover, HHcy increased hyperglycemia-induced IKCa tyrosine nitration in human micro-vascular endothelial cells. EDHF-induced vascular relaxation to L-cysteine was not altered, whereas such relaxation to NaHS was potentiated by HHcy in SMA of db/db mice which was abolished by ATP-sensitive potassium channel blocker Glycolamide but not by KCa blockers.
Conclusions
Intermediate HHcy potentiated H2S reduction via CSE-downregulation in microvasculature of T2DM mice. H2S is justified as an EDHF. Insufficient H2S impaired EDHF-induced vascular relaxation via oxidative stress and IKCa inactivation in T2DM/HHcy mice. H2S therapy may be beneficial for prevention and treatment of micro-vascular complications in patients with T2DM and HHcy.
Keywords: Hydrogen sulfide, Endothelial dysfunction, Micro-vasculature, T2DM, Calcium-activated potassium channel (KCa)
1. Introduction
Diabetes is the most prevalent metabolic disorders and is estimated to affect 400 million or 4.4% of population worldwide in the next 20 years [1], [2]. Type 2 diabetic mellitus (T2DM) is the most common form of diabetes. In adults, about 90–95% of all diagnosed cases of diabetes are T2DM. In T2DM, the micro-vascular dysfunction encompasses long-term complications, such as retinopathy, nephropathy and neuropathy which impose a major public health burden.
Endothelium plays a key role in the control of vascular homeostasis by releasing vasodilator substances, including nitric oxide (NO), prostacyclin (PGI2) and endothelium-derived hyperpolarizing factor (EDHF), and vasoconstrictor substances, such as angiotensin II, endothelin-1, thromboxane A2, and prostaglandin H2, in response to pathophysiological stimulation [3], [4]. It is generally accepted that NO predominantly controls relaxation of macro-vasculature, whereas EDHF primarily controls relaxation of micro-vasculature and becomes more important when vessel diameter decreases [5], [6], [7]
EDHF is proposed to be a substance and/or electrical signal that is synthesized or generated in and released from endothelium under pathophysiological stimuli. EDHF action is to hyperpolarize vascular smooth muscle cells (VSMCs), resulting in vascular relaxation [8], [9]. Vascular relaxation to acetylcholine (ACh) in the presence of a combination of eNOS inhibitor N(ω)-nitro-L-arginine methyl ester (L-NAME) and PGI2 inhibitor indomethacin (INDO) are used to determine EDHF-induced endothelium-dependent vascular relaxation. Although extensive studied, the nature of EDHF remains unclear. Many factors have been suggested to be EDHF which induces endothelium-dependent vascular hyperpolarization and vascular relaxation in the presence of L-NAME+INDO, such as epoxyeicosatrienoic acids (EETs), H2O2, gap junctions [10]. Very recently, study demonstrated that hydrogen sulfide (H2S) may be one of major EDHF regulating endothelial function in micro-vasculature [11]. Numerous studies supported the concept that EDHF-mediated vascular relaxation is elicited by the opening of Ca2+-activated potassium channels (KCa) in endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) [12]. The KCa family consists of small conductance KCa (SKCa, including SKCa1, SKCa2, SKCa3), intermediate conductance KCa (IKCa) and large conductance KCa (BKCa) subtypes. SKCa and IKCa predominantly expressed in ECs, whereas BKCa is preferentially located in VSMCs [13], [14]
Endothelial dysfunction (ED) is an early event in the development of vascular abnormalities prior to any visible morphological changes and is characterized by the impairment of endothelium-dependent vasodilatation. Many factors are related to ED, including diabetes, hypertension, smoke, obese and elevated plasma homocysteine level called hyperhomocysteinemia (HHcy). We have reported that HHcy impairs endothelium-dependent vascular relaxation to ACh in the presence of L-NAME + INDO in mouse micro-vasculature (small mesenteric artery, SMA) via oxidation/nitration of SKCa/IKCa [13]. Recently, we also observed that HHcy potentiates hyperglycemia-induced ED in mouse aorta via activation of µ-calpain [15]. HHcy appeared to be a stronger risk factor for cardiovascular disease (CVD) in patients with T2DM [16]. However, whether HHcy impairs endothelial function in micro-vasculature in T2DM and the underlying mechanisms have not been studied.
H2S is an endogenously produced gasotransmitter that is critical for the regulation of cardiovascular homeostasis.[17], [18], [19]. In mammalian species, H2S is produced enzymatically by cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (MPST), of which CSE is primarily expressed in the cardiovascular system, including cardiomyocytes, ECs and VSMCs [20]. It was reported that H2S production was reduced in mice with HHcy [21] and T2DM [22], [23]. A role of H2S in the pathogenesis of T2DM has been suggested, as circulating levels of H2S were found to be inversely proportional to cardiovascular complications in T2DM animals [22], [24] The negative association between T2DM and H2S is also reinforced by decreased plasma H2S levels in patients with T2DM [23], [25]. Recently, we provided strong evidence that H2S reduction is related to T2DM-induced bone marrow cell (BMC) dysfunction and impaired ischemic tissue repair [26]. Restoration of H2S production in T2DM mice improves reparative property of BMCs [26]. In this study, we examined the effects and underlying mechanisms of HHcy on T2DM-impaired endothelial function in SMA of db/db mice.
2. Materials and methods
2.1. Experimental animals and sample collections
HHcy was induced in male T2DM db/db mice (Jackson Laboratory) by feeding 8-week-old mice with our newly designed high-methionine (HM) diet (methionine, 2%, 07794, Harlan Teklad), in which folic acid and B vitamins are reduced to the sufficient basal levels,[15] for 8 weeks. Diet contains equal amount of folic acid and B vitamins but less methionine ((CT, 0.37%), 07793, Harlan Teklad) was used as control diet (CT). Non-T2DM db/+ mice were served as controls. All animals received humane care in compliance with institutional guideline and the “Guide for the Care and Use of Laboratory Animals” prepared by the “Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council”. At the end of experiments, 24 h metabolic parameters including water intake and urine excretion were collected in metabolic cages; body weight, heart weight and kidneys weight were recorded. Blood was collected by cardiac puncture. Lungs were perfused with PBS and digested with collagenase II for mouse lung endothelial cells (MLECs) isolation. Second order of SMAs were dissected for vascular reactivity assessment.
2.2. Plasma total homocysteine (t-Hcy) concentrations
t-Hcy levels were analyzed using a Biochrom 30 amino acid analyzer (Cambridge, UK) as we previously described [27], [28].
2.3. Oral glucose tolerance test (OGTT) and blood glucose
OGTT and blood glucose were analyzed as previous describe [15].
2.4. Free sulfide levels in small mesenteric arteries (SMAs)
Free sulfide in SMAs was measured by RP-HPLC after derivatization with excess monobromobimane (MBB) as stable products sulfide-dibimane (SDB) as previously described [29].
2.5. H2S production in SMAs by fluorescent probe
H2S levels were determined in fresh SMAs using a stable H2S fluorescent probe as previously described [26].
2.6. SMAs preparation for vessel reactive assessment
Mice were sacrificed under anesthesia. Mesenteric bed was removed and prepared for vascular reactive assessment as we previously described [13].
2.6.1. Vascular contractile responses
After an equilibration period, SMA rings were exposed to potassium chloride (KCl, 120 mM). Following a second round of washing and equilibration with Krebs, vascular contractile responses to cumulative additions of phenylephrine (PE, 10 nM to 33 µM) were determined.
2.6.2. Endothelium-dependent and -independent vascular relaxation responses
The presence of intact endothelium in the vascular preparations was confirmed as described previously [30]. Endothelium-dependent relaxation responses to cumulative concentrations of ACh (10 nM to 33 µM) and endothelium-independent relaxation responses to sodium nitroprusside (SNP, 1 nM to 10 µM) in rings pre-contracted with PE (1 µM) were determined.
