Abstract
Shewanella algae are Gram-negative, nonfermentative, motile bacilli, classified in the genus Shewanella in 1985. These environmental bacteria are occasionally identified in human infections, with a relatively strong association with exposure to seawater during warm seasons. This report describes a case series of 17 patients with infection correlated to S. algae in the coastal area of Romagna, Italy, from 2013 to 2016. The types of infection included otitis, pneumonia, sepsis and soft tissue (wound). Exposure to the marine environment during hot months was confirmed in 12 of 17 patients. An apparent correlation between increased severity of infection and patient age was also observed.
Keywords: Adriatic Sea, otitis, seawater, sepsis, Shewanella algae
Introduction
Shewanella species are Gram-negative, nonfermentative, motile bacilli. The first isolation occurred in 1931 by Derby and Hammer from dairy products; they named this unknown species Achromobacter putrefaciens [1]. In 1941, this species was transferred to the genus Pseudomonas under the name P. putrefaciens, and according to Shewan et al. [2], for the next three decades, they were placed in the Pseudomonas IV group. On the basis of phylogenetic studies and small subunit rDNA sequencing, these organisms were reclassified to the Vibrionaceae family and described as a new genus, Shewanella. The genus Shewanella was shown to be a monophyletic taxon within the gamma subgroup of the phylum Proteobacteria. From 16S RNA genetic analysis of genera belonging to this group emerged the existence of a new family, Shewanellaceae, containing about 50 Shewanella spp., most of which are psychrophilic and therefore of little interest to clinical microbiologists [3], [4], [5].
Bacteria of the genus Shewanella are found throughout the world, mainly in marine environments and other underwater settings; these germs have also been reported in soil, fish, meat, poultry and dairy products [6]. Shewanella spp. are an unusual cause of disease in humans; however, reports of Shewanella infections have been increasing, likely as the result of better testing. Most human Shewanella infections have been reported in warmer areas, including Southeast Asia, Southern Europe, South Africa and the Caribbean [7], [8], [9], [10]. Sporadic cases have also been reported from countries with cooler climates, including Australia, Belgium and Denmark [10]. In Denmark, S. putrefaciens and S. algae have been reported in seawater, with the frequency of occurrence correlating with sea temperature. These organisms were detected in Denmark only during July to October, when the water temperature is above 13°C [11]. The only Shewanella spp. found in human clinical specimens are S. putrefaciens and S. algae, and more than 80% of the isolates from humans reported in the literature belong to S. algae [12], [13], [14], [15]. Because these organisms are frequently isolated together with other bacteria, the pathogenic potential of Shewanella has been controversial since its very first report. In a murine pathogenicity model, S. algae was the most virulent species, and it has been speculated that the haemolytic activity of this species could play an important role in its virulence factors [16].
The major risk factor linked to Shewanella infection is an intimate association between humans and the marine environment or its contents; this correlation has been described in the literature [11], [17], [18], [19], [20], [21], [22]. S. algae has also been isolated from the heart of a dolphin with meningoencephalitis along the cost of the Adriatic Sea; indeed, this is additional evidence of the potential pathogenic role of S. algae for mammals in the marine environment [23]. The clinical picture of human infection caused by S. algae is generally quite similar to those caused by various species of the Vibrionaceae family: skin and soft tissue infections associated with ulcers or trauma; ear infections present as acute infections or acute exacerbations in chronic otitis media; and bacteraemia, the course of which in S. algae blood infection is usually benign [6]. To our knowledge, to date, only a single case of isolation of S. putrefaciens has been described from Italy. This event was related to a patient with soft tissue infection and bacteraemia who, a few days before the infection, had just returned from a holiday on the Adriatic shore [9].
This study was conducted to investigate the epidemiologic and clinical characteristics of S. algae isolates obtained from clinical specimens submitted for routine microbiologic diagnosis over a period of 3 years (2013–2016) along the Romagna Adriatic shore in northern Italy. To our knowledge, this is the first report of human infection clearly caused by S. algae in Italy.
Materials and methods
The S. algae isolates were identified from samples routinely submitted to the microbiology unit of the Great Romagna Hub Laboratory, Pievesestina-Cesena, northern Italy. Details about the standard diagnostic methods used to manage the samples positive for S. algae isolates are provided online in Supplementary Appendix S1. The identification of S. algae from the growing brown mucous colonies was obtained (99% confidence) using a VITEK MS instrument (bioMérieux, Marcy l’Etoile, France). The antimicrobial susceptibility of S. algae isolates was determined with a VITEK 2 system (bioMérieux). The Etest was used as a confirmatory test for susceptibility to carbapenems and was interpreted following European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines for other non-Enterobacteriaceae glucose nonfermenting Gram-negative bacilli.
