Abstract
Cholangiocarcinoma (CCA) is one of the most common hepatic and biliary malignancies, accounting for about 3% of all gastrointestinal tumors. GATA5 is a transcription factor capable of suppressing the development of various human cancer types. Transcriptional inactivation and CpG island (CGI) methylation of GATA3 and GATA5, two members of the GATA family of transcription factors, have been observed in some human cancers. But whether high-density CGI methylation of GATA5 is associated with the clinical course of CCA patients has not been clarified. Herein, we observed reduced expression of GATA5 in CCA tissues compared with noncancerous tissues. Treatment with the demethylating agent 5-aza-2'-deoxycytidine restored GATA5 expression in CCA cell lines. Furthermore, GATA5 expression was downregulated after treatment with IL-6 in human intrahepatic biliary epithelial cells. Upregulated GATA5 inhibited CCA cell growth and metastasis. Mechanistically, GATA5 suppressed CCA cell growth and metastasis via Wnt/β-catenin pathway. Specific β-catenin inhibitor or siRNA abolished the discrepancy of the proliferation and metastasis capacity between GATA5-overexpression CCA cells and their control cells, which further confirmed that Wnt/β-catenin was required in GATA5-inhibited CCA cell growth and metastasis.
Introduction
Cholangiocarcinoma (CCA) is a prevalent bile duct malignancy with poor prognosis [1], [2]. According to the anatomic location, CCA is classified into three types: intrahepatic CCA, hilar CCA, and extrahepatic CCA [3]. The mean 5-year survival rate for CCA is estimated to be less than 5% if the cancer is detected in the late stage [4]. Surgical resection is the mainstay of treatment at present but with unsatisfactory outcomes due to high recurrence and metastasis [5], [6]. Therefore, clarification of the underlying mechanism and development of optional therapeutic targets for the sake of early detection and treatment are of great importance in reducing the disease-specific mortality.
The GATA gene family is composed of zinc finger transcription factors capable of binding to the GATA motif present in the promoters of certain genes [7]. GATA1, GATA2, and GATA3 are known to play important roles in cellular lineage determination [8], while GATA4, GATA5, and GATA6 are believed to be involved in the development of endoderm-derived organs such as the heart and gut [9]. In early embryonic development, GATA5 helps produce sufficient cardiac muscle precursor cells to differentiate into the final myocardial cells. In addition, it regulates other genes necessary for successful heart development [10]. In adults, GATA5 regulates epithelial cell differentiation [11]. However, altered expression of GATA5 protein was reported to be associated with tumorigenesis in gastric and colon cancers, suggesting that GATA5 may function as a putative tumor suppressor gene [12], [13].
Recent studies showed that aberrant DNA methylation is one of the most remarkable characteristics of malignant cells [14]. CpG island (CGI) is a genomic region containing a high frequency of cytosine-guanine sites. Its hypermethylation in gene promoters is believed to be an alternative mechanism underlying transcriptional silencing of critical genes involved in carcinogenesis-related processes [15]. Hypermethylation of CpG sites in the promoter region of certain TSGs may worsen the prognosis and increase the tumor-node-metastasis stage and metastasis in CCA patients [16].A previous study revealed that GATA5 expression was lost in ovarian and gastric cancers and that the chromosomal region of GATA5 (20q13.2-q13.3) locus was frequently deleted in various types of human cancer [17]. Additional studies showed that promoter methylation contributed to the loss of GATA5 expression during the progression of pancreatic, non–small cell lung, esophageal, and renal cancers, which in turn altered the typical expression patterns of numerous downstream gene networks with antineoplastic properties [18], [19], [20]. However, the function of GATA5 in CCA is poorly understood.
In this study, we identified GATA5 as a tumor suppressor that was epigenetically silenced in CCA tissues. In addition, GATA5 downregulation was associated with decreased survival time of CCA patients. We also characterized Wnt/β-catenin as the downstream pathway of GATA5. Our results highlight the important role of GATA5 in inhibiting the proliferation and metastasis of CCA.
Materials and Methods
Patients and Samples
Tissue samples were collected from 152 CCA patients admitted in the Department of Hepatobiliary surgery, Navy General Hospital (Beijing, China). The time between surgery and death was defined as overall survival, the time between surgery and recurrence was defined disease-free survival, and non–HCC-related death was scored as recurrence. If recurrence was not diagnosed, patients were censored on the date of the last follow-up. The procedure of human sample collection was approved by the Ethic Committee of Department of Hepatobiliary Surgery, Navy General Hospital.