2.6.3. EDHF-induced vascular relaxation to ACh
EDHF-induced vascular relaxation responses to ACh were determined in the presence of NG-nitro-L-arginine methyl ester hydrochloride (L-NAME, 100 µM, a NOS inhibitor that blocks NO production) plus indomethacin (INDO, 10 µM, a non-selective cyclooxygenase (COX) inhibitor that blocks the formation of PGI2) for 30 min. To dissect the mechanisms underlying EDHF-induced relaxation, SMAs were incubated with either a non-selective KCa inhibitor TEA (1 mM), or a selective SKCa inhibitor Apamin (1 µM) and/or a selective IKCa inhibitor Tram34 (1 µM) for 30 min in the presence of INDO and L-NAME, respectively. Further, NS309 (1 µM, 30 min), IKCa/SKCa opener, was used to determine the role of IKCa and SKCa in T2DM (db/db mice)- and T2DM/HHcy (db/db mice fed with HM diet)-impaired EDHF-induced endothelium-dependent vascular relaxation in the presence of L-NAME+INDO as we described previously [31].
The role of oxidative stress in T2DM/HHcy-impaired EDHF-induced relaxation was examined by pre-incubating the SMAs with antioxidants polyethylene glycol (PEG)-superoxide dismutase (PEG-SOD, 150 U/ml) for 1 h before testing vascular relaxation responses to ACh in the presence of L-NAME+INDO. To examine the role of insufficient H2S production on T2DM/HHcy-impaired EDHF-induced relaxation to ACh, SMAs were treated with a stable H2S donor DATS (5 µM) for 30 min before testing vascular relaxation responses to ACh in the presence of L-NAME+INDO.
2.6.4. EDHF-induced vascular relaxation by H2S or L-cysteine
EDHF-induced vascular relaxation in response to NaHS (10–60 µM) or L-cysteine (10 µM) were determined in the presence of L-NAME (100 µM) and INDO (10 µM) for 30 min. To dissect the role of KCa, the SMAs were incubated with either TEA (1 mM), Apamin (1 µM) or Tram34 (1 µM) for 30 min in the presence of INDO and L-NAME, respectively.
Moreover, to assess the role of ATP-activated potassium channel (KATP) in T2DM- and T2DM/HHcy-impaired ED, vascular relaxation response to H2S was examined in the presence of KATP inhibitor glybenclamide (GLB, 10 µM, 30 min), L-NAME and INDO.
2.7. Isolation of mouse lung endothelial cells (MLECs)
MLECs were isolated and identified as described previously [15], [32]
2.8. Superoxide production in SMAs and MLECs
Superoxide generation in fresh SMA segments and MLECs were measured by staining with dihydroethidium (DHE) as described previously [13], [15]
2.9. IKCa tyrosine nitration in SMAs by immunohistochemistry
Tyrosine nitration is a marker of oxidative stress. To explore whether IKCa is oxidized under T2DM/HHcy condition in SMAs, we examined the co-localization of IKCa and 3-nitrotyrosine (3-NT) as described previously [13].
2.10. Cell culture
Human cardiac micro-vascular endothelial cells (HCMVECs, CC7030, Lonza Inc, Allendale, USA) were cultured in EGM®− 2 MV medium [13], [26]. HCMVECs were treated with D-glucose (25 mM) with or without DL-homocysteine (DL-Hcy, 500 mM) for 48 h [15], [26]
2.11. Western blot and immunoprecipitation
IKCa oxidation was determined in HCMVECs treated with D-glucose with or without DL-Hcy for 48 h by immunoprecipitation of 200 µg protein with a mouse monoclonal antibody against 3-NT (SC32757, Santa Cruz) followed by blotting with antibodies against IKCa (H-120, SC32949, Santa Cruz).
2.12. Chemicals
All chemicals, if not specified above, were purchased from Sigma-Aldrich (St. Louis, MO).
2.13. Statistics
In vitro studies were repeated at least 3 times with triplicates/group/experiment. Results are expressed as the mean ± SEM. For statistical comparison of single parameters, independent t-test was used for two groups (unpaired) and one way ANOVA (Tukey) with adjustment was performed for multiple groups. A probability value p < 0.05 was considered to be significant.
3. Results
3.1. Body, plasma total Hcy and blood glucose levels, glucose tolerance
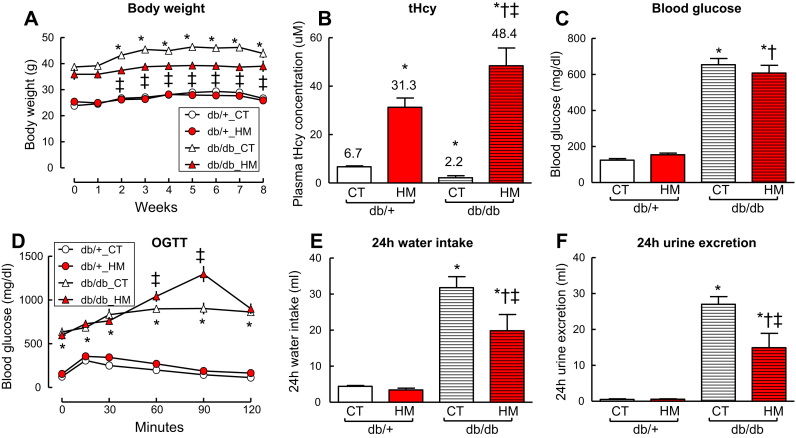
Body weight was reduced in db/db mice with HHcy (Fig. 1A, p < 0.05). Plasma total Hcy (t-Hcy) level was decreased in diabetic db/db mice compared with that in non-diabetic db/+ (2.15 ± 0.88 and 6.74 ± 0.36 μM, respectively, Fig. 1B, p < 0.05). The HM diet elevated t-Hcy level to 31.3 ± 3.8 μM in db/+ mice and 48.4 ± 3.4 μM in db/db mice (Fig. 1B, p < 0.05). Blood glucose level was significant higher in db/db mice (654 ± 35.1 mg/dl) compared with that in non-diabetic db/+ mice (124 ± 8.5 mg/dl) (Fig. 1C). HHcy did not change blood glucose levels in both db/+ mice and db/db mice, but aggravated glucose intolerance in db/db mice (Fig. 1C and D, p < 0.05).
Fig. 1.
HHcy aggravated glucose intolerance and reduced body weight of db/db mice. A. Body weight. B. Total plasma homocysteine (tHcy) levels. C. Blood glucose levels. D. Oral glucose tolerance test (OGTT). E. 24 h water intake. F. 24 h urine excretion. At the age of 8-week, db/+ and db/db mice were fed with a high methionine diet (HM, methionine: 2% w/w) for 8 weeks. db/+ and db/db mice fed with a control diet (CT, methionine: 0.37% w/w) served as controls. n = 5–10, * p < 0.05 vs db/+ mice on CT diet (db/+_CT); †p < 0.05 vs db/+ mice on HM diet; ‡p < 0.05 vs db/db mice on CT diet (db/db_CT). HHcy, hyperhomocysteinemia.
3.2. Heart and kidneys weights, and 24 h metabolic parameters
Heart weights were not changed by either T2DM, HHcy, or the combination (Supplementary Fig. A to C). HM diet-induced intermediate HHcy, defined as plasma tHcy level between 30 and 100 µM [33], [34], reduced 24 h water intake and urine excretion, but increased kidneys weights in db/db mice (Fig. 1E to F and Supplementary Fig. D to F, p < 0.05). Whether HHcy aggravated renal damage in db/db mice needs further investigation.
3.3. HHcy had no significant effect on SMA contractile response to KCl
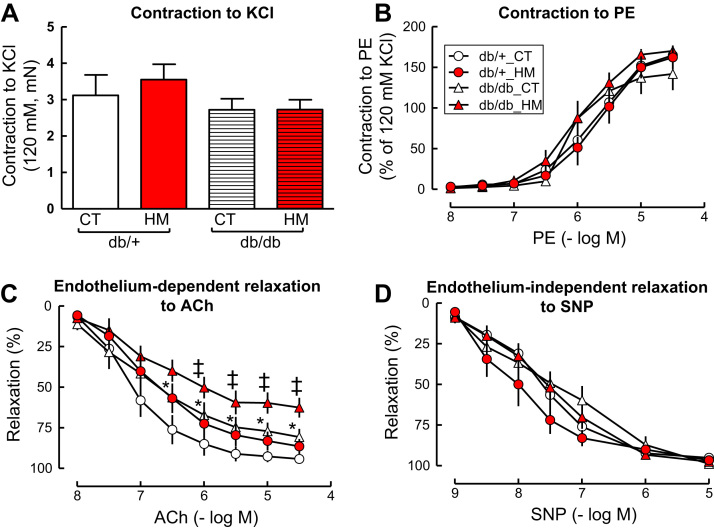
HHcy did not change vascular contractile response to the single dose of KCl in SMA from either db/db or db/+ mice (Fig. 2A). The db/db mice had a trend of reduced contraction to KCl compared to such response in the db/+ mice when fed a control diet. Intermediate HHcy had no significant effect on vascular contraction to KCl compared to their parallel control group on control diet. Contractile response to PE was not changed by either T2DM and/or HHcy (Fig. 2B).