Results
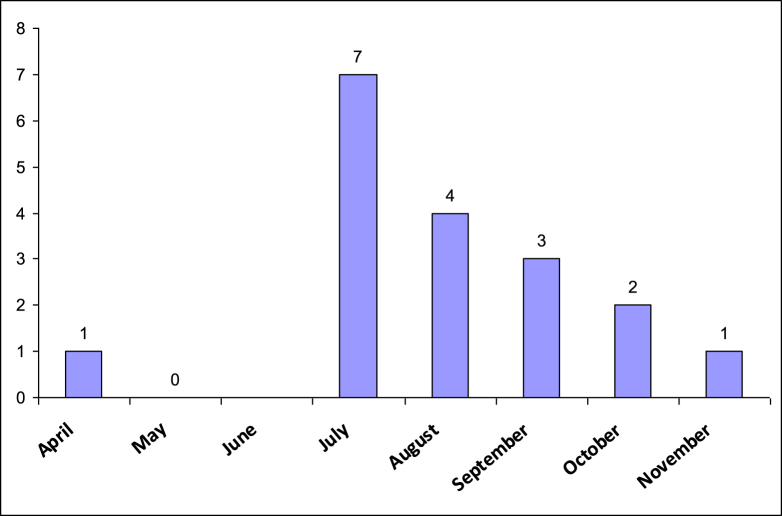
A total of 18 S. algae strains were isolated from 17 immunocompetent patients seeking care at the healthcare facilities of the Great Romagna area in northern Italy from 1 July 2013 to 30 November 2016. The Great Romagna area is located along the coast of the western Adriatic Sea. The microbiology unit of the Great Romagna Hub Laboratory serves the entire network of these public facilities. All the isolates were found during April to November, with a peak in July (Fig. 1). The S. algae strains were identified in the following samples: blood cultures (n = 5), skin and soft tissue swabs (n = 5), ear swabs (n = 5), bronchial aspiration (n = 1) and sputum (n = 2). In one patient, S. algae was isolated from two different types of specimens: blood culture and bronchial aspiration.
Fig. 1.
Seasonal trends of Shewanella algae isolates.
Most patients with S. algae infection were older than 60 years (n = 12), with a mean age of 68 years. One patient was 43 years old, and the remaining four were children (mean age, 7 years and 6 months). Nine patients were female and eight were male.
Recent (<4 weeks) exposure to seawater along the local coast of the Adriatic Sea was reported for 13 patients. No precise information is available in the scientific literature regarding the incubation time of S. algae infections, and consequently we can only hypothesize that for these 13 subjects, the infection was a direct consequence of contact with polluted seawater. All of the paediatric patients had otitis externa. The adult patients had different clinical pictures: five had skin and soft tissue infections, five had bacteraemia, two had acute pneumonia and one had external otitis. It is noteworthy that S. algae was associated with other pathogens in nine of the 17 patients. All patients had a positive final outcome, with the exception of a 92-year-old woman with sepsis, who died 8 days after S. algae was cultured from a blood sample. Table 1 summarizes the clinical picture of each patient.
Table 1.
Clinical characteristics of 17 patients with Shewanella algae infection
| Patient | Age/sex | Additional microorganisms isolated from same sample | Clinical picture | Exposure to seawater within 4 weeks | Final outcome |
|---|---|---|---|---|---|
| 1 | 6/F | None | Otitis externa | Yes | Positive |
| 2 | 7/M | Vibrio alginolyticus | Otitis externa | Yes | Positive |
| 3 | 8/F | V. alginolyticus | Otitis externa | Yes | Positive |
| 4 | 9/F | Klebsiella oxytoca | Otitis externa | Yes | Positive |
| 5 | 71/M | None | Otitis externa | Yes | Positive |
| 6 | 83/M | None | Acute pneumonia | No | Positive |
| 7 | 75/M | None | Acute pneumonia | Yes | Positive |
| 8 | 80/M | None | Sepsis | No | Positive |
| 9 | 92/F | None | Sepsis | No | Died from acute sepsis |
| 10 | 82/F | None | Sepsis | Yes | Positive |
| 11a | 68/F | None; Pseudomonas aeruginosa | Sepsis; acute pneumonia | Yes | Positive |
| 12 | 78/M | None | Sepsis | No | Positive |
| 13 | 70/F | Staphylococcus aureus, Acinetobactecter baumannii, Escherichia coli | Skin and soft tissue infection | Yes | Positive |
| 14 | 73/F | Myroides spp. | Skin and soft tissue infection | Yes | Positive |
| 15 | 65/F | Staphylococcus aureus | Skin and soft tissue infection | No | Positive |
| 16 | 66/M | Morganella morganii | Skin and soft tissue infection | Yes | Positive |
| 17 | 43/M | None | Skin and soft tissue infection | Yes | Positive |
Only patient from whom S. algae was isolated from two different samples (blood and bronchial aspiration).