Cell Lines and Cell Culture
TFK-1 and HuCCT-1 cells were cultured with Dulbecco's modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM L-glutamine, and 25 μg/ml of gentamicin and maintained at 37°C in a 5% CO2 incubator. Normal biliary epithelial cells (HIBEpic) were grown in RPMI 1640 (Gibco, Carlsbad, CA) medium supplemented with 10% FBS (Gibco, Carlsbad, CA) and 1% penicillin/streptomycin (Beijing Solarbio Science & Technology Co., Beijing, China) at 37°C and 5% CO2. The culture was dissociated with 0.5% trypsin and moved to a new six-well plate twice a week. The lentivector expressing GATA5 or control was generated as described previously [21]. TFK-1 or HuCCT1 GATA5 and their control stable cell lines were established using lentivirus infection.
Cell Migration Assays
For cell migration experiments, 2×105 cells were seeded into the upper chamber of a polycarbonate Transwell in serum-free DMEM. The lower chamber was added with DMEM containing 20% FBS as chemoattractant. The cells were incubated for 12 hours, and the chamber was fixed. Cell count is expressed as the average number of the cells in each field.
Cell Invasion Assays
For cell invasion experiments, 2×105 cells were seeded into the upper chamber of a polycarbonate Transwell in serum-free DMEM. The lower chamber was added with DMEM containing 20% FBS as chemoattractant. The cells were incubated for 36 hours, and the chamber was fixed. Cell count is expressed as the average number of the cells in each field.
Real-Time Polymerase Chain Reaction (PCR)
The cell RNA was extracted by using Trizol reagent (Invitrogen, 15596-018) as described previously [22]. Total cDNAs were synthesized by ThermoScript RT-PCR system (Invitrogen, 11146-057). The GAT5 primer sequences were forward: 5′-TGCAGTCATCACAGACTTAC-3′, reverse: 5′-CACAGATTCAGCTAGAACAA-3′.
Western Blotting Assays
The cells were collected with cell lysis buffer and then disposed as described previously [23]. Twenty protein micrograms of the cell extracts was subjected to Western Blot with one of the antibodies against GATA5, β-catenin, and GAPDH (Cell Signaling Technology, Danvers, MA).
Luciferase Reporter Assays
CCa cells were transfected with β-catenin promoter luciferase reporter in combination with the pRL-TK vector (Promega, E2241, Madison, WI) as an internal control. The dual luciferase assay kit was purchased from Promega (0000060417). The luciferase activities were determined using a luminometer (Wallac 1420 Victor 2 multilabel counter system) as described in previous studies [24].
Transient Transfection of Small Interference RNA
CCA cells were seeded into a six-well plate until they reached 80% to 90% confluence. Transfection of si-β-catenin or its negative control was performed in each well in the absence of serum with small interfering RNA transfection reagent according to the manufacturer’s instructions (Polyplus, Illkirch, France). The sequence of si-β-catenin is as follows: 5′-GUGCUAUCUGUCUGCUCUA-3′.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Statistical analysis was carried out using t test or Bonferroni multiple-comparisons test: *P<.05. A P value less than .05 was considered statistically significant.
Results
The Expression of GATA5 Is Reduced in Human CCA Tissues
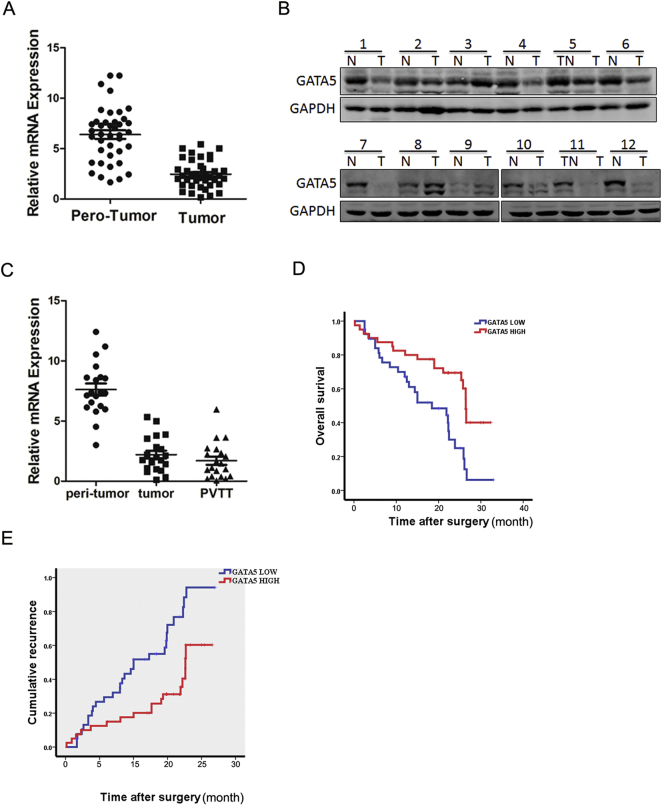
To explore the role of GATA5 in CCA progression, the expression of GATA5 in the human CCA tissues was detected. As shown in Figure 1A, GATA5 mRNA expression was markedly decreased in 77.5% CCA cases (31/40) compared with that in the paired nontumorous tissues, with higher GATA5 transcripts observed in 22.5% patients. Moreover, Western bolt assays also observed downregulation of GATA5 expression in the tumor tissues and upregulation in the surrounding normal tissues (Figure 1B). CCA patients always had a high rate of metastasis, and the metastatic focus was a prognostic factor of poor prognosis of the patients. As expected, GATA5 transcripts were observably increased in the metastatic foci compared with the primary CCA tissues, indicating that GATA5 played an important role in CCA metastasis (Figure 1C).