Fig. 2.
HHcy aggravated T2DM-impaired endothelium-dependent vascular relaxation to ACh in SMA of db/db mice. A and B. Vascular contractile response to potassium chloride (KCl, A) and phenylephrine (PE, B). C. Endothelium-dependent vascular relaxation to acetylcholine (ACh). D. Endothelium-independent vascular relaxation to sodium nitroprusside (SNP). Small mesenteric arterial rings were pre-contracted with phenylephrine (1 µM) and examined for relaxation response to cumulative additions of ACh or SNP. n = 5–10, *p < 0.05 vs db/+ mice on CT diet (db/+ _CT); ‡p < 0.05 vs db/db mice on CT diet (db/db_CT). SMAs, small mesenteric arteries.
3.4. HHcy potentiated T2DM-impaired endothelium-dependent vascular relaxation to ACh
Endothelium-dependent vascular relaxation responses to accumulative concentrations of ACh were impaired in SMAs of db/db mice compared with that of db/+ mice (Fig. 2C, maximal relaxation: 80.8 ± 5.0 and 94.3 ± 2.7, respectively, p < 0.05). HM diet-induced intermediate HHcy had a trend of impairing vascular relaxation to ACh in db/+ mice and significantly aggravated T2DM-impaired relaxation to ACh in db/db mice (Fig. 2C, maximal relaxation: 62.7 ± 6.1, p < 0.05). Neither T2DM nor HHcy alone or a combination of T2DM and HHcy affected endothelium-independent vascular relaxation to SNP in SMAs (Fig. 2D).
3.5. HHcy aggravated T2DM-impaired vascular relaxation to ACh in the presence of eNOS and COX inhibitors
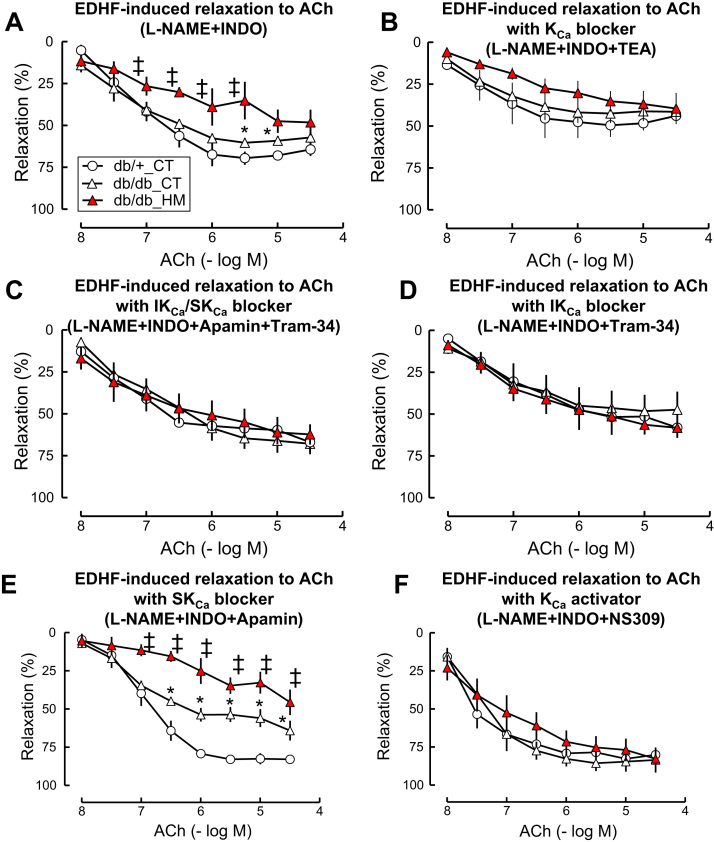
It is generally accepted that EDHF predominantly controls vascular relaxation in micro-vasculature [5], [6], [7]. Because that HM diet-induced intermediate HHcy had no effect either on endothelium-dependent or endothelium-independent relaxation in SMAs of db/+ mice, we examined the effect of HHcy on T2DM/HHcy-impaired EDHF-induced relaxation to ACh in the SMAs of db/db mice. We found that EDHF-induced relaxation to ACh was impaired in db/db mice compared to that in db/+ mice (maximal relaxation: 60.4 ± 2.1 and 69.6 ± 3.9, respectively, Fig. 3A, p < 0.05), and that HM diet-induced HHcy further aggravated T2DM-impaired EDHF-induced relaxation to ACh in db/db mice (maximal relaxation: 48.3 ± 7.7% (p < 0.05)).
Fig. 3.
HHcy aggravated impaired EDHF-induced relaxation to ACh in SMA of db/db mice via IKCainhibition. EDHF-induced relaxation was determined by pretreated the SMA with NOS inhibitor L-NAME (100 µM) and COX inhibitor (INDO, 10 µM) and indicated inhibitors, precontracted with phenylephrine (1 µM), and examined for relaxation to acetylcholine (ACh). A. EDHF-induced relaxation to ACh. B. EDHF-induced relaxation in the presence of SKCa/IkCa blocker TEA (1 mM). C. EDHF-induced relaxation in the presence of SKCa blocker Apamin (1 μM) and IKc blocker Tram-34 (1 μM). D. EDHF-induced relaxation in the presence of IkCa blocker Tram-34. E. EDHF-induced relaxation in the presence of SKca blocker Apamin. F. EDHF-induced relaxation in the presence of SKCa/IKCa activator NS309 (10 μM). n = 5–10. *p < 0.05 vs db/+ mice on CT diet (db/+_CT); ‡p < 0.05 vs db/db mice on CT diet (db/db_CT). COX, cyclooxygenase; HHcy, hyperhomocysteinemia; INDO, indomethacin; L-NAME, NG-Nitro-L-arginine methyl ester; NOS, NO synthase.
3.6. HHcy worsen T2DM-impaired EDHF-induced vascular relaxation to ACh via IKCa inactivation
Because EDHF induces endothelium-dependent vascular relaxation to ACh is mainly via KCa opening in EC [12], we tested the effect of different KCa blockers on EDHF-induced vascular relaxation to ACh. We found a non-selective KCa blocker TEA completely diminished either T2DM- or T2DM pluc HHcy-impaired EDHF-induced vascular relaxation to ACh (Fig. 3B). IKCa blocker Tram-34 or the combination of IKCa blocker Tram-34 and SKCa blocker Apamine but not Apamine alone also diminished T2DM- and T2DM/HHcy-impaired EDHF-induced relaxation to ACh mice (Fig. 3C to E) in SMAs. Moreover, we found that NS309, a non-selective activator of KCa, completely restored EDHF-induced relaxation responses to ACh in db/db and db/db/HHcy mice (Fig. 3F, p < 0.05), suggesting that inactivation of IKCa, but not SKCa, plays a major role in HHcy-potentiated diabetes-impaired EDHF-induced vascular relaxation in db/db and db/db/HHcy mice.