All the S. algae isolates were susceptible to third-generation cephalosporins and gentamycin. In addition, amikacin, carbapenems and piperacillin/tazobactam showed very good activity against most of the isolated strains. Amoxicillin/clavulanic acid was efficacious against <50% of the isolates (5/13). Phosphomycin showed no activity against the seven strains evaluated. The in vitro activity of tigecycline and colistin was only evaluated against five and six isolates, respectively. The antimicrobial susceptibility data of the 17 S. algae isolates are detailed in Table 2.
Table 2.
Antibiotic susceptibility of 17 strains of Shewanella algae
| Antibiotic | MIC (mg/L), mean (range) | No. of clinically sensitive isolates of S. algae strains/no. tested |
|---|---|---|
| Amikacin | ≤2 (≤2 to 4) | 15/17 |
| Amoxicillin/clavulanic acid | 16 (≤2 to ≥32) | 5/13 |
| Cefepime | ≤1 (≤1) | 17/17 |
| Cefotaxime | ≤1 (≤1) | 16/16 |
| Ceftazidime | ≤1 (≤1) | 16/16 |
| Ciprofloxacin | ≤0.25 (≤0.25 to 2.0) | 15/16 |
| Phosphomycin | ≥256 (≥256) | 0/7 |
| Gentamycin | ≤1 (≤1) | 17/17 |
| Imipenem | 1 (≤0.25 to ≥32) | 14/17 |
| Meropenem | 1 (≤0.25 to 4.0) | 16/17 |
| Piperacillin/tazobactam | ≤4 (≤4 to ≥128) | 15/16 |
| Tigecycline | ≤0.5 (≤0.5) | 5/5 |
MIC, minimum inhibitory concentration.
Discussion
Shewanella spp. infections have been reported worldwide. To our knowledge, this is the first report of infections caused by S. algae in Italy, where a single sporadic case of bacteraemia associated with soft tissue infection by S. putrefaciens was described 14 years ago [9]. The most frequent clinical features of S. algae–related human disease include soft tissue infections, otitis externa, invasive bacteraemia and sepsis with hepatic and biliary involvement. Because Shewanella spp. are mostly environmental microbes whose pathogenic mechanisms remain to be completely elucidated, most cases of human infection develop in people with underlying diseases, and infection is often related to massive exposure to seawater [6].
Bacteria that today is included in the genus Shewanella were first described in 1931 [1] as Achromobacter putrefaciens. Later they were classified as P. putrefaciens, and finally the novel genus Shewanella was proposed in 1985 [6]. The only Shewanella spp. relevant for human diseases so far are S. putrefaciens and S. algae, with a higher frequency of reported cases due to the latter species. This potentially higher pathogenic capability for humans has been correlated with the production of a haemolysin [24]. Most automated identification systems based on biochemical reactions are unable to distinguish between S. putrefaciens and S. algae because the latter species is not included in their database [7]. However, today, identification is based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, as these systems include the S. algae spectrum in their database [25]. This new ability to correctly and precisely identify S. algae has likely contributed to the increased frequency of descriptions of infections caused by S. algae in the last decade [26]. Our study describes a series of 17 cases of infection due to S. algae detected from 2013 to 2016 along the shores of northern Adriatic Sea in Romagna, Italy.