Figure 1.
Expression of GATA5 in human CCA tissues.
(A) Real-time PCR analysis of GATA5 expression in 40 pairs of CCA (T) and paratumor normal tissues (N).
(B) Western blot assay of GATA5 expression in 12 pairs of CCA (T) and paratumor normal tissues (N).
(C) Comparison of GATA5 transcripts in paired paratumor normal and CCA tissues, and metastasis foci using real-time PCR (n=20).
(D & E). RT-PCR and the expression of GATA5 were scored in 80 human CCA samples. Overall survival (P=.032) and disease-free survival (P=.041) after surgery were compared between “low-GATA5 “ group (n=40) and “high-GATA5” group (n=40), P<.05.
According to the GATA5 mRNA level, the 80 CCA patients were equally divided into a “high-GATA5” group and a “low-GATA5” group. The survival time in “low-GATA5” group was shorter than that in “high-GATA5” group (Figure 1, D and E). Collectively, GATA5 could be a good prognostic factor for CCA patients.
GATA5 Gene Is Methylated in CCA
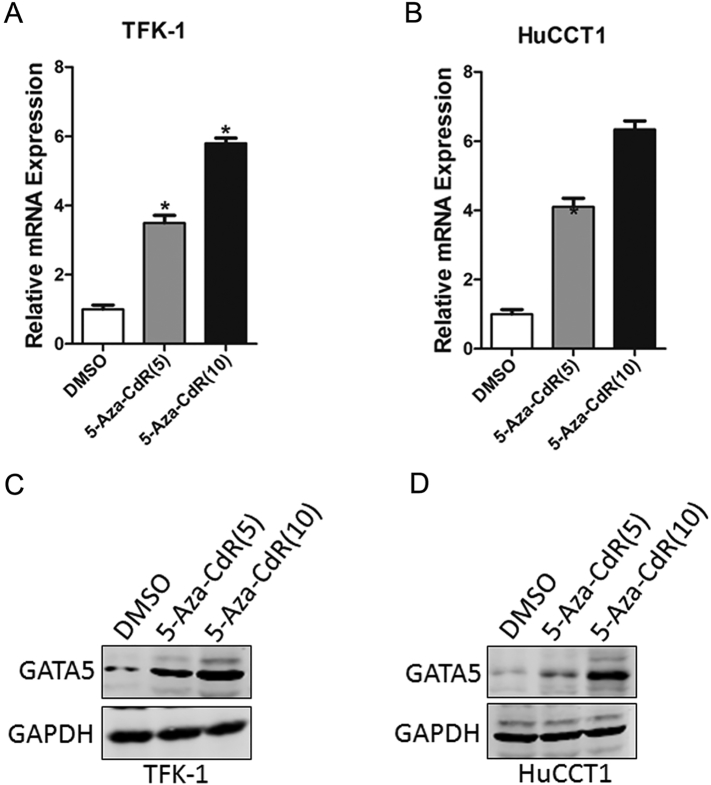
Knowing that aberrant hypermethylation of CGI in the 5′ regulatory region is a major cause of tumor suppressor silencing in cancer, TFK-1 and HuCCT1 cells with relative low levels of GATA5 expression were treated with different concentrations of DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine (5-Aza-dC) to determine whether GATA5 downregulation was associated with DNA methylation in CCA. Treatment of CCA cells with 5-Aza-dC led to a dose-dependent restoration of both GATA5 mRNA and protein expressions, suggesting that there was a negative correlation between DNA methylation and GATA5 expression (Figure 2).
Figure 2.
Aberrant CpG methylations associated with downregulation of GATA5.
(A) TFK-1 cells were treated with 5-Aza-dC (μM), and GATA5 mRNA level was evaluated by RT-PCR.
(B) HuCCT-1 cells were treated with 5-Aza-dC (μM), and GATA5 mRNA level was measured by RT-PCR.
(C) TFK-1 cells were treated with 5-Aza-dC (μM), and GATA5 protein level was evaluated by RT-PCR.
(D) HuCCT-1 cells were treated with 5-Aza-dC (μM), and GATA5 protein level was measured by RT-PCR.