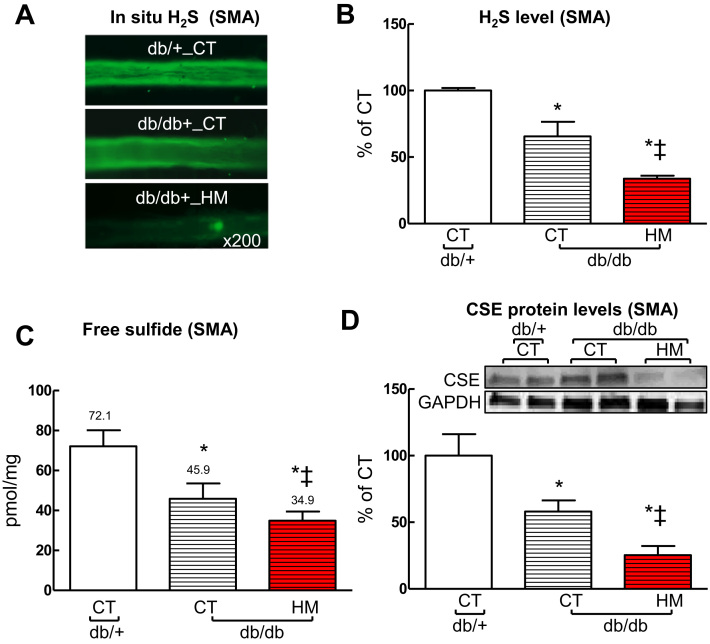
3.7. HHcy aggravated T2DM-induced H2S deficiency and CSE protein reduction in SMAs of db/db mice
We examined H2S production in SMA with two different methodologies. Using a florescent probe, we found that H2S production was decreased to 65% in db/db mice compared with that in db/+ mice (p < 0.05) and further reduced to 33.7% by the addition of HHcy (Fig. 4A and B, p < 0.05). Using RP-HPLC detection, free sulfide levels were reduced to 63.7% in SMAs of db/db mice (45.9 pM/mg) which were further reduced to 48.4% by the addition of HHcy (34.9 pM/mg) compared to that in db/+ mice (72.1 pM/mg), respectively (Fig. 4C, p < 0.05). We and others recently reported that cystathionine β-lyase (CSE) is a dominant H2S-synthesizing enzyme in cardiovascular system [19], [35], [36]. Thus we examined the effect of T2DM alone and a combination of T2DM and HHcy on CSE level in SMA. We found that CSE protein levels were decreased to 58% (p < 0.05) in SMAs of db/db mice which were further reduced to 25.4% (p < 0.05) by HHcy (Fig. 4D, p < 0.05).
Fig. 4.
HHcy potentiated H2S deficiency and CSE downregulation in SMA of db/db mice. A. Reprehensive images of intracellular H2S production in SMAs stained with fluorescent probe sulfidefluor 7AM (SF-7AM, 25 µM, for 30 min). B. Quantification of Intracellular H2S production in SMAs. C. Free sulfide levels in SMAs measured by reversed phase HPLC. D. CSE protein levels in SMAs. n = 3–5 *p < 0.05 vs db/+ mice on CT diet (db/+_CT); ‡p < 0.05 vs db/db mice on CT diet (db/db_CT). CSE, cystathionine γ-lyase.
3.8. H2S donor DATS rescued T2DM/HHcy-induced oxidative stress in SMAs and mouse lung endothelial cells (MLECs)
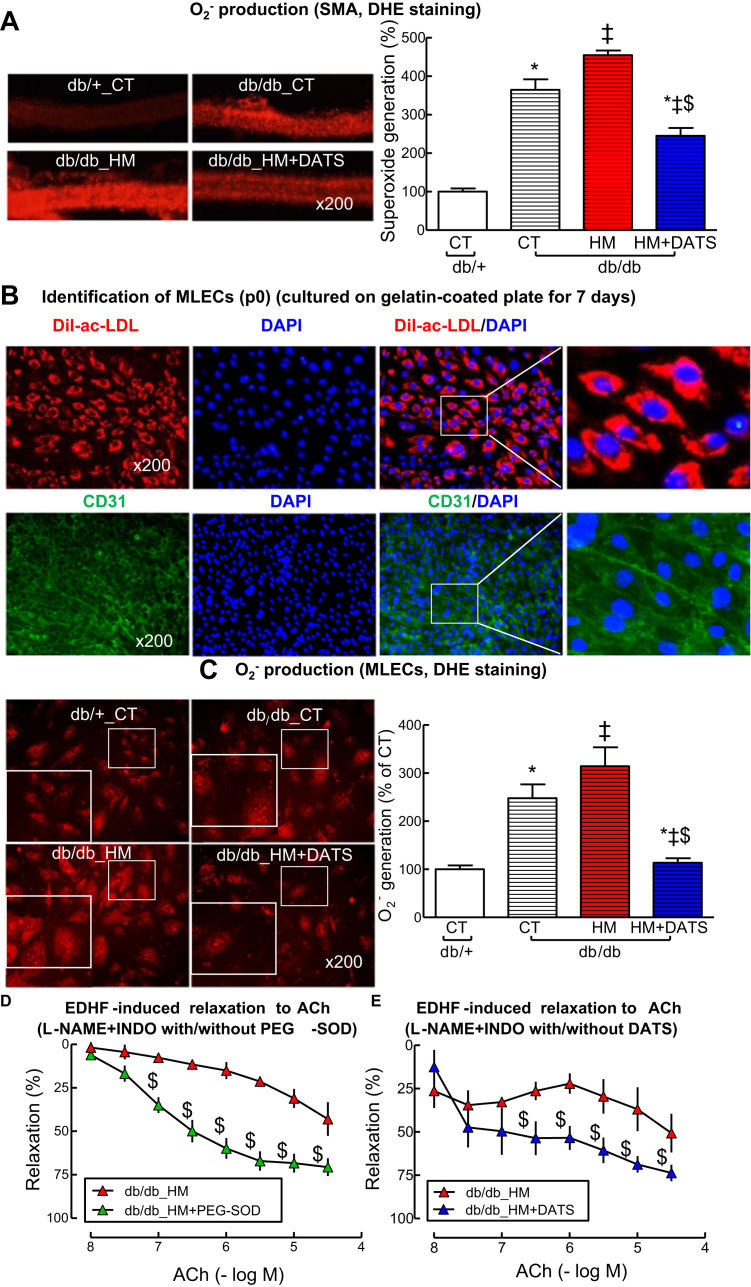
Insufficient H2S has been suggested to be one of the potential sources of oxidative stress in diseases conditions [37]. Here, we examined in situ superoxide (O2-) generation in SMAs, and found that O2- was increased in db/db SMAs by 365%, further elevated by HHcy to 455% (Fig. 5A, p < 0.05). To explore the role of insufficient H2S on T2DM/HHcy-induced oxidative stress, we applied DATS, a stable H2S donor, and found that DATS effectively reduced O2- levels to 245% in SMAs from db/db/HHcy mice (Fig. 5A, p < 0.05). O2- production was increased to 248% in MLECs from db/db and further elevated by HHcy to 314% (Fig. 5C, p < 0.05) which was completely reversed by DATS to 113% (Fig. 5C, p < 0.05). Finally, we found that antioxidant PEG-SOD and DATS improved EDHF-induced relaxation to ACh in SMAs of db/db HHcy mice (maximal relaxation: 43.1–70.1% and 50.7–73.8%, respectively, Fig. 5D and E, p < 0.05). These data suggest that H2S deficiency is, at least in part, a source of oxidative stress in microvasculature and responsible for T2DM/HHcy-impaired EDHF-induced relaxation.
Fig. 5.
H2S donor DATS rescued HHcy-induced oxidative stress and -impaired EDHF-induced relaxation to ACh in SMA of db/db mice with HHcy. A. Representative images (Left panel) and quantifications (Right panel) of in situ O2- production in SMAs stained with DHE. The SMAs from db/db mice fed with HM diet were treated with or without DATS (5 µM) for 30 min before DHE staining. B. Identification of primary mouse lung endothelial cells (MLECs) isolated from lung of db/+ and db/db by Dil-ac-LDL uptake and CD31 staining. C. Representative images (Left panel) and quantifications of O2- production (Right panel) in mouse MLECs (DHE staining). The MLECs from db/db mice fed with HM diet were treated with or without DATS (5 µM) for 30 min. D. EDHF-induced relaxation to ACh in SMAs of db/db mice fed with HM diet in the presence of L-NAME+INDO (30 min) with or without PEG-SOD (150 U/ml) for 1 h. E EDHF-induced relaxation to ACh in SMAs of db/db mice fed with HM diet in the presence or L-NAME+INDO with or without DATS (5 µM) for 30 min. Then the SMAs were contracted with phenylephrine (1 µM), and examined for relaxation to ACh. n = 3–5. *p < 0.05 vs db/+ mice on CT diet (db/+_CT); ‡p < 0.05 vs db/db mice on CT diet (db/db_CT); $p < 0.05 vs db/db mice on HM diet (db/db_HM). DATS, diallyl trisulfide; DHE, dihydroethidium. PEG-SOD, polyethylene glycol superoxide dismutase.