As reported previously in other geographical areas, human cases of S. algae infection are overwhelmingly linked with exposure to the marine environment. The mode of contact reported in the literature includes recreational or occupational exposure, consumption of seafood, introduction of Shewanella species into puncture wounds caused by marine life (sea urchins, fish) or direct exposure to seawater. In our study, more than 70% (12/17) of the patients reported massive contact with seawater within 4 weeks of the isolation of S. algae. This fact also correlates with the time period during which S. algae has been identified (April to November, with a peak from July to September), which corresponds to the months with the highest frequency of sea-bathing activities along the coast of the northern Adriatic Sea (Fig. 1). Because not all human cases of infection by S. algae are linked to exposure to seawater, and because other studies have not reported such a strong association, other possible ways to acquire this infection have been hypothesized [6]. Such hypothetical modes of transmission could also apply to the five patients in this series who did not report contact with the marine environment. The presence of S. algae in the marine environment correlates with water temperature [10]. In the northern Adriatic Sea, the average temperature is above 13°C from April until November, with a peak (constantly above 20°C) from June to September detected since 2011. This climatic feature is compatible with a possible increase of S. algae concentration into seawater (http://www.isprambiente.gov.it).
The isolation of S. algae was frequently associated with other pathogenic or potentially pathogenic microbes (8/17 cases); this was more relevant in cases of less invasive infection (3/5 otitis externa, 4/5 wound infection) than in cases of bacteraemia or acute pneumonia (1/6). This fact clearly suggests that in superficial infections, the pathogenic role of S. algae is less defined because the pathogen is part of a mixed infecting flora and is a member of the microbiota found in the deeper part of skin and soft tissue and wound infections. However, S. algae likely plays a relevant pathogenic role on its own in invasive infections. As previously described in a Danish study [12], S. algae causes otitis, particularly in younger children, whereas invasive infections apparently develop only in the older population. Most of the cases of Shewanella-related bacteraemia are community acquired [27], [28], and in the absence of underlying complications, these infection have an overall benign clinical course [28], [29], [30], as was indeed found in the population we studied. In our cases series, only one patient died from sepsis due to S. algae. This unfavourable outcome was correlated with the extreme age (92 years) of the patient.
S. algae was also identified in specimens from infected wounds; this is a common site for isolation of Shewanella spp., which are frequently found as members of the polymicrobial flora in skin and soft tissue infections [10], [21], [22], [30]. This characteristic is likely linked to the exposure of broken skin to environmental bacteria; in this limited cases series, four of five patients with wound infections reported recent exposure to the marine environment.
Acute pneumonia is infrequently caused by S. algae. Patients can come into contact via the respiratory route in near-drowning events in saltwater, via head submersion resulting from recreational activities or via environmental exposure due to trauma [31]. In this study, only two strains were found as the unique pathogen in sputum samples, thus confirming the relatively infrequent role of S. algae in acute respiratory infections. The in vitro antimicrobial susceptibility of S. algae was reviewed in 2013 by Vignier et al. [10], who found that most isolates were sensitive to third-generation cephalosporin, piperacillin/tazobactam, ciprofloxacin and gentamycin. The isolates that we evaluated showed almost identical patterns of antimicrobial sensitivity. Erythromycin, chloramphenicol and tetracycline, although reported in the literature as effective against S. algae [10], [28], were not included in our “Antimicrobial Susceptibility Testing (AST)” evaluation because they do not belong the first line of drugs used in clinical practice for the standard of cure for the illnesses caused by S. algae. As reported in the literature, S. algae showed a propensity towards resistance to carbapenems [30]. The mechanism of resistance may be related to a carbapenem-hydrolyzing Amber class D β-lactamase [32]. This feature was confirmed among the isolates described in this study that were susceptible to imipenem (14/17 susceptible isolates), with meropenem being more effective (16/17 susceptible isolates).
This study is novel in that it provides evidence for the first time of the pathogenic role played by S. algae in Italy; in particular, it confirms that isolation of this microbe mainly occurs in patients exposed to the marine environment when the seawater temperature is elevated. Although the number of patients is small and we could therefore not perform statistical analysis, we found an association between age and the severity of the S. algae infection. Otitis was the predominant clinical feature in children, whereas invasive infections and infections of skin and soft tissue were prevalent in older subjects. Moreover, the isolation of S. algae in clinical samples only during the summer period correlates with data reported in the literature, which demonstrate a connection between an increase in water temperature and the frequency of identification of this organism in human infections. S. algae should be considered an emerging opportunistic pathogen linked to environmental conditions in the coastal area of Romagna.