IL-6 Is Required for GATA5 Downregulation in CCA Cells
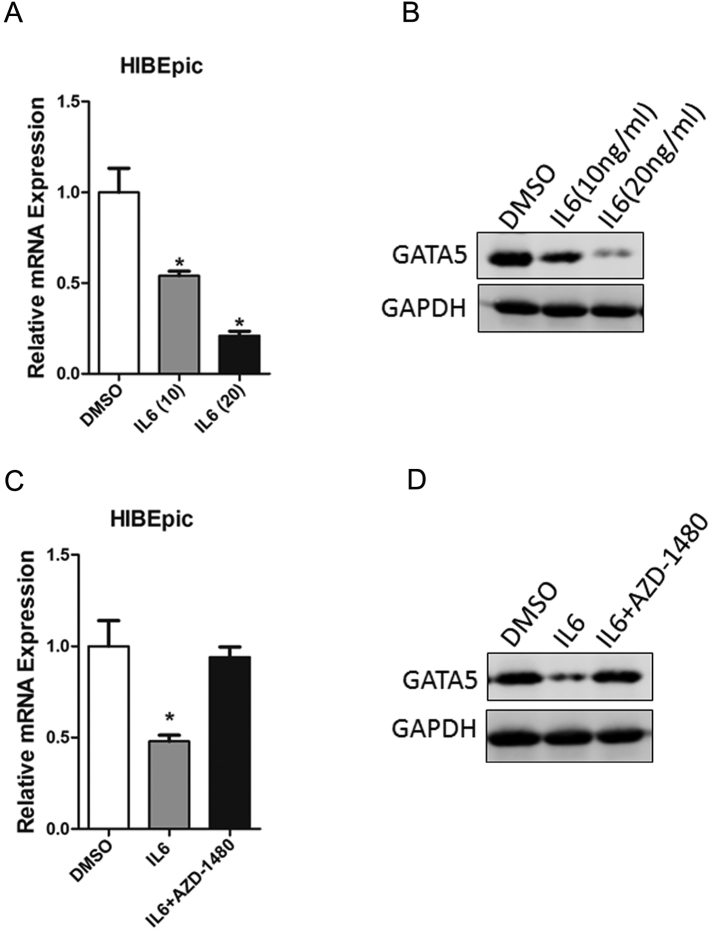
Knowing that chronic inflammatory stress in the biliary ducts may promote tumorigenesis by inducing aberrant DNA methylation in cultured cells and that IL-6 is a proinflammatory cytokine that mediates chronic inflammation and may play an important role in inflammation-driven CCA, we determined whether IL-6 was involved in GATA5 downregulation in CCA cells. As expected, GATA5 expression was significantly decreased after treatment of human intrahepatic biliary epithelial cells (HIBEpic) with various concentrations of IL-6 (Figure 3, A and B). Moreover, IL-6–triggered GATA5 downregulation was reversed by the JAK2/STAT3 inhibitor AZD-1480 (Figure 3, C and D), suggesting that IL-6/STAT3 signaling could decrease GATA5 expression in HIBEpic.
Figure 3.
IL-6 was required for GATA5 down regulation.
(A) HIBEpic cells were treated with IL-6 (ng/ml), and GATA5 mRNA level was evaluated by RT-PCR.
(B) HIBEpic cells were treated with IL-6 (ng/ml), and GATA5 protein level was checked by RT-PCR.
(C) HIBEpic cells were pretreated with AZD1480 (10 μM) for 30 minutes and then stimulated with IL-6 (10 ng/ml) for an additional 48 hours followed by real-time PCR analysis.
(D) HIBEpic cells were pretreated with AZD1480 (10 μM) for 30 minutes and then stimulated with IL-6 (10 ng/ml) for an additional 48 hours followed by Western blot analysis.