3.9. HHcy potentiated IKCa oxidation/tyrosine nitration in micro-vascular endothelial cells
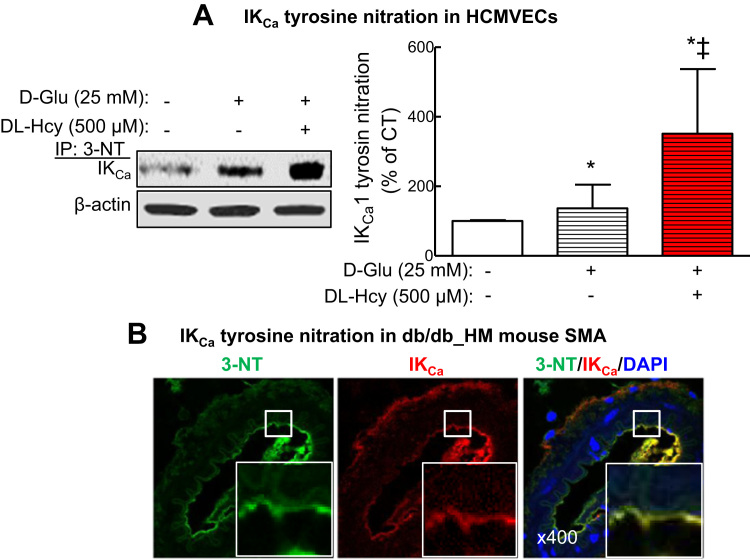
One of the major potential mechanisms leading to inactivation of KCa is oxidative stress [13], [38]. We used HCMVEC as a relevant cellular model for studying the underlying mechanisms of T2DM and T2DM/HHcy-mediated IKCa inactivation in EC. By immunoprecipitation with antibody of 3-NT, a marker of oxidative stress, followed with IKCa Western blot, we found that IKCa oxidation was increased by high glucose (25 mM D-glucose) by 1.37-fold (Fig. 6A, p < 0.05), and by the combination of D-glucose plus DL-Hcy by 3.51-fold (Fig. 6A, p < 0.05) in HCMVECs. Finally, we confirmed such IKCa oxidation induction in the endothelium by co-immunostaining of 3-NT and IKCa in the cross sections of mouse SMA (Fig. 6B). These data suggest that HHcy aggravated T2DM-induced IKCa oxidation in micro-vascular ECs.
Fig. 6.
HHcy potentiated hyperglycemia-induced tyrosine nitration of IKCa. A. IKCa levels in HCMVECs after immunoprecipitation with 3-NT antibody. HCMVECs were treated with D-glucose (D-Glu, 25 mM) or D-Glu plus DL-homocysteine (DL-Hcy, 500 µM) for 48 h. *p < 0.05 vs HCMVECs treated with vehicle; ‡p < 0.05 vs HCMVECs treated with D-Glu). B. Representative images of co-staining of 3-nitrotyrosine (3-NT, green) and IKCa (red) and DAPI (blue) in SMAs of db/db mice fed with HM diet. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.10. NaHS caused EDHF-induced vascular relaxation, which was enhanced in T2DM SMAs and potentiated by HHcy via ATP-activated potassium channel (KATP) activation
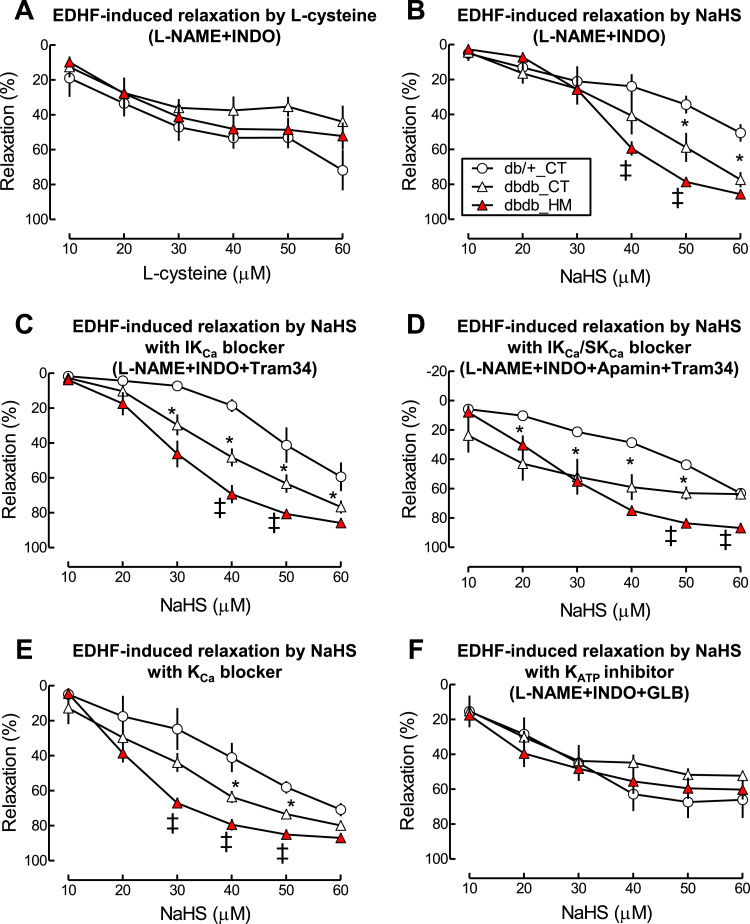
H2S has been suggested to be one of EDHF causing EC and SMC hyperpolarization via KCa and KATP [39]. We hypothesized that insufficient H2S is responsible for T2DM/HHcy-impaired EDHF-induced vascular relaxation in micro-vasculature of mice. To validate this hypothesis, we firstly tested whether reduced H2S in T2DM/HHcy is responsible for impaired EDHF-induced relaxation by examining EDHF-induced relaxation to L-cysteine, a precursor of endogenous H2S for 30 min. Surprisingly, we found that L-cysteine can cause EDHF-induced vascular relaxation, which was not altered in db/db and db/db/HHcy mice (Fig. 7A), suggesting that L-cysteine display EDHF function but not directly contribute to H2S deficiency related EDHF dysfunction in T2DM and T2DM/HHcy mice. Next we examined EDHF-induced vascular relaxation to exogenous H2S. We found that vascular relaxation to NaHS in the presence of L-NAME plus INDO was enhanced in db/db mice compared with that in db/+ mice (maximal relaxation: 77.4 ± 4.4 vs 50.6 ± 5.0, Fig. 7B, p < 0.05). HHcy further enhanced EDHF-induced relaxation to NaHS in db/db mice to a greater extend (maximal relaxation: 85.7 ± 1.2, p < 0.05). Increased sensitivity to NaHS in T2DM/HHcy SMA was abolished by KATP blocker GLB, but not changed in the presence of three KCa blockers (Fig. 7C to F). Our findings suggest that the increased sensitivity of SMAs to H2S in SMAs of T2DM and T2MD/HHcy mice is largely due to KATP activation, and may play a compensatory effect for insufficient endogenous H2S production.
Fig. 7.