Conflict of interest
None declared.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.nmni.2018.01.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Derby H.A., Hammer H.A. Bacteriology of butter. IV. Bacteriological studies on surface taint butter. Iowa Agric Exp Stn Res Bull. 1931;145:389–416. [Google Scholar]
- 2.Shewan J.M., Hobbs G., Hodgkiss W. A determinative scheme for the identification of certain genera of gram-negative bacteria, with special reference to Pseudomonadaceae. J Appl Bacteriol. 1960;23:379–390. [Google Scholar]
- 3.MacDonell M.T., Colwell R.R. Phylogeny of the Vibrionaceae, and recommendation for two new genera, Listonella and Shewanella. Syst Appl Microbiol. 1985;6:171–182. [Google Scholar]
- 4.Gauthier G., Gauthier M., Christen R. Phylogenetic analysis of the genera Alteromonas, Shewanella, and Moritella using genes coding for small-subunit rRNA sequences and division of the genus Alteromonas into two genera, Alteromonas (emended) and Pseudoalteromonas gen. nov., and proposal of twelve new species combinations. Int J Syst Bacteriol. 1995;45:755–761. doi: 10.1099/00207713-45-4-755. [DOI] [PubMed] [Google Scholar]
- 5.Ivanova E.P., Flavier S., Christen R. Phylogenetic relationships among marine Alteromonas-like proteobacteria: emended description of the family Alteromonadaceae and proposal of Pseudoalteromonadaceae fam. nov., Colwelliaceae fam. nov., Shewanellaceae fam. nov., Moritellaceae fam. nov., Ferrimonadaceae fam. nov., Idiomarinaceae fam. nov. and Psychromonadaceae fam. nov. Int J Syst Evol Microbiol. 2004;54:1773–1788. doi: 10.1099/ijs.0.02997-0. [DOI] [PubMed] [Google Scholar]
- 6.Janda J.M., Abbott S.L. The genus Shewanella: from the briny depths below to human pathogen. Crit Rev Microbiol. 2014;40:293–312. doi: 10.3109/1040841X.2012.726209. [DOI] [PubMed] [Google Scholar]
- 7.Holt H.M., Gahrn-Hansen B., Bruun B. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect. 2005;11:347–352. doi: 10.1111/j.1469-0691.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein R., Oren I. Soft tissue infections caused by marine bacterial pathogens: epidemiology, diagnosis, and management. Curr Infect Dis Rep. 2011;13:470–477. doi: 10.1007/s11908-011-0199-3. [DOI] [PubMed] [Google Scholar]
- 9.Pagani L., Lang A., Vedovelli C., Molino O., Rimenti G., Pristeran R. Soft tissue infection and bacteremia caused by Shewanella putrefaciens. J Clin Microbiol. 2003;41:2240–2241. doi: 10.1128/JCM.41.5.2240-2241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vignier N., Barreau M., Olive C., Baubion E., Théodose R., Hochedez P. Human infection with Shewanella putrefaciens and S. algae: report of 16 cases in Martinique and review of the literature. Am J Trop Med Hyg. 2013;89:151–156. doi: 10.4269/ajtmh.13-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gram L., Bundvad A., Melchiorsen J., Johansen C., Fonnesbech V.B. Occurrence of Shewanella algae in Danish coastal water and effects of water temperature and culture conditions on its survival. Appl Environ Microbiol. 1999;65:3896–3900. doi: 10.1128/aem.65.9.3896-3900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt H.M., Søgaard P., Gahrn-Hansen B. Ear infections with Shewanella alga: a bacteriologic, clinical and epidemiologic study of 67 cases. Clin Microbiol Infect. 1997;3:329–334. doi: 10.1111/j.1469-0691.1997.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 13.Nozue H., Hayashi T., Hashimoto Y., Ezaki T., Hamasaki K., Ohwada K. Isolation and characterization of Shewanella alga from human clinical specimens and emendation of the description of S. alga Simidu et al., 1990, 335. Int J Syst Bacteriol. 1992;42:628–634. doi: 10.1099/00207713-42-4-628. [DOI] [PubMed] [Google Scholar]