Overexpression of GATA5 Suppresses CCA Cell Proliferation and Metastasis
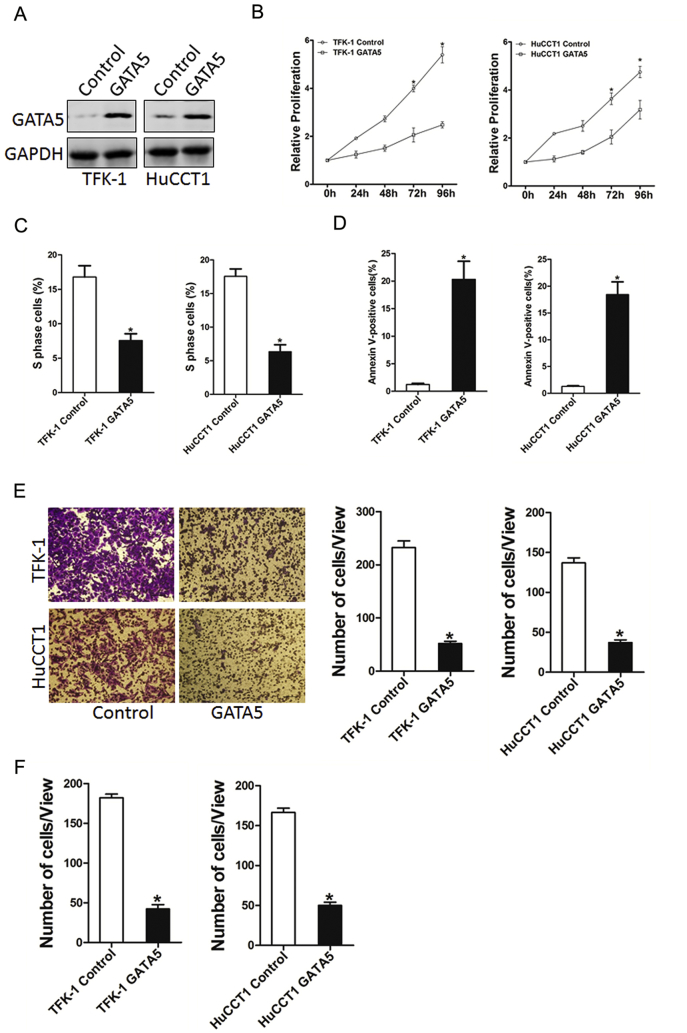
To further elucidate the role of GATA5 in CCA cells growth and metastasis, we stably overexpressed GATA5 in TFK-1 and HuCCT-1 cells. Immunoblotting revealed that GATA5 was overexpressed in TFK-1 and HuCCT-1 cells (Figure 4A). As expected, GATA5 overexpression inhibited the proliferation of CCA cells markedly (Figure 4, B and C). In addition, GATA5 overexpression induced CCA cell apoptosis (Figure 4D). Moreover, Transwell assay showed that the migration ability was impaired in GATA5-overexpressed CCA cells (Figure 4E). Consistently, the invasion capacity was also destroyed in GATA5-overexpressed CCA cells (Figure 4F). Collectively, our results demonstrate that GATA5 suppressed the growth and metastatic potential of CCA cells.
Figure 4.
GATA5 overexpression suppresses CCA cell proliferation and metastasis.
(A) TFK-1 and HuCCT1 cells were infected with GATA5 overexpression lentivirus or vector virus, and the stable transfectants were determined by Western bolt assay.
(B) Cell proliferation was measured using CCK-8 assays in TFK-1 or HuCCT1 cells stably overexpressing GATA5.
(C) Cell cycle was assessed by flow cytometry in TFK-1 or HuCCT1 cells stably overexpressing GATA5.
(D) Cell apoptosis was detected by flow cytometry in TFK-1 or HuCCT1 cells stably overexpressing GATA5.
(E) Migration assay was performed utilizing polycarbonate membrane inserts in a 24-well plate.
(F) The invasive properties of TFK-1 GATA5 or HuCCT1 GATA5 and their control cells were analyzed using Matrigel-coated Boyden chamber.
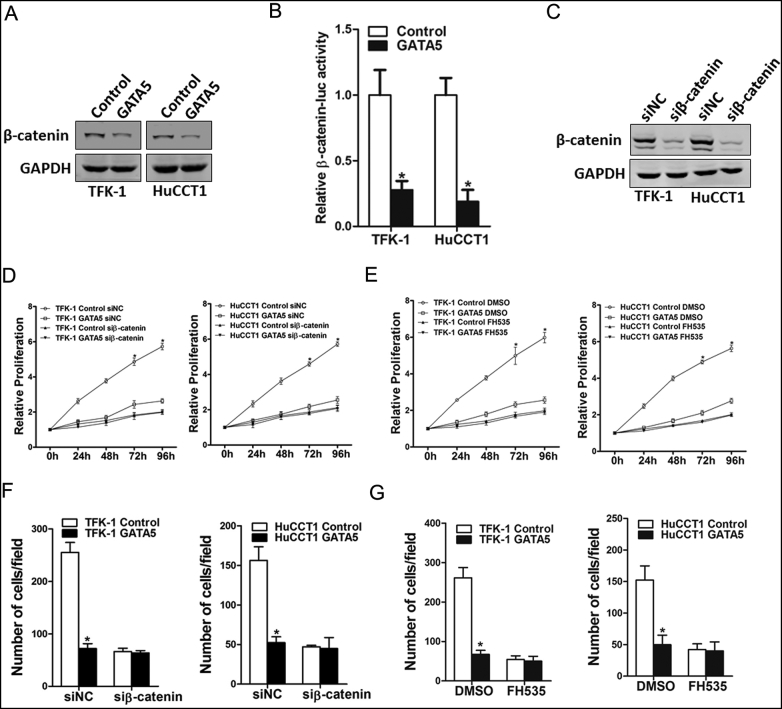
GATA5 Inhibits CCA Cell Progression via Wnt/β-Catenin Cascades
Ample evidence shows that Wnt/β-catenin signaling plays a pivotal role in cell growth, metastasis, and apoptosis [25]. Our data showed that β-catenin molecule was apparently inactivated in both TFK-1 and HuCCT-1 GATA5 cells (Figure 5A). β-Catenin reporter assay further confirmed the effect of GATA5 on β-catenin activation (Figure 5B). More importantly, blockage of β-catenin by specific β-catenin inhibitor or siRNA diminished the distinct growth capacity between GATA5 overexpression CCA cells and control cells (Figure 5, C-E) and eliminated the discrepancy of metastasis between GATA5 overexpression CCA cells and control cells (Figure 5, F and G), suggesting that GATA5 suppressed CCA cell progression by inhibiting Wnt/β-catenin signaling.