HHcy sensitized H2S donor NaHS-induced vascular relaxation in SMA of db/db mice via activation of KATP. EDHF-induced relaxation in SMA was determined by pretreated the SMA with NOS inhibitor L-NAME (100 µM) and COX inhibitor (INDO, 10 µM) and indicated inhibitors, precontracted with phenylephrine (1 µM), and examined for relaxation to L-cysteine or NaHS. A. EDHF-induced relaxation by L-cysteine (10 µM). B. EDHF-induced relaxation by NaHS. C. EDHF-induced relaxation to NaHS in the presence of IKCa inhibitor Tram-34 (1 μM). D. EDHF-induced relaxation to NaHS in the presence of IKCa inhibitor Tram-34 and SKCa inhibitor Apamin (1 μM). E. EDHF-induced relaxation to NaHS in the presence of KCa inhibitor TEA (1 mM). F. EDHF-induced relaxation to NaHS in the presence of KATP inhibitor glibenclamide (GLB, 3 µM). n = 5–8 *p < 0.05 vs db/+ mice on CT diet (db/+_CT); ‡p < 0.05 vs db/db mice on CT diet (db/db_CT).
4. Discussion
We investigated the single and combined effects of HHcy and T2DM on endothelial function of mouse micro-vasculature SMA and reported four major novel findings. Firstly, T2DM worsens HHcy induced by HM diet. Secondly, HHcy aggravated T2DM-impaired EDHF-induced vascular relaxation to ACh in SMA via IKCa inactivation. Thirdly, H2S deficiency, due to downregulation of CSE, contributes to IKCa oxidation. Fourthly, H2S and L-cysteine display EDHF function and could be EDHF. We propose that administration of H2S may open a novel avenue for prevention and therapeutics of CVD in patients with T2DM and HHcy.
We used db/+ mice as the non-diabetic control and found that db/+ mice on 8 week HM diet developed intermediate HHcy (tHcy = 31.3 µM), which neither impaired endothelium-dependent relaxation to ACh, nor affected vascular contraction to KCl, PE and the endothelium-independent relaxation to SNP (Fig. 1). This is in good accordance with our previous findings showing that diet-induced mild HHcy (22.0 µM) did not impair endothelial function in non-diabetic mice, but intermediate HHcy (tHcy = 52.6 µM) aggravated hyperglycemia-impaired endothelial function in the aorta of STZ-treated mice [15]. We also reported that severe HHcy (tHcy = 169.5 µM) impaired endothelium-dependent vascular relaxation to Ach, as well as EDHF-induced relaxation, in SMAs of non-diabetic mice [13]. In the present study, we induced intermediate HHcy in db/db mice by using our own designed HM diet fed and examined its effect on vascular reactivity with a focus on EDHD-induced vascular relaxation in the micro-vasculature.
We discovered that HHcy aggravated impaired EDHF-induced relaxation to Ach in SMA of db/db mice, determined by measuring endothelium-dependent vascular relaxation to ACh in the presence of L-NAME and INDO, a standard approach for EDHF-induced relaxation study [6], [8], [13]. EDHF is defined as substance or electrical signal generated in and released from endothelium thereby hyperpolarizes VSMC resulting in vascular relaxation [9]. It is generally agreed that EDHF is the major contributor for micro-vasculature relaxation.
Although the nature of EDHF remains unclear, the endothelium-dependent vascular relaxation to ACh in the presence of L-NAME+INDO, are suggested to be initiated by the activation of endothelial IKCa and SKCa which result in endothelial cell (EC) hyperpolarizationf [45]. Hyperpolarization of endothelium is then transferred to VSMC by synthesizing or generating signals capable of diffusing through BKCa and KATP in VSMC or myoendothelial gap junctions resulting in vascular relaxation. Although both IKCa and SKCa regulate EDHF-induced vascular relaxation, plenty of evidence showed that IKCa plays a major role in the regulation of microcardiovascular homeostasis in mice and humans [46]. Previously, inactivation of IKCa-related ED was reported in SMA of db/db mice [8]. We also demonstrated that HHcy impairs EDHF-induced vascular relaxation to ACh in SMA by inhibiting SKCa/IKCa activities via oxidation and tyrosine nitration related mechanisms [13]. In this study, we observed that EDHF-induced relaxation to ACh was inhibited by a non-selective KCa blocker TEA and intermediate-conductance KCa blocker (IKCa) Tram-34, but not by small-conductance KCa (SKCa) blocker Apamin. Our findings suggest that HHcy potentiates T2DM-impaired EDHF function in SMAs via IKCa inactivation.
Our study confirmed that H2S is an effective EDHF, because that L-cysteine and NaHS, precursor and donor of H2S, induced vascular relaxation to ACh in the presence of L-NAME plus INDO (Fig. 7). However, the detail molecular mechanisms of H2S-induced vascular relaxation in the presence of L-NAME+INDO need further investigation. H2S has been suggested as a new type of EDHF [40]. H2S induced cell hyperpolarization measured by patch clamp [41]. H2S-induced vascular relaxation is endothelium-dependent. Removal of endothelium completely abolished the NaHS-induced relaxation in human and rat mesenteric arteries [42], [43]. Vascular relaxation to ACh in SMAs was virtually abolished in the presence of L-NAME+INDO in CSE-deleted mice [11], [39]. We demonstrated that the levels of H2S, free sulfide and CSE protein were all reduced and further potentiated by intermediate HHCy in T2DM mice SMA, similar and associated with impaired EDHD-induced SMA relaxation. These data lead us to hypothesize that reduced CSE, the key enzyme for H2S generation, may be the cause of H2S reduction, which is responsible for T2DM-induced and HHcy-potentiated impairment of EDHF-induced relaxation. Our results are in good accordance with recent findings that vascular relaxation response to NaHS was enhanced in the aorta of non-obese diabetic mice [22], and in polyamory arteries of STZ-treated diabetic mice [44].
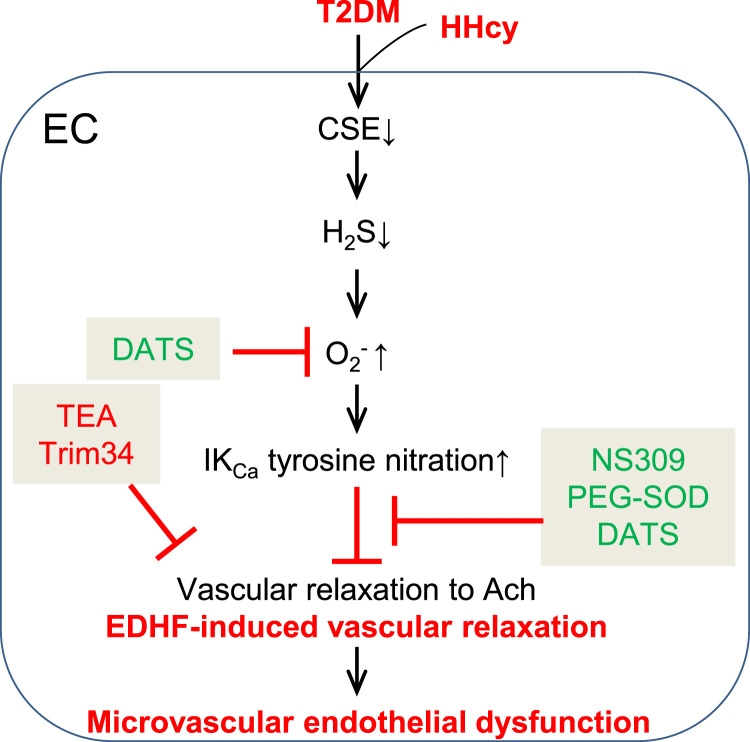
Previously, we and others reported that oxidative stress is one of the major sources for IKCa inactivation [13]. Here we show that H2S is a powerful antioxidant. We employed two methods to measure H2S levels, in situ staining with a H2S probe and analytical quantitation of free sulfide using HPLC. We found that HHcy potentiated H2S and free sulfide reduction in the SMAs of diabetic db/db mice, and that H2S donor DATS rescued T2DM/HHcy-induced superoxide production in SMAs and MLECs (Fig. 5). In summary, our H2S studies demonstrate that: 1) HHcy aggravated H2S reduction, CSE downregulation and oxidative stress in SMAs of T2DM mice; 2) HHcy aggravated T2DM-impaired EDHF function, at least in part, via oxidation/inactivation of IKCa; 3) DATS, a stable H2S donor, rescued oxidative stress in SMAs and MLECs of T2DM mice; 4) Antioxidant PEG-SOD and DATS improved EDHF function in SMAs of T2DM/HHcy mice. These findings allow us to propose a working model of T2DM/HHcy-induced ED in micro-vasculature. We conclude that HHcy aggravated H2S reduction via CSE suppression in diabetic micro-vascular ECs, leading to IKCa oxidation/inactivation and ED (Fig. 8).