- 14.Khashe S., Janda J.M. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens. J Clin Microbiol. 1998;36:783–787. doi: 10.1128/jcm.36.3.783-787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel B.F., Jorgensen K., Christensen H., Olsen J.E., Gram L. Differentiation of Shewanella putrefaciens and Shewanella alga on the basis of whole-cell protein profiles, ribotyping, phenotypic characterization, and 16S rRNA gene sequence analysis. Appl Environ Microbiol. 1997;63:2189–2199. doi: 10.1128/aem.63.6.2189-2199.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma K.K., Kalawat U. Emerging infections: Shewanella—a series of five cases. J Lab Physicians. 2010;2:61–65. doi: 10.4103/0974-2727.72150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heller H.M., Tortora G., Burger H. Pseudomonas putrefaciens bacteremia associated with shellfish contact. Am J Med. 1990;88:85–86. doi: 10.1016/0002-9343(90)90139-5. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal S.L., Zuger J.H., Apollo E. Respiratory colonization with Pseudomonas putrefaciens after near-drowning in salt water. Am J Clin Pathol. 1975;64:382–384. doi: 10.1093/ajcp/64.3.382. [DOI] [PubMed] [Google Scholar]
- 19.Leong J., Mirkazemi M., Kimble F. Shewanella putrefaciens hand infection. Aust N Z J Surg. 2000;70:816–817. doi: 10.1046/j.1440-1622.2000.01962.x. [DOI] [PubMed] [Google Scholar]
- 20.Bulut C., Ertem G.T., Gökcek C., Tulek N., Bayar M.A., Karakoc E. A rare cause of wound infection: Shewanella putrefaciens. Scand J Infect Dis. 2004;36:692–694. doi: 10.1080/00365540410022620. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y.S., Liu Y.C., Yen M.Y., Wang J.H., Wann S.R., Cheng D.L. Skin and soft-tissue manifestations of Shewanella putrefaciens infection. Clin Infect Dis. 1997;25:225–229. doi: 10.1086/514537. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez H., Vogel B.F., Gram L., Hoffmann S., Schaebel S. Shewanella alga bacteremia in two patients with lower leg ulcers. Clin Infect Dis. 1996;22:1036–1039. doi: 10.1093/clinids/22.6.1036. [DOI] [PubMed] [Google Scholar]
- 23.Di Renzo L., Di Francesco G., Profico C., Di Francesco C.E., Ferri N., Averaimo D. Vibrio parahaemolyticus– and V. alginolyticus–associated meningo-encephalitis in a bottlenose dolphin (Tursiops truncatus) from the Adriatic coast of Italy. Res Vet Sci. 2017;115:363–365. doi: 10.1016/j.rvsc.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Goyal R., Kaur N., Thakur R. Human soft tissue infection by the emerging pathogen Shewanella algae. J Infect Dev Ctries. 2011;5:310–312. doi: 10.3855/jidc.1436. [DOI] [PubMed] [Google Scholar]
- 25.Tang T.H., Cheng N.H., Ho R.T., Chan H.S., Lam K.W., Xavier J. Shewanella-related bacteremia and Fournier’s gangrene: a case report. Open Forum Infect Dis. 2016;3:ofw148. doi: 10.1093/ofid/ofw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hau H.H., Gralnick J.A. Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol. 2007;61:237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- 27.Brink A.J., Van Straten A., Van Rensburg A.J. Shewanella (Pseudomonas) putrefaciens bacteriemia. Clin Infec Dis. 1995;20:1327–1332. doi: 10.1093/clinids/20.5.1327. [DOI] [PubMed] [Google Scholar]
- 28.To K.K., Wong S.S., Cheng V.C., Tang B.S., Li I.W., Chan J.F. Epidemiology and clinical features of Shewanella infection over an eight-year period. Scand J Infect Dis. 2010;42:757–762. doi: 10.3109/00365548.2010.490562. [DOI] [PubMed] [Google Scholar]
- 29.Otsuka T., Noda T., Noguchi A., Nakamura H., Ibaraki K., Yamaoka K. Shewanella infection in decompensated liver disease: a septic case. J Clin Gastroenterol. 2007;42:87–90. doi: 10.1007/s00535-006-1957-0. [DOI] [PubMed] [Google Scholar]
- 30.Tsai M.S., You H.L., Tang Y.F., Liu J.W. Shewanella soft tissue infection: case report and literature review. Int J Infect Dis. 2008;12:e119–e124. doi: 10.1016/j.ijid.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Jorens P.G., Goovaerts K., Ieven M. Shewanella putrefaciens isolated in a case of ventilator-associated pneumonia. Respiration. 2004;71:199–201. doi: 10.1159/000076686. [DOI] [PubMed] [Google Scholar]
- 32.Kim D.M., Kang C.L., Lee C.S., Kim H.B., Kim E.C., Kim N.J. Treatment failure due to emergence of resistence to carbapenem during therapy for Shewanella algae bacteremia. J Clin Microbiol. 2006;44:1172–1174. doi: 10.1128/JCM.44.3.1172-1174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.