Figure 5.
GATA5 suppresses CCA cell proliferation and metastasis through Wnt/β-catenin pathway.
(A). The protein status of β-catenin in TFK-1 or HuCCT1 GATA5 and their control cells was checked by Western bolt assay.
(B). Luciferase activity in the lysates of TFK-1 or HuCCT1 cells transfected with β-catenin response element-luciferase reporter plasmid (β-catenin-luc) was measured (normalized by Renilla luciferase activity).
(C) TFK-1 or HuCCT1 cells were transfected with si-β-catenin or negative control, followed by Western bolt assay.
(D) TFK-1 or HuCCT1 GATA5 and their control cells were transfected with si-β-catenin or negative control, respectively, followed by CCK8 assay.
(E) The proliferation of TFK-1 or HuCCT1 GATA5 and their control cells in the presence of FH535 (40 μM) or DMSO, respectively, was measured using the CCK8 assay.
(F) TFK-1 or HuCCT1 GATA5 and their control cells were transfected with si-β-catenin or negative control, respectively, followed by migration assay.
(G). Migration assay was performed in TFK-1 or HuCCT1 GATA5 and their control cells with FH535 (40 μM) or DMSO treatment.
Discussion
Cholangiocarcinoma is a bile duct cancer composed of mutated epithelial cells originating in the bile ducts that drain bile from the liver into the small intestine [26]. CCA is a rapidly lethal cancer and is usually incurable unless the primary tumor and any metastasis are removed completely by surgery [27]. However, most CCA cases were detected in the advanced stage and therefore inoperable at the time of diagnosis [28]. One of the reasons for failure to detect CCA in the early stage is the lack of biomarkers to indicate the initiation and progression of the disease [29], and therefore, it is urgent to identify novel biomarkers to improve early diagnosis and explore therapeutic targets for CCA patients [30]. In the present study, we for the first time identified that reduced expression of GATA5 correlated well with the poor prognosis of CCA patients, suggesting that GATA5 might be a novel prognostic biomarker for CCA patients.
GATA5 is a gene involved in cell and tissue differentiation and gastrointestinal development. Being a lineage-restricted transcription factor, GATA5 is usually expressed in the stem cell compartment in the gut and is upregulated when these cells undergo terminal differentiation [31]. Previous studies demonstrated that loss of GATA4 and GATA5 expression was due to gene promoter methylation in gastric, ovarian, lung, esophageal, and pancreatic cancers [32]. Nevertheless, the role of GATA5 in CCA remains unclear. In the current study, it was demonstrated that the methylation of GATA5 promoters was in accordance with previous studies. Consistently, we found that GATA5 gene overexpression suppressed CCA proliferation and metastasis in vitro.
It was hypothesized that chronic inflammatory stress in the biliary ducts may promote tumorigenesis by inducing aberrant DNA methylation in cultured cells. In the present study, we performed a systematic evaluation to verify the hypothesis for the sake of improving our current understanding about the molecular mechanisms operative in the biliary ducts, hoping that it could provide additional insights into the “epigenetic field effect,” which is believed to play an important role in the genesis of certain human cancers [33]. To simulate a model of chronic inflammation, we used IL-6, an activator of the OSCC-associated STAT3 cascade, to simulate inflammation in a panel of CCA cell lines. Our data showed that GATA5 expression could be upregulated by IL6 stimulation in HIBEpic.
Abnormal activation of Wnt/β-catenin signaling pathway was observed in most CCA cases and some neoplasia lesions [34]. This abnormal activation is believed to trigger or contribute to carcinogenesis by regulating the expression of large numbers of genes in tumor cells [35]. Although aberrant activation of Wnt/β-catenin signaling is known to play an unequivocal role in numerous cancers, it is a tremendous challenge to identify effective Wnt inhibitors for clinical use in cancers [36]. It was found in this study that GATA5 played a negative role in CCA cells by inhibiting CCA proliferation and metastasis through deactivating Wnt/β-catenin signaling. In addition, these effects could be attenuated by specific β-catenin siRNA or inhibitor FH535.