Fig. 8.
Mechanism of HHcy-potentiated diabetes-impaired EDHF-induced vascular relaxation. Working model of T2DM/HHcy-induced endothelial dysfunction in micro-vasculature.
The underlying mechanisms of H2S as an antioxidant remain unclear. It may be related with: 1, quenching free radicals as a chemical reductant; 2, scavenging free radical via nonenzymatic antioxidants; 3, activating antioxidant enzymes; 4, inhibiting mitochondrial free radicals production [37], We previously reported that HHcy induced oxidative stress in SMA [13] and enhanced hyperglycemia-induced superoxide generation in aortic arteries [15], [47]. The current studies provide additional evidence suggesting that insufficient H2S is likely, at least one of mediators of oxidative stress in T2DM/HHcy which induces ED in micro-vasculature.
Together with the effect of D-glucose and D-glucose/DL-Hcy on induction of IKCa oxidation in HCMVECs (Fig. 6), we believe that deficient H2S production/oxidative stress and tyrosine nitration of IKCa play important role in impaired EDHF function in T2DM/HHcy mice. Restoration of H2S bioavailability may open a novel avenue for prevention and treatment of micro-vascular complications in patients with T2DM/HHcy. Details studies to explore underlying mechanisms and effects of deficient H2S on IKCa oxidation and inactivation may lead to the identification of novel therapeutic targets.
Previous studies showed that H2S induces EC and VSMC hyperpolarization via opening IKCa/SKCa in ECs and BKCa/KATP in VSMCs, respectively [39], [48], [49]. Selective IKCa and SKCa channel blockers, Charybdotoxin and Apamin inhibited H2S-induced vascular relaxation in SMA of CSE knockout mice [39] and aorta of Sprague-Dawley rats [49], but failed to influence the concentration-dependent relaxation induced by NaHS in human mesenteric arteries [42]. Moreover, Non-specific KCa blocker TEA completely inhibited vascular relaxation to NaHS in the presence of L-NAME+INDO in rat cerebral arteries.[50]. Interestingly, we discovered an enhanced EDHF relaxation to NaHS in T2DM/HHcy mice, which was not inhibited by any of KCa channel blockers (Fig. 7 B-E). Because that KATP may also mediate H2S-induced relaxation [39], we also tested the effect of KATP blocker glibenclamide (GLC) and found that GLC rescued enhanced relaxation to NaHS in T2DM/HHcy mice. We then conclude that KATP channel activation is responsible for enhanced EDHF relaxation to NaHS in SMA in TD2M and TD2M/HHcy mice. This difference, compared to previous findings, may be related with the size and type of vessels, and species. Moreover, because enhanced relaxation to exogenous H2S (NaHS) in the presence of L-NAME plus INDO in T2DM and T2DM/HHcy mice was inhibited by KATP blocker GLC, we suggest that KATP is the major target of H2S in VSMCs which was sensitized by T2DM and HHcy. Our findings support the previous concept that H2S targets KATP in VSMCs thus hyperpolarizes VSMCs leading to vasorelaxation [49]. Our findings are also in line with recent studies that H2S induced vascular relaxation in KATP-dependent manner in rat aortic arteries and SMAs [8], [43]. KATP blocker GLB reduced vascular relaxation response to H2S in rat SMAs [43] and KATP opener pinacidil induced vascular relaxation in rat SMAs whereas KATP blocker GLB inhibited H2S induced vascular relaxation [49]. The enhanced vascular relaxation to H2S in SMAs of T2DM and T2DM/HHcy mice in the presence of L-NAME plus INDO may be a complementary effect to overcome insufficient H2S production and ED in micro-vasculature under metabolic disorder. It would be ideal to examine whether KCl can completely block vascular relaxation response to H2S in the presence of L-NAME+INDO thus confirm H2S as an EDHF regulating endothelial function under T2DM/HHcy condition. It is an ongoing effort in our laboratory to continually explore mechanisms underlying H2S-induced endothelium-dependent vascular relaxation.
In addition, we observed some resistant relaxations to ACh in the presence of KCa blockers and NO/PGI2 inhibitors (Fig. 3B). Several potential factors may contribute to these resistant relaxation, including: 1) NO, PGI2 and KCa channels may not be completely inhibited, because of the dosage limitation; 2) ACh may stimulate other relaxation in the presence of NO/PGI2 inhibitors independent from KCa; 3) Other residual relaxant factors independent from NO, PGI2 and KCa. Future studies should explore other EDHF relaxation factors and identify novel therapeutic targets.
In good accordance with our previous study that HM induced higher plasma total Hcy (t-Hcy) levels in mice with hyperglycemia [15], we also observed that the plasma t-Hcy level is higher in db/db mice fed with a HM diet compared that in db/+ mice (Fig. 1B). The interaction between HHcy and hyperglycemia/T2DM is not known and need to be addressed in the future studies.
Acknowledgements
No potential conflicts of interest relevant to this article were reported.
Acknowledgments
Funding
This work was supported in part by the NIH grants HL67033, HL77288, HL82774, HL-110764, HL130233, HL131460, DK104114 and DK113775 (H.W.); HL091983, HL053354, HL108795 and HL126186 (R.K.); National Science Foundation of China Grants number 81330004 and 91639204 (JY), and by American Heart Association grant SDG16390004 (ZJ.C.) and 13BGIA16500030 (ZJ.C).
Author contribution
Conceived and designed the experiments: ZJC, RK and HW. Performed the experiments: ZJC, XQS, XHJ, HMS, PF and MC. Analyzed the data: ZJC and HW. Contributed reagents/materials/analysis tools: ZJC, YJ, RK, CK, XFY, RK and HW. Wrote the paper: ZJC and HW. ZJC and HW are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.02.006.
Contributor Information
Zhongjian Cheng, Email: zjcheng@temple.edu.
Hong Wang, Email: hong.wang@temple.edu.
Appendix A. Supplementary material
Supplementary material
References
- 1.Gregg E.W., Li Y., Wang J., Burrows N.R., Ali M.K., Rolka D., Williams D.E., Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 2.Unwin N., Gan D., Whiting D. The IDF Diabetes Atlas: providing evidence, raising awareness and promoting action. Diabetes Res. Clin. Pract. 2010;87:2–3. doi: 10.1016/j.diabres.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Z., Yang X., Wang H. Hyperhomocysteinemia and endothelial dysfunction. Curr. Hypertens. Rev. 2009;5:158–165. doi: 10.2174/157340209788166940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feletou M., Vanhoutte P.M. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler. Thromb. Vasc. Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 6.Triggle C.R., Ding H. Endothelium-derived hyperpolarizing factor: is there a novel chemical mediator? Clin. Exp. Pharmacol. Physiol. 2002;29:153–160. doi: 10.1046/j.1440-1681.2002.03632.x. [DOI] [PubMed] [Google Scholar]
- 7.Kohler R., Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int. 2007;72:145–150. doi: 10.1038/sj.ki.5002303. [DOI] [PubMed] [Google Scholar]
- 8.Pannirselvam M., Ding H., Anderson T.J., Triggle C.R. Pharmacological characteristics of endothelium-derived hyperpolarizing factor-mediated relaxation of small mesenteric arteries from db/db mice. Eur. J. Pharmacol. 2006;551:98–107. doi: 10.1016/j.ejphar.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 9.Luksha L., Agewall S., Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202:330–344. doi: 10.1016/j.atherosclerosis.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 10.McGuire J.J., Ding H., Triggle C.R. Endothelium-derived relaxing factors: a focus on endothelium-derived hyperpolarizing factor(s) Can. J. Physiol. Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- 11.Tang G., Yang G., Jiang B., Ju Y., Wu L., Wang R. H(2)S is an endothelium-derived hyperpolarizing factor. Antioxid. Redox Signal. 2013;19:1634–1646. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 12.Ji C., Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J. Gastroenterol. 2004;10:1699–1708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Z., Jiang X., Kruger W.D., Pratico D., Gupta S., Mallilankaraman K., Madesh M., Schafer A.I., Durante W., Yang X., Wang H. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118:1998–2006. doi: 10.1182/blood-2011-01-333310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br. J. Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Z., Jiang X., Pansuria M., Fang P., Mai J., Mallilankaraman K., Gandhirajan R.K., Eguchi S., Scalia R., Madesh M., Yang X., Wang H. Hyperhomocysteinemia and hyperglycemia induce and potentiate endothelial dysfunction via mu-calpain activation. Diabetes. 2015;64:947–959. doi: 10.2337/db14-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoogeveen E.K., Kostense P.J., Beks P.J., Mackaay A.J., Jakobs C., Bouter L.M., Heine R.J., Stehouwer C.D. Hyperhomocysteinemia is associated with an increased risk of cardiovascular disease, especially in non-insulin-dependent diabetes mellitus: a population-based study. Arterioscler. Thromb. Vasc. Biol. 1998;18:133–138. doi: 10.1161/01.atv.18.1.133. [DOI] [PubMed] [Google Scholar]
- 17.Calvert J.W., Jha S., Gundewar S., Elrod J.W., Ramachandran A., Pattillo C.B., Kevil C.G., Lefer D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King A.L., Polhemus D.J., Bhushan S., Otsuka H., Kondo K., Nicholson C.K., Bradley J.M., Islam K.N., Calvert J.W., Tao Y.X., Dugas T.R., Kelley E.E., Elrod J.W., Huang P.L., Wang R., Lefer D.J. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., Snyder S.H., Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannigan K.L., Agbor T.A., Blackler R.W., Kim J.J., Khan W.I., Verdu E.F., Ferraz J.G., Wallace J.L. Impaired hydrogen sulfide synthesis and IL-10 signaling underlie hyperhomocysteinemia-associated exacerbation of colitis. Proc. Natl. Acad. Sci. USA. 2014;111:13559–13564. doi: 10.1073/pnas.1413390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brancaleone V., Roviezzo F., Vellecco V., De Gruttola L., Bucci M., Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br. J. Pharmacol. 2008;155:673–680. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain S.K., Bull R., Rains J.L., Bass P.F., Levine S.N., Reddy S., McVie R., Bocchini J.A. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid. Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert J.P., Nicholson C.K., Amin H., Amin S., Calvert J.W. Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med. Gas Res. 2014;4:20. doi: 10.1186/s13618-014-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteman M., Gooding K.M., Whatmore J.L., Ball C.I., Mawson D., Skinner K., Tooke J.E., Shore A.C. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Z., Garikipati V.N., Nickoloff E., Wang C., Polhemus D.J., Zhou J., Benedict C., Khan M., Verma S.K., Rabinowitz J.E., Lefer D., Kishore R. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation. 2016;134:1467–1483. doi: 10.1161/CIRCULATIONAHA.116.022967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L., Jhee K.H., Hua X., DiBello P.M., Jacobsen D.W., Kruger W.D. Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ. Res. 2004;94:1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D., Jiang X., Fang P., Yan Y., Song J., Gupta S., Schafer A.I., Durante W., Kruger W.D., Yang X., Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen X., Pattillo C.B., Pardue S., Bir S.C., Wang R., Kevil C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mervaala E.M., Teravainen T.L., Malmberg L., Laakso J., Vapaatalo H., Karppanen H. Cardiovascular effects of a low-dose combination of ramipril and felodipine in spontaneously hypertensive rats. Br. J. Pharmacol. 1997;121:503–510. doi: 10.1038/sj.bjp.0701166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.E. Brondum, H. Kold-Petersen, U. Simonsen, C. Aalkjaer: NS309 restores EDHF-type relaxation in mesenteric small arteries from type 2 diabetic ZDF rats. British Journal of Pharmacology, 159, 154-165. [DOI] [PMC free article] [PubMed]
- 32.Kobayashi M., Inoue K., Warabi E., Minami T., Kodama T. A simple method of isolating mouse aortic endothelial cells. J. Atheroscler. Thromb. 2005;12:138–142. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- 33.Welch G.N., Loscalzo J. Homocysteine and atherothrombosis. N. Engl. J. Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 34.Kang S.S., Wong P.W., Malinow M.R. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu. Rev. Nutr. 1992;12:279–298. doi: 10.1146/annurev.nu.12.070192.001431. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Z., Garikipati V., Nickoloff E., Wang C., Polhemus D.J., Zhou J., Benedict C., Khan M., Verma S.K., Rabinowitz J.E., Lefer D., Kishore R. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.116.022967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolluru G.K., Bir S.C., Yuan S., Shen X., Pardue S., Wang R., Kevil C.G. Cystathionine gamma-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc. Res. 2015;107:590–600. doi: 10.1093/cvr/cvv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Z.Z., Liu Y., Bian J.S. Hydrogen sulfide and cellular redox homeostasis. Oxid. Med. Cell. Longev. 2016;2016:6043038. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dal-Ros S., Oswald-Mammosser M., Pestrikova T., Schott C., Boehm N., Bronner C., Chataigneau T., Geny B., Schini-Kerth V.B. Losartan prevents portal hypertension-induced, redox-mediated endothelial dysfunction in the mesenteric artery in rats. Gastroenterology. 2010;138:1574–1584. doi: 10.1053/j.gastro.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 39.Mustafa A.K., Sikka G., Gazi S.K., Steppan J., Jung S.M., Bhunia A.K., Barodka V.M., Gazi F.K., Barrow R.K., Wang R., Amzel L.M., Berkowitz D.E., Snyder S.H. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R. Hydrogen sulfide: a new EDRF. Kidney Int. 2009;76:700–704. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- 41.Mustafina A.N., Yakovlev A.V., Gaifullina A., Weiger T.M., Hermann A., Sitdikova G.F. Hydrogen sulfide induces hyperpolarization and decreases the exocytosis of secretory granules of rat GH3 pituitary tumor cells. Biochem. Biophys. Res. Commun. 2015;465:825–831. doi: 10.1016/j.bbrc.2015.08.095. [DOI] [PubMed] [Google Scholar]
- 42.Materazzi S., Zagli G., Nassini R., Bartolini I., Romagnoli S., Chelazzi C., Benemei S., Coratti A., De Gaudio A.R., Patacchini R. Vasodilator activity of hydrogen sulfide (H2S) in human mesenteric arteries. Microvasc. Res. 2016;109:38–44. doi: 10.1016/j.mvr.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Y., Ndisang J.F., Tang G., Cao K., Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 44.Denizalti M., Bozkurt T.E., Akpulat U., Sahin-Erdemli I., Abacioglu N. The vasorelaxant effect of hydrogen sulfide is enhanced in streptozotocin-induced diabetic rats. Naunyn-Schmiede's Arch. Pharmacol. 2011;383:509–517. doi: 10.1007/s00210-011-0601-6. [DOI] [PubMed] [Google Scholar]
- 45.Brahler S., Kaistha A., Schmidt V.J., Wolfle S.E., Busch C., Kaistha B.P., Kacik M., Hasenau A.L., Grgic I., Si H., Bond C.T., Adelman J.P., Wulff H., de Wit C., Hoyer J., Kohler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 46.Toyama K., Wulff H., Chandy K.G., Azam P., Raman G., Saito T., Fujiwara Y., Mattson D.L., Das S., Melvin J.E., Pratt P.F., Hatoum O.A., Gutterman D.D., Harder D.R., Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J. Clin. Investig. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kishore R., Benedict C., Cheng Z. mu-Calpain as a novel target for impairment of nitric oxide-mediated vascular relaxation in diabetes: a mini review. J. Mol. Genet. Med.: Int. J. Biomed. Res. 2015;9 doi: 10.4172/1747-0862.1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao W., Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 50.Han J., Chen Z.W., He G.W. Acetylcholine- and sodium hydrosulfide-induced endothelium-dependent relaxation and hyperpolarization in cerebral vessels of global cerebral ischemia-reperfusion rat. J. Pharmacol. Sci. 2013;121:318–326. doi: 10.1254/jphs.12277fp. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material