Here, we first showed that GATA5 was downregulated in CCA tissues, which in turn inhibited the growth and metastasis of CCA cells, accompanied by dysregulation of Wnt/β-catenin signaling in vitro. These findings not only shed a new light on the mechanism of CCA progression but suggest a potential prognostic marker and a possible therapeutic target against CCA.
Competing Interests
All authors declare no competing interests.
Acknowledgements
This work was supported by grand from the Navy General Hospital of Cultivate Innovation Fund (CXPY201512).
References
- 1.Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 2.Mu X, Pradere JP, Affò S, Dapito DH, Friedman R, Lefkovitch JH, Schwabe RF. Epithelial transforming growth factor-beta signaling does not contribute to liver fibrosis but protects mice from cholangiocarcinoma. Gastroenterology. 2016;150(3):720–733. doi: 10.1053/j.gastro.2015.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyson GL, Ilyas JA, Duan Z, Green LK, Younes M, El-Serag HB, Davila JA. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci. 2014;59(12):3103–3110. doi: 10.1007/s10620-014-3276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan D, Huang S, Berger E, Liu L, Gross N, Heinzmann F, Ringelhan M, TO Connor, Stadler M, Meister M. Kupffer cell-derived Tnf Triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell. 2017;31(6):771–789. doi: 10.1016/j.ccell.2017.05.006. [e6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee GW, Kang JH, Kim HG, Lee JS, Lee JS, Jang JS. Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol. 2006;29(2):127–131. doi: 10.1097/01.coc.0000203742.22828.bb. [DOI] [PubMed] [Google Scholar]
- 6.Lafaro KJ, Cosgrove D, Geschwind JFH, Kamel I, Herman JM, Pawlik TM. Multidisciplinary care of patients with intrahepatic cholangiocarcinoma: updates in management. Gastroenterol Res Pract. 2015;2015:860861. doi: 10.1155/2015/860861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275(50):38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 8.Patient PK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12(4):416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 9.Laverriere AC, MacNeill C, Mueller C, Poelmann RE, Burch JB, Evans T. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem. 1994;269(37):23177–23184. [PubMed] [Google Scholar]
- 10.Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki J, Niwa H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16(7):784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X, Sedgwick T, Shi YB, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol. 1998;18(5):2901–2911. doi: 10.1128/mcb.18.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellebrekers DM, Lentjes MH, Bosch SM, Melotte V, Wouters KA, Daenen K, Smits KM, Akiyama Y, Yuasa Y, Sanduleanu S. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res. 2009;15(12):3990–3997. doi: 10.1158/1078-0432.CCR-09-0055. [DOI] [PubMed] [Google Scholar]
- 13.Sobota RS, Kodaman N, Mera R, Piazuelo MB, Bravo LE, Pazos A, Zabaleta J, Delgado AG, El-Rifai W, Morgan DR. Epigenetic and genetic variation in GATA5 is associated with gastric disease risk. Hum Genet. 2016;135(8):895–906. doi: 10.1007/s00439-016-1687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Y, Zou YM, Lei D, Huang Y, Li W, Mo Z, Hu Y. Promoter DNA methylation analysis reveals a novel diagnostic CpG-based biomarker and RAB25 hypermethylation in clear cell renel cell carcinoma. Sci Rep. 2017;7(1):14200. doi: 10.1038/s41598-017-14314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessaman JD, Selker EU. Induction of H3K9me3 and DNA methylation by tethered heterochromatin factors in Neurospora crassa. Proc Natl Acad Sci U S A. 2017;114(45):E9598–E9607. doi: 10.1073/pnas.1715049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AW, Ng LM. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mžik M, Chmelařová M, John S, Laco J, Slabý O, Kiss I, Bohovicová L, Palička V, Nekvindová J. Aberrant methylation of tumour suppressor genes WT1, GATA5 and PAX5 in hepatocellular carcinoma. Clin Chem Lab Med. 2016;54(12):1971–1980. doi: 10.1515/cclm-2015-1198. [DOI] [PubMed] [Google Scholar]
- 18.Peters I, Gebauer K, Dubrowinskaja N, Atschekzei F, Kramer MW, Hennenlotter J, Tezval H, Abbas M, Scherer R, Merseburger AS. GATA5 CpG island hypermethylation is an independent predictor for poor clinical outcome in renal cell carcinoma. Oncol Rep. 2014;31(4):1523–1530. doi: 10.3892/or.2014.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo MZ, House MG, Akiyama Y, Qi Y, Capagna D, Harmon J, Baylin SB, Brock MV, Herman JG. Hypermethylation of the GATA gene family in esophageal cancer. Int J Cancer. 2006;119(9):2078–2083. doi: 10.1002/ijc.22092. [DOI] [PubMed] [Google Scholar]
- 20.Wen XZ, Akiyama Y, Pan KF, Liu ZJ, Lu ZM, Zhou J, Gu LK, Dong CX, Zhu BD, Ji JF. Methylation of GATA-4 and GATA-5 and development of sporadic gastric carcinomas. World J Gastroenterol. 2010;16(10):1201–1208. doi: 10.3748/wjg.v16.i10.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang RY, Chen L, Chen HY, Hu L, Li L, Sun HY, Jiang F, Zhao J, Liu GMY, Tang J. MUC15 inhibits dimerization of EGFR and PI3K-AKT signaling and is associated with aggressive hepatocellular carcinomas in patients. Gastroenterology. 2013;145(6):1436–1448. doi: 10.1053/j.gastro.2013.08.009. [e1-12] [DOI] [PubMed] [Google Scholar]
- 22.Li XF, Chen C, Xiang DM, Qu L, Sun W, Lu XY, Zhou TF, Chen SZ, Ning BF, Cheng Z. Chronic inflammation-elicited liver progenitor cell conversion to liver cancer stem cell with clinical significance. Hepatology. 2017;66(6) doi: 10.1002/hep.29372. [DOI] [PubMed] [Google Scholar]
- 23.Han T, Xiang DM, Sun W, Liu N, Sun HL, Wen W, Shen WF, Wang RY, Chen C, Wang X. PTPN11/Shp2 overexpression enhances liver cancer progression and predicts poor prognosis of patients. J Hepatol. 2015;63(3):651–660. doi: 10.1016/j.jhep.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Xiang DM, Sun W, Ning BF, Zhou TF, Li XF, Zhong W, Cheng Z, Xia MY, Wang X, Deng X. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2017 doi: 10.1136/gutjnl-2016-313392. [[Epub ahead of print] IF=16.6] [DOI] [PubMed] [Google Scholar]
- 25.Xiang D, Cheng Z, Liu H, Wang X, Han T, Sun W, Li XF, Yang W, Chen C, Xia MY. Shp2 promotes liver cancer stem cell expansion by augmenting beta-catenin signaling and predicts chemotherapeutic response of patients. Hepatology. 2017;65(5):1566–1580. doi: 10.1002/hep.28919. [DOI] [PubMed] [Google Scholar]
- 26.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WMC, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl. 6):VI1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XF, Tang K, Sui LL, Xu G. Cholangiocarcinoma: present status and molecular aspects of diagnosis. Oncol Res. 2014;22(4):177–183. doi: 10.3727/096504015X14343704124386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao D, Kunam VK, Li X. A review of the clinical diagnosis and therapy of cholangiocarcinoma. J Int Med Res. 2014;42(1):3–16. doi: 10.1177/0300060513505488. [DOI] [PubMed] [Google Scholar]
- 29.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zender S, Nickeleit I, Wuestefeld T, Sörensen I, Dauch D, Bozko P, El-Khatib M, Geffers R, Bektas H, Manns MP. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2016;30(2):353–356. doi: 10.1016/j.ccell.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Messaoudi S, He Y, Gutsol A, Wight A, Hébert RL, Vilmundarson RO, Makrigiannis AP, Chalmers J, Hamet P, Tremblay J. Endothelial Gata5 transcription factor regulates blood pressure. Nat Commun. 2015;6:8835. doi: 10.1038/ncomms9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujiwara Y, Emi M, Ohata H, Kato Y, Nakajima T, Mori T, Nakamura Y. Evidence for the presence of two tumor suppressor genes on chromosome 8p for colorectal carcinoma. Cancer Res. 1993;53(5):1172–1174. [PubMed] [Google Scholar]
- 33.Gasche JA, Hoffmann J, Boland CR, Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer. 2011;129(5):1053–1063. doi: 10.1002/ijc.25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Wan M, Xu Y, Li Z, Leng K, Kang P, Cuia Y, Jianga X. Long noncoding RNA PCAT1 regulates extrahepatic cholangiocarcinoma progression via the Wnt/beta-catenin-signaling pathway. Biomed Pharmacother. 2017;94:55–62. doi: 10.1016/j.biopha.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Larriba MJ, González-Sancho JM, Barbáchano A, Niell N, Ferrer-Mayorga G, Muñoz A. Vitamin D is a multilevel repressor of Wnt/b-catenin signaling in cancer cells. Cancers (Basel) 2013;5(4):1242–1260. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao C, Chen G, Kuan SF, Zhang DH, Schlaepfer DD, Hu Jing. FAK/PYK2 promotes the Wnt/beta-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3beta. Elife. 2015;4(15 Supplement) doi: 10.7554/eLife.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]